Abstract
The interaction between gravitropism and phototropism was analyzed for sporangiophores of Phycomyces blakesleeanus. Fluence rate-response curves for phototropism were generated under three different conditions: (a) for stationary sporangiophores, which reached photogravitropic equilibrium; (b) for sporangiophores, which were clinostated head-over during phototropic stimulation; and (c) for sporangiophores, which were subjected to centrifugal accelerations of 2.3g to 8.4g. For blue light (454 nm), clinostating caused an increase of the slope of the fluence rate-response curves and an increase of the maximal bending angles at saturating fluence rates. The absolute threshold remained, however, practically unaffected. In contrast to the results obtained with blue light, no increase of the slope of the fluence rate-response curves was obtained with near-ultraviolet light at 369 nm. Bilateral irradiation with near-ultraviolet or blue light enhanced gravitropism, whereas symmetric gravitropic stimulation caused a partial suppression of phototropism. Gravitropism and phototropism appear to be tightly linked by a tonic feedback loop that allows the respective transduction chains a mutual influence over each other. The use of tropism mutants allowed conclusions to be drawn about the tonic feedback loop with the gravitropic and phototropic transduction chains. The results from clinostating mutants that lack octahedral crystals (implicated as statoliths) showed that these crystals are not involved in the tonic feedback loop. At elevated centrifugal accelerations, the fluence-rate-response curves for photogravitropic equilibrium were displaced to higher fluence rates and the slope decreased. The results indicate that light transduction possesses a logarithmic transducer, whereas gravi-transduction uses a linear one.
Light and the earth's gravitational field represent, for plants and fungi, the most important environmental cues for development and orientation in space (Braun, 1997; Hangarter, 1997; Kern et al., 1997; Sack, 1997). In the case of grass coleoptiles, shoots, and the Phycomyces blakesleeanus sporangiophore, the gravitropic response is negative, i.e. the organisms bend away from the source of the earth's gravitational field. In contrast, phototropism is generally positive, i.e. the bending occurs in the direction of the unilateral light. During phototropic bending an organism is also subjected to a gravitropic stimulation, and therefore phototropic organisms must be able to evaluate the two conflicting stimuli and find a compromise between them. One obvious way to assess the effect of gravity on phototropism is the elimination of the gravitropic stimulus either in outer space or by the use of a clinostat.
Shen-Miller and Gordon (1967) used a clinostat rotating about a horizontal axis to investigate the effect of gravity on the phototropism of Avena sativa coleoptiles. Pulse-induced phototropism of grass coleoptiles is characterized by the well-known fluence-response curves with their characteristic first, second, and third positive curvatures (Briggs, 1960; Blaauw and Blaauw-Jansen, 1970a, 1970b). Horizontal clinostating of oat coleoptiles, after the light stimulus, increased the maximum of the first positive response, which was attributed by the authors to the removal of the counteracting gravitropic stimulus and to alterations in the production, transport of auxin, and the tissue sensitivity to auxin (Shen-Miller and Gordon, 1967).
With continuous horizontal unilateral light acting over long periods of time, fluence rate-response curves for photogravitropic equilibrium are obtained that are less complex than the time-dependent fluence-response curves mentioned above. In the investigation of P. blakesleeanus phototropism, such equilibrium curves have been generated extensively in the study of action spectroscopy (e.g. Varjú et al., 1961; Galland, 1983; Galland and Lipson, 1985a, 1985b; Campuzano et al., 1996). These curves can be fitted to simple hyperbolic functions and are characterized by wavelength-dependent thresholds (about 10−9 W m−2 near 450 nm), wavelength-dependent slopes, and, at saturating fluence rates, maximal bending angles of about 70° from the vertical. During photogravitropic equilibrium, the sporangiophore reacts to two stimuli: the unilateral light and the unilateral gravitropic stimulus. The two stimuli guide the sporangiophore to two different directions. The absolute threshold and the slope of the fluence rate-response curves of photogravitropic equilibrium will therefore depend on the nature of the interaction between the light and the gravitropic stimulus, which is, however, not well understood.
One needs to distinguish between two different types of interactions: vectorial interactions and tonic interactions. During unilateral irradiation, the positive phototropism and the negative gravitropism add up vectorially in accordance to their subjective strength and thus constitute an example for vectorial interaction. Superimposed on this vectorial interaction is also a tonic interaction. A tonic effect of light manifests as a modification of the graviresponse where it is irrelevant whether the light is given symmetrically or asymmetrically with respect to the irradiated organism. Blue light below 3 × 10−3 W m−2, for example, enhances the negative gravitropism of the sporangiophore of P. blakesleeanus (Dennison, 1964). In the moss Ceratodon purpureus, blue light changes the negative gravitropism of protonemata to positive gravitropism (Lamparter et al., 1998), whereas red light mediated by the photoreceptor phytochrome inhibits gravitropism (Kern and Sack, 1999). Red light-regulated gravitropism occurs in roots of maize (Lu et al., 1996). Evidence for a tonic effect of gravistimulation on phototropism has been unknown so far, but will be shown in this work.
To characterize the interplay between gravitropism and phototropism, we measured fluence rate-response curves for phototropism under conditions where the gravitropic stimulus was neutralized by clinostating the sporangiophores while they were exposed to the unilateral light. In addition we increased the gravitropic stimulus by subjecting sporangiophores on a centrifuge to accelerations exceeding 1g and generated fluence-response curves of phototropism under these conditions.
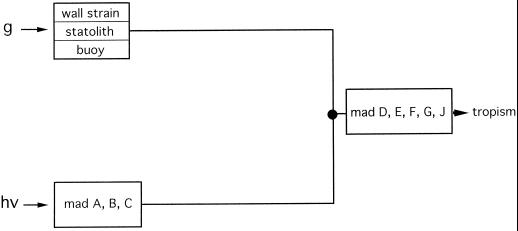
To investigate the mode of interaction between gravitropism and phototropism more specifically, we made use of tropism mutants that show either enhanced or reduced gravitropism. The gravitropic and phototropic transduction chains possess different inputs but share elements in common at the output, which comprise the gene products of the genes madD, E, F, G, and H (Fig. 1). A notable feature of the gravitropism of P. blakesleeanus is the fact that it reacts to different gravitropic stimuli. These are the wall strain (bending stress or flexure) in horizontal sporangiophores (Dennison, 1961), the action of sedimenting protein crystals (statoliths), and floating lipid globules (Schimek et al., 1999a, 1999b). Three types of gravitropism mutants are presently available: (a) mutants without protein crystals, which show reduced gravitropism but normal phototropism (Schimek et al., 1999a, 1999b); (b) mutants affected in the genes mad D, E, F, G, and J, which show reduced gravitropism, phototropism, and also avoidance response (Fig. 1; Bergman et al., 1973; Campuzano et al., 1996); and (c) an hypertropic mutant, C5 geo-10 (see below), which shows enhanced gravitropism (Ootaki et al., 1995; Schimek et al., 1999a, 1999b). It was of particular interest to see whether or not the gravitropism mutant, A909 madJ, and the hypergravitropic mutant, C5 geo-10, could mimic the photogravitropic behavior of the wild type during elevated centrifugal accelerations. The hypertropic property of this mutant at 1g is equivalent to the behavior of the wild type at elevated centrifugal acceleration. One should thus expect that the geo-10 mutation should affect the phototropism in a similar way as do elevated centrifugal accelerations in the wild type.
Figure 1.
Transduction chains for gravitropism and phototropism of the sporangiophore of P. blakesleeanus. Negative gravitropism in response to the earth's gravitational acceleration or to centrifugal acceleration (g) is elicited by wall strain (i.e. bending stress or flexure) of the sporangiophore by sedimenting protein crystals and by floating lipid globules (Schimek et al., 1999a). Phototropism is elicited by unilateral light (hν). Class-2 mutants (madD, E, F, G, and J) are defective in phototropism and gravitropism. Class-1 mutants (madA, B, and C) are defective only in phototropism (Bergman et al., 1973). Further explanations in the introductory portion of the paper.
Our results indicate that the gravitropic and phototropic transduction chains interact through a tonic feedback loop of mutual influence, which is essential for the establishment of a wavelength-dependent photogravitropic bending angle.
RESULTS
Photogravitropic Equilibrium during Clinostating
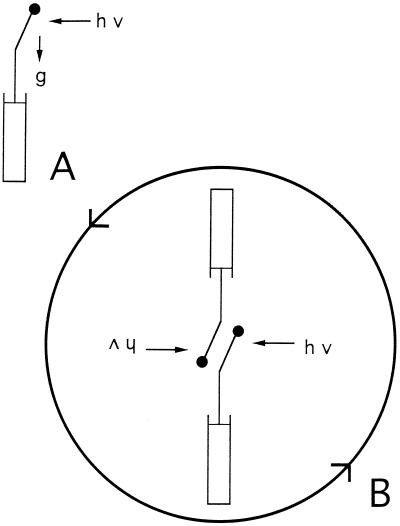
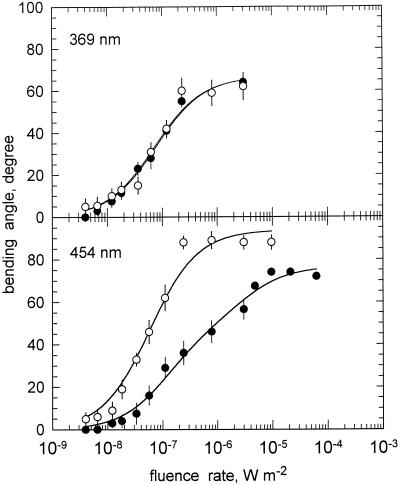
To see the effect of gravitropism on the bending angle reached during photogravitropic equilibrium, we compared fluence rate-response curves that were generated either with static sporangiophores or with sporangiophores that were clinostated head-over (Fig. 2). When sporangiophores were irradiated with unilateral monochromatic light (454 nm) for 8 h, a photogravitropic equilibrium angle was reached that depended on the fluence rate. The threshold for the photogravitropic bending angle was near 6 × 10−9 W m−2 and the maximal bending angle was about 72° (Fig. 3, bottom, black circles). When the sporangiophores were clinostated head-over during the 8-h exposure to unilateral light, then the fluence rate-response curve for phototropism was much steeper than the one obtained for the static condition, and the maximal bending angle was increased to 90°; the threshold was only slightly lowered (Fig. 3, bottom). The clinostating thus caused an increase of the bending angle compared to the static condition. This was expected, because the clinostating removed the gravitropic stimulus that counteracts the unilateral light stimulus.
Figure 2.
A, Mode of irradiation for reaching photogravitropic equilibrium (8 h of unilateral irradiation, stationary sporangiophores, and lamp). B, Mode of irradiation for reaching phototropic equilibrium with clinostating (8 h of unilateral irradiation). During clinostating, sporangiophores and lamp rotate head-over at a speed of 3 rpm. The relative position of the base of the sporangiophores and the lamp does not change during the experiment.
Figure 3.
The effect of clinostating on the fluence rate-response curves for phototropism of the P. blakesleeanus wild-type NRRL1555. Sporangiophores were exposed for 8 h to monochromatic, unilateral light of the indicated wavelengths and the bending angle in the plane of bending (polar angle) was then determined. ●, No clinostating. ○, With clinostating. Top panel, 369 nm; bottom panel, 454 nm. se of 10 to 15 determinations. For symbols without error bar, the se was smaller or equal to the size of the symbol. The solid lines connecting the experimental points represent the fits to the hyperbolic function: y = ax/(x + b) + cx/(x + d) (Eq. 1).
When fluence rate-response curves were measured at 369 nm, however, we observed no difference between the two experimental conditions (Fig. 3, top). With near-UV light, the clinostating had apparently no effect.
Tonic Light Effect on Gravitropism
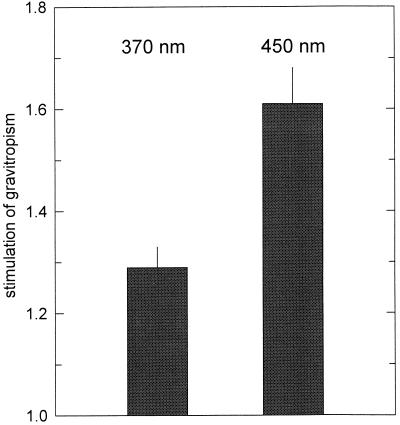
The observation that clinostating had no effect when near-UV light was applied (Fig. 3) suggested that light could exert a tonic effect on gravitropism. To test directly for such a possible effect, we irradiated horizontally positioned sporangiophores bilaterally with horizontal monochromatic light (such that the sporangiophores were in phototropic balance) and compared the gravitropic bending of the irradiated samples with dark controls (Fig. 4). At 10−7 W m−2, near-UV light (370 nm) enhanced gravitropism about 1.3-fold, whereas blue light (450 nm) enhanced it about 1.6-fold.
Figure 4.
The effect of symmetric irradiation on the negative gravitropism of wild-type sporangiophores. Horizontal sporangiophores were irradiated bilaterally with horizontal monochromatic light (370 and 450 nm) at a fluence rate of 10−7 W m−2. After 4 h, the gravitropic bending angles were determined and compared to dark controls, which were made at the same time in the same room. Stimulation of gravitropism is defined as: bending angle(light)/bending angle(dark). Results and se of 25 to 35 determinations.
Photogravitropic Equilibrium of a Statolith Mutant during Clinostating
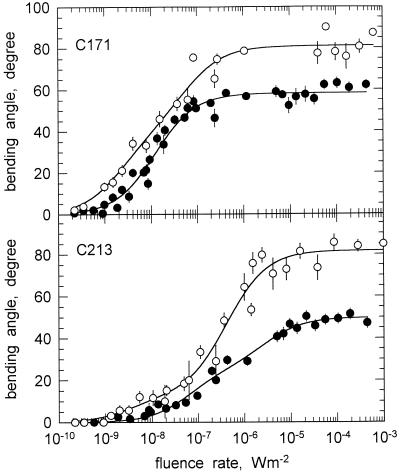
To better assess the effect of clinostating with respect to the different gravitropic stimuli (Fig. 1), we tested an albino mutant (C213 carA geo-3) that lacks the sedimenting protein crystals (statoliths) and compared it to an albino strain (C171 carAcarR) that contains them. The white mutant C171, which lacks β-carotene, is phototropically normal and displays a photogravitropic threshold slightly lower than that of the wild type (Fig. 5, top). Also, the maximal bending angles obtained for fluence rates above 10−6 W m−2 were practically identical to those obtained with the wild type. In contrast with wild type, clinostating did not significantly increase the slope of the fluence-response curve but did increase the maximal bending angle. The threshold was lowered by the clinostating.
Figure 5.
The effect of clinostating on the fluence rate-response curves (461 nm) for phototropism of the white mutants C171 and C213 (no octahedral crystals). Same experimental conditions as in Figure 3. ●, No clinostating. ○, With clinostating. For symbols without error bar, the se was smaller or equal to the size of the symbol. The solid lines connecting the experimental points represent the fits to the hyperbolic function: y = ax/(x + b) + cx/(x + d) (Eq. 1).
Mutant C213 carA geo-3 was different from the strains described above in that the threshold for photogravitropic equilibrium was raised slightly (Fig. 5, bottom). The effect of clinostating was, however, the same as in the other strains. Even in C213, clinostating caused the characteristic double effect of increasing the slope of the fluence rate-response curve and, at the same time, increasing the maximal bending angle by about 35° (Fig. 5, bottom).
Photogravitropic Equilibrium of Two Tropism Mutants during Clinostating
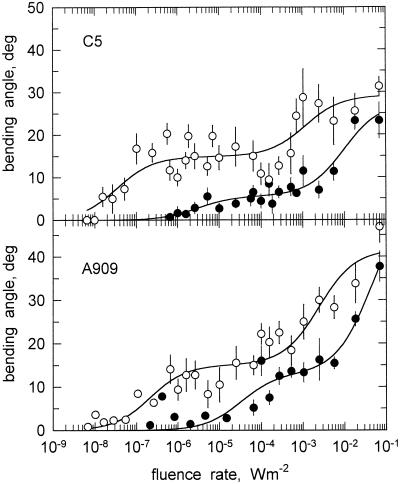
Two additional gravitropism mutants that were tested for the effect of clinostating contained octahedral protein crystals. One mutant, C5 carB geo-10, is hypergravitropic (Ootaki et al., 1995; Schimek et al., 1999a, 1999b). Figure 6 (top) shows that the photogravitropic threshold of this mutant was raised to about 10−6 W m−2. Apparently, this mutant is nightblind and senses only imperfectly blue light. Clinostating the sporangiophores of C5 caused an increase of the slope and an increase of the maximal bending angle to about 20° to 30°. At the same time, the threshold was restored to about 10−8 W m−2, which is practically identical to the wild-type value (Fig. 6, top).
Figure 6.
The effect of clinostating on the fluence rate-response curves (461 nm) for phototropism of two gravitropism mutant. Top panel, Hypergravitropic mutant C5 carB geo-10. Bottom panel, Gravitropism mutant A909 madJ. Same experimental conditions as in Figure 3. ●, No clinostating. ○, With clinostating. se of 10 to 15 determinations. For symbols without error bar, the se was smaller or equal to the size of the symbol. The solid lines connecting the experimental points represent the fits to the hyperbolic function: y = ax/(x + b) + cx/(x + d) (Eq. 1).
Mutant A909 madJ has a raised photogravitropic threshold and substantially reduced gravitropism and avoidance response (Campuzano et al., 1996). The threshold for photogravitropic equilibrium of this mutant was about 2 × 10−5 W m−2 and the maximal bending angle under stationary conditions was merely 38° (Fig. 6, bottom). Clinostating of the sporangiophores of this mutant caused an increase of the slope of the fluence-response curve and an increase of the maximal bending angle by about 10° (Fig. 6, bottom). Clinostating restored the threshold to about the value of the wild type. Clinostating did not, however, increase the maximal bending angle to the same high values (some 70°) as the wild type.
Photogravitropic Equilibrium at Elevated g: Centrifugation Experiments
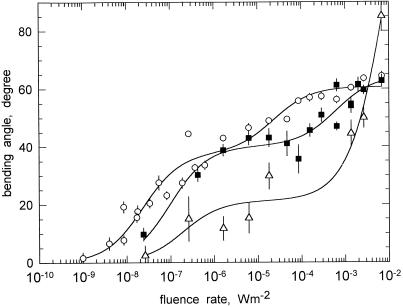
To investigate how the angle of photogravitropic equilibrium depends on the strength of the gravitational acceleration, phototropism experiments were done on a centrifuge that generated centrifugal net accelerations ranging from 2.3g to 8.4g. As detailed in “Materials and Methods,” sporangiophores were mounted on the horizontal disc of the centrifuge and were exposed to a beam of unilateral broad-band blue light that had a width of 2 cm. Because the sporangiophores passed this beam only once per revolution, they were thus subject to continual pulsed stimulus light. The illumination beam was arranged to strike the sporangiophores at 90° from the direction of the net acceleration vector, r (Fig. 7). The fluence rate-response curves that were obtained with wild-type sporangiophores are shown in Figure 8. It is apparent that an increase of the acceleration between 2.3g and 8.4g alters the fluence rate dependence in that the slopes become more shallow. In addition, the extrapolated thresholds increased with increasing acceleration (Fig. 8).
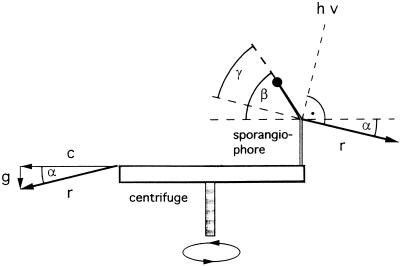
Figure 7.
Determination of the photogravitropic bending angle (γ) as measured on a centrifuge (see “Materials and Methods”). Without unilateral irradiation (hν), the sporangiophore would grow opposite to the resultant (r) of the centrifugal force (c) and the earth gravitational acceleration (g); it would thus be inclined at an angle α above the horizontal centrifuge disc. In the example shown in this figure, the centrifugal acceleration, c, is 4.1g and the resultant, r, is 4.2g. The angle α between c and r is 13.7°. The vector of the unilateral light (hatched line, hν) is perpendicular to r. The angle γ represents the angle of photogravitropic equilibrium. It is determined by subtracting α from the measured bending angle β (protractor reading).
Figure 8.
Fluence rate-response curves for photogravitropic equilibrium at different net accelerations. Sporangiophores were mounted on a centrifuge disc and were subjected to variable centrifugal accelerations while they were irradiated unilaterally with broad-band blue light (Fig. 7; see “Materials and Methods”). ○, 2.3g; ▪, 4.2g; ▵, 8.4g. Accelerations are net values. The bending angle of photogravitropic equilibrium was determined after 4.5 h as shown in Figure 7. The solid lines connecting the experimental points represent the fits to the hyperbolic function: y = ax/(x + b) + cx/(x + d) (Eq. 1).
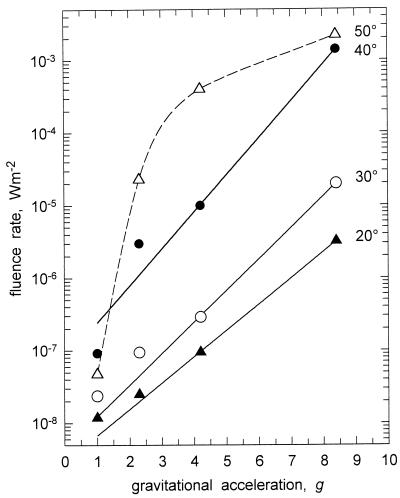
The dependence of the angle of photogravitropic equilibrium on centrifugal acceleration is shown in Figure 9. It is apparent that for responses between 20° and 40° bending, there exists a log-linear relationship. Although the acceleration increases linearly, the resulting fluence rate, which is required to elicit the given reference response, increases logarithmically (Fig. 9).
Figure 9.
Relation between net gravitational acceleration (g) and the phototropic bending angle of wild-type sporangiophores for 20°, 30°, 40°, and 50° bending. Sporangiophores were irradiated with broad-band blue light. The values for 2.3g, 4.2g, and 8.4g were obtained from this work (Fig. 8). The data for 1g were obtained from Bergman et al. (1973). The solid lines connecting the experimental points represent the fits to the exponential function: y = a × exp(bx) (Eq. 2). The dashed line connecting the data points for the reference response of 50° is a spline fit provided by the plotting program.
DISCUSSION
There are several investigations on the interaction between gravity and light. Practically all of these studies were devoted to assessing the effect of light on gravitropism (Lu et al., 1996; Lamparter et al., 1998; Kern and Sack, 1999). For example, it has been known for over 100 years that the gravitropic latency of P. blakesleeanus sporangiophores and A. sativa coleoptiles increases after a short preillumination (Czapek, 1895; Pilet, 1956). Blue, red, and far-red light irradiation prior to the gravitropic stimulation partially suppressed the gravitropism of corn coleoptiles (Wilkins and Goldsmith, 1964; Hild, 1977). Because the fluence dependence for this blue-light effect resembled that of phototropism, it appeared likely that the same photoreceptor mediates phototropism and the suppression of gravitropism. The blue light-mediated suppression followed a time course similar to that of the tonic effect of blue light on phototropism in A. sativa coleoptiles, thus suggesting adaptation processes that could be shared by the light and gravity responses (Blaauw and Blaauw-Jansen, 1970b; Hild, 1977). The gravitropic response of P. blakesleeanus sporangiophores when centrifuged at 4.22g was enhanced by broad-band blue light at fluence rates below 3 × 10−3 W m−2 and completely inhibited at fluence rates above 10−2 W m−2 (Dennison, 1964). In the present work, however, the gravitropic response to 1g was not affected by steady bilateral illumination at 450 nm and fluence rates of 10−7 W m−2 and 2 × 10−3 W m−2. The discrepancy between these two results remains unexplained, though the presence of red observation light in the earlier experiments may be significant.
The mentioned investigations provide a basis for understanding how light influences gravitropism. They do not, however, address the reciprocal question of how gravitropic stimulation affects phototropism. This question is particularly pertinent for the understanding of photogravitropic equilibrium. A clinostat, in which bending organisms are rotated vertically to the axis of the clinostat, provides an easy way to neutralize the effect of gravity and thus the gravitropic response. If provisions are made that the actinic unilateral light rotates along with the organism, the apparatus should enable one to dissect the complex response of photogravitropic equilibrium and to distinguish the gravitropic from the phototropic component. To our knowledge, fluence rate-response curves for phototropism were never before generated under conditions in which the gravitropic stimulus was neutralized by clinostating.
A fluence-response curve for the phototropism of oat coleoptiles was generated under conditions in which the coleoptiles were clinostated horizontally after the application of the unilateral light pulse (Shen-Miller and Gordon, 1967). In line with the observations presented in this work, clinostating caused an increase of the phototropic bending angle. However, the authors measured the response only for a limited fluence range of the first positive bending without determining the threshold. It therefore remained unknown whether clinostating resulted in a shift of the threshold.
Relation between Gravitational Acceleration and Phototropism
The effect of clinostating on P. blakesleeanus wild type and the carotene-lacking mutants, C171 and C213, was manifested in two ways: (a) an increase of the slope of the fluence rate-response curves, and (b) and increase of the maximal bending angle.
The fact that the threshold remained practically unaffected was anticipated. At the threshold no (asymmetric) gravitropic stimulus is exerted on a stationary vertical sporangiophore and, as a consequence, clinostating should remain without an effect. This expectation was largely borne out by the results obtained with the wild type and strain C213 for blue light. The somewhat surprising—even though small—increase of the sensitivity, particularly in C171 (Fig. 5), remains presently unexplained.
For fluence rates above threshold, clinostating did increase the bending and thus the slope of the fluence rate-response curve (Figs. 3 and 5). This observation was in line with our expectation, because clinostating eliminated the counteracting effect of the negative gravitropism so that light could act with a greater efficiency.
The second notable effect of clinostating was the increase of the maximal bending angle (plateau of the fluence rate-response curves; Figs. 3 and 5). This effect of clinostating indicates that the maximal bending angle is, under normal conditions, limited by the action of gravity. This conclusion is novel and differs from previous explanations that rested on the argument that no further bending beyond 70° would be possible, because the incidence angle of the unilateral light would be too small for causing positive bending (Dennison, 1965; Bergman et al., 1969). Because phototropism requires the unilateral light to be focused at the distal side of the sporangiophore, it was reasoned, on the ground of optical arguments, that the focus advantage should disappear for very small angles of incidence (Dennison, 1965). The fact that 90° bending can nevertheless be obtained with clinostating indicates, then, that the explanation of the limiting angle must be sought not in the optics of the sporangiophore but, rather, in the counteracting effect of gravity.
Gravitropism and Phototropism Are Linked via a Tonic Feedback Loop
For the analysis of photogravitropic equilibrium, it is essential to distinguish between vectorial and non-vectorial, i.e. tonic, responses. The fact that clinostating did not have an effect on the shape of the fluence rate-response curve of phototropism when near-UV light of 369 nm was applied (Fig. 3) indicated that light could exert a tonic effect. Bilateral irradiation of horizontal sporangiophores resulted indeed in an enhancement of gravitropism (Fig. 4).
The complex behavior of the wild type and the mutants is explained in the framework of a tropism model, which represents an extension of the traditional model shown in Figure 1. The extended model involves the novel feature of a tonic feedback loop, which links the gravitropic and phototropic transduction chains (Fig. 10). The tonic feedback loop consists of three regulatory elements: (a) a tonic enhancer (+, dashed line), which exerts a light-mediated stimulation of gravitropism; (b) the negative regulator geo-10, which dampens the gravitropic transduction chain; and (c) a tonic inhibitor (-, dashed line), which inhibits the phototropic transduction chain. A gravistimulation (either unilateral or multilateral as on the clinostat) generates a moderate tonic inhibition of the phototropic transduction chain, the extent of which depends on the activity of the negative regulator geo-10. The geo-10 element has the function of dampening the vectorial transduction chain of gravitropism, causing a concomitant down-regulation of the tonic inhibitor. Light (either unilateral or bilateral) stimulates tonically the gravitropic transduction chain and counteracts the geo-10 element. Blue light at threshold-fluence rates enhances gravitropism more effectively than near-UV light (Fig. 4), and thus the tonic inhibition is substantially larger than in near-UV light.
Figure 10.
Extended model of the transduction chains for gravitropism and phototropism and their putative interconnections via the tonic feedback loop. The mutation geo-10 confers hypergravitropism (Ootaki et al., 1995). The gravitropic and the phototropic transduction chains are linked through the tonic feedback loop consisting of three elements: (a) a tonic enhancer (+, dashed line), which exerts a light-mediated stimulation of gravitropism; (b) the negative regulator geo-10, and (c) a tonic inhibitor (-, dashed line), which acts on the phototropic transduction chain. Irradiation with blue light (450 nm) causes strong tonic enhancement and strong tonic inhibition that are symbolized by large numbers. Near-UV light (370 nm) causes only moderate tonic effects (small numbers). Vectorial interactions of the transduction chains are indicated by solid lines. Further explanations in the “Discussion.” The other symbols and mutants are as in Figure 1.
The tonic feedback loop explains the observation that clinostating causes an increase of the slope for blue light (454 nm), whereas it is ineffective for near-UV light (369 nm; Fig. 3). For blue light, the negative tonic effect is relatively large (Fig. 10), but also the vectorial gravitropic input is large. This generates a situation in which a large vectorial gravitropic input is counteracted by an only moderate phototropism input. Blue-light elicited phototropism is, therefore, more efficient when gravitropism is neutralized by clinostating. In contrast, for near-UV light, the negative tonic effect is moderate, but the vectorial gravitropic input is also moderate. Here a modest vectorial gravitropic input is counteracted by a strong phototropism input. The net result for unilateral irradiation with near-UV light is a lack of increase of the phototropic bending angle, despite the neutralization of gravitropism by clinostating (Fig. 3).
The tonic feedback loop also offers an explanation for the phenotype of the hypergravitropic mutant C5 and for the allocation of the geo-10 mutation in the transduction chains (Fig. 10). The defect of mutant C5 cannot be located near the output of the transduction chain, because the bending is very efficient, i.e. the cell wall is not stiff as it appears to be in class-2 mutants (madD–J). Clearly, the defect must reside near the input on the branch of the gravitropism transduction chain (Fig. 10). The hypergravitropic phenotype is best explained by the assumption that geo-10 represents a negative regulator exerting a suppressing (dampening) effect on the vectorial transduction chain, which in turn represents the input for the negative tonic element. Elimination of the geo element by the geo-10 mutation thus causes hypergravitropism and an enhancement of the negative feedback on the phototropism chain. As a result, the effectiveness of the light input becomes reduced. The fluence rate-response curve of phototropism (454 nm) of C5 geo-10 remains shallow even with clinostating, i.e. when the vectorial effect of gravitropism is neutralized (Fig. 6). Clinostating itself, however, represents a continual tonic (multilateral) gravistimulation, which, in C5, is not reduced by the geo-10 element as it is in the wild type, so that the negative feedback in C5 exceeds that of the wild type during clinostating. As a result unilateral blue light is less effective in C5 than in the wild type.
The negative tonic effect that gravistimulation exerts over phototropism might also be responsible for the log-linear relationship between phototropism and gravitropism (see below).
Quantitative Relationship between Light and Gravitational Stimulation
It is apparent from Figure 9 that a linear increase of the gravitational acceleration requires an exponential increase of the fluence rate in order to elicit a given constant bending angle. For bending angles up to 40°, the data can be fitted to a simple exponential function, y = a × exp(bx) where y is the fluence rate of the unilateral blue light, x is the centrifugal acceleration, and a and b are adjustable parameters (Eq. 2). For higher bending angles, the relationship becomes more complex, but the fluence rate still grows exponentially with the centrifugal acceleration (Fig. 9, dashed line). It is concluded that the light-transduction chain possesses a logarithmic transducer (Bergman et al., 1973), whereas the gravi-transduction chain possesses a linear one (Fig. 9). Logarithmic transduction of the light stimulus corresponds to the fact that the environmental irradiance changes during the course of a d by 10 to 12 orders of magnitude. In contrast, the earth's gravitational field is constant and the gravitational stimulus that a sporangiophore experiences varies between the values 0 and 1, depending on the angle between the vertical and the axis of the sporangiophore (sine rule).
Behavior of Gravitropism Mutants Lacking Octahedral Crystals
The mutant C213 carA geo-3 displays a reduced gravitropic responsiveness. This defect has been associated with the lack of so-called octahedral crystals in vacuoles of the sporangiophore (Ootaki and Wolken, 1973; Schimek et al., 1999a, 1999b). The paracrystalline inclusions are probably statoliths for gravitropism. The phototropic sensitivity of mutant C213 had not been determined previously. We found that this strain is slightly less light sensitive than the albino mutant C171 and about the same as the wild type (Fig. 5). Besides a partial loss of light sensitivity, mutant C213 also displayed a reduction of the maximal bending angle.
Clinostating had a similar effect on the crystal-lacking strain C213 as on the other strains, i.e. clinostating caused an increase of the slope and also an increase of the maximal bending angle. These results were counterintuitive, because one could have expected that the weakened gravitropic response in C213 should enable the unilateral light to act more efficiently. The lack of the statoliths, one might reason, should be equivalent to the effect of clinostating. For this reason, we had actually expected that crystal-lacking mutants would display an increased slope of the fluence rate-response curves of photogravitropic equilibrium (static conditions). This was, however, clearly not the case, as the crystal-lacking mutants had the same slopes as the crystal-containing strains. We conclude from these observations that the protein crystals do not play an essential role in the interaction between gravitropism and phototropism. Because of the redundant gravitropic input (Figs. 1 and 10), the other two gravistimuli (lipid globules and wall strain) may substitute for the lack of protein crystals.
Hypergravitropic and Gravitropic Defective Mutants Mimic Elevated Centrifugal Acceleration
The behavior of the mutants C5 and A909 is reminiscent of the behavior of the wild type on the centrifuge. At high centrifugal accelerations (8.4g) the wild type manifests an elevated threshold and a biphasic fluence rate-response curve. A similar pattern is found also for the mutants (Fig. 6). At lower centrifugal accelerations (2.3g) the threshold of the wild type decreases and the slope increases. Clinostating of the mutants has a similar effect as a reduction of the centrifugal acceleration in the wild type.
The hypergravitropic mutant C5 geo-10 behaves as if the gravitropic stimulus at normal earth's gravity (1g) was amplified, resulting in hypergravitropism. Clinostating of C5 lowers the threshold to almost the wild-type value, indicating that the light sensitivity of the hypergravitropic mutant is not basically affected. The slope reduction with clinostating was explained in the framework of the tonic feedback loop (see above). In C5, without clinostating, the phototropism has to counteract the vectorial as well as the excessive negative tonic effect of the hypergravitropic mutation and, as a result, the fluence rate-response curve of C5 shows an elevated threshold and a reduced slope in comparison to that of the wild type (Fig. 6). The effect of the geo-10 mutation in C5 is thus equivalent to augmenting the centrifugal acceleration in the wild type.
The gravitropic defective mutant, A909 madJ (class 2), showed similar fluence-response curves as the hypertropic mutant C5 (Fig. 6). The madJ mutation must reside on a branch of the transduction chain that is common to both gravitropism and phototropism, because the bending response is affected for both types of tropism (Campuzano et al., 1996). Despite its different genetic defect, the phenotype of A909 madJ is nevertheless similar to that of the hypergravitropic mutant. Because of the reduced bending capacity of class-2 mutants, the effectiveness of the unilateral light is reduced, causing concomitantly a reduced slope. Clinostating cannot fully restore the normal slope, because it cannot restore the bending capacity and because of the above-mentioned tonic effect that suppresses phototropism (Fig. 1).
Wavelength Dependence and Consequences for Action Spectroscopy
Action spectra for phototropism have been generated either on the basis of fluence-response curves, as in the case of grass coleoptiles (Shropshire and Withrow, 1958; Thiman and Curry, 1960), or on the basis of fluence rate-response curves, as in the case of P. blakesleeanus (Varjú et al., 1961; Galland, 1983; Galland and Lipson, 1985b). Thus, the action spectra are not free from artifacts caused by the interference of gravitropism. The use of a clinostat enables one to generate fluence rate-response curves for phototropism, which are free of the gravitropic component.
Action spectra for photogravitropic equilibrium (based on fluence rate-response curves as in this work) differ from those obtained with the phototropic balance method (Curry and Gruen, 1959; Galland and Lipson, 1985a) in that the near-UV light maximum is larger than that at 450 nm and that minor maxima near 415 and 490 nm become apparent (Varjú et al., 1961; Galland and Lipson, 1985b; Hohl et al., 1992). The reasons for these obvious differences were never really understood. It had been surmised that the gravitropic response may interfere with phototropism and thus distort the true phototropism action spectra. The data shown in Figure 3 fully support this conjecture. With clinostating, the relative effectiveness of the blue light (454 nm) increases about 5- to 7-fold when a reference response of 40° bending is chosen. In contrast, the relative effectiveness of near-UV light does not increase by clinostating (Fig. 3). These data indicate that clinostating (i.e. the neutralization of gravitropism) increases specifically the maximum of the photogravitropic action spectrum in blue-light region relative to that in near-UV light. Thus, the action spectrum with clinostating looks much more akin to the absorption spectrum of a typical flavin than the action spectrum of photogravitropic equilibrium (Galland, 1983). This result is very satisfying, because action spectra of phototropic balance, in which gravitropism does not play a role, likewise look flavin-like (Curry and Gruen, 1959; Delbrück and Shropshire, 1960; Galland and Lipson, 1985a). We thus conclude that gravity is the major cause for the discrepancies in the shapes of action spectra of phototropic balance and photogravitropic equilibrium.
MATERIALS AND METHODS
Strains
The standard wild-type strain of Phycomyces blakesleeanus (Burgeff) NRRL1555 (-) was originally obtained from the Northern Regional Research Laboratories (Peoria, IL). The mutants described in this paper are listed in Table I.
Table I.
Strains of P. blakesleeanus (Burgeff) used in this work
| Strain | Genotype | Phenotype | Reference |
|---|---|---|---|
| NRRL1555 | (-) | Wild type | Bergman et al. (1973) |
| C5 | carB10 geo-10 (-) | White, hypergravitropic | Meissner and Delbrück (1968) |
| C213 | carA5 geo-3 (-) | White, no crystals, defective tropisms | Schimek et al. (1999a, 1999b) |
| C171 | carA30 carR21 (-) | White | Ootaki et al. (1973) |
| A909 | madJ407 (-) | Defective tropisms | Campuzano et al. (1996) |
Mutant strains were derived from the wild-type NRRL1555 by mutagenesis with nitrosoguanidine. Genetic designations: geo indicates abnormal gravitropism; car indicates absence of β-carotene; (-) denotes the mating type. Crystals refer to the octahedral crystals (Ootaki and Wolken, 1973), which probably serve as statoliths of gravitropism (Schimek et al., 1999a, 1999b).
Growth Conditions
Sporangiophores were grown in glass shell vials (1-cm diameter × 4-cm height; Flachbodengläser, AR Klarglas, Münnerstädter Glaswarenfabrik, Münnerstadt, Germany) on a synthetic medium (so-called SIV medium described by Sutter [1975]). Each shell vial was inoculated with five to 10 heat-induced spores (at 49°C for 10 min). Until the appearance of stage 4b-sporangiophores (i.e. with sporangium), the material was kept in a temperature-controlled room (21°C) under white fluorescent light of moderate fluence rate (0.7 W m−2). Sporangiophores used for the centrifuge experiments were grown on potato dextrose agar medium under incandescent light, as previously described (Dennison, 1964).
Measurement of Fluence Rate-Response Curves for Phototropism
The photogravitropic equilibrium of stage-4 sporangiophores was measured in a threshold box, as described previously (Campuzano et al., 1996). This home-designed box contained 10 individual cubicles in which 10 to 12 vials with one or two sporangiophores each could be placed. Unilateral light was deflected into these cubicles by beamsplitters. The fluence rates from the first to the tenth cubicle decreased stepwise approximately 2-fold per step. A halogen-mirror lamp (50 W, Q50MR 16/NSP [EXT], type narrow spot, General Electric, Cleveland) provided horizontal collimated light that passed through the filter-holder box and entered the threshold box.
The entire threshold box, including the lamp, was mounted in a metal frame with an attached electrical motor, which allowed rotation around its long axis. This allowed the sporangiophores to be rotated head-over (3 rpm) while they were continually irradiated unilaterally (Fig. 2). The glass shell vials with the sporangiophores were kept in place by special tight-fitting plastic holders (rubber cushioned) that could be fixed inside the threshold box. The irradiations with unilateral light lasted for 8 h. After completion of the irradiation, the phototropic bending angle was determined with a protractor. The bending angle in the plane of the bending sporangiophore, i.e. the polar angle, was measured.
Optical Equipment and Measurement of Irradiances
We used interference filters (8–12 nm one-half-band width, Schott Glaswerke, Mainz, Germany), neutral density filters (type NG, Schott Glaswerke), and heat-absorbing filters (type KG1, 5 mm, Schott Glaswerke). Fluence rates were determined with a UV-enhanced photodiode (BN-9102–4, Gigahertz-Optik, Puchheim, Germany) and a calibrated readout instrument (Optometer P-9201, Gigahertz-Optik).
Centrifugation Experiments
The centrifuge that was used for the establishment of photogravitropic equilibrium at centrifugal accelerations at 2.1g, 4.1g, and 8.3g was described previously (Dennison, 1961). The resulting net accelerations (r, Fig. 7), i.e. taking into account the earth gravitational field, were 2.3g, 4.2g, and 8.4g respectively. The axis of rotation was vertical (Fig. 7). Ten sporangiophores were mounted in holders at a radius of 20 cm on the centrifuge disc of 61 cm in diameter. The disc was covered by a single cylindrical cover of Plexiglas (Rohm and Haas, Philadelphia), with a diameter of 56 cm and a height of 13 cm.
The light source was as described by Dennison (1964). Briefly, the optical train consisted of a 100-W tungsten lamp, a 2-cm-thick water-filled glass cell (to absorb infrared radiation), a 5–61 blue glass filter (Corning Glass Works, Corning, NY), a 211-mm focal length lens adjusted to give a parallel output beam, and a rectangular aperture of 2 by 5 cm. The illumination beam was arranged to strike the sporangiophore at 90° from the direction of the net acceleration vector, corresponding to the usual photogravitropic setup at 1g, in which the illumination direction is horizontal (Fig. 7).
For the 2.1g series, the net acceleration was 2.3g, directed downwards at an angle of 25.9° from horizontal, and the illumination beam was 25.9° from vertical (Fig. 7). For the 4.1g series, the net acceleration was 4.2g, directed downwards at an angle of 13.675° from horizontal, and the illumination beam was 13.675° from vertical. For the 8.3g series, the net acceleration was 8.4g, directed downwards at an angle of 6.87° from horizontal, and the illumination beam was 6.87° from vertical.
The illumination intensity was varied by changing the lamp voltage. Calibration was carried out as described in Dennison (1964). For the 2.1g series (centrifugal acceleration), intensities in the range from 6.14 × 10−7 to 9.86 × 10−10 W m−2 were obtained by using a neutral density filter in the beam, which allowed the lamp to be operated in a higher voltage range, where the settings were more reproducible.
The sporangiophores were rotated on the centrifuge disc at a radius of 20 cm, and they passed through the 2-cm width of the beam once per revolution. Therefore, the average intensity experienced by the sporangiophores was 2/40 π, or 1/62.832 of the measured beam intensity. The centrifuge speeds used for 2.1g, 4.1g, and 8.3g were 95.4, 135.4, and 192.6 rpm, respectively, so that the sporangiophores experienced illumination that was pulsed at 1.59, 2.26, and 3.21 flashes per second, respectively.
Measurements of the angle of sporangiophore growth (β; Fig. 7) were taken by a long focus lens-microscope system whose optic axis was tangent to the circle of rotation (Fig. 1; Dennison, 1961). Illumination was provided by a strobe light covered with a red filter. The strobe was triggered by a sliding spring on the centrifuge disc as it came into contact with one of the 10 pins set into a Plexiglas block mounted under the centrifuge disc. The experimenter took angle readings by successively switching to each of 10 positions (two sets of five) and using a protractor eyepiece to measure the angle of the upper 1 mm of the sporangiophore. If the sporangiophore appeared to be oscillating around an equilibrium position, the cross hair was set tangent to the frozen waves, which often extended to 4 mm below the growing zone, or even farther. The 10 observations were routinely carried out within a 5-min interval, thus allowing 30 s for each measurement. The photogravitropic angles plotted in Figure 8 were determined after 4.5 h.
Since the microscope axis was tangent to the circle of rotation, the phototropic angle was measured in the radial plane. In some cases, usually at the lowest intensities, the sporangiophore's final orientation deviated significantly from this plane. Such declinations, or aiming errors, were qualitatively estimated by eye while looking down on the sporangiophore as they rotated on the centrifuge, but no systematic aiming error data were collected.
Data Analysis
The fluence rate-response curves shown in Figures 3, 5, 6, and 8 were fitted to the equation:
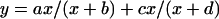 |
1 |
where x is the fluence rate in watts per square meter, y is the bending angle and a, b, c, and d are adjustable parameters. The single components ax/(x + b) and cx/(x + d) represent hyperbolas when y is plotted as a function of x. When y is plotted as a function of log x, as done in Figures 3, 5, 6, and 8, one obtains a symmetrical sigmoidal curve for each component. The theory for the use of hyperbolic functions in the context of photobiology, specifically for fluence rate-response curves, is given by Hartmann (1977). The data in Figure 9 were fitted to the equation:
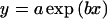 |
2 |
where x is the centrifugal acceleration (expressed as multiples of g), y is the fluence rate of unilateral blue light to elicit a given phototropic bending angle, and a and b are adjustable parameters. The error-weighted fitting routines were provided by the program SigmaPlot 4.0 (SPSS, Chicago).
ACKNOWLEDGMENTS
We thank Siegrid Völk and Marko Göttig for excellent technical assistance.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. Ga 173/7–1).
LITERATURE CITED
- Bergman K, Burke PW, Cerdá-Olmedo E, David CN, Delbrück M, Foster KW, Goodell EW, Heisenberg M, Meissner G, Zalokar M, Dennison DS, Shropshire W., Jr Phycomyces. Bacteriol Rev. 1969;33:99–157. doi: 10.1128/br.33.1.99-157.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Eslava AP, Cerdá-Olmedo E. Mutants of Phycomyces with abnormal phototropism. Mol Gen Genet. 1973;123:1–16. doi: 10.1007/BF00282984. [DOI] [PubMed] [Google Scholar]
- Blaauw OH, Blauw-Jansen G. The phototropic responses of Avena coleoptiles. Acta Bot Neerl. 1970a;19:755–763. [Google Scholar]
- Blaauw OH, Blauw-Jansen G. Third positive (c-type) phototropism in the Avena coleoptile. Acta Bot Neerl. 1970b;19:764–776. [Google Scholar]
- Braun M. Gravitropism in tip-growing cells. Planta. 1997;203:S11–S19. doi: 10.1007/pl00008098. [DOI] [PubMed] [Google Scholar]
- Briggs WR. Light dosage and phototropic responses of corn and oat coleoptiles. Plant Physiol. 1960;35:961–962. doi: 10.1104/pp.35.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campuzano V, Galland P, Alvarez MI, Eslava AP. Blue-light receptor requirement for gravitropism, autochemotropism and ethylene response in Phycomyces. Photochem Photobiol. 1996;63:686–694. doi: 10.1111/j.1751-1097.1996.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Curry GM, Gruen HG. Action spectra for the positive and negative phototropism of Phycomyces sporangiophores. Proc Natl Acad Sci USA. 1959;45:797–804. doi: 10.1073/pnas.45.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czapek F. Über Zusammenwirken von Heliotropismus und Geotropismus. Sitzungsber Oesterr Akad Wiss Math-Natwiss Kl Abt I Biol Wiss Erdwiss. 1895;104:337–375. [Google Scholar]
- Delbrück M, Shropshire W., Jr Action and transmission spectra of Phycomyces. Plant Physiol. 1960;35:194–204. doi: 10.1104/pp.35.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison DS. Tropic responses of Phycomyces sporangiophores to gravitational and centrifugal stimuli. J Gen Physiol. 1961;45:23–38. doi: 10.1085/jgp.45.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison DS. The effect of light on the geotropic responses of Phycomyces sporangiophores. J Gen Physiol. 1964;47:651–665. doi: 10.1085/jgp.47.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison DS. Steady state phototropism in Phycomyces. J Gen Physiol. 1965;48:393–408. doi: 10.1085/jgp.48.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland P. Action spectra of photogeotropic equilibrium in Phycomyces wild type and three behavioral mutants. Photochem Photobiol. 1983;37:221–228. doi: 10.1111/j.1751-1097.1985.tb03493.x. [DOI] [PubMed] [Google Scholar]
- Galland P, Lipson ED. Action spectra for phototropic balance in Phycomyce blakesleeanus: dependence on reference wavelength and intensity range. Photochem Photobiol. 1985a;41:323–329. doi: 10.1111/j.1751-1097.1985.tb03492.x. [DOI] [PubMed] [Google Scholar]
- Galland P, Lipson ED. Modified action spectra of photogeotropic equilibrium in Phycomyces blakesleeanus with defects in genes madA, madB, madC, and madH. Photochem Photobiol. 1985b;41:331–335. doi: 10.1111/j.1751-1097.1985.tb03493.x. [DOI] [PubMed] [Google Scholar]
- Hangarter RP. Gravity, light and plant form. Plant Cell Environ. 1997;20:796–800. doi: 10.1046/j.1365-3040.1997.d01-124.x. [DOI] [PubMed] [Google Scholar]
- Hartmann K. Aktionsspektrometrie. In: Hoppe W, Lohmann W, Markl H, Ziegler H, editors. Biophysik—Ein Lehrbuch. Berlin: Springer-Verlag; 1977. pp. 197–222. [Google Scholar]
- Hild V. Wirkung von Vorbestrahlung mit Rot—oder Blaulicht auf die geotropische Empfindlichkeit von MaisKoleoptilen. Planta. 1977;133:309–314. doi: 10.1007/BF00380694. [DOI] [PubMed] [Google Scholar]
- Hohl N, Galland P, Senger H, Eslava AP. Altered pterin patterns in photoreceptor mutants of Phycomyces blakesleeanus with defective madI gene. Bot Acta. 1992;105:441–448. [Google Scholar]
- Kern VD, Mendgen K, Hock B. Flammulina as a model system for fungal graviresponses. Planta. 1997;203:S23–S32. doi: 10.1007/pl00008111. [DOI] [PubMed] [Google Scholar]
- Kern VD, Sack FD. Irradiance-dependent regulation of gravitropism by red light in protonemata of the moss Ceratodon purpureus. Planta. 1999;209:299–307. doi: 10.1007/s004250050636. [DOI] [PubMed] [Google Scholar]
- Lamparter T, Hughes J, Hartmann E. Blue light- and genetically-reversed gravitropic response in protonemata of the moss Ceratodon purpureus. Planta. 1998;206:95–102. doi: 10.1007/s004250050378. [DOI] [PubMed] [Google Scholar]
- Lu Y-T, Hidaka H, Feldmann LJ. Characterization of a calcium/calmodulin-dependent protein kinase homolog from maize roots showing light-regulated gravitropism. Planta. 1996;199:18–24. doi: 10.1007/BF00196876. [DOI] [PubMed] [Google Scholar]
- Meissner G, Delbrück M. Carotene and retinal in Phycomyces mutants. Plant Physiol. 1968;43:1279–1283. doi: 10.1104/pp.43.8.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootaki T, Ito K, Abe M, Lazarova G, Miyazaki A, Tsuru T. Parameters governing gravitropic response of sporangiophores in Phycomyces blakesleeanus. Mycoscience. 1995;36:263–270. [Google Scholar]
- Ootaki T, Lighty AC, Delbrück M, Hsu W-J. Complementation between mutants of Phycomyces deficient with respect to carotenogenesis. Mol Gen Genet. 1973;121:57–70. doi: 10.1007/BF00353693. [DOI] [PubMed] [Google Scholar]
- Ootaki T, Wolken JJ. Octahedral crystals in Phycomyces: II. J Cell Biol. 1973;57:278–288. doi: 10.1083/jcb.57.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilet PE. Sur l' apogéotropisme du Phycomyces. Experientia. 1956;12:148–149. [Google Scholar]
- Sack FD. Plastids and gravitropic sensing. Planta. 1997;203:S63–S68. doi: 10.1007/pl00008116. [DOI] [PubMed] [Google Scholar]
- Shen-Miller J, Gordon SA. Gravitational compensation and the phototropic response of oat coleoptiles. Plant Physiol. 1967;42:352–360. doi: 10.1104/pp.42.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimek C, Eibel P, Grolig F, Horie T, Ootaki T, Galland P. Gravitropism in Phycomyces: a role for sedimenting protein crystals and floating lipid globules. Planta. 1999a;210:132–142. doi: 10.1007/s004250050662. [DOI] [PubMed] [Google Scholar]
- Schimek C, Eibel P, Horie T, Galland P, Ootaki T. Protein crystals in Phycomyces sporangiophores are involved in graviperception. Adv Space Biol. 1999b;24:687–696. doi: 10.1016/s0273-1177(99)00400-7. [DOI] [PubMed] [Google Scholar]
- Shropshire W, Jr, Withrow RB. Action spectrum of phototropic tip curvature of Avena. Plant Physiol. 1958;33:360–365. doi: 10.1104/pp.33.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter RP. Mutations affecting sexual development in Phycomyces blakesleeanus. Proc Natl Acad Sci USA. 1975;72:127–130. doi: 10.1073/pnas.72.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiman KV, Curry GM. Phototropism and phototaxis. In: Florkin M, Mason HS, editors. Comparative Biochemistry: A Comparative Treatise. 1: Sources of Free Energy. New York: Academic Press; 1960. pp. 243–309. [Google Scholar]
- Varjú D, Edgar L, Delbrück M. Interplay between the reactions to light and to gravity in Phycomyces. J Gen Physiol. 1961;45:47–58. doi: 10.1085/jgp.45.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins MB, Goldsmith MHM. The effect of red, far-red, and blue light on the geotropic response of coleoptiles of Zea mays. J Exp Bot. 1964;15:600–615. [Google Scholar]