Abstract Abstract
During the course of a study on the functional biodiversity of the mycobiota inhabiting rainforests in Thailand, a fungal strain was isolated from a plant sample and shown to represent an undescribed species, as inferred from a combination of morphological and molecular phylogenetic methods. Molecular phylogenetic analyses, based on four DNA loci, revealed a phylogenetic tree with the newly generated sequences clustering in a separate branch, together with members of the Sulcatisporaceae (Pleosporales, Ascomycota). The Thai specimen morphologically resembled Neobambusicola strelitziae in having pycnidial conidiomata with phialidic conidiogenous cells that produce both fusoid-ellipsoid macroconidia and subcylindrical microconidia. However, the new fungus, for which the name Pseudobambusicola thailandica is proposed, differs from N. strelitziae in having conidiomata with well-defined necks, the presence of globose to subglobose thick-walled cells adjacent to conidiomata and the production of chlamydospores in culture. When cultures of P. thailandica, growing on water agar, were confronted with Caenorhabditis elegans nematodes, worms approaching the fungal mycelia were killed. This observation gave rise to a study of its secondary metabolites and six novel and two known compounds were isolated from submerged cultures of P. thailandica. The structures of metabolites 1–6, for which the trivial names thailanones A–F are proposed, were elucidated using a combination of spectral methods, including extensive 1 and 2D NMR analysis and high resolution mass spectrometry. Compounds 4 and 8 showed strong nematicidal and weak antifungal activity, whereas all other tested compounds showed moderate to weak nematicidal activity but no significant effects in the serial dilution assay against various fungi and bacteria. Compounds 1 and 8 also inhibited growth of the pathogenic basidiomycete Phellinus tremulae in a plate diffusion assay.
Keywords: Antifungal agent, deoxyphomalone, monocerin, nematode-antagonism, nematicide, phylogeny
Introduction
Fungi are regarded as prolific sources of secondary metabolites with prominent and selective biological activities that can serve as a basis for development of new antimicrobials, agrochemical pesticides and other useful compounds (Bills and Gloer 2016, Karwehl and Stadler 2017). In particular, the mycobiota of tropical countries are widely unexplored and can still yield a plethora of novel chemical entities. In recent years, many novel compounds with, for example, antimicrobial (Helaly et al. 2016, 2017, Richter et al. 2016, Kuephadungphan et al. 2017), cytotoxic (Surup et al. 2017) and antioxidative (Kuhnert et al. 2015) effects were isolated in the authors’ laboratory from tropical fungi. Furthermore, fungi represent a rich source of nematicidal compounds because they are both prey and natural antagonists of nematodes. Thus, understanding the chemical basis for fungi nematode interactions offers natural biocontrol strategies (Anke et al. 1995). According to Degenkolb and Vilcinskas (2016), approximately 700 species of nematophagous fungi have been described so far and four ecophysiological categories have been proposed. However, little has been done to screen for metabolites in nematophagous fungi or, for that measure, nematicidal metabolites in other fungi since the first studies of this kind during the 1990s (Stadler et al. 1993a, b, 1994).
Environmentally compatible and low-cost alternatives to chemical control measures for phytoparasitic nematodes are urgently needed and these must not affect vertebrates, crops and other non-target organisms. Highly specific, preferably soil-borne antagonists are best suited for this purpose (Degenkolb and Vilcinskas 2016).
In this context, fungi isolated from nature were examined for morphological features and by ITS sequencing. The strains that turned out to belong to well-studied, ubiquitous mycotoxin-producing genera (in particular Trichocomaceae and Hypocreaeae) were discarded. Those strains that belong to less studied phylogenetic lineages were selected for studies of their antagonistic activities. They were first tested using a water agar assay to detect nematicidal effects and, in parallel, extracts were prepared and checked in an agar plate diffusion assay for antifungal and nematicidal activities. Herein, the authors report the discovery of a new genus and species Pseudobambusicola thailandica and its six novel and two known secondary metabolites, including their isolation, structure elucidation and biological activity.
Materials and methods
Fungal isolation
During a fungal exploration in Thailand in 2015, an unrecognised fungus was found growing on a twig of an unidentified plant. The twig was incubated in a damp chamber and treated according to Castañeda-Ruiz et al. (2016). Single conidial isolates were established from sporulating conidiomata in Petri dishes containing water agar (WA; Difco agar 5 g, tap water 1 l). Colonies were sub-cultured on potato carrot agar (PCA; potatoes 20 g; carrots 20 g; agar 20 g; distilled water 1 l) and oatmeal agar (OA; oatmeal 30 g; agar 18 g; distilled water 1 l) as described previously (Hernández-Restrepo et al. 2017). Herbarium type material and the ex-type strain are maintained at the BIOTEC Bangkok herbarium (BBH) and at the BIOTEC culture collection (BCC; both Pathum Thani, Thailand), respectively.
Morphology
Morphological features were characterised from colonies growing on OA or on synthetic nutrient-poor agar (SNA; Nirenberg 1976) supplemented by fragments of autoclaved pine needles and incubated at 25 °C under continuous near-ultraviolet light to promote sporulation. Colony colours were assessed according to the charts of Rayner (1970). Micromorphological descriptions and measurements for 30 replicates of relevant features were carried out from mature conidiomata and conidia mounted in lactic acid 90%. Photomicrographs were made following Hernández-Restrepo et al. (2017).
DNA isolation, amplification and sequences analyses
Genomic DNA was extracted from fungal colonies growing on MEA using the Wizard® Genomic DNA purification kit (Promega, Madison, USA) following the manufacturer’s protocols. The nuclear rDNA operon spanning the 3’ end of the 18S nrRNA gene, the first internal transcribed spacer (ITS1), the 5.8S nrRNA gene, the second ITS region (ITS2) and approximately 900 bp of the 5’ end of the large subunit of the nrRNA gene (LSU), part of the RNA polymerase II second largest subunit gene (rpb2) and part of the translation elongation factor 1-α gene (tef1) were amplified following Hernández-Restrepo et al. (2016). The programme SeqMan Pro v. 10.0.1 (DNASTAR, Madison, WI, USA) was used to obtain consensus sequences for each DNA region. Blast searches using ITS and LSU sequences were performed and the closest matches and related taxa were retrieved from GenBank and included in the phylogenetic analyses (Table 1, See Suppl. material 1). Alignments were produced with MAFFT v. 7 (Katoh and Standley 2013), checked and refined using MEGA v. 6 (Tamura et al. 2013) and SequenceMatrix (Vaidya et al. 2011). Individual alignments for each locus and the concatenated four-loci dataset were analysed by maximum likelihood (ML) with gamma model of rate heterogeneity using the RAxML HPC BlackBox v. 8.2.8 (Stamatakis 2014) online server of the Cipres Science gateway portal (Miller et al. 2010). The maximum likelihood search option was used to search for the best-scoring tree after bootstrapping. By default, the RAxML BlackBox calculates statistical support for branches by rapid bootstrap analyses of 1000 replicates (Stamatakis 2014). Bootstrap support (bs) values ≥ 70 % were considered significant. Incongruence amongst datasets was tested by visual inspection of all groups with ≥ 70 % bs in partial trees of each locus to search for potentially conflicting groups. A Markov Chain Monte Carlo (MCMC) algorithm was used to generate phylogenetic trees with Bayesian probabilities from the concatenated four-loci dataset using MrBayes v. 3.2.6 (Ronquist et al. 2012). Two analyses of four MCMC chains were run from random trees, trees were sampled every 100 generations and 25 % of them were discarded as the burn-in phase. Posterior probabilities (pp) were determined from the remaining trees. The sequences generated during this study and the alignments used in the phylogenetic analyses were deposited in GenBank and TreeBASE, respectively.
Table 1.
Isolates and GenBank accession numbers used in the phylogenetic analyses.
| Taxa | Strain number1 | GenBank accession numbers2 | References | |||
|---|---|---|---|---|---|---|
| ITS | LSU | rpb2 | tef1 | |||
| Alternaria tenuissima | CBS 918.96 | – | KC584311 | KC584435 | KC584693 | Woudenberg et al. 2013 |
| Bambusicola didymospora | MFLUCC 10-0557 | KU940116 | KU863105 | KU940163 | KU940188 | Dai et al. 2017 |
| B. loculata | MFLU 15-0056 | KP761732 | KP761729 | KP761715 | KP761724 | Dai et al. 2015 |
| B. pustulata | MFLUCC 15-0190 | KU940118 | KU863107 | KU940165 | KU940190 | Dai et al. 2017 |
| B. splendida | MFLUCC 11-0611 | KU940121 | KU863110 | KU940168 | – | Dai et al. 2017 |
| Coniothyrium palmicola | CBS 161.37 | JX681086 | JX681086 | – | – | Verkley et al. 2014 |
| Dendrothyrium longisporum | CBS 824.84 | JX496115 | JX496228 | – | – | Verkley et al. 2014 |
| Dydimella exigua | CBS 183.55 | NR135936 | EU754155 | GU357800 | KR184187 | De Gruyter et al. 2009, Schoch et al. 2009, Kim et al. 2016 |
| Keissleriella culmifida | KT 2308 | – | AB807591 | – | AB808570 | Tanaka et al. 2015 |
| K. quadriseptata | KT 2292 | NR145135 | AB807593 | – | AB808572 | Tanaka et al. 2015 |
| Latorua caligans | CBS 576.65 | NR132923 | KR873266 | – | – | Crous et al. 2015a |
| Leptosphaeria doliolum | CBS 505.75 | JF740205 | GQ387576 | KY064035 | GU349069 | De Gruyter et al. 2013, Schoch et al. 2009 |
| Lophiostoma arundinis | AFTOL-ID 1606 | – | DQ782384 | DQ782386 | DQ782387 | Schoch et al. 2009 |
| Macrodiplodiopsis desmazieri | CBS 140062 | NR132924 | KR873272 | – | – | Crous et al. 2015a |
| Magnicamarosporium iriomotense | KT 2822 | AB809640 | AB807509 | – | AB808485 | Tanaka et al. 2015 |
| Massarina phragmiticola | CBS 110446 | – | DQ813510 | – | – | Kodsueb et al. 2007 |
| Montagnula bellevaliae | MFLUCC 14-0924 | KT443906 | KT443902 | – | – | Hongsanan et al. 2015 |
| M. scabiosae | MFLUCC 14-0954 | KT443907 | KT443903 | – | – | Hongsanan et al. 2015 |
| Murilentithecium clematidis | MFLUCC 14-0562 | KM408757 | KM408759 | KM454447 | KM454445 | Wanasinghe et al. 2014 |
| Neobambusicola strelitziae | CBS 138869 | NR 137945 | KP004495 | – | MG976037 | Crous et al. 2014, this study |
| Palmiascoma gregariascomum | MFLUCC 11-0175 | KP744452 | KP744495 | KP998466 | – | Liu et al. 2015 |
| Parabambusicola bambusina | H 4321 | – | AB807536 | – | AB808511 | Tanaka et al. 2015 |
| Paraconiothyrium brasiliense | CBS 122851 | JX496036 | JX496149 | – | – | Verkley et al. 2014 |
| Phoma herbarum | CBS 615.75 | NR135967 | EU754186 | KP330420 | KR184186 | Aveskamp et al. 2009, Chen et al. 2015 |
| Pleurophoma ossicola | CPC 24985 | KR476737 | KR476770 | – | – | Crous et al. 2015b |
| Polyschema congolensis | CBS 542.73 | – | EF204502 | EF204486 | – | Shenoy et al. 2010 |
| P. terricola | CBS 301.65 | – | EF204504 | EF204487 | – | Shenoy et al. 2010 |
| Pseudobambusicola thailandica sp. nov. | BCC 79462 | MG926559 | MG926560 | MG926561 | MG926562 | This study |
| Pseudoleptosphaeria etheridgei | CBS 125980 | NR111620 | JF740291 | – | – | De Gruyter et al. 2013 |
| Pseudoxylomyces elegans | KT 2887 | – | AB807598 | – | AB808576 | Tanaka et al. 2015 |
| Setoseptoria arundinacea | KT 552 | – | AB807574 | – | AB808550 | Tanaka et al. 2015 |
| Stemphylium vesicarium | CBS 191.86 | KC584239 | JX681120 | KC584471 | KC584731 | Woudenberg et al. 2013 |
| Sulcatispora acerina | KT 2982 | LC014597 | LC014610 | – | LC014615 | Tanaka et al. 2015 |
| S. berchemiae | KT 1607 | AB809635 | AB807534 | – | AB808509 | Tanaka et al. 2015 |
| Trematosphaeria pertusa | CBS 122368 | NR132040 | FJ201990 | FJ795476 | KF015701 | Zhang et al. 2009 |
1 BCC: BIOTEC Culture Collection, Thailand; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at CBS; KT and H: Culture collection of K. Tanaka and K. Hirayama, housed at the National Institute of Agrobiological Science, Japan (MAFF); MFLU: Mae Fah Laung University Herbarium, Chiang Rai, Thailand; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand.
2 ITS: internal transcribed spacer regions 1 & 2 including 5.8S nrRNA gene; LSU: large subunit of the nrRNA gene, rpb2: partial RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-α gene. Sequences generated in the present study are in bold.
Chromatography and spectral methods
1D and 2D nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance III 700 spectrometer with a 5 mm TXI cryoprobe (1H 700 MHz, 13C 175 MHz) and a Bruker Avance III 500 (1H 500 MHz, 13C 125 MHz) spectrometer, UV spectra were recorded with a Shimadzu UV-2450 UV−Vis spectrophotometer and optical rotations were measured on a Perkin-Elmer 241 polarimeter. Analytical HPLC was carried out on an Agilent 1200 Series, equipped with degasser, binary pump SL, autosampler and connected to a diode array detection/light scattering detector Corona Ultra RS. A Waters C18 Acquity UPLC BEH column (2.1 × 50 mm, 1.7 μm) was used as stationary phase. The mobile phase consisted of H2O + 0.1% formic acid (solvent A) and acetonitrile + 0.1% formic acid (solvent B) with the following gradient: 0–0.5 min 5% B, 0.5–20 min 100% B, 20–30 min 100% B; injection volume was 2 µl, flow rate 600 µl/min.
HPLC-ESI-MS spectra were recorded on an ion trap mass spectrometer [scan range 100–2000 m/z, capillary voltage 4000 V, dry temperature 250 °C] (amaZon speed, Bruker) and HR-ESIMS spectra on a time-of-flight (TOF) MS [scan range 250–25000 m/z, capillary voltage 4500 V, dry temperature 200 °C] (MaXis, Bruker). In parallel, UV/Vis spectra in the range of 200–600 nm were recorded.
Chemicals and solvents were obtained from AppliChem GmbH (Darmstadt, Germany), Avantor Performance Materials (Deventer, Netherlands), Carl Roth GmbH & Co. KG (Karlsruhe, Germany) and Merck KGaA (Darmstadt, Germany) in analytical and HPLC grade.
Fermentation and extraction
A seed culture was prepared as follows: five mycelial plugs (0.5 × 0.5 cm2) were cut from actively growing colonies maintained on YM 6.3 agar (malt extract 10 g/l, D-glucose 4 g/l, yeast extract 4 g/l, agar 20 g/l, pH 6.3 before autoclaving) and placed into a 500 mL Erlenmeyer flask containing 200 mL Q6½ medium (D-glucose 2.5 g/l, glycerol 10 g/l, cotton seed flour 5 g/l, pH 6.3) and incubated on a rotary shaker for 96 hours at 24 °C and 140 rpm. 20 mL of the seed culture were added into 10 × 1000 ml sterile Erlenmeyer flasks with 500 ml of Q6 ½ medium (5 l total) and incubated on a rotary shaker (288 hours, 24 °C, 140 rpm).
Biomass and supernatant were separated by means of centrifugation and filtration. The mycelia were extracted twice with acetone (2 l), the extract was evaporated in vacuo and the remaining aqueous phase extracted with equal amounts of ethyl acetate three times. One percent (1 %) of Amberlite XAD-16N was given to the culture broth and stirred for 1 h. After filtration, the XAD resin was extracted as described above. 220 mg and 88 mg of mycelial and supernatant crude extracts were obtained, respectively.
Isolation of the compounds 1–8
The supernatant crude extract was fractionated on preparative HPLC (Gilson GX270 Series HPLC system). The reversed phase C18 column (Nucleodur 150/40, 10 µm, 110 Å; with a precolumn VP 100/10; Macherey-Nagel) was used as a stationary phase and the mobile phase was composed of deionised water + TFA 0.05 % (Milli-Q, Millipore, Schwalbach, Germany; solvent A) and acetonitrile (ACN) + TFA 0.05 % (solvent B). The fractionation was accomplished with the following gradient: 15 % of B isocratic for 5 min, followed by a linear increase to 80 % B over 30 min, afterwards increasing to 100% B in 5 min and thereafter isocratic conditions at 100 % for 5 min. In total, 7 compounds were obtained from the supernatant crude extract: Compound 1 (thailanone A; 1 mg) was obtained at the retention time tR = 6 min, compound 2 (thailanone B; 1 mg) at tR = 4.3 min, compound 3 (thailanone C; 1.3 mg) at tR = 6.4 min, compound 4 (thailanone D; 1 mg) at tR = 8.1 min; compound 5 (thailanone E; 4.2 mg) at tR = 8.2, compound 6 (thailanone F; 1.6 mg) at tR = 8.6 min) and compound 7; monocerin (7.8 mg) at tR = 9.1 min. The mycelial crude extract was chromatographed in a similar manner as described above, yielding 77.8 mg of deoxyphomalone (8, tR = 11.2 min) but none of the other compounds.
Evaluation of antimicrobial activities
Minimum inhibitory concentrations (MIC) of compounds 1–8 were determined in serial dilution assays against Bacillus subtillis DSM10, Mucor plumbeus MUCL 49355 and Candida tenuis MUCL 29892 as described previously by Chepkirui et al. (2016). The assays were carried out in 96-well microtiter plates in YMG (yeast-malt-glucose) medium for filamentous fungi and yeasts and MH (Müller-Hinton) medium for the bacterium. For all tested compounds, the starting concentration was 100 µg/mL and final 0.78 µg/mL.
Water agar plate assay
The fungal cultures were tested in the water agar plate assay against Caenorhabditis elegans nematodes (wild type strain, see Ashrafi et al. 2017), in a similar manner as previously described by Stadler et al. (1994). After 3–7 days, nematicidal effects became visible by many dead and immotile nematodes in the vicinity of the mycelia. Fungal colonies exhibiting toxic effects were selected for submerged cultivation and production/isolation of nematicidal compounds.
Microtiter plate assay for nematicidal activities
The nematicidal activity against C. elegans of all isolated compounds was determined by a slightly modified method (Stadler et al. 1994, Kuephadungphan et al. 2017 and Ashrafi et al. 2017). C. elegans was inoculated monoxenically on nematode agar (soy peptone 2 g/l, NaCl 1 g/l, agar-agar 20 g/l) and, after autoclaving, the following ingredients were added as sterile filtered solutions: cholesterol (1 mg/mL dissolved in EtOH) 0.5 ml, 1M CaCl2 1 mll, 1M MgSO4 1 ml, 40 mM potassium phosphate buffer 12.5 ml; pH 6.8) with living Escherichia coli DSM498 (1 ml of a suspension containing approximately 10 cells/ml, pre-inoculated for 12 h at 37 °C) and the plates were incubated at 21 °C for 4–5 days. Thereafter, nematodes were washed down from the plates with M9 buffer (3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl and, after autoclaving, the addition of 1 ml 1 M MgSO4). Finally, a nematode suspension of approximately 500 nematodes/ml in M9 buffer was prepared and used in the microtiter plate assay.
The assay was performed in 24-well microtiter plates at four concentrations (100, 50, 25 and 12.5 µg/ml) for each compound. Ivermectin was used as a positive control, while methanol was used as a negative control. The plates were incubated at 20 °C in the dark and nematicidal activity was recorded after 18 h of incubation and expressed as LD90 (i.e. concentration causing over 90 % immobility of the nematodes).
Antifungal activity assay against Phellinus tremulae
Growth inhibition of Phellinus tremulae CBS 123.40 for compounds 1–8 was tested according to the modified protocol published by Ayer and Jimenez (1994). The assay was performed in 24-well microtiter plates where 1 mL of YM agar was added in each well and thereafter the compounds were dissolved in methanol (100, 50, 25 and 12.5 µg/ml) and added to the wells. Shortly after the media solidified, 0.5 × 0.5 mm2 agar plugs of actively growing colonies of Ph. tremulae CBS 123.40, grown on a YM 6.3 agar plate, were placed in each well of the microtiter plate. Nystatin and methanol were used as positive and negative controls, respectively, together with control wells without additives. Inhibition of the radial growth of the colonies of Ph. tremulae CBS 123.40 relative to the control was recorded as a positive result. The radial growth was measured after 3, 5, 7 and 9 d.
Phytotoxic activity assay
Phytotoxic activities were carried out by germination and seedling growth bioassay against Setaria italica and Lepidum sativum according to the protocol from Anke et al. (1989). The amount of 100 µg/paper disc of compound was tested; as a positive control herbicide methyl vilogen dichloride hydrate was used. The negative controls were the seeds only and the solvent alone (the one used for dissolving the compounds).
Results and discussion
Molecular phylogenetic analysis
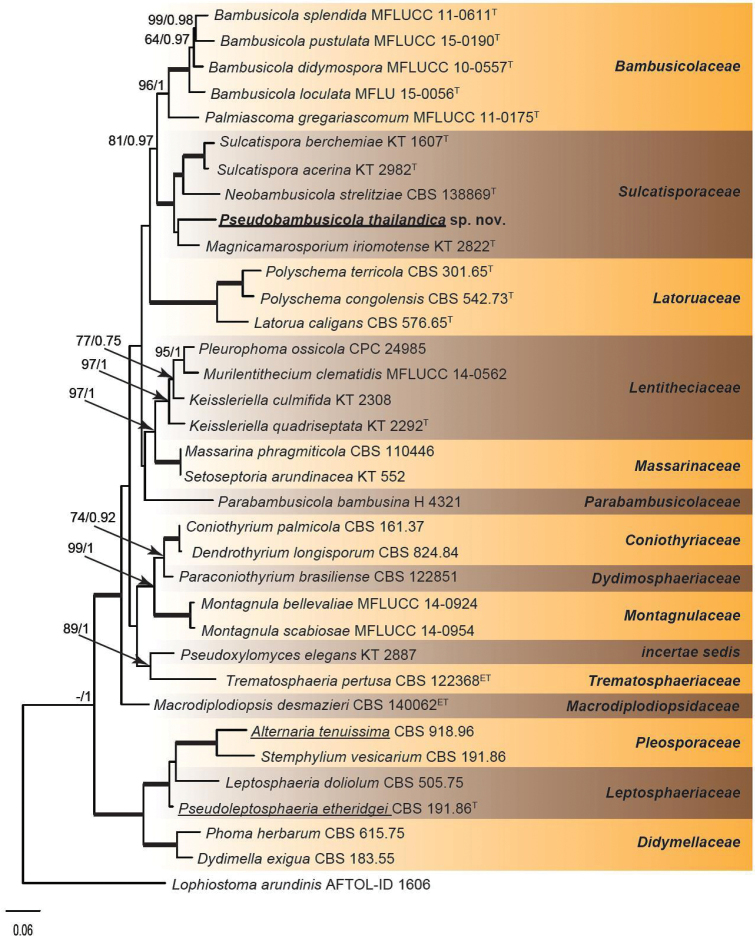
The combined dataset consisted of 35 taxa with 3126 characters of which 396 bp corresponded to ITS, 853 bp to LSU, 904 bp to rpb2 and 973 bp to tef1. The alignment had 100% representation for LSU, 74% for ITS, 46% for rpb2 and 57% for tef1. The phylogenetic tree (Fig. 1) shows two fully supported main clades, corresponding to the sub-orders Massarineae and Pleosporineae (Pleosporales, Dothideomycetes). In the Massarineae, eleven clades representing families are shown, i.e., Bambusicolaceae (96%, 1 pp), Coniotyriaceae (100%, 1 pp), Didymosphaeriaceae, Latoruaceae (100%, 1 pp), Lentitheciaceae (97%, 1 pp), Macrodiplodiopsidaceae, Massarinaceae (100%, 1 pp), Montagnulaceae (100%, 1 pp), Parabambusicolaceae, Sulcatisporaceae (100%, 1 pp) and Trematosphaeriaceae and an additional subclade comprising Pseudoxylomyces elegans. In the phylogenetic tree (Fig. 1), the sequence data of the new species indicate a systematic position in an independent branch in the Sulcatisporaceae close to Magnicamarosporium eriomotense without any support.
Figure 1.
Phylogenetic tree (RAxML) inferred from the DNA sequence data of four loci (ITS, LSU, tef1 and rpb2) of Pseudobambusicola thailandica and related species in Pleosporales (Dothideomycetes). The new taxon is indicated in bold. Taxa reported to produce deoxyphomalone are indicated by an underlined. Maximum likelihood bootstrap values ≥ 70 % and Bayesian posterior probabilities ≥ 0.95 are shown at the nodes and the scale bar indicates the number of expected mutations per site. Clades with 100 BML and 1 PP are indicated by thickened lines . The tree was rooted to Lophiostoma arundinis (AFTOL-ID 1606). T = ex-type strain; ET = epitype strain.
Taxonomy
Pseudobambusicola
Hern.-Restr. & Crous gen. nov.
MB824299
Etymology.
The name reflects its morphological similarity of the type species to the asexual morphs of Bambusicola and Neobambusicola.
Type species.
Pseudobambusicola thailandica Hern.-Restr. & Crous.
Diagnosis.
Differs from Neobambusicola in having conidiomata with a neck, the presence of globose to subglobose thick-walled cells adjacent to the conidiomata and the production of chlamydospores in culture.
Mycelium composed of hyaline to pale brown, septate, smooth to slightly verruculose, hyphae. Conidiomata pycnidial, semi- or entirely immersed in the agar, solitary or aggregated, erumpent, globose with a neck, opening via central ostiole, dark brown, surrounded by dark brown, smooth to slightly verruculose hyphae, at the base globose to subglobose, thick-walled cells often present. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic with periclinal thickening at the conidiogenous locus, subcylindrical to ampulliform, hyaline, smooth. Conidia exposed in white, mucous drops at the ostioles of the pycnidia, composed by macro- and microconidia. Macroconidia produced in white, mucous heads, solitary, fusoid-ellipsoid, apex bluntly to subobtusely rounded, tapering to a distinctly truncate base, prominently guttulate, hyaline, smooth, 0–3-septate. Microconidia produced in the same pycnidia as macroconidia, solitary, oblong to cuneiform, non-guttulate to slightly guttulate, hyaline, smooth, aseptate. Chlamydospores brown, terminal at the tips of vegetative hyphae, in chains. Sexual morph not observed.
Pseudobambusicola thailandica
Hern.-Restr. & Crous sp. nov.
MB824300
Etymology.
The epithet refers to Thailand, where this species was collected.
Type.
THAILAND. Lop Buri Province: Chai Badan, Wang Kan Lueang Arboretum, Wang Kan Lueang Waterfall, on twig (unidentified), 14 Jul 2015, M. Hernández-Restrepo, MHR 1534 (holotype: BBH 42022!, culture ex-type BCC 79462!).
Description of fungal structures on SNA.
Mycelium composed by hyaline to pale brown, septate, smooth to slightly verruculose, hyphae, 1–2.5 µm wide. Conidiomata pycnidial, semi- or entirely immersed in the agar, solitary or aggregated, erumpent, globose, sometimes with a neck, opening via central ostiole, dark brown, 63–360 µm diam., sometimes with a cylindrical neck 50–125 × 40–50 µm, opening via central ostiole; at the base of the conidiomata are often present globose to subglobose cells, thick-walled, 5–9 µm wide; conidiomata surrounded by dark brown, smooth to slightly verruculose hyphae, 2–2.5 µm wide. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic with periclinal thickening, subcylindrical to ampulliform, hyaline, smooth, 6.5–7 × 2.5–4 µm. Conidia exposed in white, mucous drops at the ostiole of pycnidia, composed by macro- and microconidia. Macroconidia produced in white, mucous heads, solitary, fusoid-ellipsoid, apex bluntly to subobtusely rounded, tapering to a distinctly truncate base, mostly straight, but sometimes slightly curved, prominently guttulate, hyaline, smooth, 0–3-septate, 10–20 × 2–4(–6) µm. Microconidia produced in the same pycnidia with macroconidia, solitary, oblong to cuneiform, non-guttulate to slightly guttulate, hyaline, smooth, aseptate, 2–4(–5.5) × 1–2 µm, apex rounded, base truncate. Chlamydospores brown, terminal, in chains, 16–38 × 5–6 µm. Sexual morph not observed.
Culture characteristics.
Colonies on OA at 25 °C reaching 24 mm diam. in 2 weeks, elevated, with dense cottony mycelium at the centre, mouse grey, margin whitish, effuse to fimbriate; reverse dark mouse grey.
Notes.
Pseudobambusicola is introduced here for a pycnidial coelomycete producing two kinds of conidia. Morphologically, it is similar to the species of Bambusicola and Neobambusicola. However, asexual morphs in Bambusicola are characterised by brown or pale brown conidia and annellidic rather than phialidic conidiogenous cells and hyaline conidia as in Pseudobambusicola (Dai et al. 2012, 2017). Neobambusicola is a monotypic genus erected for N. strelitziae, first described from South Africa growing on necrotic leaf tissue associated with Phyllachora strelitziae (Phyllachoraceae, Phyllachorales, Sordariomycetes) (Crous et al. 2014). Both genera are similar in having pycnidial conidiomata and phialidic conidiogenous cells that produce fusoid-ellipsoid macro- and subcylindrical microconidia. However, in the new genus, the conidiomata are surrounded by dark brown, smooth to slightly verruculose hyphae and, in mature conidiomata, a cylindrical neck is often present; furthermore, chlamydospores can be present in culture. Although both genera belong to the Sulcatisporaceae (Pleosporales, Dothideomycetes), they are placed in different clades, Neobambusicola is more closely related to Sulcatispora (100 %, 1 pp), while Pseudobambusicola was placed in a distinct branch with Magnicamarisporium (Fig. 1). Additionally, based on LSU, ITS and tef1 sequences, P. thailandica is 97 % (KP004495) and 83 % (KP004467) and 93 % similar to N. strelitziae, respectively.
Figure 2.
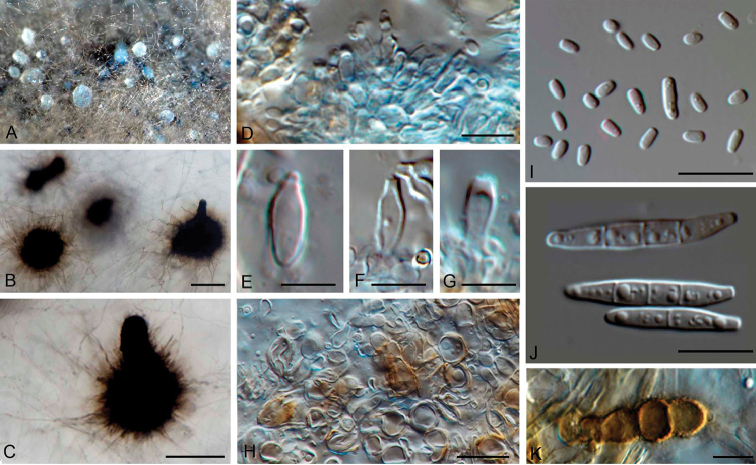
Pseudobambusicola thailandica (BCC 79462) on SNA. A Colony overview B–C Pycnidia D–G Conidiogenous cells H globose to subglobose cells, thick-walled, at the base of the conidiomata I Microconidia J Macroconidia K Chlamydospores. Scale bars: 200 µm (B), 100 µm (C), 10 µm (D, H, I–K), 5 µm (E–G).
Water agar plate assay
Out of 66 fungal strains investigated, 18 exhibited antagonistic activity towards nematodes in the water agar assay. Of those, 3 strains produced compounds with nematicidal activity in submerged culture, while in 5 strains, antimicrobial activity was observed. Extracts from P. thailandica (BCC 79462) submerged fermentation displayed strong activity towards nematodes and were subjected to extensive chromatographic studies as described in the Experimental part.
Structure elucidation of compounds 1–8
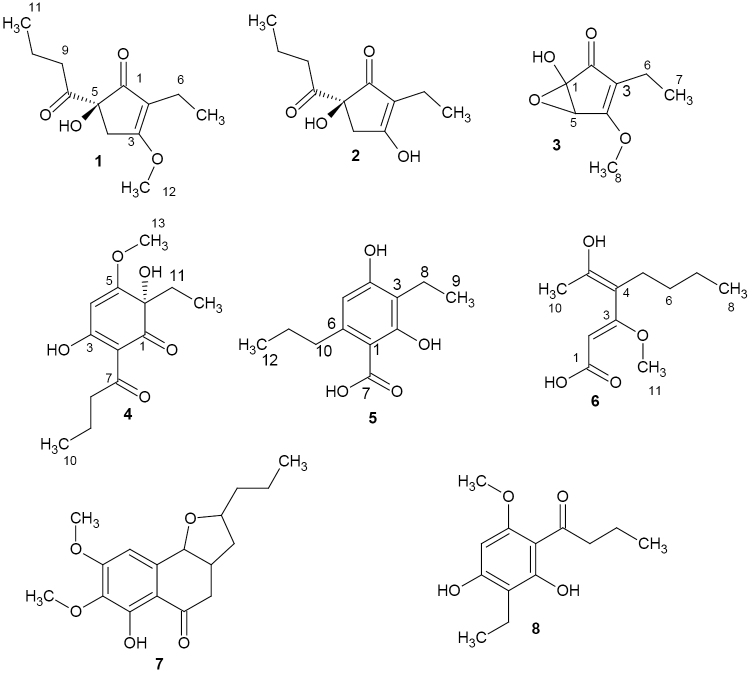
Fractionation of the crude extracts obtained from submerged cultures of P. thailandica (BCC 79462) resulted in the identification of six previously undescribed polyketides for which the authors propose the trivial names thailanones A–F (1–6) and two known compounds, monocerin and deoxyphomalone, 7 and 8 (see chemical structures in Fig. 3). The NMR spectroscopic data are compiled in Tables 2 and 3 and the spectra and chromatograms are compiled in the Suppl. material 1.
Figure 3.
Chemical structures of thailanones A–F (1–6), monocerin (7) and deoxyphomalone (8).
Table 2.
NMR spectroscopic data for compounds 1–3 in D6-acetone (1H NMR at 700 MHz; 13C at 500 MHz).
| 1 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | 13C | DEPT | 1H/HSQC | 13C | 1H/HSQC | DEPT | ||
| 1 | 202.6 | C | 202.6 | 83.9 | C | |||
| 2 | 120.2 | C | 119.0 | 194.1 | C | |||
| 3 | 187.9 | C | 191.2 | 120.3 | C | |||
| 4 | 38.9 | CH2 | 2.61 (s),3.29 (s) | 43.8 | 2.41 (s)2.88 (s) | 170.1 | C | |
| 5 | 86.3 | C | 84.4 | 58.3 | CH | 3.46 (s) | ||
| 6 | 15.5 | CH2 | 2.11 (q), J= 7.53 Hz | 15.2 | 2.11 (q), J= 7.53 Hz | 16.7 | CH2 | 2.16 (q), J=7.53 Hz |
| 7 | 12.7 | CH3 | 0.93 (t), 7.53 Hz | 12.8 | 0.93 (t), 7.53 Hz | 13.2 | CH3 | 0.78 (t), J=7.53 Hz |
| 8 | 210.7 | C | 210.1 | 57.4 | CH3 | 3.84 (s) | ||
| 9 | 40.1 | CH2 | 2.61 (m), 2.74(m) | 38.9 | 2.53 (dt), J=7.10, 17.96 Hz2.68 (m), J=7.10, 17.96 Hz | |||
| 10 | 17.8 | CH2 | 1.55 (m) | 17.6 | 1.55 (m) | |||
| 11 | 14.0 | CH3 | 0.88 (t), 7.42 Hz | 13.9 | 0.86 (t), 7.31 Hz | |||
| 12 | 58.3 | CH3 | 4.05 (s) | |||||
Table 3.
NMR spectroscopic data for compounds 4–6 in D6-acetone (1H NMR at 700 MHz; 13C at 500 MHz).
| 4 | 5 | 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | 13C | DEPT | 1H/HSQC | 13C | DEPT | 1H/HSQC | 13C | DEPT | 1H/HSQC |
| 1 | 196.36 | C | – | 104.2 | C | 164.3 | C | ||
| 2 | 106.4 | C | – | 164.7 | C | 88.7 | CH | 5.42(s) | |
| 3 | 190.6 | C | – | 116.1 | C | 171.9 | C | ||
| 4 | 96.0 | CH | 5.56 (s) | 160.7 | C | 112.3 | C | ||
| 5 | 177.9 | C | – | 111.1 | CH | 6.36(s) | 25.0 | CH2 | 2.35 (t), J=7.74 Hz |
| 6 | 79.1 | C | – | 146.2 | C | 32.8 | CH2 | 1.40 (p), J=7.31Hz | |
| 7 | 203.2 | C | – | 174.7 | C | 23.6 | CH2 | 1.34 (m), | |
| 8 | 41.5 | CH2 | 2.92 (m) | 16.8 | CH2 | 2.64 (q), J=7.53 Hz | 14.7 | CH3 | 0.91 (t), J=7.31 Hz |
| 9 | 19.34 | CH2 | 1.65 (m) | 13.7 | CH3 | 1.08 (t), J=7.53 Hz | 159.3 | C | |
| 10 | 14.25 | CH3 | 0.96 (t), J=7.31Hz | 39.3 | CH2 | 2.86 (t), 7.31Hz | 17.7 | CH3 | 2.19 (s) |
| 11 | 36.12 | CH2 | 1.79 (dq), J=7.53, 13.34 1.92 (dq), J=7.53, 13.34 | 26.0 | CH2 | 1.59 (sext), J=7.31 Hz | |||
| 12 | 8.2 | CH3 | 0.79 (t), J=7.53 | 14.6 | CH3 | 0.93 (t), J=7.31 | |||
| 13 | 57.7 | CH3 | 3.94 (s) | ||||||
| OH | 4.44 (s) | ||||||||
Compound 1 (thailanone A) was isolated as a white solid from the supernatant with the molecular formula C12H18O4 and four degrees of unsaturation established from the HRMS data. 13C and DEPT NMR data revealed the presence of 12 carbons in the molecule: three methyl groups, four methylene groups and five quaternary carbons (Table 2). 1H NMR spectra on the other hand revealed the presence of two methyl triplets at δ 0.88 (H-11) and δ 0.93 (H-7) together with a methoxy group singlet resonating at δ 4.05 (H-12).
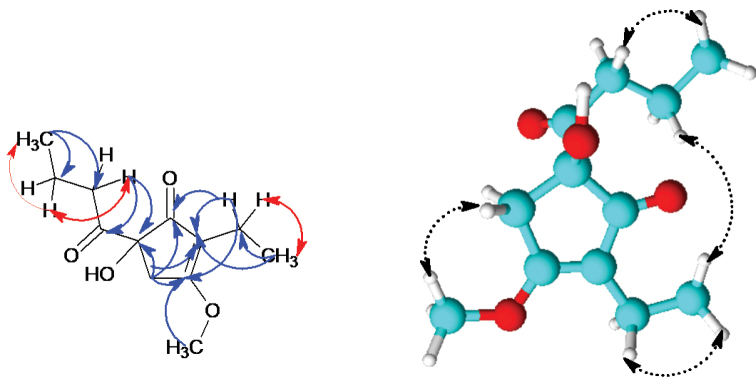
HMBC correlations of H-4 to C-1/C-2/C-3-C-5/C-6, H-6 to C-1/C-2/C-2/C-7 and H-9 to C-5/C-8/C-10/C-11 indicated the presence of an isohumulone moiety differing in the ring substitution (Fig. 4). Furthermore, HMBC correlations between H-11 to C-10/C-9 and H-7 to C-2/C-6 were observed. These correlations were further supported by the COSY correlations observed between H-10 and H-9/H-11 and H-6 and H-7. The methoxy proton H-12 showed a HMBC correlation to C-3. Therefore, the structure of compound 1 was established as 5-butanoyl-2-ethyl-5-hydroxy-3-methoxycyclopent-2-en-1-one.
Figure 4.
COSY, HMBC and ROESY correlations of 1.
Compound 2 (thailanone B) was obtained from the supernatant as a white solid. From the HR mass spectrum, its molecular formula was deduced as C11H16O4 with four degrees of unsaturation. Analysis of the 1H NMR and 13C NMR spectra of 2 suggested a closely related structure to that of 1 with the difference being the absence of the methoxy group at C-3. Further, the HMBC and COSY correlations observed were similar to those observed for 1. Hence, the structure was elucidated as (5S)-5-butanoyl-2-ethyl-3,5-dihidroxycyclopent-2-en-1-one.
The white solid compound 3 (thailanone C) with the molecular formula C8H10O4 and 4 degrees of unsaturation deduced from HR mass spectrum was isolated from the supernatant. The 1D and 2D NMR data of 3 suggested that the molecule has a closely related structure to 1 with one of the side chains missing. Analysis of the 1H NMR spectrum indicated the presence of a triplet at δ 0.78 (H-7) and a singlet at δ 3.84 (H-8) for methyl and methoxy groups, respectively. A COSY correlation was observed between H-6/H-7. Further, H-7 exhibited HMBC correlations to C-3/C-6, while H-6 was correlating to C-2/C3/C-4/C-7 in the HMBC spectra. H-5 on the other hand showed HMBC correlations to C-1/C-2/C-3/C-4. The epoxide ring was assigned based on the chemical shifts of C-1 (δ 86.3) and C-5 (δ 58.3) and also the established molecular formula. The methoxy group showed HMBC a correlation to C-4 (δ 170.1). The structure of 3 was established as 3-ethyl-1-hydroxy-4-methoxy-6-oxabicyclo[3.1.0]hex-3-en-2-one.
Compound 4 (thailanone D) was isolated as white solid. The molecular formula C13H18O5 was deduced from the HRMS data. 13C and DEPT NMR data indicated the presence of two methyl groups, a methoxy group, three methylene groups and five quaternary carbons in the molecule (Table 3). The 1H NMR spectra revealed 2 methyl triplets at δ 0.79 (C-12) and δ 0.96 (C-10). In addition, a methoxy singlet was observed at δ 3.94 (C-13). Networks of COSY correlations were observed between H-9 and H-8/H-10 and H-11 to H-12. In the HMBC spectra, H-4 showed correlations to C-2/C-3/C-5/C-6. Proton H-8 was correlating to C-2/C-7/C-9 /C-10, while the methyl protons H-10 were correlating to C-8/C-9 in the HMBC spectra. Further, H-11 showed HMBC correlations to C-1/C-5/C-6/C-12. The hydroxy group singlet at δ 4.44 showed HMBC correlations to C-1/C-5/C-6/C-11. Cross peaks in the ROESY spectra between the methoxy proton H-13 (δ 3.94) and the aromatic proton H-4 (δ 5.56) were observed. No ROESY correlations were observed between the hydroxy group proton (δ 4.44) and H-11/H-12/H-13. Hence the relative stereochemistry at C-6 can be assigned as S. The structure of compound 4 was elucidated as (6S)-2-butanoyl-6-ethyl-3,6-dihidroxy-5-methoxycyclohexa-2,4-dien-1-one.
The white solid compound 5 (thailanone E) showed the molecular formula C12H16O4 as deduced from HRMS data. The 1D and 2D NMR data of 5 suggested a closely related structure to 4 with the difference being in the ring substitution: The C-7 to C-10 chain and the carbon resonating δ 79.1 in 4 were missing. Analysis of the COSY spectra revealed correlations of H-8 to H-9 and H-11 to H-10/H-12. HMBC correlations of H-5 to C-1/C-3/C-4/C-10, H-8 to C-2/C-3/C-4/C-9, H-10 to C-1/C-5/C-6/C-11/C-12 and H-12 to C-10/C-11 were observed. Hence, the structure of the compound 5 was elucidated as 3-ethyl-2,4-dihydroxy-6-propylbenzoic acid.
Compound 6 (thailanone F) was obtained from the supernatant as a white solid with the molecular formula C11H18O4 established from HRMS data. Analysis of the 1H NMR data revealed a methyl group triplet at δ 0.91 (H-8), a methyl group singlet at δ 2.19 (H-10) and a methoxy singlet at 3.87 (H-11). HMBC correlations of H-2 to C-1/C-3/C-4 and H-10 to C-4/C-9 were recorded. Furthermore, HMBC correlations between H-5 and C-3/C-4/C-6/C-7/C-9, H-6 and C-4/C-5/C-7/C-8, H-7 and C-5/C-6/C-8 and H-8 and C-6/C-7 were observed. These correlations were further supported by the COSY correlations of H-6 to H-5/H-7 and H-7 to H-6/H-8. Cross peaks between H-2 (5.42) and methoxy protons H-11 (δ 3.87) were not observed in the ROESY spectra, indicating that the olefinic bond at position two had E configuration. The olefinic bond between C-4 (δ 112.3) and C-9 (δ 159.3) was assigned E configuration, since H-5 (δ 2.35) and H-10 (δ 2.19) also did not correlate in the ROESY spectra. The structure of the compound 6 was established as (2Z, 4E)-4-(1-hydroxyethylidene)-3-methoxyoct-2-enoic acid.
Monocerin (7) and deoxyphomalone (8) were identified by comparing their NMR and HRMS data with those reported in literature (Aldridge et al. 1970, Ayer and Jimenez 1994, respectively). Monocerin was reported before as a potent herbicide and insecticide against Johnson grass and woolly aphids, respectively (Grove et al. 1979, Robeson et al. 1982), while deoxyphomalone has been reported from other pleosporalean fungi like Alternaria. To the best of the authors’ knowledge, it has not been reported previously from a species of the Sulcatisporaceae.
Biological activity
The results of the biological assays that were performed to detect antibacterial, antifungal and nematicidal activities are summarised in Table 4. Compound 6 was moderately active against M. plumbeus with MIC of 25 µg/ml, while deoxyphomalone (8) exhibited moderate activities against B. subtilis and M. plumbeus with MIC values of 12.5 and 25 µg/ml, respectively. Compounds 1–6 and 8 were also the only compounds with significant activities against M. plumbeus. The results by Ayer and Jimenez (1994), regarding the antifungal activity of phomalone and its deoxy derivative, were also repeated in the serial dilution assay. Compounds 2–7 failed to significantly inhibit growth of Ph. tremulae, whereas the new phomalone derivative 1 showed moderate inhibition and was more weakly active than the known compound 8. No phytotoxic effects in plant germination and seedling growth bioassay with S. italica and L. sativum at 100 μg/paper disk were observed for any of tested compounds.
Table 4.
Biological activities of compounds 1–8.
| Compounds | Antimicrobial activity MIC (µg/mL) | Nematicidal activity LD90 (µg/mL) | Antifungal activity (% growth inhibition at ≤ 12.5 µg/mL) | |
|---|---|---|---|---|
| Bacillus subtillis DSM10 | Mucor plumbeus MUCL 49355 | Caenorhabditis elegans | Phellinus tremulae CBS 123.40 | |
| Thailanone A (1) | ≤ 50 | – | ≤ 50 | 50 |
| Thailanone B (2) | – | – | ≤ 25 | 28.6 |
| Thailanone C (3) | – | – | ≤ 25 | 31.4 |
| Thailanone D (4) | – | ≤ 25 | ≤ 12.5 | 28.6 |
| Thailanone E (5) | – | – | ≤ 50 | 25. |
| Thailanone F (6) | – | – | ≤ 25 | 41.4 |
| Monocerin (7) | – | – | – | 28.6 |
| Deoxyphomalone (8) | ≤ 12.5 | ≤ 25 | ≤ 12.5 | 50 |
| Standards | ||||
| Nystatin # | – | ≤ 0.782 | – | 100 |
| Ciprofloxacin †† | ≤ 0.78 | – | – | – |
| Ivermectin ‡‡ | – | – | ≤ 12.5 | – |
| Methanol | – | – | – | – |
No activity against Candida tenuis, Setaria italica and Lepidum sativum was observed for any of tested compounds up to concentrations of 100 µg/mL. # Nystatin-antifungal reference; †† Ciprofloxacin-antibacterial reference; ‡‡ Ivermectin-nematicidal reference.
Compound 7 was reported as a potent herbicide and insecticide against Johnson grass and woolly aphids, respectively (Grove et al. 1979, Robeson et al. 1982). It was first isolated from a fungus described as Phoma etheridgei (Hutchison et al. 1994) and recently, 7 was also isolated from Alternaria tenuissima (Pleosporaceae) and the bioactivity was tested against E. coli (Anyanwu and Sorensen 2015). It has previously been reported to be active against the pathogenic basidiomycete Phellinus tremulae, which infects different species of poplar (Ayer and Jimenez 1994). This fungus causes extensive damage to hardwoods in North America and, in Canada, Ph. tremulae seriously reduces the economic value of Populus tremuloides (Trifonov et al. 1992). This prompted the authors to re-evaluate the compound in an antifungal assay against Ph. tremulae, in which all purified metabolites from P. thailandica were tested.
Moreover, the phytotoxic activity of terrein and congeners on plant growth and induction of lesions on fruit surfaces were previously investigated by Zaehle et al. (2014). Terrein, a major metabolite of Aspergillus terreus, resembles 1 and 7 in its chemical structure. The authors therefore performed a phytotoxicity assay, but they did not find significant effects of these compounds on germination and shoot/root elongation.
Conclusion
In the course of this investigation of the fungal specimens collected in the rainforest of Thailand, several nematode-antagonistic strains were detected. The use of nematodes as test organisms can detect bioactivity from the compounds that are not detected by whole-cell-based screening for antimicrobial activities. As an outcome of the antihelmintic screening, six new compounds (thailanones 1–6) and two known compounds, deoxyphomalone (7) and monocerin (8) were isolated and further evaluated regarding their antifungal activity. Even though these results are just preliminary and the biological activities of the new compounds are rather moderate, they are very likely to play an important chemo-ecological role in the natural habitat of the fungal producer organisms, e.g. to protect against nematode predation. The authors have not yet tried to detect the metabolites on water agar in the presence of nematodes because of the experimental limitations that would first need to be overcome. The moderate activity of the new compounds (as compared to, for example, the standard ivermectin, which is at least ten times more active) probably precludes their adoption as a nematicidal agent that could serve as a candidate for an antihelmintic drug or an agrochemical nematicide. On the other hand, the fungus might turn out to be a candidate for a biocontrol agent to act as an antagonist of pathogenic nematodes and fungi.
Supplementary Material
Acknowledgments
This project has received funding from the European Union’s Horizon 2020 research and innovation programme (RISE) under the Marie Skłodowska-Curie grant agreement No 645701, Project acronym “GoMyTri” (lead beneficiaries JJL, PWC and MS). Financial support by the German Academic Exchange Service (DAAD) and the Kenya National Council for Science and Technology (NACOSTI), who provided a personal PhD stipend for CC is gratefully acknowledged. We are grateful to Cäcilia Bergmann, Vanessa Stiller and Anke Skiba for expert technical assistance and to Christel Kakoschke for recording NMR spectra.
Citation
Rupcic Z, Chepkirui C, Hernández-Restrepo M, Crous PW, Luangsa-ard JJ, Stadler M (2018) New nematicidal and antimicrobial secondary metabolites from a new species in the new genus, Pseudobambusicola thailandica. MycoKeys 33: 1–23. https://doi.org/10.3897/mycokeys.33.23341
Funding Statement
European Union’s Horizon 2020 research and innovation programme (RISE) under the Marie Skłodowska-Curie grant agreement No 645701, Project acronym “GoMyTri” Kenya National Council for Science and Technology (NACOSTI)and German Academic Exchange Service (DAAD)
References
- Ahmed SA, van de Sande WW, Stevens DA, Fahal A, van Diepeningen AD, Menken SB, de Hoog GS. (2014) Revision of agents of black-grain eumycetoma in the order Pleosporales. Persoonia 33: 141–154. https://doi.org/10.3767/003158514X684744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge DC, Turner WB. (1970) MetaboIites of Helminthosporium monoceras : Structures of monocerin and related benzopyrans. Journal of the Chemical Society 18: 2598–2600. https://doi.org/10.1039/J39700002598 [DOI] [PubMed] [Google Scholar]
- Anke H, Hillen-Maske E, Steglich W. (1989) 9 ß-hydroxymarasmic acid and other sesquiterpenoids from submerged cultures of a basidiomycete. Zeitschrift für Naturforschung 44C: 7– 11.
- Anke H, Stadler M, Mayer A, Sterner O. (1995) Secondary metabolites with nematicidal and antimicrobial activity from nematophagous fungi and ascomycetes. Canadian Journal of Botany 73(Suppl. 1): S932–S939. https://doi.org/10.1139/b95-341
- Anyanwu CS, Sorensen JL. (2015) Secondary metabolites from a strain of Alternaria tenuissima Isolated from Northern Manitoba soil. Natural Products Communications 10(1): 39–42. [PubMed] [Google Scholar]
- Aveskamp MM, Verkley GJ, de Gruyter J, Murace MA, Perelló A, Woudenberg JH, Groenewald JZ, Crous PW. (2009) DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 101(3): 363–382. https://doi.org/10.3852/08-199 [DOI] [PubMed] [Google Scholar]
- Ashrafi S, Helaly SE, Schroers HJ, Stadler M, Richert-Poeggeler KR, Dababat AA, Maier W. (2017) Ijuhya vitellina sp. nov., a novel source for chaetoglobosin A, is a destructive parasite of the cereal cyst nematode Heterodera filipjevi PLoS ONE 12(7): e0180032. https://doi.org/10.1371/journal.pone.0180032 [DOI] [PMC free article] [PubMed]
- Ayer WA, Jimenez LD. (1994) Phomalone, an antifungal metabolite of Phoma etheridgei. Canadian Journal of Chemistry 72: 2326–2332. https://doi.org/10.1139/v94-296 [Google Scholar]
- Bills GF, Gloer JB. (2016) Biologically active secondary metabolites from the Fungi Microbiology Spectrum 4(6). https://doi.org/10.1128/microbiolspec.FUNK-0009-2016 [DOI] [PubMed]
- Boerema GH, de Gruyter J, Noordeloos ME, Hamers MEC. (2004) Phoma Identification Manual – Differentiation of Specific and Infra-Specific Taxa in Culture. CABI Publishing, Wallingford, 470 pp https://doi.org/10.1079/9780851997438.0000 [Google Scholar]
- Castañeda-Ruiz RF, Heredia G, Gusmao LFP, Li DW. (2016) Fungal diversity of Central and South America. In: De-Wei L. (Ed.) Biology of microfungi. Springer International Publishing, Switzerland, 197–218. https://doi.org/10.1007/978-3-319-29137-6_9
- Chen Q, Zhang Z, Zhang G, Cai L. (2015) A polyphasic approach to characterise two novel species of Phoma (Didymellaceae) from China. Phytotaxa 197: 267–281. http://dx.doi.org/10.11646/phytotaxa.197.4.4 [Google Scholar]
- Chepkirui C, Richter C, Matasyoh JC, Stadler M. (2016) Monochlorinated calocerins A-D and 9-oxostrobilurin derivatives from the basidiomycete Favolaschia calocera, Phytochemistry 132: 95–101. https://doi.org/10.1016/j.phytochem.2016.10.001 [DOI] [PubMed]
- Crous PW, Carris LM, Giraldo A, Groenewald JZ, Hawksworth DL, Hernández-Restrepo M, Jaklitsch WM, Lebrun MH, Schumacher RK, Stielow JB, van der Linde EJ, Vilcāne J, Voglmayr H, Wood AR. (2015a) The Genera of Fungi – fixing the application of the type species of generic names – G 2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus 6: 163–198. https://doi.org/10.5598/imafungus.2015.06.01.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Schumacher RK, Summerell BA, Giraldo A, Gené J, Guarro J, Wanasinghe DN, Hyde KD, Camporesi E, Gareth Jones EB, Thambugala KM, Malysheva EF, Malysheva VF, Acharya K, Álvarez J, Alvarado P, Assefa A, Barnes CW, Bartlett JS, Blanchette RA, Burgess TI, Carlavilla JR, Coetzee MP, Damm U, Decock CA, den Breeÿen A, de Vries B, Dutta AK, Holdom DG, Rooney-Latham S, Manjón JL, Marincowitz S, Mirabolfathy M, Moreno G, Nakashima C, Papizadeh M, Shahzadeh Fazeli SA, Amoozegar MA, Romberg MK, Shivas RG, Stalpers JA, Stielow B1, Stukely MJ, Swart WJ, Tan YP, van der Bank M, Wood AR, Zhang Y, Groenewald JZ. (2014) Fungal Planet description sheets: 281–319. Persoonia 33: 212–289. https://doi.org/10.3767/003158514X685680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, Hernández-Restrepo M, Sutton DA, Acharya K, Barber PA, Boekhout T, Dimitrov RA, Dueñas M, Dutta AK, Gené J, Gouliamova DE, Groenewald M, Lombard L, Morozova OV, Sarkar J, Smith MT, Stchigel AM, Wiederhold NP, Alexandrova AV, Antelmi I, Armengol J, Barnes I, Cano-Lira JF, Castañeda Ruiz RF, Contu M, Courtecuisse PR, da Silveira AL, Decock CA, de Goes A, Edathodu J, Ercole E, Firmino AC, Fourie A, Fournier J, Furtado EL, Geering AD, Gershenzon J, Giraldo A, Gramaje D, Hammerbacher A, He XL, Haryadi D, Khemmuk W, Kovalenko AE, Krawczynski R, Laich F, Lechat , Lopes UP, Madrid H, Malysheva EF, Marín-Felix Y, Martín MP, Mostert L, Nigro F, Pereira OL, Picillo B, Pinho DB, Popov ES, Rodas Peláez CA, Rooney-Latham S, Sandoval-Denis M, Shivas RG, Silva V, Stoilova-Disheva MM, Telleria MT, Ullah C, Unsicker SB, van der Merwe NA, Vizzini A, Wagner HG, Wong PT, Wood AR, Groenewald JZ. (2015b) Fungal Planet description sheets: 320–370. Persoonia 34: 167–266. https://doi.org/10.3767/003158515X688433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DQ, Bahkali AH, Bhat DJ, Zhao RL, Hyde KD. (2015) Bambusicola loculata sp. nov. (Bambusicolaceae) from bamboo. Phytotaxa 213: 122–130. http://dx.doi.org/10.11646/phytotaxa.213.2.5 [Google Scholar]
- Dai DQ, Bhat DJ, Liu JK, Chukeatirote E, Zhao RL, Hyde KD. (2012) Bambusicola, a new genus from bamboo with asexual and sexual morphs. Cryptogamie Mycologie 33: 363–379. https://doi.org/10.7872/crym.v33.iss3.2012.363 [Google Scholar]
- Dai DQ, Phookamsak R, Wijayawardene NN, Li WJ, DJ Bhat, Xu JC, Taylor JE, Hyde KD, Chukeatirote E. (2017) Bambusicolous fungi. Fungal Diversity 82: 1–105. https://doi.org/10.1007/s13225-016-0367-8 [Google Scholar]
- de Gruyter J, Aveskamp MM, Woudenberg JH, Verkley GJ, Groenewald JZ, Crous PW. (2009) Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycological Research 113: 508–519. https://doi.org/10.1016/j.mycres.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Degenkolb T, Vilcinskas A. (2016) Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: Metabolites from nematophagous ascomycetes. Applied Microbiology and Biotechnology 100: 3799–3812. https://doi.org/10.1007/s00253-015-7233-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove JF, Pople M. (1979) Metabolic products of Fusarium larvarum Fuckel. The fusarentins and the absolute configuration of monocerin. Journal of the Chemical Society, Perkin Transactions 1: 2048–2051. https://doi.org/10.1039/P19790002048 [Google Scholar]
- Helaly SE, Kuephadungphan W, Phongpaichit S, Luangsa-ard JJ, Rukachaisirikul V, Stadler M. (2017) Five unprecedented secondary metabolites from the spider parasitic fungus Akanthomyces novoguineensis Molecules 22: 991 https://doi.org/10.3390/molecules22060991 [DOI] [PMC free article] [PubMed]
- Helaly SE, Richter C, Thongbai B, Hyde KD, Stadler M. (2016) Lentinulactam, a hirsutane sesquiterpene with an unprecedented lactam modification. Tetrahedron Letters 57: 5911–5913. https://doi.org/10.1016/j.tetlet.2016.11.075 [Google Scholar]
- Hernández-Restrepo M, Gené J, Castañeda-Ruiz RF, Mena-Portales J, Crous PW, Guarro J. (2017) Phylogeny of saprobic microfungi from Southern Europe. Studies in Mycology 86: 53–97. https://doi.org/10.1016/j.simyco.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Restrepo M, Schumacher RK, Wingfield MJ, Ahmad I, Lei Cai L, Duong TA, Edwards J, Josepa Gené J, Johannes Z, Groenewald JZ, Sana Jabeen S, Khalid AN, Lombard L, Madrid H, Marin-Felix Y, Marincowitz S, Miller AN, Rajeshkumar KC, Rashid A, Sarwar S, Stchigel AM, Taylor PWJ, Zhou N, Crous PW. (2016) Fungal Systematics and Evolution: FUSE 2. Sydowia 68: 193–230. https://doi.org/10.12905/0380.sydowia68-2016-0193 [Google Scholar]
- Hongsanan S, Hyde KD, Bahkali AH, Camporesi E, Chomnunti P, Ekanayaka H, Gomes AAM, Hofstetter V, Jones EBG, Pinho DB, Pereira OL, Tian Q, Wanasinghe DN, Xu JC, Buyck B. (2015) Fungal Biodiversity Profiles 11–20. Cryptogamie Mycologie 36: 355–380. https://doi.org/10.7872/crym/v36.iss3.2015.355 [Google Scholar]
- Hutchinson LJ, Chakravarty P, Hiratsuka Y. (1994) Phoma etheridgei sp. nov. from black galls and cankers of trembling aspen (Populus tremuloides) and its potential role as a bioprotectant against the aspen decay pathogen Phellinus tremulae. Canadian Journal of Botany 72: 1424–1431. https://doi.org/10.1139/b94-175 [Google Scholar]
- Karwehl S, Stadler M. (2017) Exploitation of fungal biodiversity for discovery of novel antibiotics. In: Stadler M, Dersch P. (Eds) How to overcome the antibiotic crisis – Facts, challenges, technologies & future perspective. Current Topics in Microbiology and Immunology 398: 303–338. https://doi.org/10.1007/82_2016_496 [DOI] [PubMed]
- Katoh K, Standley DM. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. https://doi.org/10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Peever TL, Park JJ, Park CM, Gang DR, Xian M, Davidson JA, Infantino A, Kaiser WJ, Chen W. (2016) Use of metabolomics for the chemotaxonomy of legume-associated Ascochyta and allied genera. Scientific Reports 6: 20192. https://doi.org/10.1038/srep20192 [DOI] [PMC free article] [PubMed]
- Kodsueb R, Lumyong S, Ho WH, Hyde KD, Mckenzie EHC, Reewon J. (2007) Morphological and molecular characterization of Aquaticheirospora and phylogenetics of Massarinaceae (Pleosporales). Botanical Journal of the Linnean Society 155: 283–296. https://doi.org/10.1111/j.1095-8339.2007.00685.x [Google Scholar]
- Kuephadungphan W, Helaly SE, Daengrot C, Phongpaichit S, Luangsa-ard JJ, Rukachaisirikul V, Stadler M. (2017) Akanthopyrones A–D, α-pyrones bearing a 4-O-methyl-β-D-glucopyranose moiety from the spider-associated ascomycete Akanthomyces novoguineensis Molecules 22: e1202. https://doi.org/10.3390/molecules22071202 [DOI] [PMC free article] [PubMed]
- Kuhnert E, Surup F, Herrmann J, Huchd V, Müller R, Stadler M. (2015) Rickenyls A–E, antioxidative terphenyls from the fungus Hypoxylon rickii (Xylariaceae, Ascomycota). Phytochemistry 118: 68–73. https://doi.org/10.1016/j.phytochem.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Liu JK, Hyde KD, Jones EBG, Ariyawansa HA, Bhat DJ, Boonmee S, Maharachchikumbura SSN, McKenzie EHC, Phookamsak R, Phukhamsakda C, Shenoy BD, Abdel-Wahab MA, Buyck B, Chen J, Chethana KWT, Singtripop C, Dai DQ, Dai YC, Daranagama DA, Dissanayake AJ, Doilom M, D’souza MJ, Fan XL, Goonasekara ID, Hirayama K, Hongsanan S, Jayasiri SC, Jayawardena RS, Karunarathna SC, Li WJ, Mapook A, Norphanphoun C, Pang KL, Perera RH, Peršoh D, Pinruan U, Senanayake IC, Somrithipol S, Suetrong S, Tanaka K, Thambugala KM, Tian Q, Tibpromma S, Udayanga D, Wijayawardene NN, Wanasinghe DN, Wisitrassameewong K, Zeng XY, Abdel-Aziz FA, Adamčík S, Bahkali AH, Boonyuen N, Bulgakov T, Callac P, Chomnunti P, Greiner K, Hashimoto A, Hofstetter V, Kang JC, Lewis D, Li XH, Liu XZ, Liu ZY, Matsumura M, Mortimer PE, Rambold G, Randrianjohany E, Sato G, Sri-Indrasutdhi V, Tian CM, Verbeken A, von Brackel W, Wang Y, Wen TC, Xu JC, Yan JY, Zhao RL, Camporesi E. (2015) Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Diversity 72: 1–197. https://doi.org/10.1007/s13225-015-0324-y [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE), November 14, 2010, New Orleans, Louisiana, 1–8. https://doi.org/10.1109/GCE.2010.5676129
- Nirenberg HI. (1976) Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 169: 1–117. [Google Scholar]
- Rayner RW. (1970) A mycological colour chart. British Mycological Society. Commonwealth Mycological Institute, Kew, Surrey.
- Richter C, Helaly SE, Thongbai B, Hyde KD, Stadler M. (2016) Pyristriatins A and B: pyridino-cyathane antibiotics from the Basidiomycete Cyathus cf. striatus Journal of Natural Products 79: 1684–1688. https://doi.org/10.1021/acs.jnatprod.6b00194 [DOI] [PubMed] [Google Scholar]
- Robeson DJ, Strobel GA. (1982) Monocerin, a phytotoxin from Exserohilum turcicum (≡ Drechslera turcica). Agricultural and Biological Chemistry 46(11): 2681–2683. https://doi.org/10.1080/00021369.1982.10865494. [Google Scholar]
- Ronquist F, Teslenko M, Mark van der P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EW, Burgess TI, de Gruyter J, de Hoog GS, Dixon LJ, Grube M, Gueidan C, Harada Y, Hatakeyama S, Hirayama K, Hosoya T, Huhndorf SM, Hyde KD, Jones EB, Kohlmeyer J, Kruys A, Li YM, Lücking R, Lumbsch HT, Marvanová L, Mbatchou JS, McVay AH, Miller AN, Mugambi GK, Muggia L, Nelsen MP, Nelson P, Owensby CA, Phillips AJ, Phongpaichit S, Pointing SB, Pujade-Renaud V, Raja HA, Plata ER, Robbertse B, Ruibal C, Sakayaroj J, Sano T, Selbmann L, Shearer CA, Shirouzu T, Slippers B, Suetrong S, Tanaka K, Volkmann-Kohlmeyer B, Wingfield MJ, Wood AR, Woudenberg JH, Yonezawa H, Zhang Y, Spatafora JW. (2009) A class-wide phylogenetic assessment of Dothideomycetes Studies in Mycology 64: 1–15–S10. https://doi.org/10.3114/sim.2009.64.01 [DOI] [PMC free article] [PubMed]
- Shenoy BD, Jeewon R, Wang H, Amandeep K, WH Ho, Bhat DJ, Crous PW, Hyde KD. (2010) Sequence data reveals phylogenetic affinities of fungal anamorphs Bahusutrabeeja, Diplococcium, Natarajania, Paliphora, Polyschema, Rattania and Spadicoides Fungal Diversity 44: 161–169. https://doi.org/10.1007/s13225-010-0059-8
- Stadler M, Anke H, Sterner O. (1993a) Linoleic acid — The nematicidal principle of several nematophagous fungi and its production in trap-forming submerged cultures. Archives of Microbiology 160: 401–405. https://doi.org/10.1007/BF00252228 [Google Scholar]
- Stadler M, Anke H, Sterner O. (1993b) New biologically active compounds from the nematode-trapping fungus Arthrobotrys oligospora Zeitschrift für Naturforschung 48c: 843–850. https://doi.org/10.1515/znc-1993-11-1205
- Stadler M, Mayer A, Anke H, Sterner O. (1994) Fatty acids and other compounds with nematicidal activity from cultures of basidiomycetes. Planta Med 60: 128–132. https://doi.org/10.1055/s-2006-959433 [DOI] [PubMed] [Google Scholar]
- Stamatakis A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. https://doi.org/10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surup F, Kuhnert E, Böhm A, Wiebach V, Solga D, Engler H, Pendzialek T, Berkessel A, Stadler M, Kalesse M. (2017) The rickiols, 20-, 22-, and 24-membered macrolides from the ascomycete Hypoxylon rickii. Chemistry-A European Journal 24: 2200–2213. https://doi.org/10.1002/chem.201704928 [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. https://doi.org/10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifonov LS, Chakravarty P, Hiratsuka Y, Ayer WA. (1992) Antifungal activity of metabolites of Peniophora polygonia against the aspen decay fungus Phellinus tremulae. European Journal of Plant Pathology 22: 441–448. https://doi.org/10.1111/j.1439-0329.1992.tb00318.x [Google Scholar]
- Vaidya G, Lohman DJ, Meier R. (2011) SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27: 171–180. https://doi.org/10.1111/j.1096-0031.2010.00329.x [DOI] [PubMed] [Google Scholar]
- Verkley GJ, Dukik K, Renfurm R, Goker M, Stielow JB. (2014) Novel genera and species of coniothyrium-like fungi in Montagnulaceae (Ascomycota). Persoonia 32: 25–51. https://doi.org/10.3767/003158514X679191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanasinghe DN, Gareth Jones EB, Camporesi E, Boonmee S, Ariyawansa HA, Wijayawardene NN, Mortimer PE, Xu J, Yang JB, Hyde KD. (2014) An exciting novel member of Lentitheciaceae in italy from Clematis vitalba. Cryptogamie Mycologie 35: 323–337. https://doi.org/10.7872/crym.v35.iss4.2014.323 [Google Scholar]
- Woudenberg JH, Groenewald JZ, Binder M, Crous PW. (2013) Alternaria redefined. Studies in Mycology 75: 171–212. https://doi.org/10.3114/sim0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehle C, Gressler M, Shelest E, Geib E, Hertweck C, Brock M. (2014) Terrein biosynthesis in Aspergillus terreus and its impact on phytotoxicity. Chemistry & Biology 21(6): 719–731. https://doi.org/10.1016/j.chembiol.2014.03.010 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Fournier J, Pointing SB, Hyde KD. (2008) Are Melanomma pulvis-pyrius and Trematosphaeria pertusa congeneric? Fungal Diversity 33: 47–60.
- Zhang Y, Wang HK, Fournier J, Crous PW, Jeewon R, Pointing SB, Hyde KD. (2009) Towards a phylogenetic clarification of Lophiostoma/Massarina and morphologically similar genera in the Pleosporales. Fungal Diversity 38: 225–251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.