Introduction
Type 1 diabetes (T1D) is an autoimmune disease caused by destruction of insulin producing β-cells in the pancreas. Standard of care therapy consists of life long symptomatic insulin treatment and in rare and severe cases patients undergo islet transplantation (1). Until today, autologous hematopoietic stem cell transplantation (aHSCT) proved to be the only intervention therapy for T1D reaching complete and sometimes even lasting remission (2–7). In spite of many other immunotherapies assessed around the globe, none matched the clinical efficacy of aHSCT (8, 9). Indeed, aHSCT had insulin-independency as primary end-point, rather than delayed loss of insulin production or decreased insulin needs. aHSCT is already widely and successfully used as a treatment for hematological malignancies (10, 11). Interestingly, one diabetic patient, when treated with aHSCT for multiple myeloma, became insulin independent (12). aHSCT was evaluated as a treatment for several autoimmune disorders as well, such as rheumatoid arthritis (13), systemic sclerosis (14, 15), multiple sclerosis (16), and juvenile idiopathic arthritis (17). By 2012, up to 3,000 aHSCT had been performed for autoimmune diseases (18). Yet, in the case of T1D, aHSCT remains controversial (19–21).
Indeed, the use of aHSCT as a strategy to cure T1D has been received with mixed enthusiasm. Concerns were raised about the short follow-up, the possibility that a positive effect of aHSCT may be attributable to a honeymoon phase and the absence of a placebo-treated trial arm for comparison (19, 21). Furthermore, the ethics of including minors in the trial was being questioned (19). Although valid at the time, these concerns have all since been addressed, as will become evident in the following paragraphs.
aHSCT in T1D
The rationale behind using aHSCT in autoimmune diseases is to halt autoimmune destruction of the targeted tissue and reestablish tolerance. While the mechanism by which this is achieved remains incompletely resolved, the importance of a diverse T-cell receptor repertoire (22), thymus reactivation (23), and the number of regulatory T-cells (Treg) has been established (24).
The first evidence to demonstrate that aHSCT can reestablish tolerance in new-onset T1D patients comes from Voltarelli et al. (25, 26). Recent-onset (<6 weeks) T1D patients were included to undergo aHSCT with mobilized [cyclophosphamide (2.0 g/m2) and granulocyte colony-stimulating factor (10 µg/kg/day)] peripheral blood-derived hematopoietic stem cells after an intermediate-intensity conditioning regimen consisting of cyclophosphamide (200 mg/kg total) and rabbit antithymocyte globulin (4.5 mg/kg total). Similar mobilization and conditioning regimes were used in other discussed studies, unless mentioned otherwise. In total, 25 patients were included, of which 21 were treated according to protocol and became insulin independent, for a median of 43 months (2); a result unmatched by any intervention therapy up until this point. These results were substantiated independently around the world, accomplishing insulin independence in all studies, with maximum insulin independence ranging from 38 to 56 months and increasing with further follow-up (3–7). These studies prove that aHSCT is a promising therapy for T1D, while providing crucial and unique metabolic and immunological data of T1D patients in remission (27, 28).
Balancing the Risk of aHSCT with the Risk of Diabetes-Associated Complications
Depending on the intensity of the conditioning regime, aHSCT can cause a wide range of complications. In the T1D trials (2–7), these ranged from relatively mild symptoms such as febrile neutropenia, nausea, and alopecia to more severe complications such as de novo autoimmunity and systemic infections, which in one case resulted in an unfortunate death (7). Temporal oligospermia was witnessed in some of the studies, but not all. Of note, multiple children have been conceived after aHSCT. Apart from these complications, there is also a concern of increased risk of malignancies after aHSCT, particularly myelodysplasia. With allotransplantation, this risk is well established and can be attributed to the heavy conditioning regime, while this regime is much milder in the autologous setting for autoimmune diseases. Furthermore, in contrast to aHSCT as a treatment for malignancies, stem cells of T1D patients have not sustained any damage from previous chemotherapy. Consequently, the incidence of malignancies was reported to be lower, although further prospective studies with longer follow-up and proper control groups are warranted to asses if these malignancies are aHSCT related (29).
Containment of adverse events from aHSCT is constantly improving as illustrated by decreased morbidity and mortality to <1% (30). Furthermore, in the setting of T1D, it will be performed in relatively young and otherwise fit subsets of patients with a low to intermediate conditioning regimen (2, 31), associated with reduced risk (29) without compromising treatment efficacy. This was attested by a recent trial exploring the possibility of a simplified method of aHSCT in an outpatient setting, with a conditioning regime consisting of cyclophosphamide (2.0 g/m2 total) and fludarabine (120 mg/m2 total), still reaching 44% prolonged insulin independence for up to 56 months and beyond, without significant adverse effects (4).
To make a compelling and fair case of aHSCT in T1D, the complications of aHSCT need to be juxtaposed with the short- and long-term complications of T1D. It is important to realize that acute and possibly life threatening events related to T1D and insulin treatment such as a hypoglycemic coma (32) and diabetic ketoacidosis (DKA) (33) are not uncommon. Indeed, T1D remains a deadly disease, where insulin therapy merely provides palliative care. In addition to a significantly reduced life expectancy, T1D also imposes severe and often lifelong negative impact on the quality of life of T1D patients. The major burden of the disease is caused by long-term micro- and macrovascular complications, with T1D still being a main cause of end stage renal disease and non-inherited blindness (34, 35). Even with optimal education and state-of-the-art treatment options, good glycemic control is not achieved in the vast majority of patients (36). This is of particular importance, since good glycemic control early in the course of the disease reduces long-term complications and preserves endogenous insulin production (37). Interestingly, patients that experienced a honeymoon phase showed significantly less macrovascular complications after 7 years of follow-up (38, 39). This could imply that a similar effect can be expected from an aHSCT induced prolonged period of insulin independence.
Importantly, side effects are inherent to immunotherapy. The adverse events of, for instance, DMARD, TNF blockers, sirolimus, cyclosporine, azathioprine, prednisone, thymoglobulin, alemtuzumab, or imatinib, all considered in the context of T1D, are certainly not negligible.
Clinical Outcome of aHSCT Corresponds with the Degree of islet Autoreactivity before Therapy
Currently, after almost 15 years of experience in the application of aHSCT for the treatment of T1D, much knowledge has been gained about the mechanism of action of aHSCT and, concomitantly, about which patient population benefits most (2, 3, 5–7, 27, 28, 40–42).
Earlier this year, the first aHSCT in T1D trial reported its ad hoc analysis with a mean follow-up of 67.5 months (some patients remain insulin-independent beyond 106 months) and included 25 patients (2). HLA-A2 positive patients were divided into low and high cytotoxic T lymphocytes (CTL) autoreactivity groups according to the cumulative frequencies of islet-specific CTLs at baseline. Low CTL autoreactivity associated with higher c-peptide levels after aHSCT compared with high CTL autoreactivity. Furthermore, while 83% of patients in the high CTL group had resumed insulin therapy at 24 months after aHSCT, all patients with low frequencies of islet-autoreactive CTLs at baseline remained insulin independent. In addition, patients were divided into those with “short-remission” and “prolonged remission” depending on whether they were insulin-free for less or more than 3.5 years after aHSCT, respectively. A trend was seen of persistently lower cumulative frequencies of islet-specific CTLs in the prolonged remission group compared with the short-remission group. This outcome may point that the conditioning regimen with thymoglobulin was insufficient to deplete autoreactive T-cells. Diabetes relapse could then result from clonal expansion of autoreactive CTLs that escaped the conditioning procedure. In any case, these immunological parameters associated with superior or inferior clinical outcome of aHSCT before therapy point to patient and disease heterogeneity and present a good case for personalized and precision medicine in which tailoring the conditioning therapy might lead to more effective reversal of islet autoimmunity.
Additional evidence in favor of an immunogenic heterogeneity that relates to the outcome of aHSCT came from a study of 13 patients that was conducted in China with a mean follow-up of 42 months (5). Expressing more than one preexisting autoantibody negatively correlated with the preservation of beta-cell function as quantified by c-peptide levels. Yet, a larger study including 123 patients with a mean follow-up of 16 months found no difference in baseline presence of any of the autoantibodies between responding and non-responding patients (27). Serum levels of interleukin-10, interleukin-4, transforming growth factor-β, and fasting c-peptide after aHSCT correlated with the number of infused CD34+ cells, whereas tumor-necrosis factor-α (TNF-α) and insulin doses showed an inverse relation. Furthermore, prolonged insulin-free survival was negatively correlated with baseline TNF-α levels, which may provide another suitable negative predictor of prolonged remission (3).
In summary, current clinical evidence points to heterogeneity between patients and in disease, as well as provides immune correlates of disease remission or relapse that may offer opportunity for patient selection, precision medicine, and guidance for tailored immunotherapy following aHSCT.
The Success of aHSCT in Relation to Preexisting Functional Beta-Cell Mass
Besides a baseline immune signature, post hoc analyses have revealed the importance of preexisting beta-cell mass for the outcome of aHSCT (27). One small study (5) found that the baseline c-peptide level was a positive predictor of post-aHSCT c-peptide levels, which was corroborated by other, larger studies (3). The largest study including 123 patients stratified subjects into a responder group and a non-responder group according to the presence of a post-aHSCT clinical response assessed by a β-score (27). The β-score is mainly used in the islet transplantation setting and consists of four components: fasting plasma glucose, HbA1c, c-peptide, daily insulin use or usage of oral hypoglycemic agents. The β-score was already significantly higher at baseline in responders compared with non-responders. Moreover, baseline fasting c-peptide levels proved to be an effective positive predictor of prolonged remission and the age of onset of diabetes a negative predictor. Obviously, baseline c-peptide levels are an indication of functional β-cell mass (27), although increasing evidence points to a disconnect between beta-cell mass and function in the case of diabetes (43, 44). β-Cell regeneration may occur until adolescence, after which regenerative capacity appears to stagnate (45). Indeed, early intervention within 6 weeks after diagnosis of T1D led to remission in the vast majority of cases, whereas later intervention achieved remission in less than half of the cases (42), suggesting that timely therapy matters.
The influence of DKA before aHSCT on clinical outcome could be substantial (6). Indeed, DKA at diagnosis has been associated with lower c-peptide levels, higher insulin needs and HbA1c levels, suggesting lower remaining β-cell function (46). Yet, another trial including 24 patients with 52 months as a mean follow-up found no relation between duration of insulin independence and the time from diagnosis to AHSCT, baseline c-peptide levels, nor number of CD34+ cells (7).
To summarize, patients with sufficient beta-cell function at baseline, no DKA at diagnosis, and treated early after diagnosis appear to benefit most. These characteristics all point toward the pivotal role of remaining functional beta-cell mass for success of aHSCT in T1D (27). To verify whether the age of onset matters (3), inclusion of minors in trials of aHSCT in T1D would be required. The potential capacity to regenerate their beta cells would further support considering young patients to offer this intervention therapy. Teenagers are a particularly challenging population to treat as diabetes-related distress, which is present in one-third of adolescents with T1D, is linked to poor glycemic control (47–49). Consequently, 84% of teens do not reach target HbA1c levels (36), which jeopardizes their future health with regards to increased long-term complications, but also their career perspectives (50).
Selecting Eligible Patients for aHSCT in T1D
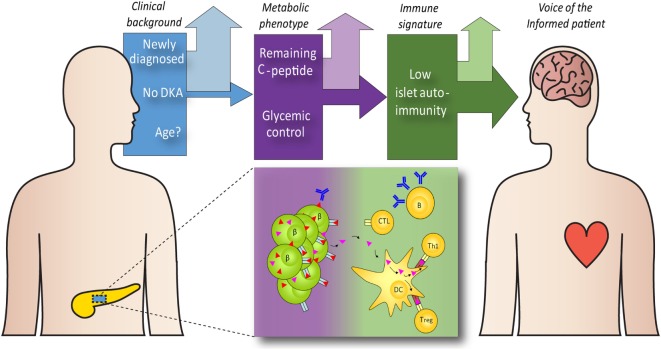
Understanding which patient groups respond better to aHSCT and why, enables us to transform aHSCT from a general therapy to personalized medicine, thus envisioning a future of aHSCT in T1D. Yet, we contend that the choice for aHSCT as therapeutic option is not confined to the care providers. The voice of the patient is equally relevant, both in terms of refusing the risk for treatment related adverse events or accepting these in favor of temporal disease remission, preservation of beta-cell function, and reduced risk of diabetic complications. In case of minors, parents face the difficult task of weighing the best therapy for the patient in consultation with the care provider, which makes careful information provision even more important. We envision a future in which care providers, in dialog with the patient and caregivers, use a framework of evidence-based risk assessment to assess whether aHSCT is a viable option (see Figure 1).
Figure 1.
Guidance on the selection of type 1 diabetes (T1D) patients for autologous hematopoietic stem cell transplantation (aHSCT). aHSCT is unlikely to benefit all T1D patients. Factors that may help selecting the preferred candidates include the clinical background [disease duration, age, and diabetic complications, such as diabetic ketoacidosis (DKA)], metabolic features [remaining functional beta-cell mass (β), glycemic control, HbA1c] and immunopathogenic features [the number and type of islet autoantibodies, the frequency and specificity of islet-autoreactive cytotoxic T lymphocytes (CTL), and other effector (Th1) and regulatory (Treg) immune cells, and cytokine profiles]. With the opportunity to identify patient subgroups with particularly great or smaller chances for clinical benefit, we propose that we engage the patient community to guide shared decision-making.
Conclusion
While aHSCT will not be the magic bullet universally curing T1D, there is a promising future for its implementation in a distinct group of patients (20). Indeed, none of the alternative intervention strategies match, or even get close to, the clinical outcome achieved in a considerable number of patients treated with aHSCT. We propose that this patient group should be identified, diligently informed and offered the possible benefits of an extended period of insulin-free and burden-free survival, while medical science continues their pursuit of developing alternative intervention strategies for those less eligible, or declining, aHSCT. T1D enters the era of personalized medicine.
Author Contributions
KM and ET performed a literature review, contributed to the design of the work, analyzed the data, and wrote and edited the manuscript. KM designed the figure. BR conceived the idea of this work. BR and SF analyzed the data, revised and edited the manuscript, and contributed to the discussion. All the authors provided approval for publication and are accountable for all aspects of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Rein Schoondorp for his help with constructing the figure.
Footnotes
Funding. BR is supported by the Wanek Family Project for Type 1 Diabetes, the Juvenile Diabetes Research Foundation (3-SRA-2014-314-M-R, 2-SRA-2014-295-Q-R, 1-SRA-2015-114-S-B, 2-SRA-2016-148-Q-R, and 2-SRA-2016-311-S-B), the Dutch Diabetes Research Foundation, Stichting DON, the Danish Diabetes Academy, and the European Commission (EU-FP7: EE-ASI; BetaCellTherapy; DIABIL-2; H2020: BCellTherapy; IMI2: INNODIA).
References
- 1.Harlan DM, Kenyon NS, Korsgren O, Roep BO, Immunology of Diabetes S Current advances and travails in islet transplantation. Diabetes (2009) 58(10):2175–84. 10.2337/db09-0476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malmegrim KC, de Azevedo JT, Arruda LC, Abreu JR, Couri CE, de Oliveira GL, et al. Immunological balance is associated with clinical outcome after autologous hematopoietic stem cell transplantation in type 1 diabetes. Front Immunol (2017) 8:167. 10.3389/fimmu.2017.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiang H, Chen H, Li F, Liu J, Su Y, Hao L, et al. Predictive factors for prolonged remission after autologous hematopoietic stem cell transplantation in young patients with type 1 diabetes mellitus. Cytotherapy (2015) 17(11):1638–45. 10.1016/j.jcyt.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 4.Cantu-Rodriguez OG, Lavalle-Gonzalez F, Herrera-Rojas MA, Jaime-Perez JC, Hawing-Zarate JA, Gutierrez-Aguirre CH, et al. Long-term insulin independence in type 1 diabetes mellitus using a simplified autologous stem cell transplant. J Clin Endocrinol Metab (2016) 101(5):2141–8. 10.1210/jc.2015-2776 [DOI] [PubMed] [Google Scholar]
- 5.Li L, Shen S, Ouyang J, Hu Y, Hu L, Cui W, et al. Autologous hematopoietic stem cell transplantation modulates immunocompetent cells and improves beta-cell function in Chinese patients with new onset of type 1 diabetes. J Clin Endocrinol Metab (2012) 97(5):1729–36. 10.1210/jc.2011-2188 [DOI] [PubMed] [Google Scholar]
- 6.Gu W, Hu J, Wang W, Li L, Tang W, Sun S, et al. Diabetic ketoacidosis at diagnosis influences complete remission after treatment with hematopoietic stem cell transplantation in adolescents with type 1 diabetes. Diabetes Care (2012) 35(7):1413–9. 10.2337/dc11-2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snarski E, Milczarczyk A, Halaburda K, Torosian T, Paluszewska M, Urbanowska E, et al. Immunoablation and autologous hematopoietic stem cell transplantation in the treatment of new-onset type 1 diabetes mellitus: long-term observations. Bone Marrow Transplant (2016) 51(3):398–402. 10.1038/bmt.2015.294 [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb PA, Quinlan S, Krause-Steinrauf H, Greenbaum CJ, Wilson DM, Rodriguez H, et al. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new-onset type 1 diabetes. Diabetes Care (2010) 33(4):826–32. 10.2337/dc09-1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet (2011) 378(9788):319–27. 10.1016/S0140-6736(11)60895-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med (2014) 371(10):895–905. 10.1056/NEJMoa1402888 [DOI] [PubMed] [Google Scholar]
- 11.Hermine O, Hoster E, Walewski J, Bosly A, Stilgenbauer S, Thieblemont C, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet (2016) 388(10044):565–75. 10.1016/S0140-6736(16)00739-X [DOI] [PubMed] [Google Scholar]
- 12.Al-Anazi KA, Bakhit K, Al-Sagheir A, AlHashmi H, Abdulbaqi M, Al-Shibani Z, et al. Cure of insulin-dependent diabetes mellitus by an autologous hematopoietic stem cell transplantation performed to control multiple myeloma in a patient with chronic renal failure on regular hemodialysis. J Stem Cell Biol Transplant (2017) 1(2):11. 10.21767/2575-7725.100011 [DOI] [Google Scholar]
- 13.Snowden JA, Passweg J, Moore JJ, Milliken S, Cannell P, Van Laar J, et al. Autologous hemopoietic stem cell transplantation in severe rheumatoid arthritis: a report from the EBMT and ABMTR. J Rheumatol (2004) 31(3):482–8. [PubMed] [Google Scholar]
- 14.van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA (2014) 311(24):2490–8. 10.1001/jama.2014.6368 [DOI] [PubMed] [Google Scholar]
- 15.Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N Engl J Med (2018) 378(1):35–47. 10.1056/NEJMoa1703327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reston JT, Uhl S, Treadwell JR, Nash RA, Schoelles K. Autologous hematopoietic cell transplantation for multiple sclerosis: a systematic review. Mult Scler (2011) 17(2):204–13. 10.1177/1352458510383609 [DOI] [PubMed] [Google Scholar]
- 17.Brinkman DM, de Kleer IM, ten Cate R, van Rossum MA, Bekkering WP, Fasth A, et al. Autologous stem cell transplantation in children with severe progressive systemic or polyarticular juvenile idiopathic arthritis: long-term follow-up of a prospective clinical trial. Arthritis Rheum (2007) 56(7):2410–21. 10.1002/art.22656 [DOI] [PubMed] [Google Scholar]
- 18.Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant (2012) 47(6):770–90. 10.1038/bmt.2011.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross LF, Philipson LH. Ethics of hematopoietic stem cell transplantation in type 1 diabetes mellitus. JAMA (2007) 298(3):285; author reply–6. 10.1001/jama.298.3.285-a [DOI] [PubMed] [Google Scholar]
- 20.Skyler JS. Cellular therapy for type 1 diabetes: has the time come? JAMA (2007) 297(14):1599–600. 10.1001/jama.297.14.1599 [DOI] [PubMed] [Google Scholar]
- 21.Gitelman SE, Haller MJ, Schatz D. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA (2009) 302(6):624; author reply 5. 10.1001/jama.2009.1099 [DOI] [PubMed] [Google Scholar]
- 22.Muraro PA, Robins H, Malhotra S, Howell M, Phippard D, Desmarais C, et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J Clin Invest (2014) 124(3):1168–72. 10.1172/JCI71691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander T, Thiel A, Rosen O, Massenkeil G, Sattler A, Kohler S, et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood (2009) 113(1):214–23. 10.1182/blood-2008-07-168286 [DOI] [PubMed] [Google Scholar]
- 24.Abrahamsson SV, Angelini DF, Dubinsky AN, Morel E, Oh U, Jones JL, et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain (2013) 136(Pt 9):2888–903. 10.1093/brain/awt182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA (2007) 297(14):1568–76. 10.1001/jama.297.14.1568 [DOI] [PubMed] [Google Scholar]
- 26.Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA (2009) 301(15):1573–9. 10.1001/jama.2009.470 [DOI] [PubMed] [Google Scholar]
- 27.Xiang H, Yang C, Xiang T, Wang Z, Ge X, Li F, et al. Residual beta-cell function predicts clinical response after autologous hematopoietic stem cell transplantation. Stem Cells Transl Med (2016) 5(5):651–7. 10.5966/sctm.2015-0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye L, Li L, Wan B, Yang M, Hong J, Gu W, et al. Immune response after autologous hematopoietic stem cell transplantation in type 1 diabetes mellitus. Stem Cell Res Ther (2017) 8(1):90. 10.1186/s13287-017-0542-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daikeler T, Tichelli A, Passweg J. Complications of autologous hematopoietic stem cell transplantation for patients with autoimmune diseases. Pediatr Res (2012) 71(4 Pt 2):439–44. 10.1038/pr.2011.57 [DOI] [PubMed] [Google Scholar]
- 30.Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R, et al. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA (2008) 299(8):925–36. 10.1001/jama.299.8.925 [DOI] [PubMed] [Google Scholar]
- 31.Swart JF, Delemarre EM, van Wijk F, Boelens JJ, Kuball J, van Laar JM, et al. Haematopoietic stem cell transplantation for autoimmune diseases. Nat Rev Rheumatol (2017) 13(4):244–56. 10.1038/nrrheum.2017.7 [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Wang R, Ganz ML, Paprocki Y, Schneider D, Weatherall J. The burden of severe hypoglycemia in type 1 diabetes. Curr Med Res Opin (2017) 34(1):171–7. 10.1080/03007995.2017.1391079 [DOI] [PubMed] [Google Scholar]
- 33.Gibb FW, Teoh WL, Graham J, Lockman KA. Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia (2016) 59(10):2082–7. 10.1007/s00125-016-4034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank RN. Diabetic retinopathy. N Engl J Med (2004) 350(1):48–58. 10.1056/NEJMra021678 [DOI] [PubMed] [Google Scholar]
- 35.Finne P, Reunanen A, Stenman S, Groop PH, Gronhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA (2005) 294(14):1782–7. 10.1001/jama.294.14.1782 [DOI] [PubMed] [Google Scholar]
- 36.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange Clinic Registry. Diabetes Care (2015) 38(6):971–8. 10.2337/dc15-0078 [DOI] [PubMed] [Google Scholar]
- 37.Nathan DM, Group DER. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care (2014) 37(1):9–16. 10.2337/dc13-2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niedzwiecki P, Pilacinski S, Uruska A, Adamska A, Naskret D, Zozulinska-Ziolkiewicz D. Influence of remission and its duration on development of early microvascular complications in young adults with type 1 diabetes. J Diabetes Complications (2015) 29(8):1105–11. 10.1016/j.jdiacomp.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 39.Marino KR, Lundberg RL, Jasrotia A, Maranda LS, Thompson MJ, Barton BA, et al. A predictive model for lack of partial clinical remission in new-onset pediatric type 1 diabetes. PLoS One (2017) 12(5):e0176860. 10.1371/journal.pone.0176860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Couri CE, de Oliveira MC, Simoes BP. Risks, benefits, and therapeutic potential of hematopoietic stem cell transplantation for autoimmune diabetes. Curr Diab Rep (2012) 12(5):604–11. 10.1007/s11892-012-0309-0 [DOI] [PubMed] [Google Scholar]
- 41.Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. Autologous hematopoietic stem cell transplantation for type 1 diabetes. Ann N Y Acad Sci (2008) 1150:220–9. 10.1196/annals.1447.048 [DOI] [PubMed] [Google Scholar]
- 42.D’Addio F, Valderrama Vasquez A, Ben Nasr M, Franek E, Zhu D, Li L, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in new-onset type 1 diabetes: a multicenter analysis. Diabetes (2014) 63(9):3041–6. 10.2337/db14-0295 [DOI] [PubMed] [Google Scholar]
- 43.van Megen KM, Spindler MP, Keij FM, Bosch I, Sprangers F, van Royen-Kerkhof A, et al. Relapsing/remitting type 1 diabetes. Diabetologia (2017) 60(11):2252–5. 10.1007/s00125-017-4403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med (2012) 209(1):51–60. 10.1084/jem.20111187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, et al. Formation of a human beta-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab (2012) 97(9):3197–206. 10.1210/jc.2012-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komulainen J, Lounamaa R, Knip M, Kaprio EA, Akerblom HK. Ketoacidosis at the diagnosis of type 1 (insulin dependent) diabetes mellitus is related to poor residual beta cell function. Childhood Diabetes in Finland Study Group. Arch Dis Child (1996) 75(5):410–5. 10.1136/adc.75.5.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagger V, Hendrieckx C, Sturt J, Skinner TC, Speight J. Diabetes distress among adolescents with type 1 diabetes: a systematic review. Curr Diab Rep (2016) 16(1):9. 10.1007/s11892-015-0694-2 [DOI] [PubMed] [Google Scholar]
- 48.Hood KK, Peterson CM, Rohan JM, Drotar D. Association between adherence and glycemic control in pediatric type 1 diabetes: a meta-analysis. Pediatrics (2009) 124(6):e1171–9. 10.1542/peds.2009-0207 [DOI] [PubMed] [Google Scholar]
- 49.Weissberg-Benchell J, Antisdel-Lomaglio J. Diabetes-specific emotional distress among adolescents: feasibility, reliability, and validity of the problem areas in diabetes-teen version. Pediatr Diabetes (2011) 12(4 Pt 1):341–4. 10.1111/j.1399-5448.2010.00720.x [DOI] [PubMed] [Google Scholar]
- 50.Semenkovich K, Patel PP, Pollock AB, Beach KA, Nelson S, Masterson JJ, et al. Academic abilities and glycaemic control in children and young people with type 1 diabetes mellitus. Diabet Med (2016) 33(5):668–73. 10.1111/dme.12854 [DOI] [PMC free article] [PubMed] [Google Scholar]