Abstract
Purpose
Increased vascularity is a hallmark of renal cell carcinoma (RCC). Microvessel density (MVD) is one measurement of tumor angiogenesis, however its utility as a biomarker of outcome is unknown. ECOG-ACRIN 2805 (E2805) enrolled 1,943 resected high-risk RCC patients randomized to adjuvant sunitinib, sorafenib, or placebo. We aimed to determine the prognostic and predictive role of MVD in RCC.
Methods
We obtained pre-treatment primary RCC nephrectomy tissues from 822 patients on E2805 and constructed tissue microarrays. Using quantitative immunofluorescence we measured tumor MVD as the area of CD34-expressing cells. We determined the association with disease-free survival (DFS), overall survival (OS), treatment arm and clinicopathologic variables.
Results
High MVD (above the median) was associated with prolonged OS for the entire cohort (p=0.021) and for patients treated with placebo (p=0.028). The association between high MVD and OS was weaker in patients treated with sunitinib or sorafenib (p=0.060). MVD was not associated with DFS (p=1.00). On multivariable analysis, MVD remained independently associated with improved OS (p=0.013). High MVD correlated with Fuhrman grade 1-2 (p<0.001), clear cell histology (p<0.001), and absence of necrosis (p<0.001) but not with gender, age, sarcomatoid features, lymphovascular invasion, or tumor size.
Conclusions
High MVD in resected high-risk RCC patients is an independent prognostic, rather than predictive, biomarker of improved OS. Further studies should assess whether incorporating MVD into clinical models will enhance our ability to predict outcome and if low MVD can be used for selection of high risk patients for adjuvant therapy trials.
Keywords: Renal cell carcinoma, microvessel density, prognosis, high-risk, recurrence, adjuvant therapy
INTRODUCTION
Renal cell carcinoma (RCC) is rising in incidence. In 2017, an estimated 64,000 new cases will be diagnosed and over 14,000 patients will die of RCC (1). At initial diagnosis, RCC is either confined to the kidney (65%), spread to regional lymph nodes (16%), or has already metastasized (16%), with associated 5-year survival rates of 93%, 66%, and 12%, respectively (2). There is currently no FDA-approved standard adjuvant therapy with proven long-term benefit to reduce the risk of recurrence or enhance survival for patients who undergo complete resection of localized or loco-regional RCC. Moreover, it is often difficult to identify which patients with high-risk features are likeliest to recur.
The strongest prognostic factors for RCC include stage and Fuhrman grade (3,4), which are incorporated into RCC prognostic models including the UCLA integrated staging system (UISS), which integrates American Joint Committee on Cancer (AJCC) stage, Eastern Cooperative Oncology Group (ECOG) performance status, and Fuhrman grade (5), and the Stage, Size, Grade, and Necrosis Score (SSIGN) (6,7). With the exception of tumor grade, no tissue-based prognostic markers are currently utilized in clinical practice. Even early stage RCCs pose a sizeable risk for recurrence and metastasis (8), yet no tools exist to further identify the most susceptible patients. Identification of additional biomarkers is warranted to more accurately risk-stratify RCC patients in order to intensify surveillance strategies in patients most likely to recur and to select the highest-risk patients for adjuvant therapies.
Activation of the vascular endothelial growth factor (VEGF) and other growth factor signaling pathways is known to be one basis for the highly vascular nature of clear cell RCC. The von Hippel Lindau (VHL) tumor suppressor gene is mutated in 60-80% of cases of sporadic RCC (9). Under normal and normoxic conditions, the VHL protein targets hypoxia-inducible transcription factors (HIF) for destruction via the E3 ubiquitin-ligase complex. When VHL is mutated, this complex no longer functions and HIFs accumulate, particularly in the hypoxic conditions of malignancy. HIFs regulate downstream targets including VEGF and other growth factors and are therefore highly implicated in tumor angiogenesis and tumor growth in RCC (9). It is unclear whether biomarkers characterizing tumor angiogenesis such as microvessel density (MVD) could aid in RCC prognostication or predict response to anti-angiogenic therapies. MVD has been associated with prognosis in a number of malignancies (10), however its role in RCC remains controversial.
In metastatic RCC, therapies targeting angiogenesis pathways have demonstrated clinical efficacy with the use of tyrosine kinase inhibitors (TKI) that target VEGF receptors (sunitinib, pazopanib, sorafenib, axitinib, cabozantinib, lenvatinib) or the monoclonal antibody to VEGF, bevacizumab (11). However, study of the efficacy of anti-angiogenic TKIs in the adjuvant setting has produced contradictory results (12-14), although several trials are still ongoing (NCT00492258, NCT01599754) (15). These drugs have a high rate of toxicity, and given their questionable activity in the adjuvant setting, prognostic and predictive biomarkers are needed for enhanced patient selection to improve their therapeutic window.
ECOG-ACRIN 2805 (E2805) was a phase 3 trial that studied adjuvant sunitinib, sorafenib, or placebo in patients with resected high-risk RCC (12). The trial did not demonstrate benefit from adjuvant sorafenib or sunitinib. The purpose of the current study was to determine the prognostic and predictive role of MVD in nephrectomy specimens collected from patients enrolled on E2805.
MATERIALS AND METHODS
Patients and Study Design
E2805 was a phase III clinical trial that enrolled 1,943 patients from 2006-2010 who underwent complete resection of high-risk, non-metastatic clear cell or non-clear cell RCC. The high-risk category was defined in accordance with the American Joint Committee on Cancer’s (AJCC) staging criteria as pathological stage pT1N0 high grade to pT2 (any grade) N0 and above. Patients were required to have an ECOG performance status of 0-1, normal liver and hematologic function, and a creatinine clearance of at least 30 mL per minute. No prior therapies were allowed (12). Institutional review boards at each of the 226 participating study sites approved the study and written informed consent was obtained for specimen collection. This study was conducted in accordance with the ethical guidelines outlined by U.S. Common Rule.
Treatment and Assessment of Response
Patients were randomized 1:1:1 in a double-blind fashion to receive a planned 54 weeks of adjuvant therapy with sunitinib (n=674) 50mg daily for 4 weeks of each 6-week cycle, sorafenib (n=649) 400 mg twice a day continuously, or placebo (n=647). Due to toxicity, starting doses were amended in May 2009 to 37.5 mg for sunitinib and 400 mg daily for sorafenib for the first one to two cycles and doses were escalated if toxicities were grade 2 or less. Surveillance imaging was performed with CT of the chest and CT or MRI of the abdomen and pelvis every 18 weeks while on treatment, every 6 months for two years, and yearly for 10 years. Primary endpoints included disease-free survival (DFS), including time to recurrence or development of a second primary cancer. Secondary endpoints included overall survival (OS), DFS for the clear cell RCC subgroup, and rates of adverse events. The remainder of the patient profile and study design has been previously described (12).
Tissue Microarray (TMA) Construction
Pre-treatment formalin-fixed paraffin embedded tumor biopsies were obtained from primary RCC specimens. Following formal central review, a pathologist determined a representative region from each tumor for inclusion on the TMA and avoided areas of necrosis or hemorrhage. Three cores from each tumor measuring 1 mm each in diameter were used. TMA blocks were cut into 5-µm sections and placed on glass slides using an adhesive tape-transfer system with UV cross-linking.
Immunofluorescence
The CD34 mouse monoclonal antibody (Class II Clone QBEnd 10; dilution 1:50; Dako, Santa Clara, CA) was used to measure MVD. Western blotting was performed on a panel of cell lines to determine antibody specificity and to verify binding to a single band at the anticipated molecular weight (not shown), as previously described (16).
Slides were individually stained for CD34 to determine MVD. Staining was performed as described (16-19). Sets of three slides, each containing a core from different areas of tumor for the same patient were de-paraffinized and hydrated followed by antigen retrieval in a pressure cooker containing 6.5mM sodium citrate, pH 6.0 buffer. Slides were placed in 2.5% hydrogen peroxide for 30 minutes and in 0.3% bovine serum albumin/1X Tris-buffered saline for 1 hour at room temperature. Incubation with primary antibody diluted in 0.3% bovine serum albumin/1X Tris-buffered saline was carried out overnight at 4°C. Goat anti-mouse horseradish peroxidase-decorated polymer backbone (Envision, Dako) was utilized to detect the primary antibody and was followed by incubation with Cy5-tyramide (Perkin-Elmer Life Science Products) and activated by horseradish peroxidase for target visualization. To generate the tumor mask, an antibody cocktail of mouse species was used including anti-cytokeratin (Dako, dilution 1:200), anti-CAIX (gift from Jan Zavada, dilution 1:1000), anti-CD10 (Dako, dilution 1:500) and Streptavidin HRP (Sigma-Aldrich, dilution 1:200). Anti-mouse amplification reagent (Envision, Dako) and Cy2-tyramide (Perkin-Elmer Life Science Products) were used to visualize the tumor mask staining. Slides were sealed with coverslips with ProLong Gold antifade reagent containing 4′,6-Diamidino-2-phenylindole (DAPI) (Invitrogen) which allowed visualization of nuclei.
Automated image acquisition and analysis
Images were analyzed by methods previously described (20). Briefly, monochromatic, high-resolution images for DAPI, Cy2, and Cy5 channels were captured for each histospot. A tumor mask was created by binarizing the Cy2 signal and creating an epithelial compartment. DAPI was used to identify the nuclear compartment within the tumor mask. CD34-staining cells were visualized with Cy5. MVD scores in the tumor compartment were calculated by dividing the area of CD-34 positivity by the area of tumor positivity. Histospots with limited amount of tumor tissue (<3%) were excluded from the analysis.
Data analysis
To measure MVD, we determined the area of CD34 coalescence within the tumor mask, averaged for each patient and dichotomized at the median. DFS was defined as time from registration to first recurrence, second primary malignancy, or death, with patients censored at the time of last disease assessment. OS was defined as time from registration to death. Patients were censored at the last date known alive. Survival estimates were obtained with the Kaplan-Meier method. Survival differences were assessed with a stratified log-rank test.
Associations between MVD and clinical (age, gender, and pre- and post-surgery serum calcium and LDH levels) or pathologic (RCC histologic subtype, tumor size, Fuhrman grade, presence or absence of sarcomatoid features, lymphovascular invasion, and necrosis) parameters were examined by the Fisher’s exact and Wilcoxon rank tests. Using dichotomized values for MVD, univariate stratified Cox proportional hazards methods were used to estimate unadjusted hazards ratios (HR) for DFS and OS for each marker. Seeing that MVD was found to be significant on univariate analysis for OS, we incorporated MVD into multivariable Cox models using established UISS prognostic factors for RCC, adjusting for T stage, N stage, Fuhrman grade, and performance status, as well as age, gender, and vascular invasion. For correlations of MVD with survival and clinicopathologic characteristics, the Holm-Bonferroni method was used to control the familywise error rate and determine adjusted p-values. No adjustments were made elsewhere. P-values less than or equal to 0.05 were considered statistically significant.
RESULTS
Patient Population
Adequate primary RCC tissue was available from 822 patients on E2805. Baseline patient and disease characteristics are summarized in Table 1. There were more males (65%) than females (35%) and the median age was 56. The predominant histologic subtype was clear cell RCC (82%), followed by papillary (8%) and chromophobe (4%) histologies. Roughly two-thirds (62%) of tumor specimens were classified as high Fuhrman grade (3-4). Treatment arms were distributed evenly amongst the 822 patients: sunitinib (n=273), sorafenib (n=272), and placebo (n=277).
Table 1.
Baseline patient and disease characteristics from 822 patients on E2805 for which adequate primary RCC tissue was available for analysis
| N (%) | ||
|---|---|---|
| Sex | Female | 289 (35.2) |
| Male | 533 (64.8) | |
| Age | ≤ 48 | 226 (27.5) |
| 49-56 | 220 (26.8) | |
| 57-64 | 186 (22.6) | |
| ≥ 65 | 190 (23.1) | |
| Histology | Clear Cell | 676 (82.2) |
| Chromophobe | 31 (3.8) | |
| Papillary | 64 (7.8) | |
| Other | 51 (6.2) | |
| Sarcomatoid features | No | 748 (91.0) |
| Yes | 73 (8.9) | |
| Missing | 1 (0.1) | |
| Fuhrman grade | 1/2 | 310 (37.7) |
| 3/4 | 512 (62.3) | |
| Path T-stage | 1 | 84 (10.2) |
| 2 | 236 (28.7) | |
| 3 | 491 (59.7) | |
| 4 | 11 (1.3) | |
| Path N-stage | 0 | 299 (36.4) |
| 1 | 37 (4.5) | |
| 2 | 23 (2.8) | |
| X | 463 (56.3) | |
| Arm | Sunitinib | 273 (33.2) |
| Sorafenib | 272 (33.1) | |
| Placebo | 277 (33.7) |
In comparing patient and disease characteristics of the non-TMA cohort (n=1,121) to the TMA cohort, there were slightly more chromophobe (n=80, 7% vs. n=31, 4%) and clear cell (n=864, 77% vs. n=676, 82%) RCCs (p=0.003) included as well as those of slightly higher Fuhrman grade of 3-4 (n=778, 69% vs. n=512, 62%) (p=0.001). There were otherwise no significant differences in sex, age, pathologic T or N stage, presence of sarcomatoid features, or treatment arm distribution in the non-TMA cohort compared to the TMA cohort.
Association between MVD and survival outcomes for patients on all treatment arms
Of the 822 patients, 319 (38.8%) developed disease recurrence. There were no significant differences in DFS for high vs. low MVD (HR 0.94; 95% CI 0.76-1.17; p=1.00), however high MVD was associated with improved OS (HR 0.63; 95% CI 0.47-0.85; p=0.021). Overall, high MVD conferred improved 5-year OS rates (83.7%) compared to low MVD (76.2%) (Figure 1). Representative MVD immunofluorescence in RCC nephrectomy specimens is demonstrated in Figure 2, showing examples of high and low MVD.
Figure 1.
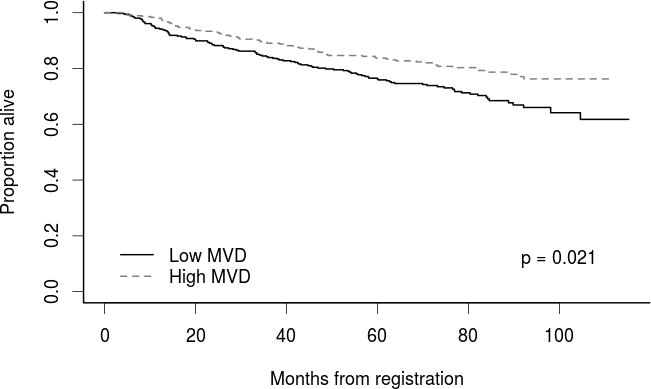
Overall survival for patients with high versus low tumor MVD
Figure 2.
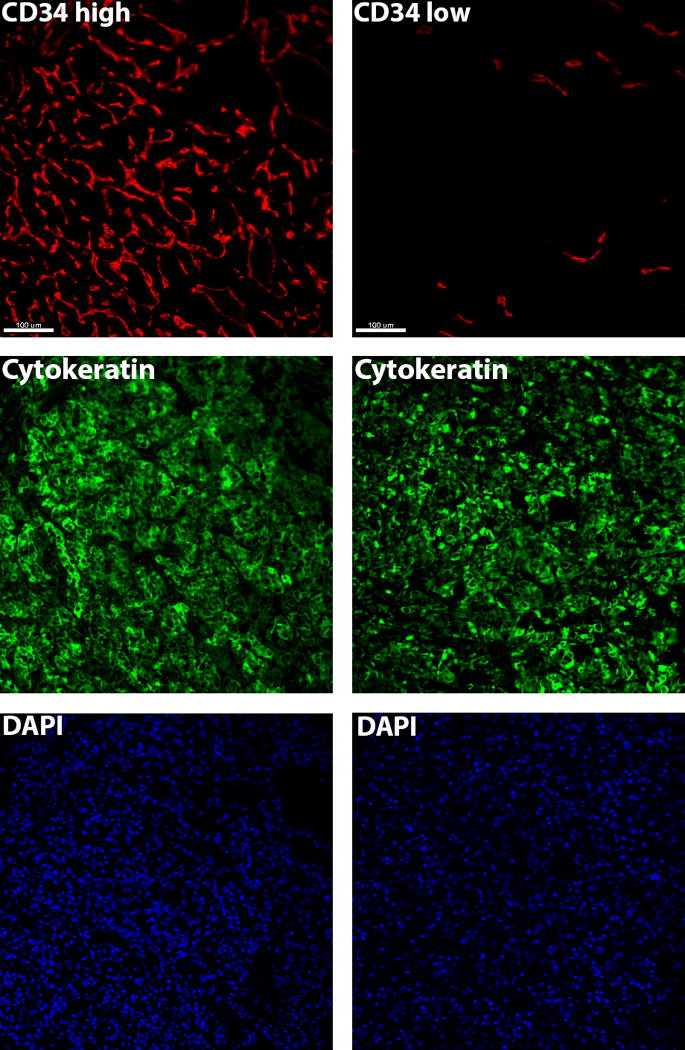
Representative immunofluorescence of high and low MVD as measured by CD34 expression in RCC nephrectomy specimens
Given the known intratumor mutational heterogeneity in RCC, we examined intratumor differences in MVD. The median log MVD was 1.29 and the median standard deviation (SD) was 0.35, with an interquartile range of 0.19-0.58. Overall, intratumor differences in MVD score were therefore fairly small, without wide variability.
MVD was divided into quartiles to further explore the strength of the association between MVD and OS for the entire cohort. As demonstrated in Figure 3, a statistically significant difference remained for OS (p=0.028), with the two higher MVD quartiles demonstrating improved OS compared to the two lower MVD quartiles. This further justifies use of the median as a cut point for MVD.
Figure 3.
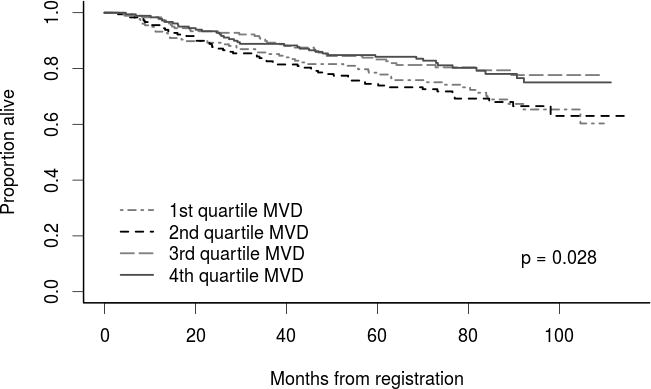
Overall survival for patients by MVD quartile
Multivariable analysis for overall survival
Because MVD was significantly associated with OS on univariate analysis, we performed multivariable analyses to identify whether MVD remained independently prognostic for OS. UISS factors including T and N stage, ECOG performance status, and tumor grade as well as age, gender, and presence of vascular invasion were assessed in a Cox model, as shown in Supplementary Table 1. MVD was then separately incorporated into the multivariable model that included these variables. MVD remained independently associated with OS (p=0.013, Table 2) and therefore improved the prognostic model.
Table 2.
Multivariable prognostic model for RCC incorporating MVD
| Variable | HR (95% CI) | p-value |
|---|---|---|
| Age ≥ median (56) | 1.67 (1.11-2.50) | 0.013 |
| Male gender | 1.20 (0.79-1.82) | 0.39 |
| Performance status (1 vs. 0) | 1.57 (0.99-2.47) | 0.054 |
| T stage (T3/T4 vs. T1/T2) | 1.63 (0.98-2.70) | 0.060 |
| N stage (N+ vs. N0/NX) | 2.95 (1.66-5.24) | <0.001 |
| Fuhrman grade (3/4 vs. 1/2) | 2.07 (1.32-3.25) | 0.001 |
| Vascular invasion | 1.52 (0.98-2.36) | 0.064 |
| MVD (high vs. low) | 0.61 (0.41-0.90) | 0.013 |
MVD and association with outcomes by treatment arm
Next, we investigated whether the association between MVD and OS was affected by treatment with sunitinib or sorafenib. MVD remained significantly associated with improved OS in patients treated on the placebo arm (p=0.028). High MVD had a weaker association with OS in patients treated with adjuvant anti-angiogenic agents (p=0.06). Overall, high MVD is prognostic in the entire cohort, more strongly in the placebo group compared to the treatment group. MVD does not serve as a predictive marker in this cohort.
Association between MVD and clinical or pathological variables
There were no significant differences in patient age, gender, or pre- or post-surgery serum calcium or LDH levels by MVD. Associations between MVD and pathological variables are shown in Figure 4. MVD was highest in RCCs of the clear cell histologic subtype, compared with other histologic types (p<0.001) (Figure 4a). There was no association between MVD and small versus large tumors when comparing T1 (≤7 cm) to T2 or larger (>7 cm) primary RCCs. MVD was significantly higher in Fuhrman grade 1-2 RCCs compared to RCCs of grade 3-4 (p<0.001) (Figure 4b) and also higher in RCCs without necrosis (p<0.001) (Figure 4c). There were no significant associations between MVD and presence of sarcomatoid features or lymphovascular invasion.
Figure 4.
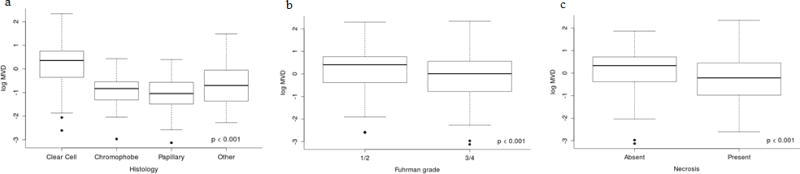
MVD and association with RCC (a) histological subtype; (b) Fuhrman Grade; and (c) tumor necrosis
DISCUSSION
We studied the prognostic and predictive value of MVD in nephrectomy specimens from patients enrolled on E2805. Our data demonstrated that MVD, as determined by area of MVD within the tumor, was an independent prognostic marker in high-risk RCC patients and appears to improve accuracy of current RCC prognostic models. The stronger association between MVD and survival in the placebo arm compared with the treatment arm indicates that MVD is a prognostic, rather than predictive, biomarker in this setting.
Surgery alone is the current mainstay of therapy for localized RCC. The majority of high-risk RCC patients undergo surveillance following surgical resection, unless enrolled on adjuvant trials. Retrospective data from over 3,600 non-metastatic RCC patients have demonstrated that about 30% recur, and up to one-third of recurrences are not captured by guideline-based surveillance practices (21). These data argue for further refinement of RCC prognostic models, perhaps through measurement of tissue-based biomarkers. The 5-year survival rates for stage I, II, and III RCCs based on the UISS prognostic model are estimated to be 94%, 67%, and 39%, respectively (5). Foreseeing which individual patients in each stage grouping will recur is a challenge. More accurate prognostic models could also better define which RCC patients should be enrolled on adjuvant therapy clinical trials. At this time, no adjuvant therapies are FDA-approved to address these recurrence statistics in high-risk RCC patients, but clinical investigations are ongoing.
To date, two major trials studying adjuvant anti-angiogenic TKIs in RCC have been published (12,13) with conflicting results, and results of a third trial were recently reported (14). The ASSURE trial (ECOG-ACRIN E2805), from which our study population was obtained, found no differences in median DFS or 5-year OS rates among high-risk RCC patients treated with adjuvant sunitinib, sorafenib, or placebo (12). In contrast, the S-TRAC trial evaluated adjuvant sunitinib compared to placebo in high-risk RCC patients and found a significant improvement in DFS for adjuvant sunitinib over placebo (6.8 vs. 5.6 years, HR 0.76, 95% CI 0.59-0.98), however OS data are not yet mature. Treatment discontinuation rates due to toxicity were high in both studies. Initial data from PROTECT, the third adjuvant RCC trial, reported a 31% decreased risk of recurrence in high risk patients who received pazopanib 800 mg daily, however the pazopanib starting dose for the trial was reduced to 600 mg daily for tolerability and did not meet the DFS endpoint (14). Other studies testing anti-angiogenic agents in the adjuvant setting have not yet matured, including trials of sorafenib (SOURCE NCT00492258) and axitinib (ATLAS NCT01599754) (15,22). There is an ongoing need to identify biomarkers that can distinguish those RCC patients at highest risk of recurrence to better tailor surveillance strategies and direct the highest risk patients towards adjuvant therapy clinical trials, given the cost and toxicities associated with TKI use.
In our study population of high-risk RCC patients from the ASSURE trial, MVD in primary nephrectomy specimens was determined to be an independent prognostic marker, with high MVD portending improved prognosis. MVD has previously been examined as a prognostic biomarker in RCC, with conflicting results. Published literature has suggested an inverse correlation of MVD with survival in RCC (23-25), no correlation (26), and even a positive association (27-31). For example, MVD was measured by CD34 in 70 primary RCCs and high MVD was associated with improved survival, however it was not independently prognostic (30). Delahunt et al. measured MVD by factor VIII expression by immunohistochemistry on 150 clear cell RCCs and higher MVD also correlated with prolonged OS (29). The disparate results in the literature are likely related to nuances in methodology including use of different markers for MVD (CD34, CD31, CD105, or von Willebrand factor), whether MVD was assessed in the center or at the perimeter of the tumor or in areas of hemorrhage or necrosis, and use of small sample sizes with potentially heterogeneous RCC patient populations (10). We used CD34 to measure MVD as it had previously been shown by us and others to be specific and reproducible for quantitating tumor vasculature (32,33). Moreover, the published series were all from single institutions, as opposed to our large cohort, multi-institutional study of patients who underwent uniform surveillance on a clinical trial.
Higher MVD in RCC is thought to reflect increased activity of the VEGF/VEGFR signaling pathway, driving tumor vascularization. However, the rate of tumor growth does not necessarily imply high MVD (10,34). High MVD in our study was associated improved OS, and likely reflects better tumor differentiation. This is consistent with other instances in which higher levels of markers involved in the malignant process are associated with better prognosis, such as improved survival of breast cancer patients whose tumors express Bcl-2 or hormone receptors (35,36). Bcl-2 and hormone receptor expression are lost in poorly differentiated breast cancers. Our study further emphasizes that RCC tumors of higher grade actually have lower MVD, indicating that high MVD might be a marker of less aggressive tumor biology.
We note that MVD was associated with OS, but not DFS. Our data indicate that MVD is purely a prognostic marker in this setting, and is likely a hallmark of better tumor differentiation. While it might be assumed that a marker of less aggressive tumors should also be associated with longer DFS, it is plausible that MVD is only important in the setting of macroscopic metastatic tumor growth, and less important for tumor cell growth in the time between nephrectomy and overt metastatic recurrence. Based on the timing of this trial, we assume that at metastatic relapse, many of these patients were treated with VEGF-receptor inhibitors, although data on subsequent treatment in this cohort of patients are not available to us. High MVD has been shown by us and others to be associated with response to VEGF-receptor inhibitors, and prolongation of OS might be due to benefit from these drugs in the metastatic setting in patients with higher MVD (37), resulting in an association between high MVD and OS but not DFS.
MVD in our study did not appear to be predictive of sensitivity to adjuvant sorafenib or sunitinib. We note, however, that this study was conducted in the setting of microscopic metastatic disease or no metastatic disease, and activity of these drugs might depend on presence of viable tumor blood vessels (10). Our measurements of MVD, particularly in resected tumors, may not necessarily distinguish higher from lower density in microscopic metastases. Pre-clinical evidence has demonstrated that poorly vascularized tumors can respond to antiangiogenic therapy just as well as highly vascularized tumors (38), but whether this concept is applicable in the adjuvant setting is not clear. Based on our data, we conclude that MVD may not be a useful marker in predicting tumor sensitivity to anti-angiogenic therapy in the adjuvant setting, but does carry independent prognostic value. Low MVD was found in a poor prognostic patient group that may be well served by enrollment on adjuvant clinical trials with agents such as immunotherapy or other investigational drugs. Patients with low MVD tumors, however, do not appear to benefit from VEGF-R targeting adjuvant therapies.
The strengths of this study are the use of a large cohort of over 800 patients, which upon literature review, is larger by far than any prior published cohort examining MVD in RCC. Moreover, this cohort is well-annotated with standardized data and represents the largest cohort enrolled on a multi-institutional adjuvant clinical trial in RCC. A shortcoming of past clinicopathologic studies on MVD has been the lack of standardized treatment in the study population. By studying tissue from patients on E2805, the variability in treatment is removed as these patients were treated in a more uniform manner. Additionally, our methods for MVD measurement are objective and automated. Median MVD score was chosen as a cutoff because this is unbiased and is justified by our data showing that the two higher MVD score quartiles had improved OS compared to the two lower MVD score quartiles. High MVD in our analysis also correlated with more favorable pathologic features including lower Fuhrman grade, clear cell histology, and absence of necrosis, which further substantiates the notion that high MVD correlates not just with OS, but also with more favorable pathologic characteristics of the tumor.
A noted limitation of our study is that data on treatment type at time of RCC relapse is unavailable for the trial, and therefore we acknowledge that this could potentially impact survival outcomes. Additionally, MVD was highest in the clear cell histologic subtype and is clearly prognostic, however further study of MVD is needed in the non-clear cell RCC histologic subtypes, which represented only 18% of the cohort.
In conclusion, high MVD is associated with improved OS in this cohort of 822 high-risk RCC patients for which primary RCC tissue was available for analysis. Although the association with survival was stronger in the placebo group than in patients treated with anti-angiogenic agents, the addition of MVD in nephrectomy specimens significantly improved a RCC prognostic model when accounting for variables contained in the validated UISS prognostic model. Further studies are warranted to confirm whether incorporating MVD into prognostic models will improve our ability to select high-risk RCC patients for enhanced surveillance and adjuvant therapy trials with agents other than anti-angiogenic TKIs, such as immunotherapy or other investigational drugs.
Supplementary Material
Statement of Translational Relevance.
Current prognostic models for patients with non-metastatic renal cell carcinoma (RCC) incorporate clinical and histological variables; none incorporate tissue-based biomarkers. Here we explored the prognostic and predictive implications of tumor microvessel density (MVD) as measured by the area of CD34-staining cells within tumors obtained from nephrectomies of high-risk RCC patients enrolled on ECOG-ACRIN 2805 who were treated with adjuvant sunitinib, sorafenib, or placebo. We found that high MVD is associated with improved prognosis, and was independent of other currently-used prognostic markers on multivariable analysis. The stronger association between high MVD and improved survival in the placebo arm compared to the treatment arm indicates that MVD is a prognostic, rather than predictive, biomarker in this setting. Further studies are warranted to confirm whether incorporating MVD into prognostic models will improve our ability to select high-risk RCC patients for enhanced surveillance programs and adjuvant therapy trials.
Acknowledgments
This work was supported by CA180820, CA180794, CA180826, and in part by NIH grants R-01 CA158167 and K24 CA172123 (H.M.K, PI). L.B.J. is also supported by the Lung Cancer Research Foundation-LUNGevity and Melanoma Research Alliance, Award #308721.
Footnotes
Conflicts of Interest: Naomi Hass consults for Exelexis, Novartis, and Pfizer. Harriet Kluger has consulted for Genentech, Alexion, Nektar and Bioclinica. Keith Flaherty previously served as a consultant to Onyx and Pfizer. All other authors report no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.SEER. Cancer Stat Facts: Kidney and Renal Pelvis Cancer. Available at: https://seer.cancer.gov/statfacts/html/kidrp.html. Accessed on 3 Apr 2017.
- 3.Rioux-Leclercq N, Karakiewicz PI, Trinh QD, Ficarra V, Cindolo L, de la Taille A, et al. Prognostic ability of simplified nuclear grading of renal cell carcinoma. Cancer. 2007;109(5):868–74. doi: 10.1002/cncr.22463. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg B, Alamdari FI, Rasmuson T, Roos G. Follow-up guidelines for nonmetastatic renal cell carcinoma based on the occurrence of metastases after radical nephrectomy. BJU Int. 1999;84(4):405–11. doi: 10.1046/j.1464-410x.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- 5.Zisman A, Pantuck AJ, Dorey F, Said JW, Shvarts O, Quintana D, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19(6):1649–57. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 6.Ficarra V, Novara G, Galfano A, Brunelli M, Cavalleri S, Martignoni G, et al. The ‘Stage, Size, Grade and Necrosis’ score is more accurate than the University of California Los Angeles Integrated Staging System for predicting cancer-specific survival in patients with clear cell renal cell carcinoma. BJU Int. 2009;103(2):165–70. doi: 10.1111/j.1464-410X.2008.07901.x. [DOI] [PubMed] [Google Scholar]
- 7.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168(6):2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 8.Russo P, Jang TL, Pettus JA, Huang WC, Eggener SE, O’Brien MF, et al. Survival rates after resection for localized kidney cancer: 1989 to 2004. Cancer. 2008;113(1):84–96. doi: 10.1002/cncr.23520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finley DS, Pantuck AJ, Belldegrun AS. Tumor biology and prognostic factors in renal cell carcinoma. The oncologist. 2011;16(Suppl 2):4–13. doi: 10.1634/theoncologist.2011-S2-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. Journal of the National Cancer Institute. 2002;94(12):883–93. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- 11.Zarrabi K, Fang C, Wu S. New treatment options for metastatic renal cell carcinoma with prior anti-angiogenesis therapy. J Hematol Oncol. 2017;10(1):38. doi: 10.1186/s13045-016-0374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas NB, Manola J, Uzzo RG, Flaherty KT, Wood CG, Kane C, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387(10032):2008–16. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravaud A, Motzer RJ, Pandha HS, George DJ, Pantuck AJ, Patel A, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. The New England journal of medicine. 2016;375(23):2246–54. doi: 10.1056/NEJMoa1611406. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Haas NB, Donskov F, Gross-Goupil M, Varlamov S, Kopyltsov E, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with locally advanced renal cell carcinoma (RCC) (PROTECT) Journal of Clinical Oncology. 2017;35(15_suppl):4507–07. doi: 10.1200/JCO.2017.73.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel DN, Figlin RA, Kim HL. Adjuvant treatment for renal cell carcinoma: do we finally have a major breakthrough? Clin Adv Hematol Oncol. 2016;14(11):907–14. [PubMed] [Google Scholar]
- 16.Aziz SA, Sznol JA, Albiges L, Zito C, Jilaveanu LB, Camp RL, et al. Microvessel area as a predictor of sorafenib response in metastatic renal cell carcinoma. Cancer Cell Int. 2014;14(1):4. doi: 10.1186/1475-2867-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy MM, DiVito KA, Sznol M, Kovacs D, Halaban R, Berger AJ, et al. Expression of tumor necrosis factor–related apoptosis-inducing ligand receptors 1 and 2 in melanoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12(12):3856–63. doi: 10.1158/1078-0432.CCR-06-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarthy MM, Pick E, Kluger Y, Gould-Rothberg B, Lazova R, Camp RL, et al. HSP90 as a marker of progression in melanoma. Ann Oncol. 2008;19(3):590–4. doi: 10.1093/annonc/mdm545. [DOI] [PubMed] [Google Scholar]
- 19.Jilaveanu LB, Zhao F, Zito CR, Kirkwood JM, Nathanson KL, D’Andrea K, et al. Expression of drug targets in patients treated with sorafenib, carboplatin and paclitaxel. PloS one. 2013;8(8):e69748. doi: 10.1371/journal.pone.0069748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8(11):1323–7. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 21.Stewart SB, Thompson RH, Psutka SP, Cheville JC, Lohse CM, Boorjian SA, et al. Evaluation of the National Comprehensive Cancer Network and American Urological Association renal cell carcinoma surveillance guidelines. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(36):4059–65. doi: 10.1200/JCO.2014.56.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker H. Sunitinib as adjuvant therapy for renal cell carcinoma. The Lancet Oncology. 2016;17(11):e485. doi: 10.1016/S1470-2045(16)30511-3. [DOI] [PubMed] [Google Scholar]
- 23.Yoshino S, Kato M, Okada K. Prognostic significance of microvessel count in low stage renal cell carcinoma. Int J Urol. 1995;2(3):156–60. doi: 10.1111/j.1442-2042.1995.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 24.Nativ O, Sabo E, Reiss A, Wald M, Madjar S, Moskovitz B. Clinical significance of tumor angiogenesis in patients with localized renal cell carcinoma. Urology. 1998;51(5):693–6. doi: 10.1016/s0090-4295(98)00019-3. [DOI] [PubMed] [Google Scholar]
- 25.Ren J, Liu H, Yan L, Tian S, Li D, Xu Z. Microvessel density and heparanase over-expression in clear cell renal cell cancer: correlations and prognostic significances. World J Surg Oncol. 2011;9:158. doi: 10.1186/1477-7819-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLennan GT, Bostwick DG. Microvessel density in renal cell carcinoma: lack of prognostic significance. Urology. 1995;46(1):27–30. doi: 10.1016/S0090-4295(99)80153-8. [DOI] [PubMed] [Google Scholar]
- 27.Yildiz E, Ayan S, Goze F, Gokce G, Gultekin EY. Relation of microvessel density with microvascular invasion, metastasis and prognosis in renal cell carcinoma. BJU Int. 2008;101(6):758–64. doi: 10.1111/j.1464-410X.2007.07318.x. [DOI] [PubMed] [Google Scholar]
- 28.Sandlund J, Hedberg Y, Bergh A, Grankvist K, Ljungberg B, Rasmuson T. Evaluation of CD31 (PECAM-1) expression using tissue microarray in patients with renal cell carcinoma. Tumour Biol. 2007;28(3):158–64. doi: 10.1159/000102980. [DOI] [PubMed] [Google Scholar]
- 29.Delahunt B, Bethwaite PB, Thornton A. Prognostic significance of microscopic vascularity for clear cell renal cell carcinoma. Br J Urol. 1997;80(3):401–4. doi: 10.1046/j.1464-410x.1997.00374.x. [DOI] [PubMed] [Google Scholar]
- 30.Imao T, Egawa M, Takashima H, Koshida K, Namiki M. Inverse correlation of microvessel density with metastasis and prognosis in renal cell carcinoma. Int J Urol. 2004;11(11):948–53. doi: 10.1111/j.1442-2042.2004.00931.x. [DOI] [PubMed] [Google Scholar]
- 31.Rioux-Leclercq N, Epstein JI, Bansard JY, Turlin B, Patard JJ, Manunta A, et al. Clinical significance of cell proliferation, microvessel density, and CD44 adhesion molecule expression in renal cell carcinoma. Hum Pathol. 2001;32(11):1209–15. doi: 10.1053/hupa.2001.28957. [DOI] [PubMed] [Google Scholar]
- 32.Kluger HM, Siddiqui SF, Angeletti C, Sznol M, Kelly WK, Molinaro AM, et al. Classification of renal cell carcinoma based on expression of VEGF and VEGF receptors in both tumor cells and endothelial cells. Laboratory investigation; a journal of technical methods and pathology. 2008;88(9):962–72. doi: 10.1038/labinvest.2008.65. [DOI] [PubMed] [Google Scholar]
- 33.Mertz KD, Demichelis F, Kim R, Schraml P, Storz M, Diener PA, et al. Automated immunofluorescence analysis defines microvessel area as a prognostic parameter in clear cell renal cell cancer. Hum Pathol. 2007;38(10):1454–62. doi: 10.1016/j.humpath.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Yao X, Qian CN, Zhang ZF, Tan MH, Kort EJ, Yang XJ, et al. Two distinct types of blood vessels in clear cell renal cell carcinoma have contrasting prognostic implications. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13(1):161–9. doi: 10.1158/1078-0432.CCR-06-0774. [DOI] [PubMed] [Google Scholar]
- 35.Nadler Y, Camp RL, Giltnane JM, Moeder C, Rimm DL, Kluger HM, et al. Expression patterns and prognostic value of Bag-1 and Bcl-2 in breast cancer. Breast Cancer Res. 2008;10(2):R35. doi: 10.1186/bcr1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroger N, Milde-Langosch K, Riethdorf S, Schmoor C, Schumacher M, Zander AR, et al. Prognostic and predictive effects of immunohistochemical factors in high-risk primary breast cancer patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12(1):159–68. doi: 10.1158/1078-0432.CCR-05-1340. [DOI] [PubMed] [Google Scholar]
- 37.Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt TJ. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int. 2011;108(10):1556–63. doi: 10.1111/j.1464-410X.2011.10629.x. [DOI] [PubMed] [Google Scholar]
- 38.Beecken WD, Fernandez A, Joussen AM, Achilles EG, Flynn E, Lo KM, et al. Effect of antiangiogenic therapy on slowly growing, poorly vascularized tumors in mice. Journal of the National Cancer Institute. 2001;93(5):382–7. doi: 10.1093/jnci/93.5.382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


