Abstract
Introduction
In 2010, the WHO recommended women living with HIV breastfeed for 12 months while taking antiretroviral therapy (ART) to balance breastfeeding benefits against HIV transmission risks. To inform the 2016 WHO guidelines, we updated prior research on the impact of breastfeeding duration on HIV‐free infant survival (HFS) by incorporating maternal ART duration, infant/child mortality and mother‐to‐child transmission data.
Methods
Using the Cost‐Effectiveness of Preventing AIDS Complications (CEPAC)‐Infant model, we simulated the impact of breastfeeding duration on 24‐month HFS among HIV‐exposed, uninfected infants. We defined “optimal” breastfeeding durations as those maximizing 24‐month HFS. We varied maternal ART duration, mortality rates among breastfed infants/children, and relative risk of mortality associated with replacement feeding (“RRRF”), modelled as a multiplier on all‐cause mortality for replacement‐fed infants/children (range: 1 [no additional risk] to 6). The base‐case simulated RRRF = 3, median infant mortality, and 24‐month maternal ART duration.
Results
In the base‐case, HFS ranged from 83.1% (no breastfeeding) to 90.2% (12‐months breastfeeding). Optimal breastfeeding durations increased with higher RRRF values and longer maternal ART durations, but did not change substantially with variation in infant mortality rates. Optimal breastfeeding durations often exceeded the previous WHO recommendation of 12 months.
Conclusions
In settings with high RRRF and long maternal ART durations, HFS is maximized when mothers breastfeed longer than the previously‐recommended 12 months. In settings with low RRRF or short maternal ART durations, shorter breastfeeding durations optimize HFS. If mothers are supported to use ART for longer periods of time, it is possible to reduce transmission risks and gain the benefits of longer breastfeeding durations.
Keywords: HIV, PMTCT, breastfeeding, modelling, maternal health, infant/child health
1. Introduction
Infants born to women living with HIV (WLHIV) are at risk for mother‐to‐child HIV transmission (MTCT) through breast milk 1, 2. Although effective, maternal antiretroviral therapy (ART) does not completely prevent transmission 3, 4. This risk can be eliminated by replacement feeding 5, 6, 7; however, in many settings, replacement feeding leads to malnutrition, diarrhoea, and respiratory illness, because infants are exposed to water contaminated with enteric pathogens and fail to receive protective maternal antibodies through breast milk 1, 8. These conditions are leading causes of infant and under‐five mortality in sub‐Saharan Africa and may account for up to 45% of deaths in children under 5 worldwide 9. In sub‐Saharan Africa, breastfeeding for durations greater than 12 months is common 10. Recommendations and practices among women with HIV vary widely, with an estimated 10‐57% of WLHIV avoiding breastfeeding altogether 5, 6, 7, 11.
To achieve this balance between the risk of HIV transmission and the risk of replacement feeding, the 2010 World Health Organization (WHO) Guidelines on HIV and Infant Feeding defined a primary goal of maximizing infant HIV‐free survival (HFS) 1. These guidelines, using two modelling analyses that outlined optimal feeding strategies as well as the cost to healthcare systems, advised that, in settings where diarrhoea, pneumonia and malnutrition are prevalent, national authorities should support mothers living with HIV whose infants were either uninfected or had an unknown status to exclusively breastfeed for the first 6 months of life, then to continue breastfeeding with complementary foods through 12 months while providing ART to mothers 1. In previous work, we used the Cost‐effectiveness of Preventing AIDS Complications (CEPAC)‐Infant model to examine breastfeeding durations that maximized HFS under various conditions 12. That analysis demonstrated the potential benefit of tailoring breastfeeding durations to maternal CD4 counts, ART availability, and mortality risk associated with replacement feeding; we concluded, however, that recommending 12 months was appropriate in resource‐limited settings as the gains from an “individualized approach” were small. New data have since emerged regarding MTCT risk as a function of maternal retention in care and adherence to ART 13, 14. We incorporated these new data and refined our analysis on the impact of context‐specific infant mortality rates and maternal ART use on optimal feeding recommendations. In 2016, in order to accommodate local breastfeeding practices that extended past 12 months and to harmonize infant feeding recommendations for women living with and without HIV, the WHO considered increasing the recommended duration of breastfeeding for women on ART from 12 to 24 months. This analysis contributed to the updated WHO guidelines on infant feeding and HIV 15.
2. Methods
2.1. Analytic overview
We used the CEPAC‐Infant model to simulate HIV‐exposed, uninfected infants through 24 months of life 12, 16. The primary outcome was 24‐month HFS, defined as the proportion of the cohort that was alive and uninfected at 24 months of life. Building upon our prior analysis, we incorporated updated MTCT data for women on ART, as well as updated neonatal, infant and under‐five mortality rates from 21 Global Plan priority countries 2, 5, 12, 13, 14, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29. We projected HFS for each breastfeeding duration from 0 (replacement‐fed from birth) through 24 months, while simultaneously varying three key parameters: the duration of maternal ART, the relative risk of infant and child mortality associated with replacement feeding compared to breastfeeding (RRRF), and background infant and child mortality rates. RRRF was modelled as a multiplier on monthly background breastfed mortality risks, applied during each month infants were replacement fed. The modelled duration of maternal ART use was intended to reflect both retention in care and ART adherence 13. The primary goals of our analysis were: (1) to determine the “optimal breastfeeding duration” or the duration that maximized 24‐month HFS for each scenario, and (2) to quantify the impact of maternal ART duration on 24‐month HFS for each combination of RRRF and breastfeeding duration. In the base case, we simulated 24 months of maternal ART, an RRRF of 3, and median infant/child mortality rates. In sensitivity analyses, we varied each of these parameters; in secondary analyses, we varied monthly MTCT rates and examined variations in RRRF with infant/child age.
2.2. CEPAC model and structure
2.2.1. CEPAC‐infant model
The CEPAC‐Infant model is a Monte Carlo microsimulation model of infant HIV infection and survival from birth through 24 months of age. The CEPAC‐Infant model is simpler in structure than the CEPAC‐Adult and CEPAC‐Paediatric models (Figure S1) 12, 30. In the CEPAC‐Infant model, HIV‐exposed, uninfected infants enter the model at birth and face three key monthly risks: (1) background mortality risks for breastfed children, which are multiplied by the RRRF for replacement‐fed children; (2) postnatal HIV infection (MTCT) risk throughout the breastfeeding period, stratified by maternal ART use and maternal CD4 count for women not on ART; and (3) maternal mortality, which leads a child to wean immediately and face the mortality risks of replacement feeding (RRRF). The primary model outcome is monthly HFS: the proportion of children alive and HIV‐uninfected at each month after birth; the model has been calibrated to data from sub‐Saharan Africa 16, 31, 32. Furthermore details about the full CEPAC‐Infant model can be found in the technical appendices of prior CEPAC‐Infant analyses 12, 30 as well as at http://web2.research.partners.org/cepac/model.php.
Because traditional measures of statistical significance (p‐values, 95% confidence intervals) do not accurately reflect precision in microsimulation model results, we instead follow International Society for Pharmacoeconomics and Outcomes Research/Society for Medical Decision Making (ISPOR/SMDM) guidance and describe the impact of uncertainty in our analysis through a series of extensive univariate and multivariate sensitivity analyses 33. A full description of our approach to 16 specific ISPOR/SMDM recommendations for analysing uncertainty in the CEPAC model is also provided in the Appendix of previous work 30.
2.3. Input parameters
2.3.1. Breastfeeding duration
We modelled breastfeeding durations of 0 to 24 months in 3‐month increments. Infants assigned to breastfeed for 0 months initiated replacement feeding immediately after birth. For all others, infants were assumed to wean immediately after the last month of breastfeeding. Breastfeeding in the first 6 months of life was modelled as exclusive breastfeeding, per WHO guidelines, and breastfeeding after 6 months of age was modelled as complementary feeding, consisting of breast milk and food 1.
2.3.2. Maternal ART duration
In the base case, we assumed that women took ART throughout the simulation (24 months), reflecting WHO “Option B+” guidelines for lifelong ART. In sensitivity analyses, we varied maternal ART duration from 0 to 24 months in 3‐month increments. Because maternal ART duration is not directly specified in the CEPAC‐Infant model structure, we adjusted model output to reflect the impact of maternal ART cessation through a series of recalculated monthly HFS risks (Material S2). We assume that MTCT risk after maternal ART cessation returns immediately to that of a mother who never received ART.
2.3.3. Relative risk of replacement feeding (RRRF)
In the model, RRRF is applied as a multiplier on monthly all‐cause infant/child mortality risk during each month children are replacement fed. We designed the RRRF to reflect the combined impact of replacement feeding on mortality, including effects of poor water quality, insufficient quantities of replacement milk, diarrhoeal disease, or pneumonia. Based on available data, RRRF was not applied during months of complementary feeding. This simulates the protective benefit of breastfeeding throughout the period of breast milk exposure; as seen in previous work, the opposite assumption – ending the protective effect of breastfeeding at 6 months of age – leads all optimal breastfeeding durations for women on ART to be 6 months 3, 5, 6, 7, 12, 34, 35, 36, 37, 38. In the base case, we modelled an RRRF of 3, a value reported in settings like Malawi and Zambia (8, 32 to 33); in sensitivity analyses, this value ranged from 1 to 6, reflecting data from various settings (Table 1) 3, 5, 6, 7, 34, 35, 36, 37, 38.
Table 1.
Selected model input parameters for the CEPAC‐Infant model
| Parameter | Value | Reference |
|---|---|---|
| I. Relative risk of mortality among replacement‐fed compared to breastfed infants (RRRF), by countrya | ||
| Kenya; Rwanda; South Africa; Côte d'Ivoire | 1.0 | 3, 5, 6, 7 |
| Botswana | 2.0 | 34 |
| Malawi | 1.8‐3.3 | 35 |
| Zambia | 2.0‐4.2 | 8, 36 |
| Uganda | 6.0 | 38 |
| II. Monthly infant mortality risk, by scenario and ageb | 49, 50 | |
| Moderate mortality (base case) | ||
| 0 to 2 months | 0.0116 | |
| 3 to 11 months | 0.0024 | |
| 12 to 24 months | 0.00067 | |
| Low mortality scenario | ||
| 0 to 2 months | 0.0082 | |
| 3 to 11 months | 0.0023 | |
| 12 to 24 months | 0.0004 | |
| High mortality scenario | ||
| 0 to 2 months | 0.0172 | |
| 3 to 11 months | 0.0034 | |
| 12 to 24 months | 0.00093 | |
| III. Monthly maternal mortality risk, by disease status and ART strategy | Projected from CEPAC‐International adult model | |
| CD4 ≤ 350/μL | ||
| No ART | 0.0078 | |
| 3‐drug ART | 0.0016 | |
| CD4 > 350/μL | ||
| No ART | 0.0024 | |
| 3‐drug ART | 0.0009 | |
| IV. Monthly postnatal mother‐to‐child transmission risk in postnatal period, by model and ART strategy | ||
| Base‐case | ||
| No ART | 0.0044 | 2, 19, 20, 22, 23, 27, 28, 29, 34, 57 |
| 3‐drug ART | 0.0019 | 2, 19, 20, 22, 23, 27, 28, 29, 34, 57 |
| Spectrum 2012 inputs | ||
| No ART | 0.0095 | 58 |
| 3‐drug ART | 0.0020 | 58 |
| Spectrum 2016 inputs (proposed) | ||
| No ART | 0.0108 | Personal communication with Spectrum team |
| 3‐drug ART | 0.0012 | Personal communication with Spectrum team |
RRRF was calculated by dividing cumulative mortality risk (at the greatest duration reported in each study) among replacement‐fed infants by cumulative mortality risk among breastfed infants.
Mortality rates are stratified by current age in each month of the simulation and reported for breastfed, HIV‐exposed, uninfected children.
In secondary analyses, we evaluated extreme RRRF values (up to 23, far higher than reported in any setting) to identify the threshold at which replacement feeding conferred such high risks that all infants, regardless of maternal ART duration, would need 24 months of breastfeeding to optimize HFS 39. We also examined scenarios in which RRRF was varied with infant/child age, based on limited data from HIV‐unexposed children suggesting that mortality risks of replacement feeding may decrease as an infant ages 40, 41. In this analysis, we decreased the at‐birth RRRF by 50% at 12 months of age, and then by another 50% at 18 months.
2.3.4. Mortality risks
To better reflect variations in infant‐mortality across a range of countries, at WHO request, we used age‐specific monthly infant/child mortality risks derived from published neonatal, infant, and under‐5 mortality rates from 21 Global Plan countries in sub‐Saharan Africa (Table 1) 18. Because breastfeeding is very common in most of the countries from which these data were derived, we have assigned these risks as the “background” mortality risk among breastfed infants; we vary these rates widely in sensitivity analyses. The median risks from all 21 countries were used in the base case analysis (a “moderate” mortality scenario). Because the RRRF and underlying background mortality rates may vary independently, we also evaluated a “low” mortality scenario and a “high” mortality scenario for our sensitivity analyses, using the 25th and 75th percentile values. Monthly maternal mortality risks were derived from previous CEPAC‐Adult modelling analyses for postpartum women (28) (Table 1).
2.3.5. Mother‐to‐child transmission risks
We derived MTCT risks from published trials in breastfeeding African women (Table 1). For infants whose mothers were not on ART, we weighted CD4‐stratified MTCT risks according to the CD4 distribution observed in the representative MTCT‐Plus cohort 17. For infants of mothers on effective ART, maternal CD4 did not impact modelled MTCT risk 2, 5, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29. In sensitivity analyses, we repeated all analyses with MTCT risks derived by the UNAIDS Reference Group for the Spectrum model in 2012 42, as well as proposed Spectrum inputs for 2016 (Nigel Rollins, personal communication); compared to base case inputs, both sets of Spectrum inputs, derived via an independent literature review, assigned slightly higher MTCT risks for women not on ART, and were similar for women on ART.
3. Results
3.1. Base case results: impact of breastfeeding duration
In the base case (RRRF=3, median infant mortality, maternal ART duration=24 months), 24‐month HFS ranged from 83.1% with replacement feeding from birth to 90.2% with breastfeeding duration of 12 months (Figure 1). Although breastfeeding for 12 months maximized HFS, breastfeeding durations ≥12 months yielded similar HFS values (89.7% to 90.2%), suggesting that a change from 12 to 24 months of breastfeeding would have minimal impact on HFS.
Figure 1.
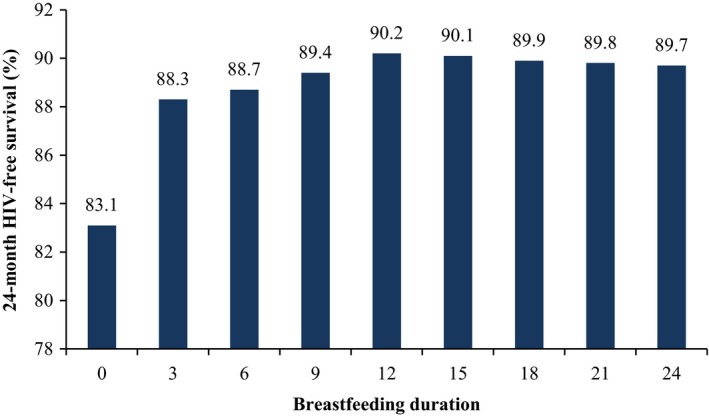
HIV‐free survival by breastfeeding duration for HIV‐exposed infants with moderate infant mortality, RRRF = 3, and maternal ART use throughout the breastfeeding period (base case). Breastfeeding duration is shown on the horizontal axis, and HIV‐free survival is shown on the vertical axis, as well as above the bar for each breastfeeding duration. RRRF, relative risk of replacement feeding; BF, breastfeeding; ART, antiretroviral therapy.
3.2. Sensitivity analyses
3.2.1. Maternal ART duration
The impact of varying maternal ART duration is shown in Table 2, section I. The base‐case (24‐month ART duration) is shown on the far right (box). The sections to the left vary breastfeeding and maternal ART duration, holding constant the RRRF (set at 3) and infant mortality (median values). For example, when the breastfeeding duration is 12 months as in the base case (bold), HFS remained 90.2% as long as maternal ART duration was also ≥12 months (yellow). For any given maternal ART duration from 3 to 12 months, HFS was usually maximized by breastfeeding for as long as maternal ART was taken, for example, with 9‐months maternal ART, by breastfeeding for 9 months (HFS 89.4%, yellow). For any given breastfeeding duration beyond 0, HFS increased substantially if maternal ART use persisted throughout the entire breastfeeding period; with 24 months of breastfeeding, for example, HFS increased from 74.1% with no ART to 89.7% with 24 months of ART (bottom row).
Table 2.
24‐month HIV‐free survival for selected breastfeeding durations, maternal ART durations, and RRRF values with moderate mortality risk (full results in Supplemental 635 Table S3)
| I RRRF = 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RR‐RF = 3 | 24‐month HIV‐free survival (%) | ||||||||
| ART duration → | 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 |
| BF 0 months | 83.1 | 83.1 | 83.1 | 83.1 | 83.1 | 83.1 | 83.1 | 83.1 | 83.1 |
| BF 3 months | 87.5 | 88.3 | 88.3 | 88.3 | 88.3 | 88.3 | 88.3 | 88.3 | 88.3 |
| BF 6 months | 87.3 | 88 | 88.7 | 88.7 | 88.7 | 88.7 | 88.7 | 88.7 | 88.7 |
| BF 9 months | 85.3 | 86 | 86.7 | 89.4 | 89.4 | 89.4 | 89.4 | 89.4 | 89.4 |
| BF 12 months | 83.4 | 84.1 | 84.7 | 87.4 | 90.2 | 90.2 | 90.2 | 90.2 | 90.2 |
| BF 15 months | 80.9 | 81.6 | 82.2 | 84.8 | 87.5 | 90.1 | 90.1 | 90.1 | 90.1 |
| BF 18 months | 78.5 | 79.2 | 79.8 | 82.3 | 84.9 | 87.4 | 89.9 | 89.9 | 89.9 |
| BF 21 months | 76.2 | 76.9 | 77.4 | 79.9 | 82.4 | 84.9 | 87.3 | 89.8 | 89.8 |
| BF 24 months | 74.1 | 74.8 | 75.3 | 77.7 | 80.2 | 82.6 | 84.9 | 87.3 | 89.7 |
| II RR‐RF = 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RR‐RF = 1 | 24‐month HIV‐free survival (%) | ||||||||
| ART duration → | 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 |
| BF 0 months | 93.8 | 93.8 | 93.8 | 93.8 | 93.8 | 93.8 | 93.8 | 93.8 | 93.8 |
| BF 3 months | 92.6 | 93.3 | 93.3 | 93.2 | 93.3 | 93.3 | 93.3 | 93.3 | 93.3 |
| BF 6 months | 91.4 | 92.1 | 92.7 | 92.7 | 92.7 | 92.7 | 92.7 | 92.7 | 92.7 |
| BF 9 months | 88.2 | 88.2 | 89.4 | 92.2 | 92.2 | 92.2 | 92.2 | 92.2 | 92.2 |
| BF 12 months | 85.1 | 85.7 | 86.3 | 89 | 91.7 | 91.7 | 91.7 | 91.7 | 91.7 |
| BF 15 months | 82.2 | 82.9 | 83.4 | 86 | 88.6 | 91.2 | 91.2 | 91.2 | 91.2 |
| BF 18 months | 79.6 | 80.1 | 80.7 | 83.2 | 85.7 | 88.2 | 90.7 | 90.7 | 90.7 |
| BF 21 months | 77 | 77.6 | 78.1 | 80.6 | 83 | 85.4 | 87.8 | 90.2 | 90.2 |
| BF 24 months | 74.7 | 75.2 | 75.7 | 78.1 | 80.4 | 82.8 | 85.1 | 87.4 | 89.7 |
| III RRRF = 6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RR‐RF = 6 | 24‐month HIV‐free survival (%) | ||||||||
| ART duration → | 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 |
| BF 0 months | 69.4 | 69.3 | 69.3 | 69.3 | 69.3 | 69.3 | 69.3 | 69.3 | 69.3 |
| BF 3 months | 80.5 | 81.3 | 81.3 | 81.3 | 81.3 | 81.3 | 81.3 | 81.3 | 81.3 |
| BF 6 months | 81.4 | 82.2 | 82.9 | 82.9 | 82.9 | 82.9 | 82.9 | 82.9 | 82.9 |
| BF 9 months | 81.2 | 82 | 82.7 | 85.4 | 85.4 | 85.4 | 85.4 | 85.4 | 85.4 |
| BF 12 months | 81 | 81.8 | 82.5 | 85.2 | 88 | 88 | 88 | 88 | 88 |
| BF 15 months | 79 | 79.7 | 80.4 | 83 | 85.8 | 88.4 | 88.4 | 88.4 | 88.4 |
| BF 18 months | 77 | 77.7 | 78.4 | 81 | 83.7 | 86.2 | 88.8 | 88.8 | 88.8 |
| BF 21 months | 75.1 | 75.9 | 76.5 | 79 | 81.7 | 84.1 | 86.7 | 89.2 | 89.2 |
| BF 24 months | 73.4 | 74.1 | 74.8 | 77.2 | 79.8 | 82.2 | 84.7 | 87.1 | 89.6 |
ART, antiretroviral therapy; BF, breastfeeding duration; RRRF, relative risk of replacement feeding; HFS, HIV‐free survival.
Bolded numbers indicate HFS at 12 months (2010 WHO Recommendation). Red box indicates base case scenario (RRRF = 3, 24‐month ART duration, median infant/child mortality). Yellow indicates maximum HFS for each ARV duration.
3.2.2. Relative risk of replacement feeding
The impact of varying Relative risk of replacement feeding (RRRF) is shown in Table 2, sections II and III, which report results for RRRF = 1 and RRRF = 6 (results for all RRRF values are in Table S3). At an RRRF of 1 (no additional mortality risk from replacement feeding), the optimal breastfeeding duration was 0 months regardless of maternal ART duration, with a 24‐month HFS of 93.8% (Section II, yellow). For all RRRF values >1, HFS was maximized by longer breastfeeding durations as RRRF increased. At a maternal ART duration of 24 months, HFS was maximized by 12 months breastfeeding if RRRF = 3 (base case, Section I, box and yellow, HFS 90.2%) and by 24 months breastfeeding if RRRF = 6 (Section III, yellow, HFS 89.6%).
The interaction between RRRF and maternal ART duration affected optimal breastfeeding duration (Figure 2A; Table S3). At low RRRF values (e.g. 2 to 3), HFS was maximized by weaning at 12 months (green) even if mothers continued on ART thereafter (and by breastfeeding for at least 3 months (red) even if ART was stopped sooner). At higher RRRF values (e.g. 4 to 6), HFS was maximized by breastfeeding for as long as maternal ART continued (and for at least 6 months at RRRF ≥4 (orange), even if ART was stopped sooner).
Figure 2.
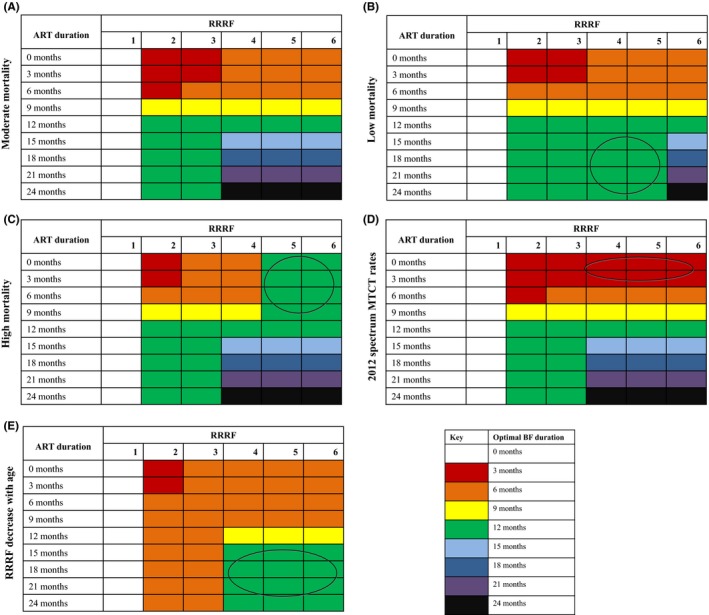
Optimal breastfeeding duration for HIV‐exposed infants by RRRF value and maternal ART duration in various settings. This figure displays the breastfeeding duration that optimizes HIV‐free survival for each combination of RRRF value and ART duration. RRRF value varies across the horizontal axis, and maternal ART duration varies across the vertical axis. Colours correspond to different breastfeeding durations, as described in the figure key. Circles indicate differences in optimal breastfeeding duration compared to the base case. (A) Results for “moderate mortality” scenario. (B) Results for “low mortality” scenario, using 25th percentile values of neonatal, infant, and under‐five mortality estimates from 21 Global Plan countries. (C) Results for “high mortality” scenario, using 75th percentile values of neonatal, infant, and under‐five mortality estimates from 21 Global Plan countries. (D) Results using MTCT rates used in the 2012 Spectrum model. Results using Spectrum 2016 rates are similar and are shown in Material S5. (E) Results for scenarios in which RRRF is reduced as children age. RRRF impact was reduced by 50% for infants who reach 12 months of age, and was again reduced by 50% for infants at 18 months of age. RRRF, relative risk of replacement feeding; BF, breastfeeding; ART, antiretroviral therapy.
3.2.3. Mortality risks
With low infant/child mortality risks, the only difference from the base case was seen at RRRF values of 4 to 5, where optimal breastfeeding durations were shorter than in the base case (Figure 2B, circle). In this scenario, optimal breastfeeding duration only exceeded 12 months if RRRF = 6 and maternal ART duration was also >12 months (blue through black). With high infant/child mortality (Figure 2C), the only difference from the base case occurred at high RRRF values and short ART durations (RRRF = 5 to 6 and ART durations ≤9 months; circle): here, optimal breastfeeding durations were longer than the base case, for example, at least 12 months (green) regardless of maternal ART duration.
3.3. Secondary analyses
3.3.1. Mother‐to‐child transmission risks
In most scenarios, use of 2012 Spectrum MTCT risk inputs resulted in optimal breastfeeding durations similar to the base case (Figure 2D). There were exceptions at higher RRRF values (≥4) when women were on ART for short durations (0 to 3 months), in these cases, the optimal breastfeeding duration was shorter than in the base case. This reflected Spectrum estimates that had higher off‐ART transmission risks and thus favoured shorter breastfeeding durations (Panel D, circle). Results with proposed 2016 Spectrum inputs were similar to those with 2012 inputs (Figure S5).
3.3.2. RRRF: Extreme values and changes with age
When RRRF declined as infants aged, the optimal breastfeeding durations were shorter than in the base case, and only exceeded 6 months if initial RRRF was ≥4 and maternal ART duration was ≥12 months (Figure 2E, yellow/green, circle). In order for the optimal breastfeeding duration to be 24 months, regardless of maternal ART duration, an RRRF value of ≥21 was needed (Material S4B, black).
4. Discussion
We used the CEPAC‐Infant model to identify the breastfeeding duration that maximizes HFS for children born to mothers living with HIV under varying conditions of infant/child mortality, replacement feeding risks and maternal ART duration. There are three primary findings from this work.
First, as mortality risks associated with replacement feeding increase, optimal breastfeeding durations increase; survival with breastfeeding begins to outweigh HIV transmission risks. In the special case of RRRF = 1, breastfeeding does not confer a mortality benefit and leads only to HIV transmission risk. For all RRRF values >1, however, optimal breastfeeding duration increases as RRRF increases. These findings are unchanged in most of the scenarios we examined. Key exceptions are lower MTCT risks for women not on ART (Spectrum inputs, influential only at high RRRF values and short ART durations); higher or lower infant mortality rates (minimally influential only at high RRRF values); and the assumption that RRRF declines with infant age (leading to shorter optimal breastfeeding durations in all scenarios when RRRF at birth is ≥2).
Second, in many scenarios, 24‐month HFS is maximized by breastfeeding durations longer than the previously WHO‐recommended 12 months. This is particularly true when RRRF values are high and maternal ART is taken throughout breastfeeding. In most scenarios, however, the difference in HFS that would result from shortening or extending breastfeeding to “optimal” durations is minimal (<1%). Similarly, several country‐specific examples favor slightly shorter breastfeeding durations. For example, Rwanda, with an RRRF value nearing 1 and low mortality rates, would have an optimal breastfeeding duration of 0 months 5. In contrast, Malawi has a moderate neonatal and infant mortality rate, yet has RRRF values near 3; with high PMTCT coverage, the optimal breastfeeding duration would be close to 6 months 35. A decision to recommend 12 versus 24 months of breastfeeding could reasonably be based on several factors, such as the important goal of harmonizing with recommendations for HIV‐uninfected women or local practices with regard to breastfeeding duration.
Third, continuation of maternal ART throughout breastfeeding is critical to maximizing child HFS, particularly if RRRF is low. For any given RRRF value and breastfeeding duration of at least 6 months, HFS is maximized when maternal ART is continued at least through the time of weaning. Breastfeeding for 24 months in the absence of long‐term maternal ART is only optimal when RRRF values are implausibly high (≥21). Supporting ART use throughout breastfeeding may be difficult; during the postpartum period, many women face medication stockouts, adherence challenges, or are lost from care 43. In those situations, however, weaning is not the answer: reliable access to replacement foods and clean water are likely to be equally challenging. Even if countries were to prioritize safe water supplies, and thus reduce their RRRF values and shorten optimal breastfeeding durations, formula can often be expensive and inaccessible for mothers and their infants. This analysis demonstrates the importance of supporting women to remain in care and on ART through breastfeeding 13. In addition, although child HFS is not affected by maternal ART use after weaning, there are substantial benefits for maternal health, and for prevention of HIV transmission to sexual partners and future children, when women remain on ART 44, 45, 46, 47.
There are several limitations to our analysis. First, we used country‐specific infant/child mortality rates from the general population, which are lower than mortality rates reported among HIV‐exposed, uninfected (HEU) infants used in our prior analysis 48, 49, 50. After adjusting for feeding status and maternal health, however, it is not known if HEU infants have higher mortality than the general population. If mortality rates among HEU infants are truly closer to those in our “high” mortality scenario, optimal breastfeeding durations will nearly always exceed 12 months. Moreover, we used “background” mortality risks to reflect the mortality rates among breastfed infants; if the true rates among breastfed infants are lower than the rates we used, our results of optimal breastfeeding durations in our base case scenario would more closely resemble a “low mortality” scenario and thus possibly favor shorter optimal breastfeeding durations. Second, our model structure requires that MTCT risks after ART discontinuation immediately equal those for women never on ART. Although there are no data on how quickly MTCT risks rise when ART is interrupted, this assumption likely overestimates MTCT risks in the months immediately after ART discontinuation. If these risks were lower, optimal breastfeeding durations would be slightly longer than reported here. Third, this analysis only examines ART duration. Our model assumes that once a woman stops taking ART, she does not reinitiate ART during breastfeeding. Unlike our prior analysis, we do not explicitly vary access or adherence to ART for women retained in care, which may fade during the postpartum period 13. Reduced ART access or adherence would increase MTCT risks and favor shorter breastfeeding durations 12. Fourth, the WHO‐recommended outcome of HIV‐free survival gives equal “importance” to HIV infection and death 1. As ART access for HIV‐infected children improves, leading to longer survival and improved quality of life, this equal weighting becomes less appropriate. Fourth, our analysis excludes costs. Several previous analyses have reported cost savings associated with breastfeeding compared to replacement feeding; however, these have primarily focused on the cost of formula milk and excluded the healthcare resources required to care for diarrhoeal disease, pneumonia, malnutrition, or HIV disease 51, 52. Including these costs could be important for understanding the programmatic requirements and feasibility of supporting either replacement or breastfeeding.
Although the RRRF value reflects a critical concept in the balance of risks and benefits needed to inform infant feeding decisions, data to inform this parameter are limited. We derived values from published research, and it may be difficult for countries or communities to predict their own RRRF values. We anticipate that most settings in sub‐Saharan Africa will have RRRF values of 2 to 3 3, 5, 6, 7, 34, 35, 36, 37, 38. Sites with good‐quality water or with comprehensive access to healthcare services for infants, may have RRRF values close to 1 7, while specific situations such as diarrhoeal outbreaks may be associated with RRRF values of 6 or greater 34, 39. Improved data on variation in replacement feeding risks over time are also needed. One analysis has reported a decrease in RRRF over time, but these data were derived from HIV‐unexposed infants outside of Africa, so their generalizability to HIV‐exposed sub‐Saharan African infants is unknown 40. If RRRF decreases as children age, we find that optimal breastfeeding only exceeds 6 months with high at‐birth RRRF (≥4) and long maternal ART duration (≥12 months).
In conclusion, this analysis provides new information regarding the impact of infant mortality, replacement feeding risks, and maternal ART duration on the feeding decisions that optimize HFS among HIV‐exposed infants. Our findings extend those of prior model‐based reports, which simulated MTCT in the absence of maternal or infant ART and examined a limited number of breastfeeding durations 53, 54, 55, 56. Like our previous analysis, this paper similarly finds that an individualized approach to identify optimal breastfeeding durations can maximize infant HFS, but confers only modest gains compared to a public health approach that suggests the same breastfeeding duration for all women with HIV taking ART 12. The current analysis, however, additionally evaluates the balance between replacement feeding risks, new information regarding ART regimens during breastfeeding and associated transmission risks, and emerging data about ART adherence and retention postpartum. Our findings in infant/child mortality risks and RRRF (as a proxy for water safety and formula access), may be used to examine our results as applicable in a wide range of settings. In many settings, breastfeeding durations longer than the 2010 WHO recommendation of 12 months would maximize child 24‐month HFS, although in settings where mortality risks associated with replacement feeding are very low, optimal breastfeeding durations are shorter than those recommendations. Importantly, however, the differences in HFS between “optimal” and recommended breastfeeding durations are small in most cases. Adherence to maternal ART throughout the entire breastfeeding period improves HFS far more than the specific breastfeeding duration itself and also leads to benefits for mothers, their partners, and their subsequent children. These findings directly informed new WHO guidance about infant feeding 15, and emphasize that programmes should focus on supporting mothers to remain in care and on ART for life, both for their own health and for the health of their children.
4.1. Study period
This analysis was performed between July and December 2015.
Competing interests
The authors declare no conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the World Health Organization.
Authors’ Contributions
DM, RLL and AC were responsible for the design of this research as well as the writing/revision of this manuscript. RS, FD, BE, KAF, VL, SL, RW and NR were instrumental in the writing and revision of this manuscript.
Supporting information
Appendix S1. Additional methods, tables, and figures.
Acknowledgements
The authors are grateful to the CEPAC‐International and CEPAC‐Paediatric research teams and the members of the HIV Infant Feeding Guidelines Committee for their feedback on study design and interpretation of model results. The authors also gratefully acknowledge the contributions of Asinath Rusibamayila and Kathleen Kelly to study design and to the development of the CEPAC‐Infant model.
Funding
Supported by the World Health Organization and the United States National Institutes of Health (Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD079214) and National Institute of Allergy and Infectious Disease (R01 AI058736, R37 AI093269)), the Harvard University Center for AIDS Research (an NIH‐funded programme, P30 AI060354), and the Steve and Deborah Gorlin Massachusetts General Hospital Research Scholar Award. The funders had no role in study design, data analysis, interpretation of results, or decision to publish.
Mallampati, D. , MacLean, R. L. , Shapiro, R. , Dabis, F. , Engelsmann, B. , Freedberg, K. A. , Leroy, V. , Lockman, S. , Walensky, R. , Rollins, N. , Ciaranello, A. .Optimal breastfeeding durations for HIV‐exposed infants: the impact of maternal ART use, infant mortality and replacement feeding risk. J Int AIDS Soc. 2018; 21(4):e25107
Contributor Information
Divya Mallampati, Email: divya.mallampati@northwestern.edu.
Andrea Ciaranello, Email: aciaranello@mgh.harvard.edu.
References
- 1. World Health Organization . Guidelines on HIV and infant feeding: Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. 2010. [cited 2016 May 13]; Available from: http://whqlibdoc.who.int/publications/2010/9789241599535_eng.pdf [PubMed]
- 2. Coutsoudis A, Pillay K, Kuhn L, Spooner E, Tsai WY, Coovadia HM; South African Vitamin A Study Group . Method of feeding and transmission of HIV‐1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS. 2001;15(3):379–87. [DOI] [PubMed] [Google Scholar]
- 3. Nduati R, John G, Mbori‐Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV‐1: a randomized clinical trial. JAMA. 2000;283(9):1167–74. [DOI] [PubMed] [Google Scholar]
- 4. White AB, Mirjahangir JF, Horvath H, Anglemyer A, Read JS. Antiretroviral interventions for preventing breast milk transmission of HIV. Cochrane Database Syst Rev 2014;10: CD011323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peltier CA, Ndayisaba GF, Lepage P, van Griensven J, Leroy V, Pharm CO, et al. Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother‐to‐child transmission in Rwanda. AIDS. 2009;23(18):2415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rollins NC, Becquet R, Bland RM, Coutsoudis A, Coovadia HM, Newell ML. Infant feeding, HIV transmission and mortality at 18 months: the need for appropriate choices by mothers and prioritization within programmes. AIDS. 2008;22(17):2349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becquet R, Bequet L, Ekouevi DK, Viho I, Sakarovitch C, Fassinou P, et al. Two‐year morbidity‐mortality and alternatives to prolonged breast‐feeding among children born to HIV‐infected mothers in Côte d'Ivoire. PLoS Med. 2007;4(1):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuhn L, Sinkala M, Semrau K, Kankasa C, Kasonde P, Mwiya M, et al. Elevations in mortality associated with weaning persist into the second year of life among uninfected children born to HIV‐infected mothers. Clin Infect Dis. 2010;50(3):437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Children: reducing mortality. 2016. [cited 2016 May 13]; Available from: http://www.who.int/mediacentre/factsheets/fs178/en/.
- 10. Ogbo FA, Agho K, Ogeleka P, Woolfenden S, Page A, Eastwood J;Global Child Health Research Interest Group . Infant feeding practices and diarrhoea in sub‐Saharan African countries with high diarrhoea mortality. PLoS ONE. 2017;12(2):e0171792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ladzani R, Peltzer K, Mlambo MG, Phaweni K. Infant‐feeding practices and associated factors of HIV‐positive mothers at Gert Sibande, South Africa. Acta Paediatr. 2011;100(4):538–42. [DOI] [PubMed] [Google Scholar]
- 12. Ciaranello AL, Leroy V, Rusibamayila A, Freedberg KA, Shapiro R, Engelsmann B, et al. Individualizing the World Health Organization public health approach to infant feeding guidelines: Optimal breastfeeding duration to maximize infant HIV‐free survival. AIDS. 2014;28 Suppl 3:S287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tenthani L, Haas AD, Tweya H, Jahn A, van Oosterhout JJ, Chimbwandira F, et al. Retention in care under universal antiretroviral therapy for HIV‐infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS. 2014;28(4):589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chikhungu LC, Bispo S, Rollins N, Siegfried N, Newell ML. HIV‐free survival at 12 ‐ 24 months in breastfed infants of HIV‐infected women on ART. Trop Med Int Health 2016; 21:820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization . U. Guideline: updates on HIV and infant feeding: the duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV. Geneva: The World Health Organization; 2016. [PubMed] [Google Scholar]
- 16. Ciaranello AL, Morris BL, Walensky RP, Weinstein MC, Ayaya S, Doherty K, et al. Validation and calibration of a computer simulation model of pediatric HIV infection. PLoS ONE. 2013; 8(12):e83389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carter RJ, Dugan K, El‐Sadr WM, Myer L, Otieno J, Pungpapong N, et al. ; MTCT Plus Initiative . CD4 + cell count testing more effective than HIV disease clinical staging in identifying pregnant and postpartum women eligible for antiretroviral therapy in resource‐limited settings. J Acquir Immune Defic Syndr. 2010;55(3):404–10. [DOI] [PubMed] [Google Scholar]
- 18. The World Bank . Indicators. [cited 2016 March 25]; Available from: http://data.worldbank.org/indicator.
- 19. Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Mwiya M, Thea DM, et al. Potential impact of new WHO criteria for antiretroviral treatment for prevention of mother‐to‐ child HIV transmission. AIDS. 2010;24(9):1374–7. [PMC free article] [PubMed] [Google Scholar]
- 20. Kesho Bora Study Group . Triple antiretroviral compared with zidovudine and single‐dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother‐to‐child transmission of HIV‐1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis, 2011; 1(3): 159. [DOI] [PubMed] [Google Scholar]
- 21. Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, et al. Maternal or infant antiretroviral drugs to reduce HIV‐1 transmission. N Engl J Med. 2010;362(24):2271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, et al. Antiretroviral regimens in pregnancy and breast‐feeding in Botswana. N Engl J Med. 2010;362(24):2282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, et al. ; ZVITAMBO study group . Early exclusive breastfeeding reduces the risk of postnatal HIV‐1 transmission and increases HIV‐free survival. AIDS, 2005. 19(7): 699–708. [DOI] [PubMed] [Google Scholar]
- 24. Leroy V, Newell ML, Dabis F, Peckham C, deVan Perre P , Bulterys M, et al. International multicentre pooled analysis of late postnatal mother‐to‐child transmission of HIV‐1 infection. Ghent International Working Group on Mother‐to‐Child Transmission of HIV. Lancet, 1998; 352(9128): 597–600. [DOI] [PubMed] [Google Scholar]
- 25. Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother‐to‐child transmission of HIV. AIDS, 2007. 21 Suppl 4: S65–71. [DOI] [PubMed] [Google Scholar]
- 26. Thomas TK, Masaba R, Borkowf CB, Ndivo R, Zeh C, Misore A, et al. ; KiBS Study Team . Triple‐antiretroviral prophylaxis to prevent mother‐to‐child HIV transmission through breastfeeding–the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med, 2011. 8(3): e1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leroy V, Karon JM, Alioum A, Ekpini ER, Meda N, Greenberg AE, et al. ; West Africa PMTCT Study Group . Twenty‐four month efficacy of a maternal short‐course zidovudine regimen to prevent mother‐to‐child transmission of HIV‐1 in West Africa. AIDS, 2002; 16(4): 631–41. [DOI] [PubMed] [Google Scholar]
- 28. Kesho Bora Study Group . Eighteen‐month follow‐up of HIV‐1‐infected mothers and their children enrolled in the Kesho Bora study observational cohorts. J Acquir Immune Defic Syndr, 2010. 54(5): 533–41. [DOI] [PubMed] [Google Scholar]
- 29. Tonwe‐Gold B, Ekouevi DK, Viho I, Amani‐Bosse C, Toure S, Coffie PA, et al. Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two‐tiered approach. PLoS Med. 2007;4(8):e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dunning L, Francke JA, Mallampati D, MacLean RL, Penazzato M, Hou T, et al. The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: a cost‐effectiveness analysis. PLoS Med. 2017;14(11):e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ciaranello AL, Morris BL, Walensky RP, Weinstein MC, Ayaya S, Doherty K, et al. Validation and calibration of a computer simulation model of pediatric HIV infection. PLoS ONE, 2013; 8(12): e83389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ciaranello A, Doherty K, Penazzato M, Lindsey JC, Harrison L, Kelly K, et al. Cost‐effectiveness of first‐line antiretroviral therapy for HIV‐infected African children less than 3 years of age. AIDS. 2015;29(10): 1247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD; ISPOR‐SMDM Modeling Good Research Practices Task Force . Model parameter estimation and uncertainty: a report of the ISPOR‐SMDM modeling good research practices task force–6. Value Health, 2012; 15(6): 835–42. [DOI] [PubMed] [Google Scholar]
- 34. Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, et al.; Mashi Study Team . Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother‐to‐child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA, 2006. 296(7): 794–805. [DOI] [PubMed] [Google Scholar]
- 35. Taha TE, Kumwenda NI, Hoover DR, Kafulafula G, Fiscus SA, Nkhoma C, et al. The impact of breastfeeding on the health of HIV‐positive mothers and their children in sub‐Saharan Africa. Bull World Health Organ. 2006;84(7):546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Mwiya M, et al. Effects of early, abrupt weaning on HIV‐free survival of children in Zambia. N Engl J Med. 2008;359(2):130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuhn L, Aldrovandi G. Survival and health benefits of breastfeeding versus artificial feeding in infants of HIV‐infected women: developing versus developed world. Clin Perinatol. 2010;37(4):843–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kagaayi J, Gray RH, Brahmbhatt H, Kigozi G, Nalugoda F, Wabwire‐Mangen F, et al. Survival of infants born to HIV‐positive mothers, by feeding modality, in Rakai, Uganda. PLoS ONE. 2008;3(12):e3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Creek TL, Kim A, Lu L, Bowen A, Masunge J, Arvelo W, et al. Hospitalization and mortality among primarily nonbreastfed children during a large outbreak of diarrhea and malnutrition in Botswana, 2006. J Acquir Immune Defic Syndr. 2010;53(1):14–9. [DOI] [PubMed] [Google Scholar]
- 40. World Health Organization . Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Lancet. 2000;355(9202):451–5. [PubMed] [Google Scholar]
- 41. Sankar MJ, Sinha B, Chowdhury R, Bhandari N, Taneja S, Martines J, et al. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta‐analysis. Acta Paediatr. 2015;104(467):3–15. [DOI] [PubMed] [Google Scholar]
- 42. Rollins N, Mahy M, Becquet R, Kuhn L, Creek T, Mofenson L. Estimates of peripartum and postnatal mother‐to‐child transmission probabilities of HIV for use in Spectrum and other population‐based models. Sex Transm Infect. 2012;88(Suppl 2):i44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kieffer MP, Mattingly M, Giphart A, van de Ven R, Chouraya C, Walakira M, et al. Lessons learned from early implementation of option B+: the Elizabeth Glaser Pediatric AIDS Foundation experience in 11 African countries. J Acquir Immune Defic Syndr. 2014;67 Suppl 4:S188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Temprano ANRS Study Group . A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–22. [DOI] [PubMed] [Google Scholar]
- 45. Townsend CL, Byrne L, Cortina‐Borja M, Thorne C, de Ruiter A, Lyall H, et al. Earlier initiation of ART and further decline in mother‐to‐child HIV transmission rates, 2000‐2011. AIDS. 2014;28(7):1049–57. [DOI] [PubMed] [Google Scholar]
- 46. Walensky RP, Ross EL, Kumarasamy N, Wood R, Noubary F, Paltiel AD, et al. Cost‐effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med. 2013;369(18):1715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Insight START Study Group . Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland‐Jones SL. HIV‐exposed uninfected children: a growing population with a vulnerable immune system? Clin Exp Immunol. 2014;176(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Becquet R, Marston M, Dabis F, Moulton LH, Gray G, Coovadia HM, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta‐analysis. PLoS ONE. 2012;7(2):e28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marston M, Becquet R, Zaba B, Moulton LH, Gray G, Coovadia H, et al. Net survival of perinatally and postnatally HIV‐infected children: a pooled analysis of individual data from sub‐Saharan Africa. Int J Epidemiol. 2011;40(2):385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ball TM, Bennett DM. The economic impact of breastfeeding. Pediatr Clin North Am. 2001;48(1):253–62. [DOI] [PubMed] [Google Scholar]
- 52. Daviaud E. Comparative costing of feeding options (as presented at the 2010 Guidelines on HIV and Infant Feeding meeting, Geneva). 2009. [cited 2016 May 10]; Available from: http://www.who.int/maternal_child_adolescent/documents/9789241599535_annex_8.pdf?ua=1.
- 53. Ross JS, Labbok MH. Modeling the effects of different infant feeding strategies on infant survival and mother‐to‐child transmission of HIV. Am J Public Health. 2004;94(7):1174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bertolli J, Hu DJ, Nieburg P, Macalalad A, Simonds RJ. Decision analysis to guide choice of interventions to reduce mother‐to‐child transmission of HIV. AIDS. 2003;17(14):2089–98. [DOI] [PubMed] [Google Scholar]
- 55. Atashili J, Kalilani L, Seksaria V, Sickbert‐Bennett EE. Potential impact of infant feeding recommendations on mortality and HIV‐infection in children born to HIV‐infected mothers in Africa: a simulation. BMC Infect Dis. 2008;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Piwoz EG, Ross JS. Use of population‐specific infant mortality rates to inform policy decisions regarding HIV and infant feeding. J Nutr. 2005;135(5):1113–9. [DOI] [PubMed] [Google Scholar]
- 57. Chigwedere P, Seage GR III, Lee TH, Essex M. Efficacy of antiretroviral drugs in reducing mother‐to‐child transmission of HIV in Africa: a meta‐analysis of published clinical trials. AIDS Res Hum Retroviruses. 2008;24(6):827–37. [DOI] [PubMed] [Google Scholar]
- 58. UNAIDS . Data tools: Spectrum/EPP. [cited 2016 May 12]; Available from: http://www.unaids.org/en/dataanalysis/datatools/spectrumepp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Additional methods, tables, and figures.


