Abstract
The androgen receptor (AR) is a ligand‐dependent transcription factor involved in the regulation of many different physiological processes. Dysfunction of AR causes diverse clinical conditions, such as testicular feminization mutation (Tfm) syndrome and prostate cancer. However, the molecular basis of the AR in these disorders largely remains unknown, as a result of a lack of genetic models. Using conditional targeting technique with Cre‐loxP system, we successfully generated null AR mutant (ARKO) mice. The ARKO males grew healthily, but they showed typical Tfm abnormalities. The ARKO males exhibited late onset of obesity with impaired bone metabolism and sexual behaviors. No overt abnormality was found in female ARKO mice, but a premature ovarian failure‐like phenotype was found with impaired folliculogenesis. Thus, andorogen/AR system supports normal reproduction as well as normal female reproduction. (Reprod Med Biol 2007; 6: 11–17)
Keywords: androgen receptor, androgen receptor knockout mouse, Cre‐loxP system, testicular feminization mutation
FUNCTION OF NUCLEAR SEX STEROID RECEPTORS IN GENE REGULATION
SEX STEROIDS EXHIBIT a wide variety of biological actions in physiological and pathological events. 1 , 2 Development of reproductive organ tumors, such as breast cancer and prostate cancer, often depends on the actions of sex hormones, but the molecular basis remains totally unknown. Nuclear sex hormone receptors (androgen receptor [AR] and estrogen receptors [ER]) are members of the nuclear hormone receptor superfamily and act as ligand‐inducible transcription factors. 3 ER and AR form homodimers and bind specific DNA elements referred as hormone responsive elements (HRE) in the target gene promoters (Fig. 1). Members of the nuclear receptor (NR) gene superfamily serve as sequence‐specific regulators in the promoters of their cognate target genes. Reflecting the spatio‐temporal expression patterns of NR in animals, a wide variety of biological events are under the control of NR‐mediated transcriptional regulations. Structurally, NR proteins can be divided into five domains, A–E 4 , 5 , 6 The highly conserved C domain acts as a DNA binding domain (DBD) which has two Zn finger motifs that recognize and stably bind to specific target DNA. The moderately conserved ligand binding domain (LBD) is mapped to the C‐terminal E domain. The N‐terminal A/B domain exhibits poor homology among NR but is responsible for ligand‐induced transactivation, together with the LBD region in NR as interacting regions for the coactivator complexes. The autonomous activation function‐1 (AF‐1) located in the A/B domain is ligand‐independent, whereas the AF‐2 in the LBD is induced upon ligand binding. 7 , 8 , 9 Unliganded LBD appears to suppress the function of the A/B domain, whereas ligand binding to the LBD is thought to evoke the function of LBD and restore the A/B domain function through as yet undescribed intramolecular alteration of the entire steroid receptor structure.
Figure 1.
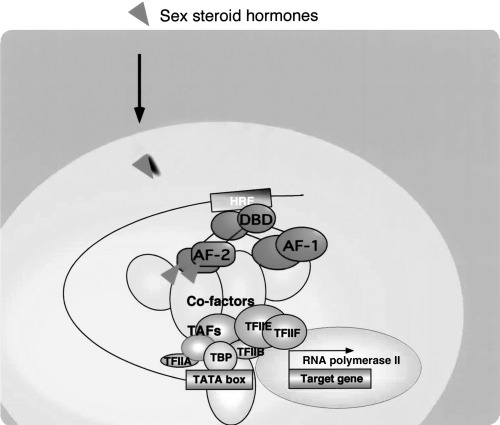
Nuclear sex hormone receptor controls expression of target genes in a hormone‐dependent manner. Sex steroid hormones are thought to exert their physiological effects through transcriptional control by the cognate nuclear receptors (NR). NR recognize and bind with the specific recognition sites, termed hormone responsive elements (HRE). Hormone binding to NR induces association with general transcription factors (GTF) and the target genes are transcribed by RNA polymerase II (RNApol II).
LIGAND‐DEPENDENT TRANSCRIPTIONAL CONTROLS BY NUCLEAR RECEPTOR REQUIRES A NUMBER OF NUCLEAR COREGULATOR COMPLEXES
LIGAND‐DEPENDENT AND ‐INDEPENDENT transcriptional control by NR requires the input of two types of coregulators with opposing functions. 10 It appears that most coregulators exist as multiprotein complexes. It is thought that three distinct classes of cofactors support NR transactivation, with two of these classes, CBP/p160 and GCN5/TRPAP complexes, containing histone acetyltransferase (HAT) enzymes. 11 , 12 , 13 , 14 , 15 The other class, DRIP/TRAP complex, is a non‐HAT coactivator complex. 16 , 17 , 18 The corepressor type complexes contain histone deacetylase (HDAC) enzymes, which along with NCoR/SMRT physically interact with NR through CoRNR motifs, and are thought to be functionally indispensable subunits in NR corepression complexes. 1 , 10 , 19 , 20 Although histone modification that is a result of HAT/HDAC activity in NR coregulator complexes, in cooperation with chromatin remodeling complexes, explains at least part of the mechanism of NR‐mediated transcriptional controls, the molecular link between NR‐mediated gene regulation and cell cycle control remains elusive. In addition to such hitone modifications, chromatin remodeling appears to be required (Fig. 2). Three subclasses of ATP‐dependent chromatin remodeling complexes have been biochemicaly identified 21 , 22 , 23 , 24 , 25 , 26 and the SWI/SNIF‐type complex is believed to support the ligand‐induced transactivation function of NR. 27 , 28 , 29
Figure 2.
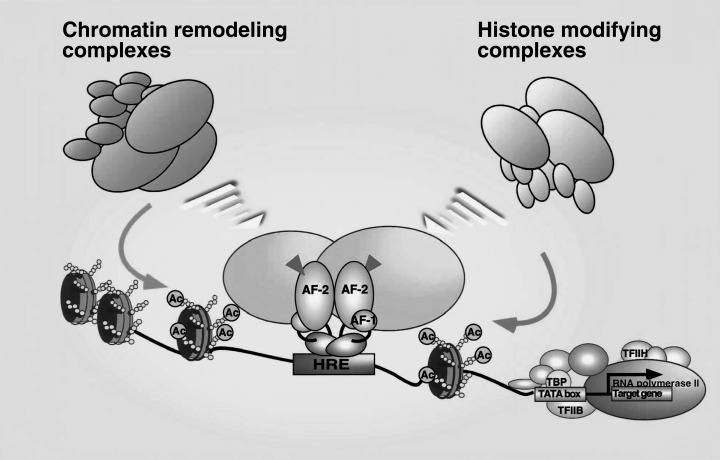
Coregulators support ligand‐dependent transcriptional controls by nuclear receptors (NR) through chromatin remodeling and histone modifications. Ligand binding positively and negatively controls gene expressions of target genes by NR through switching of coregulators; most of them form histone modifying enzyme complexes, together with histone remodeling by ATP‐dependent chromatin remodeling complexes.
ANDROGEN RECEPTOR INACTIVATION BY GENE TARGETING WITH THE CRE‐LOXP SYSTEM IN MICE
THERE WERE BASIC and technical difficulties in generating an AR knockout (ARKO) mouse (Fig. 3). The AR gene is located on the X chromosome, thereby existing as a single copy in 46, XY male 30 in which androgen exerts most profound effects. As male mice lacking the AR gene are expected to show Tfm abnormalities with complete infertility, 31 , 32 , 33 , 34 successful targeted disruption of the AR gene (which is essential for reproduction), necessarily prohibits its transmission to the next generation. It is thus impossible to generate ARKO mouse line either in nature or by conventional gene targeting method. Furthermore, because all Tfm models are genetically male, it is simply impractical to generate a genetically female animal that is homozygous for the AR gene mutation.
Figure 3.
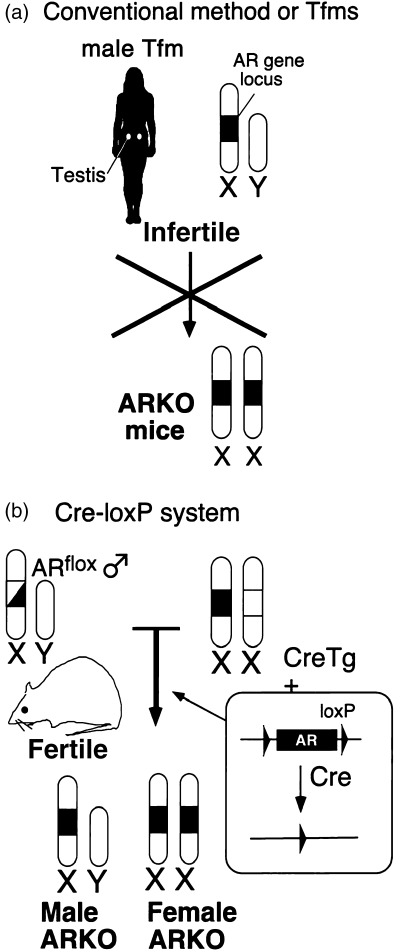
Strategy for generating ARKO mice line. (a) The androgen receptor (AR) gene is located in the X chromosome and male Tfm animals are infertile in any case, so the mutated AR gene cannot be transmitted to the next generation. (b) In the first step, floxed AR mice that carry a functional, but loxP‐flanked, AR gene are generated by introducing the loxP sites in the first exon of the AR gene by homologous recombination in embryonic stem cells. Next, by mating these mice with CMV‐Cre transgenic mice, the AR gene is disrupted during embryogenesis.
To avoid this problem, we applied a Cre‐loxP system 35 to establish the ARKO mice line (Fig. 3). We first generated the floxed AR mice, in which the AR gene locus was flanked by loxP sites. The floxed AR mice were fully fertile and expressed AR protein normally. We then crossed them with mice expressing the Cre recombinase ubiquitously under the control of the CMV promoter, and obtained male and female ARKO mice at theoretical Mendellian frequency. 36
FEMALE‐TYPICAL APPEARANCE OF MALE ARKO MICE
THE APPEARANCE OF the male and female ARKO and the wild‐type mice are shown in Figure 4. The ARKO males exhibited female‐typical external appearance, such as vagina with blind end, and clitoral‐like phallus, instead of penis and scrotum. 36 Male reproductive organs including seminal vesicle, vas deferens, epididymis and prostate were absent in ARKO males. However, no ovary and uterus were observed, although they had inguinal small testes. The histological examination of the testes showed that spermatogenesis was severely arrested. From these results, it is clear that AR is not required for the formation of testis, but essential for development of male reproductive organs and spermatogenesis.
Figure 4.
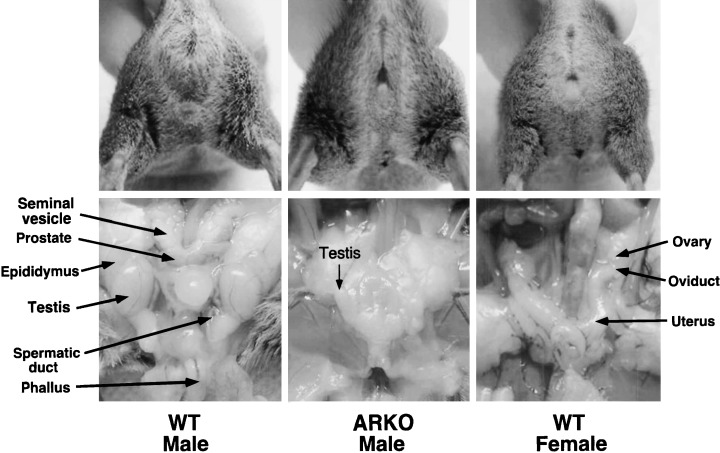
The male ARKO mice were characterized by female‐typical appearances, such as a clitoris‐like phallus and a vagina with a blind end, but also the absence of internal male and female reproductive organs, except for the presence of an atrophic testis.
Estimation of plasma hormone levels in the ARKO males showed markedly lowered androgens as well as a luteinizing hormone, but the estradiol level was almost the same as that of the wild‐type males. 36 These suggest that we can investigate the effect of androgens independently by using the ARKO mouse in that only the AR is disrupted while the estrogen receptors remain intact. Until 10 weeks‐of‐age, the ARKO males exhibited growth retardation, and the growth curve was indistinguishable from that of the wild‐type female littermates. However, thereafter, the rapid increase in the growth of ARKO males was observed, and until 12 weeks, the bodyweights of the ARKO males exceeded those of the wild‐type male littermates. 37 The late onset of the drastic increase in the growth curve in the ARKO males was understandable, as clear obesity developed (Fig. 5a). Significant increases of wet tissue weights in subcutaneous, infrarenal and intraperitorial white adipose tissues (WATS) were observed at 30 weeks‐of‐age (Fig. 5b). In contrast, no significant alteration in serum lipid parameters and food intake was observed. No obesity was seen in female ARKO mice.
Figure 5.
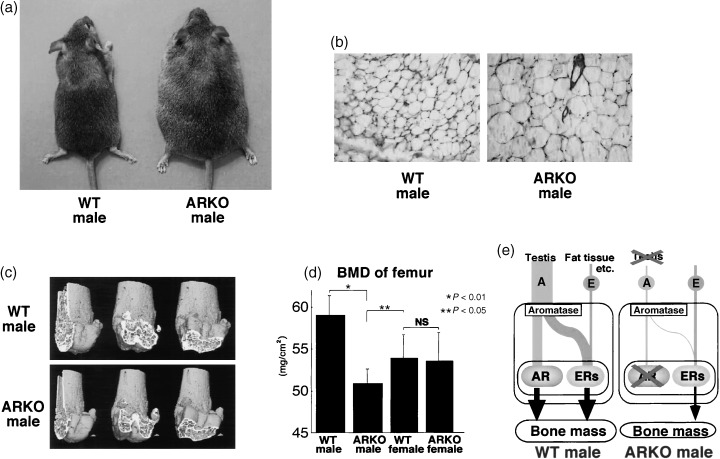
Phenotypic features of male ARKO mice. (a) External appearance of 30‐week‐old male ARKO mice. (b) Subcutaneous white adipose tissues from 30‐week‐old male. (c) Three‐dimentional computed tomography images of distal femora from representative 8‐week‐old male ARKO mice. (d) Bone loss in femur of male ARKO mice by BMD analysis. (e) Schema of skeletal sex hormone action.
IMPAIRED BONE METABOLISM AND SEXUAL BEHAVIORS IN MALE ARKO MICE
THE ARKO MALES showed a clear loss of bone mass with high bone turnover, when compared with the adult littermate males (Fig. 5c,d). 38 Such bone abnormality was not found in female ARKO mice, establishing that activated AR is essential for the male‐type bone phenotype (Fig. 5e). Likewise, male‐typical sexual behaviors were severely impaired by AR inactivation, but the sexual behaviors in female ARKO mice appeared normal. 36
PREMATURE OVARIAN FAILURE IN ARKO FEMALES
NO PHENOTYPIC ABNORMALITY was found in external and internal reproductive organs in ARKO females, but at 8 weeks, the females bore about half the normal number of pups per labor. At 40 weeks, the ARKO females became infertile. 39 Immunohistological analysis of normal ovaries detected significant expression of AR protein in growing follicles, and the ARKO ovaries at 8 weeks had impaired folliculogenesis with fewer numbers of follicles. 39 Folliculogenesis was absent in the ovaries of 40‐week‐old ARKO females, whereas the wild‐type littermate females still had active ovarian function (Fig. 6). From these findings, together with the epidemiological study that an unknown gene(s) located on the X chromosome is responsible for a hereditary disorder called premature ovarian failure (POF), 40 , 41 we believe that impaired androgen signaling might cause POF syndrome. To verify this, a clinical survey of POF patients is required to find a novel therapeutic clue.
Figure 6.
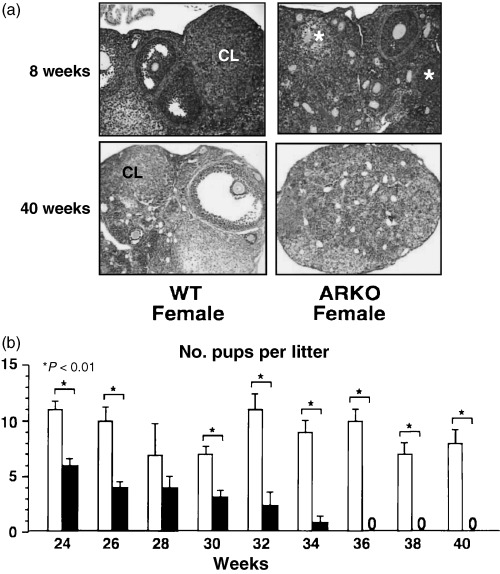
Premature ovarian failure in female ARKO mice. (a) Histology of ARKO ovary at 8 and 40 weeks‐of‐age. (b) Age‐dependent reduction in the number of pups per litter in female ARKO mice.
ACKNOWLEDGMENTS
THIS WORK WAS supported in part by a grant‐in‐aid for priority areas from the Ministry of Education, Science, Sports and Culture of Japan (S.K.).
REFERENCES
- 1. Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 1999; 20: 358–417. [DOI] [PubMed] [Google Scholar]
- 2. Wilson JD. The role of androgens in male gender role behavior. Endocr Rev 1999; 20: 726–737. [DOI] [PubMed] [Google Scholar]
- 3. Mangelsdorf DJ, Thummel C, Beato M et al. The nuclear receptor superfamily: the second decade. Cell 1995; 83: 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He B, Kemppainen JA, Voegel JJ, Gronemeyer H, Wilson EM. Activation function 2 in the human androgen receptor ligand binding domain mediates interdomain communication with the NH (2)‐terminal domain. J Biol Chem 1999; 274: 37219–37225. [DOI] [PubMed] [Google Scholar]
- 5. Watanabe M, Yanagisawa J, Kitagawa H et al. A subfamily of RNA‐binding DEAD‐box proteins acts as an estrogen receptor alpha coactivator through the N‐terminal activation domain (AF‐1) with an RNA coactivator, SRA. Embo J 2001; 20: 1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell 2002; 9: 601–610. [DOI] [PubMed] [Google Scholar]
- 7. Kato S, Endoh H, Masuhiro Y et al. Activation of the estrogen receptor through phosphorylation by mitogen‐activated protein kinase. Science 1995; 270: 1491–1494. [DOI] [PubMed] [Google Scholar]
- 8. Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell 1995; 83: 851–857. [DOI] [PubMed] [Google Scholar]
- 9. Tora L, White J, Brou C et al. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell 1989; 59: 477–487. [DOI] [PubMed] [Google Scholar]
- 10. Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 2000; 14: 121–141. [PubMed] [Google Scholar]
- 11. Yanagisawa J, Kitagawa H, Yanagida M et al. Nuclear receptor function requires a TFTC‐type histone acetyl transferase complex. Mol Cell 2002; 9: 553–562. [DOI] [PubMed] [Google Scholar]
- 12. Onate SA, Tsai SY, Tsai MJ, O'Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 1995; 270: 1354–1357. [DOI] [PubMed] [Google Scholar]
- 13. Spencer TE, Jenster G, Burcin MM et al. Steroid receptor coactivator‐1 is a histone acetyltransferase. Nature 1997; 389: 194–198. [DOI] [PubMed] [Google Scholar]
- 14. Chen H, Lin RJ, Schiltz RL et al. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 1997; 90: 569–580. [DOI] [PubMed] [Google Scholar]
- 15. Kamei Y, Xu L, Heinzel T et al. A CBP integrator complex mediates transcriptional activation and AP‐1 inhibition by nuclear receptors. Cell 1996; 85: 403–414. [DOI] [PubMed] [Google Scholar]
- 16. Naar AM, Beaurang PA, Zhou S, Abraham S, Solomon W, Tjian R. Composite co‐activator ARC mediates chromatin‐directed transcriptional activation. Nature 1999; 398: 828–832. [DOI] [PubMed] [Google Scholar]
- 17. Rachez C, Lemon BD, Suldan Z et al. Ligand‐dependent transcription activation by nuclear receptors requires the DRIP complex. Nature 1999; 398: 824–828. [DOI] [PubMed] [Google Scholar]
- 18. Fondell JD, Ge H, Roeder RG. Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex. Proc Natl Acad Sci U S A 1996; 93: 8329–8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murayama A, Kim MS, Yanagisawa J, Takeyama K, Kato S. Transrepression by a liganded nuclear receptor via a bHLH activator through co‐regulator switching. Embo J 2004; 23: 1598–1608. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Heinzel T, Lavinsky RM, Mullen TM et al. A complex containing N‐CoR, mSin3 and histone deacetylase mediates transcriptional repression [see comments]. Nature 1997; 387: 43–48. [DOI] [PubMed] [Google Scholar]
- 21. Ito T, Bulger M, Pazin MJ, Kobayashi R, Kadonaga JT. ACF, an ISWI‐containing and ATP‐utilizing chromatin assembly and remodeling factor. Cell 1997; 90: 145–155. [DOI] [PubMed] [Google Scholar]
- 22. Fyodorov DV, Kadonaga JT. The many faces of chromatin remodeling: SWItching beyond transcription. Cell 2001; 106: 523–525. [DOI] [PubMed] [Google Scholar]
- 23. Lemon B, Inouye C, King DS, Tjian R. Selectivity of chromatin‐remodelling cofactors for ligand‐activated transcription. Nature 2001; 414: 924–928. [DOI] [PubMed] [Google Scholar]
- 24. Kitagawa H, Fujiki R, Yoshimura K et al. The chromatin‐remodeling complex WINAC targets a nuclear receptor to promoters and is impaired in Williams syndrome. Cell 2003; 113: 905–917. [DOI] [PubMed] [Google Scholar]
- 25. Fujiki R, Kim MS, Sasaki Y, Yoshimura K, Kitagawa H, Kato S. Ligand‐induced transrepression by VDR through association of WSTF with acetylated histones. Embo J 2005; 24: 3881–3894. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell 2002; 108: 475–487. [DOI] [PubMed] [Google Scholar]
- 27. DiRenzo J, Shang Y, Phelan M et al. BRG‐1 is recruited to estrogen‐responsive promoters and cooperates with factors involved in histone acetylation. Mol Cell Biol 2000; 20: 7541–7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiba H, Muramatsu M, Nomoto A, Kato H. Two human homologues of Saccharomyces cerevisiae SWI2/SNF2 and Drosophila brahma are transcriptional coactivators cooperating with the estrogen receptor and the retinoic acid receptor. Nucl Acids Res 1994; 22: 1815–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Belandia B, Orford RL, Hurst HC, Parker MG. Targeting of SWI/SNF chromatin remodelling complexes to estrogen‐responsive genes. Embo J 2002; 21: 4094–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 1988; 240: 327–330. [DOI] [PubMed] [Google Scholar]
- 31. Griffin JE. Androgen resistance – the clinical and molecular spectrum. N Engl J Med 1992; 326: 611–618. [DOI] [PubMed] [Google Scholar]
- 32. McPhaul MJ. Molecular defects of the androgen receptor. J Steroid Biochem Mol Biol 1999; 69: 315–322. [DOI] [PubMed] [Google Scholar]
- 33. Takeyama K, Ito S, Yamamoto A et al. Androgen‐dependent neurodegeneration by polyglutamine‐expanded human androgen receptor in Drosophila. Neuron 2002; 35: 855–864. [DOI] [PubMed] [Google Scholar]
- 34. Quigley CA, De Bellis A, Marschke KB, El‐Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 1995; 16: 271–321. [DOI] [PubMed] [Google Scholar]
- 35. Li M, Indra AK, Warot X et al. Skin abnormalities generated by temporally controlled RXRalpha mutations in mouse epidermis. Nature 2000; 407: 633–636. [DOI] [PubMed] [Google Scholar]
- 36. Sato T, Matsumoto T, Kawano H et al. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci U S A 2004; 101: 1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sato T, Matsumoto T, Yamada T, Watanabe T, Kawano H, Kato S. Late onset of obesity in male androgen receptor‐deficient (AR KO) mice. Biochem Biophys Res Commun 2003; 300: 167–171. [DOI] [PubMed] [Google Scholar]
- 38. Kawano H, Sato T, Yamada T et al. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci U S A 2003; 100: 9416–9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shiina H, Matsumoto T, Sato T et al. Premature ovarian failure in androgen receptor‐deficient mice. Proc Natl Acad Sci U S A 2006; 103: 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laml T, Preyer O, Umek W, Hengstschlager M, Hanzal H. Genetic disorders in premature ovarian failure. Hum Reprod Update 2002; 8: 483–491. [DOI] [PubMed] [Google Scholar]
- 41. Davison RM, Davis CJ, Conway GS. The X chromosome and ovarian failure. Clin Endocrinol (Oxf) 1999; 51: 673–679. [DOI] [PubMed] [Google Scholar]


