Abstract
Purpose
Spermatogonial stem cells (SSCs) are self‐renewing cells whose progeny are committed to differentiate into spermatozoa; this is a life‐long process in male mammals. There are several methods for obtaining enriched populations of mouse SSCs, and immunological separation using surface antigens is a commonly used technique. The study of human SSCs is much less advanced.
Methods
We used biopsied human testicular tissues [obstructive azoospermia patients (n = 5) and patients who underwent a testis biopsy as part of an evaluation for infertility (n = 7)] to obtain Thy‐1+ cells. Thy‐1—a glycosyl phosphatidylinositol‐anchored surface antigen—is a marker uniquely expressed on SSCs that is used to isolate SSC‐enriched cell populations in mice. The Thy‐1+ cells from human testicular tissues were cultured in a basic system consisting of serum‐free medium and mitotically inactivated STO (SIM mouse embryo‐derived thioguanine‐ and ouabain‐resistant) cell feeders with added growth factors: glial cell line‐derived neurotrophic factor (GDNF), basic fibroblast growth factor (bFGF), and GDNF‐family receptor α1 (GFRα‐1).
Results
The Thy‐1+ cells were maintained in vitro using this system for 1 week. The Thy‐1+ cells expressed OCT3/4 and alkaline phosphatase, like mouse SSCs. They also expressed NANOG. Thy‐1+ cells injected into nude mice did not cause tumor formation over a period of at least 6 months.
Conclusions
These results support the possibility that the Thy‐1+ cell population included human SSCs, and that Thy‐1 may be a marker for human SSCs.
Keywords: Growth factors, Male infertility, Spermatogenesis, Stem cells, Thy‐1
Introduction
The rete testis is the network of testicular tubules in which the entire process of spermatogenesis takes place, and through which the spermatozoa leave the testis [1]. Spermatogonial stem cells (SSCs) are self‐renewing cells whose progeny are committed to differentiate into spermatozoa, a process that occurs throughout the life of male mammals [2]. SSCs are rare in the adult mouse testis, representing ~1 in 3,000–4,000 cells [3]. They can be identified unequivocally in a functional assay using transplantation, in which donor testis cells are injected into the seminiferous tubules of infertile recipient males [4, 5]. Under these conditions, only SSCs can undergo spermatogenesis, and they in fact restore normal spermatogenic function in these mice. Although mouse SSCs and their microenvironment have been studied over the past decade using this transplantation assay [6], the mechanisms underlying the processes of SSC self‐renewal and differentiation remain elusive. One approach to this problem is to cultivate SSCs in vitro under conditions that allow self‐renewal and induce differentiation. For this purpose, a serum‐free culture system for mouse SSCs was developed and used to evaluate the environmental factors required for their maintenance and replication [7]. One study showed that glial cell line‐derived neurotrophic factor (GDNF)‐induced cell signaling plays a central role in mouse SSC self‐renewal [8]. Another reported the long‐term survival and proliferation of SSCs when they were cultured in a proprietary medium [9].
The fate determination of stem cells is controlled to a large extent by the surrounding microenvironment, particularly the stem cell niche [10]. Although little is known about the components of the stem cell niche, the use of feeder cells is, at least for now, essential for reconstituting it in vitro [11]. The use of STO (SIM mouse embryo‐derived thioguanine‐ and ouabain‐resistant) feeder cells has improved the in vitro maintenance of mouse SSCs [12, 13].
There are several methods for obtaining enriched populations of mouse SSCs; the use of immunological separation by surface antigen expression is common [14, 15]. To obtain a highly enriched stem cell population using this method, unique cell‐surface markers for stem cells must be identified. It is important to know which stem‐cell markers are expressed throughout development, not only for their isolation, but also because these markers are often associated with the biological properties of stem cells.
Thy‐1 is a glycosyl phosphatidylinositol‐anchored surface antigen that, in the mouse testis, is expressed solely on SSCs [14]. It is also expressed on other stem cells, including hematopoietic stem cells, mesenchymal stem cells, and ES cells [16, 17, 18]. It is used to obtain mouse testis cells enriched for SSCs [7].
In this study, we investigated whether magnetic microbeads conjugated with an anti‐Thy‐1 antibody could be used to obtain Thy‐1+ cells from a cell suspension prepared from human testis, and if so, whether these cells could be cultured successfully in serum‐free medium with STO feeder layers. We obtained Thy‐1+ cells and sought to identify them by examining their phenotypic characteristics. We also studied the in vivo function of the Thy‐1+ cells by injecting them into nude mice.
Materials and methods
Human subjects
In accordance with the regulations set forth by the Human Investigations Committee of Toho University School of Medicine, informed consent was obtained from male patients being treated for infertility who visited the Reproduction Center of Toho Medical Center Omori Hospital. Testicular tissues were obtained from obstructive azoospermia patients (n = 5) and patients who underwent a testis biopsy as part of an evaluation for infertility (n = 7). We used a total of 12 testis samples in the experiments described below.
Adult human testis tissues
First, we confirmed the presence of mature sperm in the testicular tissue biopsies. The tissues were used fresh or were frozen in Quinn's Advantage™ Sperm Freezing Medium (SAGE In vitro Fertilization Inc., CooperSurgical Inc., Trumbull, CT, USA). Cell suspensions from testis tissues were prepared by enzymatic digestion [19]. In several experiments, testis cells were fractionated using Percoll (GE Healthcare UK Ltd.) to remove cellular debris and large cells. The dissociated testis cell suspension, after enzymatic digestion, was overlaid on 30% (v/v) Percoll prepared in Dulbecco's PBS containing 1% human serum (Takara, Kyoto, Japan) and spun at 600×g for 7 min at 4°C. Cells from the interphase and Percoll phase were discarded. Sedimented cells in the bottom fraction were used.
Magnetic‐activated cell sorting
Magnetic microbeads conjugated to an anti‐Thy‐1 antibody (30‐H12; Miltenyi Biotec, Gladbach, Germany) were used for magnetic‐activated cell sorting (MACS) to enrich for Thy‐1+ cells from testis cell suspensions. The procedure for obtaining Thy‐1+ cells was performed according to the manufacture's protocol with minor modifications. Dissociated testis cells were suspended in Dulbecco's PBS supplemented with 1% human serum (Takara), 10 mM HEPES (Sigma‐Aldrich, St. Louis, MO, USA), 1 mM pyruvate (Sigma), antibiotics (50 U/ml penicillin and 50 μg/ml streptomycin; Sigma), and 1 mg/ml glucose (Sigma) (PBS‐S). The dissociated cells were then fractionated by Percoll centrifugation, as described above. We counted the cells before and after Percoll treatment, and used these values to calculate cell viability after Percoll treatment (Table 1). The bottom fraction from the Percoll centrifugation was incubated with Thy‐1 microbeads for 20 min at 4°C. After being rinsed with PBS‐S, the Thy‐1+ cells were selected by passing them through an MS separation column (Miltenyi Biotec) that was placed in a magnetic field. After the column was removed from the magnetic field, the magnetically retained Thy‐1+ cells were eluted.
Table 1.
Overview of the human testicular samples, including the patient's initials, age, condition, cell count before and after Percoll treatment, viability of cells after Percoll treatment, cell count of MACS separation cells
| Patient's initial | Age | Condition | Before Percoll | After Percoll | Viability after Percoll (%) | MACS separation cells | ||
|---|---|---|---|---|---|---|---|---|
| 12 | OA | T.H | 47 | Fresh | 1.08 × 107 | 3.24 × 106 | 30.0 | 8.00 × 104 |
| 11 | OA | S.T | 56 | Fresh | 6.73 × 106 | 2.46 × 106 | 36.6 | 2.80 × 105 |
| 10 | OA | T.Z | 46 | Fresh | 1.25 × 107 | 3.64 × 106 | 29.0 | 1.80 × 105 |
| 9 | OA | H.M | 47 | Fresh | 8.47 × 106 | 2.95 × 106 | 34.8 | 4.00 × 104 |
| 8 | OA | T.S | 46 | Fresh | 6.87 × 106 | 3.56 × 106 | 51.8 | 1,10 × 105 |
| 7 | EP | T.H | 43 | Cryo | 1.93 × 106 | 2.94 × 105 | 15.2 | 5.00 × 104 |
| 6 | EP | H.D | 32 | Cryo | 8.00 × 105 | 1.20 × 105 | 15.0 | 1.00 × 105 |
| 5 | EP | O.H | 39 | Cryo | 5.30 × 105 | 8.00 × 104 | 15.0 | 4.00 × 104 |
| 4 | EP | K.S | 46 | Fresh | 1.74 × 107 | 4.84 × 106 | 27.8 | 1.92 × 105 |
| 3 | EP | O.S | 29 | Cryo | 1.00 × 106 | 1.10 × 105 | 11.0 | 2.40 × 104 |
| 2 | EP | O.S | 33 | Cryo | 1.60 × 106 | 8.00 × 104 | 5.0 | 1.41 × 104 |
| 1 | EP | K.Y | 38 | Fresh | 7.30 × 106 | 1.96 × 106 | 26.0 | 1.20 × 105 |
OA obstructive azoospermia, EP evaluation procedure
Cell culture
The basic culture system consisted of serum‐free medium and mitotically inactivated STO (SIM mouse embryo‐derived thioguanine and ouabain‐resistant) feeder cells, which were purchased (Dainippon Sumitomo Pharma, Osaka, Japan). STO cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 7% FBS, 100 μM 2‐mercaptoethanol (Sigma), 2 mM glutamine (Sigma), and antibiotics. For use as feeder cells, the STO cells were treated with 10 μg/ml mitomycin C (Sigma) for 3–4 h. The STO feeder cells were then plated in 12‐well plates in 1.0 ml of the same medium plus 0.1% gelatin (Sigma), at a density of 2 × 105 cells per well. The plated feeder cells were rinsed with Hank's balanced salt solution twice before being cultured with cells. The cells were plated at up to 1 × 105 cells per well in serum‐free medium. This medium human SSCs consisted of minimum essential medium‐alpha (MEMα; Invitrogen, Carlsbad, CA, USA), to which was added 0.2% human serum albumin (Sigma), 5 μg/ml insulin (Sigma), 10 μg/ml iron‐saturated transferrin (Sigma), 7.6 μeq/L free fatty acids [20], 3 × 10−8 M Na2SeO3 (Sigma), 10 mM HEPES (Sigma), 60 μΜ putrescine (Sigma), 2 mM glutamine (Sigma), 50 units/ml penicillin, and 50 μg/ml streptomycin (Sigma). The free fatty acids contained palmitic acid (Sigma), palmitoleic acid (Sigma), stearic acid (Sigma), oleic acid (Sigma), linoleic acid (Sigma), and linolenic acid (Sigma) at 31.0:2.8:11.6:13.4:35.6:5.6, respectively, in the 100 meq/L (ethanol) stock solution [20]. The growth factors used were human GDNF, human GFRα1, and human bFGF (all from R&D Systems, Minneapolis, MN, USA), at final concentrations of 20, 100, and 1 μg/ml, respectively. All cultures were maintained at 37°C in a humidified 5% CO2/95% air atmosphere. The medium (1.5 ml/well) was changed every 2 days.
Immunofluorescence microscopy and immunostaining
Cells were washed twice with phosphate‐buffered saline, fixed with 4% (w/v) paraformaldehyde for 20 min, permeabilized for 60 min with phosphate‐buffered saline containing 0.1% (v/v) Triton X‐100, and then blocked for 3 h with phosphate‐buffered saline containing 20% donkey serum. For immunostaining, the fixed samples were incubated with an anti‐human OCT‐3/4 polyclonal antibody (R&D systems) and anti‐human alkaline phosphatase monoclonal antibody (R&D systems), as indicated, washed three times with PBS containing 0.1% (v/v) Triton X‐100, and probed with the appropriate secondary antibodies [anti‐goat IgG antibody conjugated with Alexa 488 and anti‐mouse IgG antibody conjugated with Alexa 594, respectively (Molecular Probes, Carlsbad, CA, USA)]. We used samples #4–6 for these experiments.
Reverse‐transcription (RT)‐PCR
RT‐PCR was carried out with total RNA from cells cultured for 7 days. Total RNA was prepared using the PureLink™Micro‐to‐Midi Total RNA Purification System (Invitrogen). RT‐PCR was performed using the SuperScript™III One‐Step RT‐PCR System with Platinum® Taq DNA Polymerase (Invitrogen) for human SSC markers, using the following conditions: 55°C for 30 min for reverse transcription; 94°C for 2 min to inactivate the reverse transcriptase and activate the polymerase; 40 cycles of 94°C for 15 s, 55°C for 30 s, 68°C for 1 min; and 68°C for 5 min, for the final extension. The primers were as follows: for Thy‐1, 5′‐AGA AGG TGA CCA GCC TAA CGG‐3′ (forward) and 5′‐TCT GAG CAC TGT GAC GTT CTG (reverse), product was 324 bp; OCT3/4, 5′‐GAA GGT ATT CAG CCA AAC GAC‐3′ (forward) and 5′‐GTT ACA GAA CCA CAC TCG GA‐3′ (reverse), product was 315 bp; NANOG, 5′‐TGC AAA TGT CTT CTG CTG AGA T‐3′ (forward) and 5′‐GTT CAG GAT GTT GGA GAG TTC‐3′ (reverse), product was 285 bp; and GAPDH, 5′‐GTC CAT GCC ATC ACT GCC A‐3′ (forward) and 5′‐TTA CTC CTT GGA GGC CAT G‐3′ (reverse), product was 513 bp [21]. We used samples #1–3 and #10–12 for these experiments.
In vivo assay
The cells were harvested by trypsin treatment, collected into tubes, spun, and the pellets were suspended in DMEM. Then, 1 × 105 cells were injected into nude mice (BALB/C, 6‐weeks old, CLEA, Tokyo, Japan). We used samples #7–9 for this experiment.
Results
Thy‐1 antibody‐conjugated magnetic microbeads could be used to isolate Thy‐1+ cells from human testicular tissues
Testicular tissues were obtained from obstructive azoospermia patients and patients who underwent a testis biopsy as part of an evaluation for infertility. A total of 12 samples from human testes were obtained (Table 1). The patients’ initials, age, condition, cell numbers before and/after Percoll treatment, cell viability after Percoll, and numbers of MACS separation cells are shown in Table 1. We found that fresh tissues showed higher viability than the cryopreserved ones (Table 1). There was no clear difference in cell viability between the samples obtained from obstructive azoospermia tissues and those obtained as part of an infertility evaluation (Table 1).
We investigated whether anti‐Thy‐1 antibody‐conjugated magnetic microbeads could be used to isolate Thy‐1+ cells from human testicular tissues by MACS. After human testis cell suspensions were separated with Percoll, the bottom fractions were further separated using anti‐mouse Thy‐1 antibody‐conjugated magnetic microbeads, because there is no commercial source for anti‐human Thy‐1 antibody‐conjugated magnetic microbeads. After the MACS separation and 1 week of culture, we confirmed the presence of Thy‐1 by RT‐PCR analysis (Fig. 1). This result indicated that the anti‐mouse Thy‐1‐conjugated microbeads could be used to isolate a population of Thy‐1‐expressing cells from human testis cells.
Figure 1.
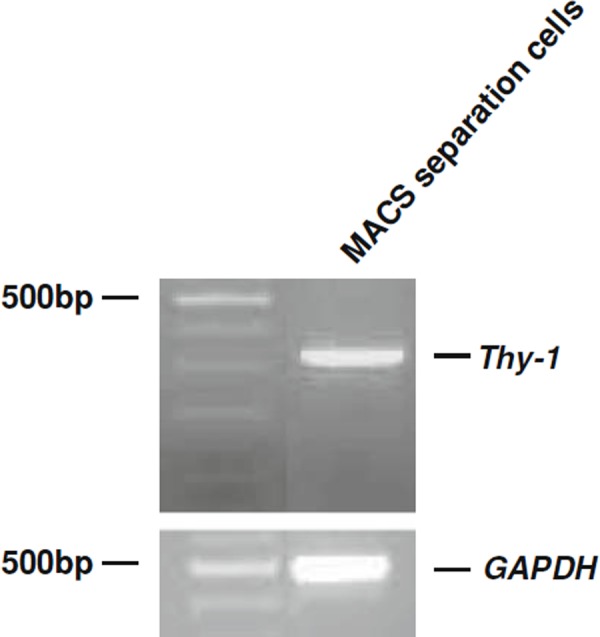
Isolation of Thy‐1+ cells by anti‐Thy‐1‐antibody‐conjugated microbeads. RT‐PCR analyses of Thy‐1 and GAPDH
Thy‐1+ cells could be cultured with serum‐free hormonally defined medium and STO feeders for 1 week but did not proliferate
We next sought to identify in vitro conditions for maintaining and/or expanding the Thy‐1+ cells ex vivo. A major objective of our work is to develop a defined culture system to facilitate the study of human SSCs and to identify external factors for their in vitro maintenance. In a previous study, a combination of serum‐free hormonally defined medium and STO feeders was found to be sufficient to support mouse SSCs [7]. Therefore, we used a basic culture system consisting of a stem cell‐enriched population, serum‐free defined culture medium, and mitotically inactivated STO feeders, similar to the system used for mouse SSCs. We found that the human Thy‐1+ cells could live on STO cells in a medium containing MEMα and no serum for 1 week (Fig. 2). The Thy‐1+ cells formed clumps macroscopically and did not proliferate during this time (Fig. 2). After 1 week, The Thy‐1+ cells floated in the medium (data not shown). These results showed that the Thy‐1+ cells could be maintained with serum‐free medium, growth factors, and STO feeders for 1 week. However, the Thy‐1+ cells did not proliferate under the same conditions that allow mouse SSC proliferation.
Figure 2.
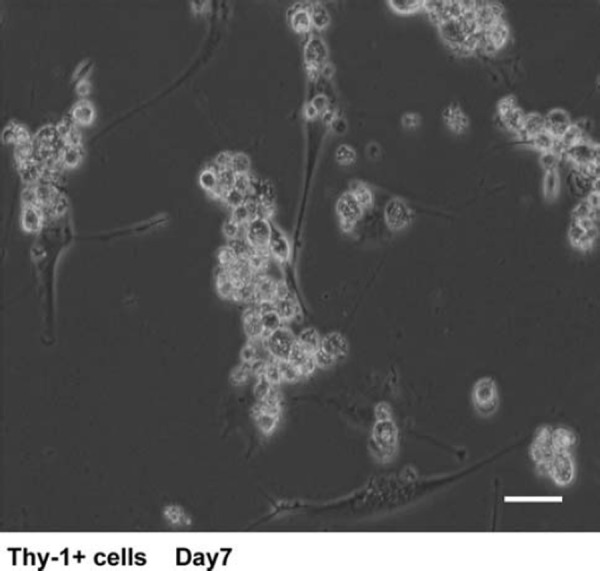
Culture of Thy‐1+ cells in serum‐free medium supplemented with GDNF, soluble GFRα1, and bFGF. Thy‐1+ cells stayed alive and formed clumps during 1 week in culture. Bars 30 μm
Thy‐1+ cells expressed the embryonic stem cell genes OCT3/4 and NANOG
We next investigated whether the human Thy‐1+ cells expressed SSC markers. It is well established that ES cells and primordial germ cells (PGCs) express high levels of alkaline phosphatase and OCT3/4, one of the POU transcription factors [22, 23]. After being induced to differentiate, these cells express lower levels of both of these molecules, eventually ceasing to express them at all. In addition, the expression of OCT3/4 is critical for the self‐renewal and pluripotency of ES cells [23, 24]. In a previous study, mouse SSCs were shown to express alkaline phosphatase and OCT3/4 [8]. We investigated the expression of these molecules by the Thy‐1+ cells, and found that both were expressed (Fig. 3a–c). We also confirmed the expression of OCT3/4 by RT‐PCR analysis using the total RNA from Thy‐1+ cell cultures (Fig. 3d). These results suggested that, based on OCT3/4 expression, the human Thy‐1+ cells had the same potential for self‐renewal as the mouse SSCs. Finally, in two previous studies, NANOG was found to be essential for the maintenance of pluripotency in ES cells [25, 26]. Mouse SSCs have been reported not to express NANOG [27]. Surprisingly, NANOG was expressed by the human Thy‐1+ cells (Fig. 3e). These results indicated that the Thy‐1+ cells expressed embryonic stem cell genes and had different characteristics from mouse SSCs.
Figure 3.
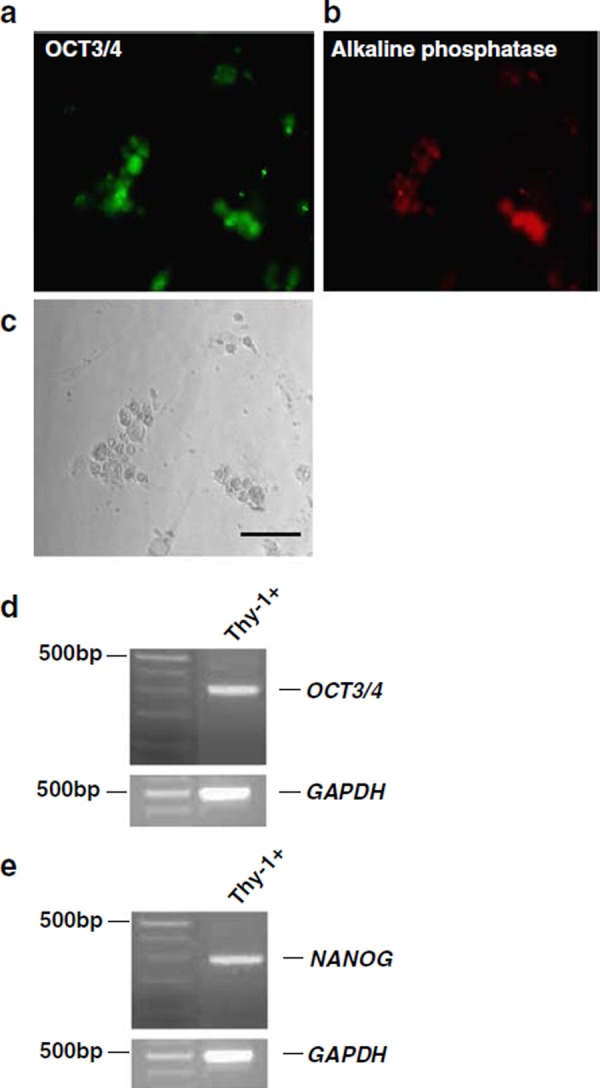
Phenotypic characteristics of cultured Thy‐1+ cells. a Immunostaining of OCT3/4 on Thy‐1+ cells. OCT3/4 immunoreactivity is shown in green (Alexa 488). b Immunostaining of alkaline phosphatase on Thy‐1+ cells. Alkaline phosphatase immunoreactivity is shown in red (Alexa 594). c Morphology of Thy‐1+ cells clumps. Bar indicates 50 μm. d RT‐PCR analysis for the expression of OCT3/4 and GAPDH in Thy‐1+ cells. e RT‐PCR analysis of the expression of NANOG and GAPDH in Thy‐1+ cells
No tumor formation was observed after the injection of Thy‐1+ cells into nude mice
We investigated whether the Thy‐1+ cells had pluripotency in vivo, by transplanting Thy‐1+ cells (1 × 105 cells) into the dorsal flanks of three nude mice. In this experiment, we used three independent samples for the injections. We observed no tumor formation macroscopically in any of Thy‐1+ cell‐treated mice, even 6 months after the injection (Fig. 4).
Figure 4.
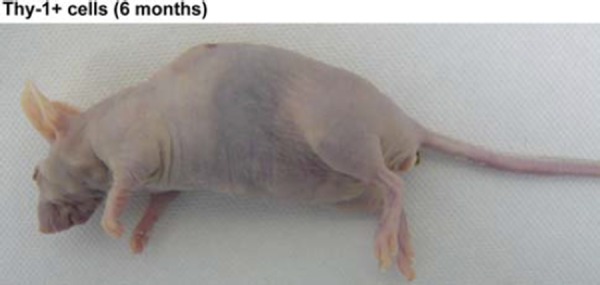
Nude mice treated with Thy‐1+ cells that had been cultured for 1 week in vitro. Nude mice treated with Thy‐1+ cells after 6 months
Discussion
The most important goals of this study were the identification and expansion of human SSCs from the adult testis in vitro. We used the same methods that are used to isolate mouse SSCs [14]. We were unable to isolate cells recognized as human SSCs using the methods for isolating mouse SSCs. However, we successfully isolated Thy‐1+ cells from human testicular tissues. We could culture these cells in serum‐free medium with STO feeders for 1 week. We found that the Thy‐1+ cells expressed embryonic stem cell genes (OCT3/4 and NANOG) and alkaline phosphatase. The Thy‐1+ cells did not form teratomas in nude mice. These results indicated that the Thy‐1+ cells potentially included human SSCs.
We found that human Thy‐1+ cells could be isolated with microbeads conjugated with anti‐mouse Thy‐1 antibodies. Presumably, microbeads conjugated with anti‐human Thy‐1 antibodies would be effective for obtaining highly enriched human Thy‐1+ cell populations from human testicular tissues. Next, we determined whether the human Thy‐1+ cells could be cultured in a serum‐free defined medium. We used a serum‐free medium because one had already been developed for culturing mouse SSCs [7]. This culture system avoids the use of contaminating somatic cells from the testis and of serum, both of which might contribute unknown factors that could induce apoptosis or differentiation of stem cells [7]. Our serum‐free culture system allowed us to culture Thy‐1+ cells for at least 1 week. We used STO cells as feeder cells and a defined set of growth factors (GDNF, bFGF, and soluble GFRα1). STO feeders can support the growth of several types of stem cells [28, 29, 30]. Because the Thy‐1+ cells did not proliferate in this system, we could not prove whether GDNF, soluble GFRα1, and bFGF were required and acted synergistically to support the Thy‐1+ cells. We also cannot rule out the possibility that other factors were produced by the feeder cells and acted on the Thy‐1+ cells. The development of a better culture system may extend the longevity of the Thy‐1+ cells.
We also found, by immunostaining, that the Thy‐1+ cells expressed the known SSC markers alkaline phosphatase and OCT3/4 [7]. Because the level of OCT3/4 influences the decision for self‐renewal or differentiation in ES cells, it may also regulate the fate determination of human SSCs. This supports the idea that the Thy‐1+ cell population included human SSCs. Although the use of the mouse cells permits in vivo transplantation to assess the multipotency of such cells [4, 5], clearly, we cannot perform the equivalent experiment in humans, for ethical reasons.
In addition, we performed RT‐PCR to confirm the identity of the Thy‐1+ cells. We confirmed that these cells expressed OCT3/4, and investigated the expression level of NANOG, which is another core transcription factor involved in maintaining pluripotency [31, 32]. In particular, NANOG's expression is more closely associated with pluripotent stem cells than is that of OCT3/4 [33]. Surprisingly, like ES cells and most mouse multipotent germline stem cells, the human Thy‐1+ cells expressed NANOG, although mouse SSCs do not express it [27]. A previous study indicated that SSCs from the mouse testis contain multipotent germ stem cells [34]. Therefore, the population of human Thy‐1+ cells may have included ES‐like pluripotent stem cells, or human SSCs may express NANOG, unlike the mouse SSCs. If the latter is the case and human SSCs are pluripotent, the interaction in vivo between Sertoli cells and SSCs in the testis may inhibit the multilineage differentiation of the SSCs, and direct their differentiation into spermatogenesis.
We also investigated whether the Thy‐1+ cells were pluripotent by injecting them into nude mice. We found that the Thy‐1+ cells did not make tumors in nude mice, for at least 6 months after the injection. It was reported that SSCs do not generate tumors when transplanted into nude mice, whereas ES cells produce highly invasive teratocarcinomas when injected into these mice [8]. We concluded that the human Thy‐1+ cells did not have the potential for pluripotency.
In conclusion, the Thy‐1+ cells we isolated from human testis may have contained human SSCs. However, we could not identify human SSCs using the methods that have been established for mouse SSC isolation. Thus, we found that human SSCs and mouse SSCs have different characteristics. It is reported that 1024 spermatocytes and thus 4096 haploid spermatids can be generated from each A single spermatogonium entering differentiation in mice and rats [35]. In humans, only four spermatocytes, leading to the production of 16 spermatids, are derived from each initial division of a germline progenitor [35]. Thus, the premeiotic steps of spermatogenesis differ in different species of mammals [35]. The types, numbers, and degree of efficiency of spermatogonial stem cell systems vary widely in different species of mammals. Even though the in vitro culture of mouse SSCs has been achieved by several groups, the isolation and culture of human SSCs has been difficult because of these differences. In this study, we confirmed that Thy‐1+ cells from the testes have the potential to include human SSCs, although more research is needed to confirm this possibility. The establishment of methods for the isolation and culture of human SSCs is important to gain a better understanding of these cells and to develop treatments for male infertility.
Acknowledgments
We thank S. Kanbe, M. Nagata, F. Yamabe, K. Takasugi, K. Kataoka, T. Ohira, N.Tanaka, N.ishii, M. Kurita, H. Hara, T. Ishii, and Y. Sasaki for assistance. This work was supported by a research grant from APSSM and a research grant from the Suzuki Urological Foundation.
References
- 1. Russell LD. Ettlin R, Sinha Hikim AP, Clegg ED Histological and histopathological evaluation of the testis, 1990. Clearwater: Cache River Press; [Google Scholar]
- 2. Meistrich ML, Beek MEAB Desjardins C. Ewing LL. Spermatogonial stem cells. Cell and molecular biology of the testis, 1993. New York: Oxford University Press; 266–295 [Google Scholar]
- 3. Tegelenbosch RA, Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res, 1993, 290, 193–200 [DOI] [PubMed] [Google Scholar]
- 4. Brinster RL, Zimmermann JW. Spermatogenesis following male germ‐cell transplantation. Proc Natl Acad Sci USA, 1994, 91, 11298–11302 10.1073/pnas.91.24.11298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA, 1994, 91, 11303–11307 10.1073/pnas.91.24.11303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brinster RL. Germline stem cell transplantation and transgenesis. Science, 2002, 296, 2174–2186 10.1126/science.1071607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kubota H, Avarbock MR, Brinster RL. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod, 2004, 71, 722–731 10.1095/biolreprod.104.029207 [DOI] [PubMed] [Google Scholar]
- 8. Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self‐renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA, 2004, 101, 16489–16494 10.1073/pnas.0407063101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanatsu‐Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long‐term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod, 2003, 69, 612–616 10.1095/biolreprod.103.017012 [DOI] [PubMed] [Google Scholar]
- 10. Spradling A, Drummond‐Barbosa D, Kai T. Stem cells find their niche. Nature, 2001, 414, 98–104 10.1038/35102160 [DOI] [PubMed] [Google Scholar]
- 11. Moore KA, Ema H, Lemischka IR. In vitro maintenance of highly purified, transplantable hematopoietic stem cells. Blood, 1997, 89, 4337–4347 [PubMed] [Google Scholar]
- 12. Nagano M, Avarbock MR, Leonida EB, Brinster CJ, Brinster RL. Culture of mouse spermatogonial stem cells. Tissue Cell, 1998, 30, 389–397 10.1016/S0040‐8166(98)80053‐0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagano M, Ryu BY, Brinster CJ, Avarbock MR, Brinster RL. Maintenance of mouse male germ line stem cells in vitro. Biol Reprod, 2003, 68, 2207–2214 10.1095/biolreprod.102.014050 [DOI] [PubMed] [Google Scholar]
- 14. Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci USA, 2003, 100, 6487–6492 10.1073/pnas.0631767100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci USA, 2000, 97, 8346–8351 10.1073/pnas.97.15.8346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science, 1988, 241, 58–62 10.1126/science.2898810 [DOI] [PubMed] [Google Scholar]
- 17. Henderson JK, Draper JS, Baillie HS, Fishel S, Thomson JA, Moore H, Andrews PW. Preimplantation human embryos and embryonic stem cells show comparable expression of stage‐specific embryonic antigens. Stem Cells, 2002, 20, 329–337 10.1634/stemcells.20‐4‐329 [DOI] [PubMed] [Google Scholar]
- 18. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science, 1999, 284, 143–147 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- 19. Ogawa T, Arechaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol, 1997, 41, 111–122 [PubMed] [Google Scholar]
- 20. Chessebeuf M, Padieu P. Rat liver epithelial cell cultures in a serum‐free medium: primary cultures and derived cell lines expressing differentiated functions. In Vitro, 1984, 20, 780–795 10.1007/BF02618294 [DOI] [PubMed] [Google Scholar]
- 21. Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Human embryonic stem cell lines derived from single blastomeres. Nature, 2006, 444, 481–485 10.1038/nature05142 [DOI] [PubMed] [Google Scholar]
- 22. Cooke JE, Godin I, Ffrench‐Constant C, Heasman J, Wylie CC. Culture and manipulation of primordial germ cells. Methods Enzymol, 1993, 225, 37–58 10.1016/0076‐6879(93)25006‐N [DOI] [PubMed] [Google Scholar]
- 23. Pesce M, Scholer HR. Oct‐4: gatekeeper in the beginnings of mammalian development. Stem Cells, 2001, 19, 271–278 10.1634/stemcells.19‐4‐271 [DOI] [PubMed] [Google Scholar]
- 24. Smith AG. Embryo‐derived stem cells: of mice and men. Annu Rev Cell Dev Biol, 2001, 17, 435–462 10.1146/annurev.cellbio.17.1.435 [DOI] [PubMed] [Google Scholar]
- 25. Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell, 2003, 113, 631–642 10.1016/S0092‐8674(03)00393‐3 [DOI] [PubMed] [Google Scholar]
- 26. Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell, 2003, 113, 643–655 10.1016/S0092‐8674(03)00392‐1 [DOI] [PubMed] [Google Scholar]
- 27. Kanatsu‐Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, Oshimura M, Heike T, Nakahata T, Ishino F, Ogura A, Shinohara T. Generation of pluripotent stem cells from neonatal mouse testis. Cell, 2004, 119, 1001–1012 10.1016/j.cell.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 28. Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell, 1992, 70, 841–847 10.1016/0092‐8674(92)90317‐6 [DOI] [PubMed] [Google Scholar]
- 29. Resnick JL, Bixler LS, Cheng L, Donovan PJ. Long‐term proliferation of mouse primordial germ cells in culture. Nature, 1992, 359, 550–551 10.1038/359550a0 [DOI] [PubMed] [Google Scholar]
- 30. Kubota H, Reid LM. Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc Natl Acad Sci USA, 2000, 97, 12132–12137 10.1073/pnas.97.22.12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell, 2005, 122, 947–956 10.1016/j.cell.2005.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, Wong KY, Sung KW, Lee CW, Zhao XD, Chiu KP, Lipovich L, Kuznetsov VA, Robson P, Stanton LW, Wei CL, Ruan Y, Lim B, Ng HH. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet, 2006, 38, 431–440 10.1038/ng1760 [DOI] [PubMed] [Google Scholar]
- 33. Hatano SY, Tada M, Kimura H, Yamaguchi S, Kono T, Nakano T, Suemori H, Nakatsuji N, Tada T. Pluripotential competence of cells associated with Nanog activity. Mech Dev, 2005, 122, 67–79 10.1016/j.mod.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 34. Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature, 2006, 440, 1199–1203 10.1038/nature04697 [DOI] [PubMed] [Google Scholar]
- 35. Ehmcke J, Wistuba J, Schlatt S. Spermatogonial stem cells: questions, models and perspectives. Hum Reprod Update, 2006, 12, 275–282 10.1093/humupd/dmk001 [DOI] [PubMed] [Google Scholar]


