Abstract
Purpose
Although adverse health effects of environment (such as cadmium, pesticides, diesel exhaust, etc.) on the male reproductive system have been suggested, there is little experimental evidence of such an effect of atmospheric sand dust. In the present study, the effects of sand dust (mineral particles) were investigated on the male reproductive system of mice.
Methods
Two types of sand dusts (Asian sand dust and Arizona sand dust) were intratracheally administered (0.1 mg/mouse 4 times every other week) to ICR male mice and then male reproductive organ weight, daily sperm production (DSP), histological analysis and serum testosterone level were measured.
Results
Histological examination showed that interstitial edema was produced by both sand dust types, and partial vacuolation of the seminiferous tubules was detected in the exposed mice. Moreover, exposure to these natural sand dusts significantly decreased DSP. On the other hand, there was no significant differences in serum testosterone concentration.
Conclusions
These results suggest that natural sand dust‐exposure produced adverse effects on mouse male reproductive function.
Keywords: Daily sperm production, Intratracheal instillation, Male reproduction, Mineral particles, Sand dust
Introduction
Recent epidemiological studies showed a correlation between elevated levels of particulate matter (PM) and increased incidence of pulmonary infections, airway hyper‐reactivity, and male reproductive function [1, 2, 3, 4, 5]. PM2.5 (particle sizes less than 2.5 μm) and PM10 (particle sizes of 2.5–10 μm) contain a variety of particles, such as industrial dust, diesel exhaust particles (DEP), sea salt, and sand dust (mineral particles) of soil origin. It has been estimated that up to 50% of the current atmospheric dust load originates from distributed surface soil [6]. Epidemiologic studies reported that sand dust events are associated with cardiovascular and respiratory problems, as well as an increase in daily mortality in Seoul, Korea [7, 8] and Taipei, Taiwan [9]. In recent experimental studies, data showed adverse effects of sand dust on murine lungs and aggravating effects of sand dust on allergen‐induced eosinophilic inflammation in murine airways [10, 11, 12, 13]. Male fertility is inhibited by inflammatory disease [14, 15, 16]. However, there is a lack of data regarding the effects of sand dust on male reproductive function.
Airborne particles are composed of various metals, acid salts, organic pollutants, diesel exhaust particles and natural sand dust. Since diesel exhaust or carbon nanoparticles also aggravate antigen‐related airway inflammation [17] and it was shown that diesel exhaust exposure or carbon nanoparticle treatment impairs male reproductive function in mice [18, 19]; it was postulated that sand dust may also affect this organ system. Because the effects of sand dust, one of the airborne particles, on the male reproductive system have not been previously investigated, the aim of present study was to examine effects of sand dust on male reproductive function in mice by histological observation of testicular tissue and daily sperm production (DSP).
Materials and methods
Experimental animals
A total of 48 male ICR mice (5 weeks of age, weighing 23–31 g) were purchased from CLEA Japan, Inc. (Tokyo, Japan) and quarantined for 1 week. Six‐week‐old mice (weighing 25–34 g) were then used in the experiment. Mice had ad libitum access to food (CE‐2 commercial diet; CLEA Japan, Inc.) and water and housed in plastic cages lined with soft wood chips. The cages were placed in a conventional room, which was air conditioned to 23°C and 55–70% humidity with a 12‐h light/dark cycle. The study adhered to the US National Institutes of Health guidelines for the use of experimental animals. The animal care method was approved by the Animal Care and Use Committee at Oita University of Nursing and Health Science in Oita, Japan.
Materials
The present study used two types of natural sand dust, Asian sand dust (Asian SD) and Arizona sand dust (Arizona SD). Asian SD was previously collected from surface soils in the Shapatou Desert located on the southern fringe of the Tengger Desert, where dust storms occur frequently in north central China [13]. The Asian SD diameter of the samples (a total of 600 particles) was measured under a scanning electron microscope (JSM‐5800, Jeol Ltd., Tokyo, Japan). The mean distribution peak of particle diameter in Asian SD was 6 μm. The Arizona SD was purchased from Powder Technology Inc. (Woonsocket, RI). The mean distribution peak of particle diameter in Arizona SD was 6.6–8.6 μm.
Asian SD and Arizona SD were heated at 360°C for 30 min under 80% nitrogen gas in an electric heater to remove toxic materials (microbiological materials, sulfate, etc.) adhering to the particles. The amounts of LPS and β‐glucan in original Asian SD (nonheated Asian SD) sample were consistent (3.66 EU/mg and 15.2 pg/ml, respectively). However, LPS and β‐glucan were not detected in heated Asian SD, original Arizona SD, and heated Arizona SD samples. The contents of the mineral elements with oxide in Asian SD and Arizona SD were 60 and 68–78% for SiO2, 11 and 10–15% for Al2O3, 4.1 and 2–5% for Fe2O3, 1.8 and 2–4% for Na2O, 9.0 and 2–5% for CaO, 2.5 and 1–2% for MgO, 0.7 and 0.5–1% for TiO2, 2.2 and 2–5% for K2O, and 8.7 and 2–5% for less on ignition, respectively [12, 20].
Study protocol
The ICR mice were divided into 3 groups (n = 16 per group) according to exposure: control (administered vehicle alone), Asian SD and Arizona SD group. The particles were suspended in normal saline solution (Otsuka Co., Kyoto, Japan) for instillation. The instillation dose of Asian SD and Arizona SD was 0.1 mg per mouse according to a previously reported protocol [19]. A maximum deposition of approximately 30 μg into the lungs of a single mouse equals 100% deposition according to the Japanese national air quality standard for suspended particulate matter (0.1 mg/m3) to be accumulated in the lungs of one mouse per week (deposition efficiencies calculated by tidal volume, 0.15 ml per mouse, and breathing rate, 200 breaths per minute). The instillation dose (0.1 mg per mouse) of particles in the present study was 3.3 times that amount. However, the deposition rate into an alveoli, in the case of the human respiratory tract model for radiological protection, is approximately 3% at 5.5‐μm particle diameter. The instillation dose of particles in the present study was 111 times the amount of approximately 0.9 μg in the case of 3% deposition. Asian dust storms with the Asian SD aerosol intermittently come flying over Japan during March and April (2 months) [20]. Therefore, this repeated instillation method was chosen to investigate the effects of natural sand dust on male reproductive function. Mice were intratracheally (i.t.) instilled with these particles under anesthesia with 4% halothane (Takeda Chemical, Osaka, Japan) using a polyethylene tube. The control mice were instilled i.t. with 0.1 ml of normal saline solution. The instillation of these particles or normal saline solution was repeated 4 times at 2‐week intervals. At 12 weeks of age, and 24‐h after the last administration, the mice were killed by exsanguination under anesthesia induced by intraperitoneal injection of pentobarbital. Serum was prepared using CAPIJECT (Terumo Medical, NJ, USA) and frozen at −80°C until assayed for testosterone.
Body and organ weights
Body weight was recorded and the weights of the testis and epididymis bilaterally were measured for each animal. After weighing, a piece of the left testis was fixed for histological observation and the right testis was frozen at −80°C until thawed for sperm count analysis.
Light microscopy
Following exsanguination the testes of mice were fixed in Bouin's solution, dehydrated with 70–100% ethanol, and embedded in paraffin. The sections were then stained with H&E and examined under a light microscope. To compare the extent of damage to the testes, the ratio of damaged seminiferous tubules, defined as those with degenerative and necrotic changes, desquamation of seminiferous epithelium to lumen, and loss of spermatozoon were observed, then calculated per cross section of each testis. Estimation of the testicular damage was conducted by counting the number of tubular cross sections and determining the percentage of total degenerated tubules in 3 cross sections per testis [19].
Daily sperm production per gram testis
A right testis sample was weighed and the seminiferous tubules released from the tunica albuginea into a saline solution in a homogenizer tube [21]. The testis was homogenized in 5 ml of lysis buffer using a Politron unit and left to stand for 60 min at room temperature. The mix was then diluted to the desired volume and the number of sperm heads or sperm cells counted in a hemocytometer chamber. The DSP/g was calculated according to the following formula:
Sperm number/ml × volume of lysis buffer = total sperm number in testis
Total sperm number in testis/4.84* = sperm production/day
(Sperm production/day)/g testis weight = DSP/g (sperm/g/day)
*4.84 is an invariable number for calculating sperm production per day in mice.
Assay for testosterone
Serum testosterone was measured in duplicate using an enzyme‐linked immunosorbent assay (Testosterone ELISA Test Kit, Endocrine Technologies Inc., CA, USA). Absorbance was measured at 450 nm by a microplate reader (SpectraFluor, TECAN, Grodig, Austria). The concentration of testosterone was calculated using standard curves. The detection limit of the assay was 20 pg/ml.
Statistical analysis
All results are expressed as mean ± SD. Significant differences were examined by Dunnett's pair‐wise multiple comparison t‐test using KyPlot Version 5 (KyensLab Inc., Tokyo, Japan). A P‐value of < 0.05 was considered to represent statistical significance.
Results
Effect of exposure to two sand dusts on body and organ weights and serum testosterone concentration
To determine the general toxicity of sand particles, body and reproductive organ weights were measured. The Asian SD group had a significantly lower mean body weight of 93.6% relative to control (Table 1). However, there were no significant differences in the organ weights between the control and treated groups. There were no significant differences in serum testosterone concentration between the control and treated groups.
Table 1.
Effect of natural sand dust on body weight and weight of reproductive organs and serum testosterone concentration
| Control | Asian SD | Arizona SD | |
|---|---|---|---|
| Body weight (g) a | 41.2 ± 2.6 | 38.6 ± 3.0* | 43.5 ± 3.7 |
| Testis (mg) | 132.2 ± 17.4 b | 132.8 ± 19.1 | 131.5 ± 20.2 |
| (3.2 ± 0.4) c | (3.5 ± 0.5) | (2.9 ± 0.9) | |
| Epididymis (mg) | 53.3 ± 4.6 b | 51.4 ± 4.8 | 53.1 ± 6.7 |
| (1.2 ± 0.3) c | (1.3 ± 0.1) | (1.2 ± 0.3) | |
| Testosterone (ng/ml) | 4.4 ± 2.0 | 8.5 ± 7.6 | 11.2 ± 9.0 |
*P < 0.05 compared with controls
aData are mean ± SD values. n = 16
bAbsolute weight
cRelative weight (mg/g body weight)
Morphological observation of testes in mice exposed to two sand dusts
In all testes of mice treated with the sand dusts, interstitial edema was observed by light microscopy (Fig. 1b, f). Moreover, vacuolation of the seminiferous tubules was observed in the both SD‐exposed groups. The damaged tubules were scattered randomly throughout the testis (Fig. 1c–e). These effects were significantly higher in Asian SD and Arizona SD‐exposed groups than in controls (Fig. 1g). Although no remarkable morphological change was observed on Sertoli cells, Leydig cells and myoid cells of Asian SD or Arizona SD treated mice.
Figure 1.
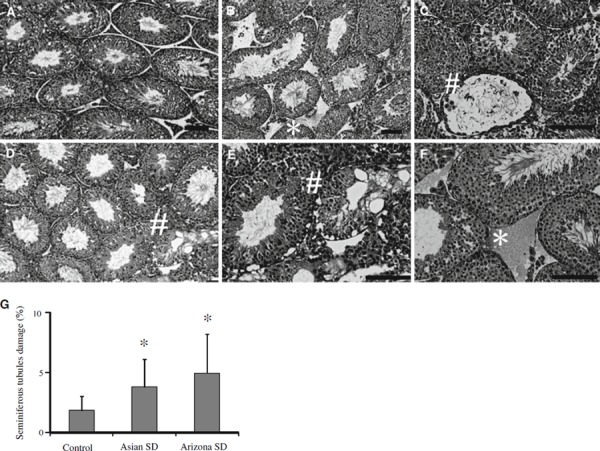
Morphological observations of the testes treated with Asian SD and Arizona SD mice. a Control; b, c Asian SD; d–f Arizona SD. Bar 100 μm. Interstitial edema was observed in the testes in b, and f (asterisk). Some degenerated seminiferous tubules were observed in the testes in c–e) (hash). g The percentage of degenerated seminiferous tubules in cross sections of treated Asian SD and Arizona SD mice. Mean ± SD (n = 16); *P < 0.05 compared with controls
Daily sperm production
The DSP was significantly lower in the Asian SD and Arizona SD treated groups. The DSP decreased by 13% in the Asian SD group (P < 0.05), by 18% in the Arizona SD group (P < 0.05) compared to the control group.
Discussion
This study focused on the influence of natural sand dusts on the murine male reproductive system. There is extensive evidence that exposure to increased levels of inhalable particulate pollutants (PM10 or PM2.5) is related to increased respiratory and cardiac morbidity, cancer and mortality [4, 22, 23, 24]. It was recently proposed that diesel exhaust (DE) may influence reproductive function [25, 26]. Exposure to 0.3, 1 or 3 mg diesel exhaust particles (DEP)/m3 for 6 months was shown to produce induction of Leydig cell degeneration, grater damaged seminiferous tubules, and reduction in daily sperm production (DSP) [18]. In our previous study, it was found that carbon nanoparticles (carbonaceous nuclei of DEP) administered at 0.1 mg/mouse at 10 one‐week intervals produced induction of Leydig cell degeneration, a reduction in DSP and an increase in the testosterone concentration in serum [19]. However, there has been no study identifying the effects of natural sand dust in the environment on the male reproductive function of mice. The natural sand dust is inhalable particulate pollutants, same as carbon nanoparticles and DEP. Carbon nanoparticle and DEP are industrial products or artifact, whereas the natural sand dust consists of natural material. It is important to determine the effects of PM10 on the male reproductive function, because natural sand dust is one of the main components of PM10. On the other hand, human semen studies imply that PM10 dust may exert a significant adverse affect on reproductive outcomes [27].
Our results indicate that two sand dust samples (Asian SD and Arizona SD) instilled i.t. markedly changed testicular morphology and decreased DSP. Thus, generalized natural sand dust (mineral particles) may aggravate the male reproductive system. Natural sand dust contains microbiological materials as well as chemical species such as polyaromatic hydrocarbons [28, 29]. In the present study, heat‐treated sand dust samples were used to exclude the effects of these elements. On the other hand, it has been estimated that silica (Si) compounds comprised 60–80% of our two sand dust samples [12, 20]. Silicon dioxide (SiO2) reduced the sperm count and induced histological changes of testes in rat [30]. Elements, such as SiO2‐containing SD, might well be related to the aggravating effects on the male reproductive system. Testicular histology demonstrated partial vacuolation of the seminiferous tubules in the Asian SD and Arizona SD groups. From an animal experimental point of view, several sand dusts considerably increase inflammation. Decreases of body weight in the SD treated mice may be associated with the inflammation [10]. Lipopolysaccharide (LPS) cause inflammation and aggravates spermatogenesis without affecting the serum testosterone levels in rats [14]. The present study also suggested that exposure to natural sand dust might induce changed testicular morphology, which may in turn have produced DSP reduction.
The present study suggests that natural sand dust exposure exerts adverse effects on mouse male reproductive function. For the present, it is not known whether sand dust poses a significant risk to the human male reproductive system. However, our data derived from the murine reproductive system suggests that various sand dusts may potentially influence spermatogenesis in men.
Acknowledgments
The authors thank Ms. Sayoko Nagayama and Ms. Kumiko Takahashi, Oita University of Nursing and Health Sciences. This work was supported in part by a Grant‐in‐Aid for Young Scientists (A) (17689007) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Pope CA, Dockery DW. Acute health effects of PM10 pollution on symptomatic and asymptomatic children. Am Rev Respir Dis, 1992, 145, 1123–1128 [DOI] [PubMed] [Google Scholar]
- 2. Thurston GD, Ito K, Kinney PL, Lippmann M. A multi‐year study of air pollution and respiratory hospital admissions in three New York State metropolitan areas: results for 1988 and 1989 summers. J Expo Anal Environ Epidemiol, 1992, 2, 429–450 [PubMed] [Google Scholar]
- 3. Tomei F, Rosati MV, Ciarrocca M, Baccolo TP, Gaballo M, Caciari T, Tomao E. Plasma cortisol levels and workers exposed to urban pollutants. Ind Health, 2003, 41, 320–326 10.2486/indhealth.41.320 [DOI] [PubMed] [Google Scholar]
- 4. Craig L, Brook JR, Chiotti Q, Croes B, Gower S, Hedley A, Krewski D, Krupnick A, Krzyzanowski M, Moran MD, Pennell W, Samet JM, Schneider J, Shortreed J, Williams M. Air pollution and public health: a guidance document for risk managers. J Toxicol Environ Health A, 2008, 71, 588–698 10.1080/15287390801997732 [DOI] [PubMed] [Google Scholar]
- 5. Vigotti MA, Chiaverini F, Biagiola P, Rossi G. Urban air pollution and emergency visits for respiratory complaints in Pisa, Italy. J Toxicol Environ Health A, 2007, 70, 266–269 10.1080/15287390600884800 [DOI] [PubMed] [Google Scholar]
- 6. Kim BG, Han JS, Park SU. Transport SO2 and aerosol over the Yellow sea. Atmos Environ, 2001, 35, 727–737 10.1016/S1352‐2310(00)00344‐7 [Google Scholar]
- 7. Kwon HJ, Cho SH, Chun Y, Lagarde F, Pershagen G. Effects of the Asian dust events on daily mortality in Seoul, Korea. Environ Res, 2002, 90, 1–5 10.1006/enrs.2002.4377 [DOI] [PubMed] [Google Scholar]
- 8. Chen YS, Yang CY. Effects of Asian dust storm events on daily hospital admissions for cardiovascular disease in Taipei, Taiwan. J Toxicol Environ Health A, 2005, 68, 1457–1464 10.1080/15287390590967388 [DOI] [PubMed] [Google Scholar]
- 9. Chen YS, Sheen PC, Chen ER, Liu YK, Wu TN, Yang CY. Effects of Asian dust storm events on daily mortality in Taipei, Taiwan. Environ Res, 2004, 95, 151–155 10.1016/j.envres.2003.08.008 [DOI] [PubMed] [Google Scholar]
- 10. Ichinose T, Nishikawa M, Takano H, Sera N, Sadakane K, Mori I, Yanagisawa R, Oda T, Tamura H, Hiyoshi K, Quan H, Tomura S, Shibamoto T. Pulmonary toxicity induced by intratracheal instillation of Asian yellow dust (Kosa) in mice. Environ Toxicol Pharmacol, 2005, 20, 48–56 10.1016/j.etap.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 11. Ichinose T, Sadakane K, Takano H, Yanagisawa R, Nishikawa M, Mori I, Kawazato H, Yasuda A, Hiyoshi K, Shibamoto T. Enhancement of mite allergen‐induced eosinophil infiltration in the murine airway and local cytokine/chemokine expression by Asian sand dust. J Toxicol Environ Health A, 2006, 69, 1571–1585 10.1080/15287390500470833 [DOI] [PubMed] [Google Scholar]
- 12. Ichinose T, Yoshida S, Sadakane K, Takano H, Yanagisawa R, Inoue K, Nishikawa M, Mori I, Kawazato H, Yasuda A, Shibamoto T. Effects of asian sand dust, Arizona sand dust, amorphous silica and aluminum oxide on allergic inflammation in the murine lung. Inhalation Toxicol, 2008, 20, 685–694 10.1080/08958370801935133 [DOI] [PubMed] [Google Scholar]
- 13. Hiyoshi K, Ichinose T, Sadakane K, Takano H, Nishikawa M, Mori I, Yanagisawa R, Yoshida S, Kumagai Y, Tomura S, Shibamoto T. Asian sand dust enhances ovalbumin‐induced eosinophil recruitment in the alveoli and airway of mice. Environ Res, 2005, 99, 361–368 10.1016/j.envres.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 14. O'Bryan MK, Schlatt S, Phillips DJ, Kretser DM, Hedger MP. Bacterial lipopolysaccharide‐induced inflammation compromises testicular function at multiple levels in vivo. Endocrinology, 2000, 141, 238–246 10.1210/en.141.1.238 [DOI] [PubMed] [Google Scholar]
- 15. Liew SH, Meachem SJ, Hedger MP. A stereological analysis of the response of spermatogenesis to an acute inflammatory episode in adult rats. J Androl, 2007, 28, 176–185 10.2164/jandrol.106.000752 [DOI] [PubMed] [Google Scholar]
- 16. Spiess AN, Feig C, Schulze W, Chalmel F, Cappallo‐Obermann H, Primig M, Kirchhoff C. Cross‐platform gene expression signature of human spermatogenic failure reveals inflammatory‐like response. Hum Reprod, 2007, 22, 2936–2946 10.1093/humrep/dem292 [DOI] [PubMed] [Google Scholar]
- 17. Ichinose T, Takano H, Sadakane K, Yanagisawa R, Kawazato H, Sagai M, Shibamoto T. Differences in airway‐inflammation development by house dust mite and diesel exhaust inhalation among mouse strains. Toxicol Appl Pharmacol, 2003, 187, 29–37 10.1016/S0041‐008X(02)00038‐8 [DOI] [PubMed] [Google Scholar]
- 18. Yoshida S, Sagai M, Oshio S, Umeda T, Ihara T, Sugamata M, Sugawara I, Takeda K. Exposure to diesel exhaust particles affects the male reproductive systems of mice. Int J Androl, 1999, 22, 307–315 10.1046/j.1365‐2605.1999.00185.x [DOI] [PubMed] [Google Scholar]
- 19.Yoshida S, Hiyoshi K, Ichinose T, Takano H, Oshio S, Sugawara I, Takeda K, Shibamoto T. Effect of nanoparticles on the male reproductive system of mice. Int J Androl. 2008 (in press). [DOI] [PubMed]
- 20.Ichinose T, Yoshida S, Hiyoshi K, Sadakane K, Yasuda A, Shibamoto T. The effects of microbial materials adhered to Asian sand dust on allergic lung inflammation. Arch Environ Contam Toxicol. 2008;20:685–94. [DOI] [PubMed]
- 21. Joyce KL, Porcelli J, Cooke PS. Neonatal goitrogen treatment increases adult testis size and sperm production in the mouse. J Androl, 1993, 14, 448–455 [PubMed] [Google Scholar]
- 22. Krewski D, Burnett R, Jerrett M, Pope CA, Rainham D, Calle E, Thurston G, Thun M. Mortality and long‐term exposure to ambient air pollution: ongoing analyses based on the American Cancer Society cohort. J Toxicol Environ Health A, 2005, 68, 1093–1109 10.1080/15287390590935941 [DOI] [PubMed] [Google Scholar]
- 23. Vedal S, Brauer M, White R, Petkau J. Air pollution and daily mortality in a city with low levels of pollution. Environ Health Persp, 2003, 111, 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hong YC, Leem JH, Ha EH. Air pollution and daily mortality in Inchon, Korea. J Korean Med Sci, 1999, 14, 239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoshida M, Yoshida S, Sugawara I, Takeda K. Maternal exposure to diesel exhaust decreases expression of steroidogenic factor‐1 and mullerian inhibiting substance in the murine fetus. J Health Sci, 2002, 48, 317–324 10.1248/jhs.48.317 [Google Scholar]
- 26. Yoshida S, Ono N, Tsukue N, Oshio S, Umeda T, Takano H, Takeda K. In utero exposure to diesel exhaust increased accessory reproductive gland weight and serum testosterone concentration in male mice. Environ Sci, 2006, 13, 139–147 [PubMed] [Google Scholar]
- 27. Sram RJ, Binkova B, Rossner P, Rubes J, Topinka J, Dejmek J. Adverse reproductive outcomes from exposure to environmental mutagens. Mutat Res, 1999, 428, 203–215 [DOI] [PubMed] [Google Scholar]
- 28. Choi JC, Lee M, Chun Y, Kin J, Oh S. Chemical composition and source signature of spring aerosol in Seoul, Korea. J Geophys Res, 2001, 106, 18067–18074 10.1029/2001JD900090 [Google Scholar]
- 29. Mori I, Nishikawa M, Tanimura T, Quan H. Change in size distribution and chemical composition of Kosa (Asian dust) aerosol during long‐range transport. Atmos Environ, 2003, 37, 4253–4263 10.1016/S1352‐2310(03)00535‐1 [Google Scholar]
- 30. Fan YO, Zhang YH, Zhang XP, Liu B, Ma YX, Jin YH. Comparative study of nanosized and microsized silicon dioxide on spermatogenesis function of male rats. Wei Sheng Yan Jiu, 2006, 35, 549–553 [PubMed] [Google Scholar]


