Abstract
Obesity, which disturbs lipid and glucose metabolism, is a recent medical concern. It threatens human health and also has adverse effects on reproductive functions by causing insulin resistance/hyperinsulinemia, especially in women with polycystic ovary syndrome (PCOS). For PCOS patients to prevent these adverse effects, it is important to take into account improving their lifestyles by exercise and proper diets. The relationship between insulin resistance/hyperinsulinemia and reproductive disorders should be understood as fully as possible in order to provide effective treatment. It is well known that insulin resistance and compensatory hyperinsulinemia can be triggered by obesity with visceral fat accumulation. Hyperinsulinemia affects granulosa cells in small follicles and theca cells. This condition induces early response to luteinizing hormones on granulosa cells of small follicles and causes premature differentiation of these cells, which eventually results in anovulation. For improvement of anovulation because of hyperinsulinemia, insulin‐sensitizing agents (biguanide and thiazolidinedione derivatives) are useful. Hyperinsulinemia may adversely affect the endometrial functions and environment, and evoke implantation disturbance. Treatment with an insulin‐sensitizing agent (metformin) improves the levels of glycodelin, insulin‐like growth factor binding protein 1, and blood flow in spiral arteries during the peri‐implantation period. It supports endometrial function, improves the endometrial environment, and facilitates embryo implantation. The rate of early pregnancy loss during the first trimester is 30–50% in women with PCOS, which is threefold higher than for normal women. Metformin treatment improves the levels of insulin, the homeostasis model assessment for insulin resistance, and plasminogen activator inhibitor activity, and decreases early pregnancy loss. It goes without saying that lifestyle change is fundamental for improving reproductive performance in addition to treatment with insulin‐sensitizing agents.
Keywords: Hyperinsulinemia, Insulin resistance, Insulin sensitizer, Lifestyle, Reproductive disorders
Introduction
There is great concern about the high prevalence of obesity and overweight worldwide, especially in Western countries. A high obesity rate is prevalent in Okinawa prefecture, Japan [1]. Obesity is known to cause disturbances of lipid and glucose metabolism. The serious health threats of diabetes, cardiovascular disease, cancers, and anovulation are the principle manifestations of obesity [2, 3, 4, 5].
As for obesity and reproduction, weight increase is correlated with a higher frequency of menstrual disorders, infertility, gestational diabetes, and other significant sequelae [6]. Infertile women with polycystic ovary syndrome (PCOS) were first described by Stein and Leventhal [7]. PCOS is characterized by chronic anovulation, infertility, and a typical sonographic appearance of the ovaries. Thus, an important cause of infertility in women with PCOS is anovulation [8]. Of the women with PCOS, 40–50% frequently experience insulin resistance/hyperinsulinemia, especially obese women [9]. Obesity or overweight is usually more prevalent in infertile/subfertile women with PCOS. Although the importance of obesity/overweight and PCOS status is yet to be fully understood, recent evidence suggests that body mass index (BMI) contributes significantly to the severity of many problems, such as the risk of miscarriage [10, 11]. Furthermore, weight reduction has been reported to improve reproductive function in overweight and obese infertile women, and an insulin‐sensitizing drug such as biguanide (metformin) also improves the menstrual cycle and ovulation in women with PCOS [12, 13, 14]. Thus, understanding the relationship between insulin resistance/hyperinsulinemia and reproductive disorders in infertile women living a modern lifestyle is important.
Insulin resistance/hyperinsulinemia and visceral adipose tissue
Insulin resistance is defined as the decreased ability of insulin to stimulate glucose disposal into target tissues or a reduced glucose response to a given amount of insulin. Hyperinsulinemia is a compensatory response to this target tissue resistance. It is well known that insulin resistance and compensatory hyperinsulinemia are triggered by obesity. There are two types of fat distribution in obese subjects: subcutaneous fat and intra‐abdominal visceral fat. A number of clinical studies demonstrate the contribution of visceral fat accumulation to the development of metabolic disorders, including glucose intolerance and hyperlipidemia [15, 16, 17, 18, 19]. Kissenbah et al. [20] and Matsuzawa [21] clearly demonstrate that visceral fat obesity has greater insulin resistance than subcutaneous fat obesity.
Several mechanisms of insulin resistance are hypothesized, such as deficient insulin action [22], β‐cell dysfunction [23], increased insulin secretion in response to dietary stimuli [24, 25], and decreased hepatic clearance of insulin [26]. Matsuzawa [27] postulates that insulin resistance in visceral fat obesity is exacerbated by an increased supply of free fatty acid (FFA). He also states that adipose tissues directly secrete multiple bioactive molecules (adipocytokines), such as tumor necrosis factor α (TNF‐α), leptin, and plasminogen activator inhibitor 1 (PAI‐1), which plays an important role in the development of metabolic disorders. Namely, visceral fat accumulation causes an increase in FFA and adipocytokines (probably TNF‐α), resulting in insulin resistance.
Insulin resistance/hyperinsulinemia and hyperandrogenism
Hyperinsulinemia correlates positively with the presence of hyperandrogenism in lean and obese women with PCOS [28, 29, 30, 31]. Hyperandrogenism is understood to result from both increased adrenal and ovarian androgen production [23, 32, 33, 34]. Insulin, by acting via its receptor, appears to promote ovarian and adrenal androgen biosynthesis [35, 36], amplifying luteinizing hormone (LH)‐induced androgen production by theca cells and resulting in hyperandrogenemia [37, 38]. Amelioration of hyperinsulinemia leads to a marked decline in circulating androgens to a normal level [39]. Hyperinsulinemia may also upregulate insulin‐like growth factor‐1 (IGF‐1) receptors, which are potent stimulators of LH‐induced androgen synthesis and suppression of IGF‐binding protein 1 (IGF‐BP1) production by the liver [40, 41]. Additionally, insulin may have an inhibitory effect on hepatic sex hormone‐binding globulin (SHBG) production [42], thereby increasing the bioactive androgen, which produces virilization.
Insulin resistance/hyperinsulinemia and ovulatory disorder
The characteristic sonography of the ovary in PCOS shows the growth arrest of the antral follicles after reaching a diameter between 5 and 8 mm. Franks et al. [43] reported that the arrest of small follicles may be caused by premature activation of LH‐mediated terminal differentiation of granulosa cells. In the a normal menstrual cycle, granulosa cells of the dominant follicle become responsive to LH in the mid‐follicular phase at a follicular diameter of 10 mm [44]. In the preovulatory phase, LH enhances steroidogenesis and triggers terminal differentiation of granulosa cells so that the growth of follicles is arrested. On the other hand, granulosa cells from follicles as small as 4 mm in diameter of anovulatory PCOSs are responsive to LH. This response to LH is remarkably amplified by insulin [43]. The premature activation of granulosa cells by LH induces terminal differentiation, resulting in the arrest of follicle growth [9].
The relationship between insulin resistance/hyperinsulinemia and PCOS is summarized in Fig. 1. Hyperinsulinemia may amplify the production of androgen on theca cells, resulting in virilization. Also, an excess amount of androgen converts to estrogen. Converted estrogen increases LH secretion via GnRH and by a direct effect on the pituitary gland. Insulin also acts on granulosa cells of small follicles and amplifies premature responsiveness to LH. Increasing LH leads to the terminal differentiation of granulosa cells in small follicles and causes early arrest of follicular growth, which results in anovulation. Hyperinsulinemia affects two types of cells. In theca cells, hyperinsulinemia increases the secretion of androgens and results in virilization. An excess amount of androgen converts to estrogen, resulting in elevation of LH. Hyperinsulinemia initiates the response of granulosa cells to LH during the premature stage and leads to premature differentiation of granulosa cells. It results in anovulation.
Figure 1.
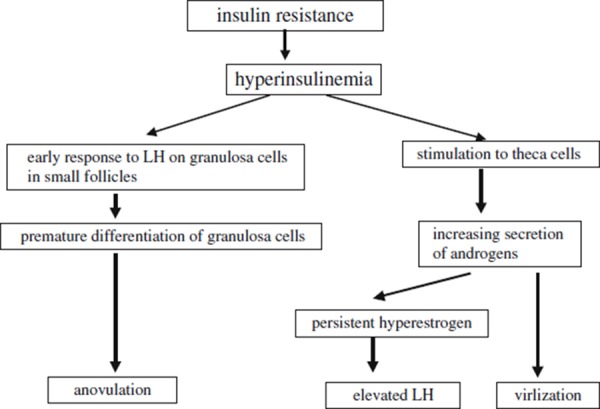
The relationship between insulin resistance/hyperinsulinemia and PCOS
Ovulatory disorder and insulin‐sensitizing agents
In general, treatment for ovulatory disorders attributed to obesity and/or insulin resistance is by lifestyle changes such as dietary changes and increased physical activities [45]. At the same time, other approaches to treat these patients include the use of insulin‐sensitizing agents, namely, biguanide (metformin) and thiazolidinedione derivative (troglitazone). Metformin has been used for anovulatory patients with PCOS. This treatment has been highly successful, with ovulation rates as high as 46% [46]. Thiazolidinedione derivative (troglitazone) is also used for ovulation induction in PCOS. Thiazolidinedione derivative (troglitazone) improves the ovulation rate by 57% in PCOS patients compared with 12% in placebo‐treated patients [47]. The functional mechanisms of the two agents are known to be different. Metformin is an oral biguanide, category B drug for pregnant women that has been approved for treatment of type 2 diabetes mellitus. It is thought to affect multiple metabolic pathways, decreasing glucose absorption, suppressing hepatic glucose output and gluconeogenesis, and also decreasing the BMI [48, 49, 50]. In addition, metformin directly inhibits androgen production in human thecal cells [51]. Side effects are rare, and gastrointestinal disturbances, such as abdominal pain and nausea, rarely cause discontinuation of treatment. On the other hand, troglitazone is a thiazolidinedione derivative used for the treatment of type 2 diabetes mellitus. It has a more potent glucose‐lowering effect and favorable effects on abdominal lipid levels, including the decrease of circulating triglyceride and FFA levels. Troglitazone may affect and differentiate adipocytes via peroxisome proliferator‐activated receptor γ (PPARγ). As a result, differentiated adipocytes regulate insulin sensitivity and improve insulin resistance [52]. The reported side effects are hepatic disturbance and edema, but these are tolerable. At present, there is uncertainty about which types of insulin‐sensitizing agents to select for specific patients. Therefore, high molecular weight (HMW) adiponectin is the focus because HMW adiponectin is secreted from adipocytes and acts on increasing insulin sensitivity in target organs [53]. Decreased HMW adiponectin has been found to play a crucial and causal role in obesity‐linked insulin resistance [54]. Namely, HMW adiponectin reflects the pathophysiological condition of insulin resistance. Recently, based on the properties of biguanide and thiazolidinedione derivative and also levels of HMW adiponectin, anovulatory patients with PCOS have been treated. The obtained results showed a high ovulatory rate, a high pregnancy rate, and a low abortion rate (unpublished data). However, further precise investigations are necessary concerning the choice of insulin‐sensitizing agents.
Insulin resistance/hyperinsulinemia and implantation disorder
Recently, it has been reported that hyperinsulinemia may adversely affect the endometrial functions and environment, and evoke implantation disturbance and/or early pregnancy loss [55]. Jakubowicz et al. [56] studied glycodelin, IGF‐BP1, and uterine vascularity and blood flow in PCOS patients with metformin and placebo treatment. Glycodelin is a specific protein synthesized by secretary/decidualized endometrial glands. Circulating glycodelin may reflect endometrial function, such as endometrial maturation and inhibition of the endometrial immune response to the embryo [57, 58, 59, 60, 61]. IGF‐BP1 is a protein that appears to facilitate adhesion processes at the feto‐maternal interface and may play an important role in the peri‐implantation period [62, 63]. The concentration of glycodelin and IGF‐BP1 in the luteal phase is highly increased by three‐ to fourfold by metformin treatment compared with placebo. Also, metformin treatment increases vascular penetration and decreases the blood flow of spiral arteries, demonstrating a 20% decrease in the resistance index. These results indicate that improvement of hyperinsulinemia may support endometrial function and amelioration of the endometrial environment, resulting in facilitation of embryo implantation.
Insulin resistance/hyperinsulinemia and early pregnancy loss
The rates of early pregnancy loss, defined as miscarriage during the first trimester, have been reported to be 30–50% in women with PCOS, which is threefold higher than in normal women [64, 65]. In addition, 36–82% of women with recurrent early pregnancy loss have PCOS [66]. According to Jakubowicz et al. [67], the rate of early pregnancy loss with metformin treatment was 8.8% compared to 41.9% in the control group with PCOS. Glueck reports that metformin treatment decreases early pregnancy loss in current (on metformin) and previous (no metformin) pregnancy with PCOS [68]. Additionally, metformin treatment improves the levels of insulin, homeostasis model assessment for insulin resistance (HOMA‐IR), and PAI‐Fx. This insulin‐lowering effect of metformin treatment might result in protection from early pregnancy loss. Furthermore, metformin treatment during pregnancy in women with PCOS is safely associated with a reduction of first‐trimester spontaneous abortions and is not teratogenic, having no adverse effects on the biological and physical conditions of baby.
Conclusion
Recently, the obesity rate has been a great concern in Okinawa, Japan, as Western dietary habits are becoming more popular [69]. Moreover, as signs of metabolic syndrome, insulin resistance and hyperinsulinemia are among the major issues. These conditions are highly related to lifestyle‐related diseases such as diabetes, cardiovascular disease, and cancer in middle‐aged people [2]. For young women, these conditions also threaten their reproductive systems. Concerning metabolic syndrome, properly obtaining a nutritional and metabolic balance in daily life becomes increasingly crucial not only for middle‐aged and older women, but also for young adults in order to increase their fecundity. In fact, some studies showed that the usage of insulin‐sensitizing agents alone is not sufficient for treating infertility associated with PCOS [70]. Furthermore, according to Mathur et al. [71], there are no clear data showing that using metformin alone reduces pregnancy loss or improves the pregnancy rate. Thus, it is possible to suggest that combining insulin‐sensitizing agent therapy with an improved lifestyle may greatly support women with PCOS and improve their pregnancy outcomes. In addition, exercise and diet have been reported to improve insulin resistance and reproductive performance [72]. Some are of the opinion that PCOS is one of the primary symptoms of lifestyle diseases among women. Regarding the present report, gynecologists should emphasize healthy lifestyles for women as part of their medical care.
The relationship between differentiation of visceral adipocytes and reproductive processes is shown in Fig. 2 [73].
Figure 2.
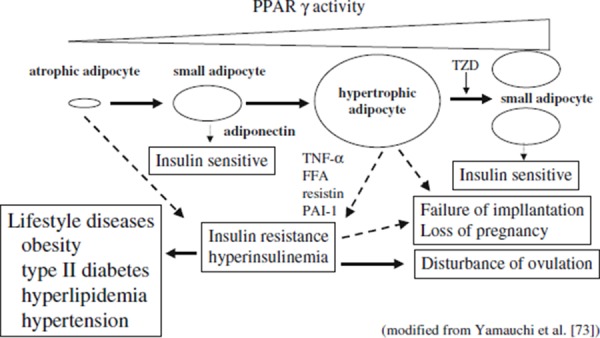
The relationship between differentiation of visceral adipocytes and reproductive processes. Small adipocyte secretes adiponectin, which facilitates insulin sensitivity. Hypertrophic adipocyte secretes FFA, TNF‐α, resistin and PAI‐1, which suppress insulin sensitivity and result in insulin resistance and hyperinsulinemia. This status may evoke disorders such as disturbance of ovulation, failure of implantation and loss of pregnancy. If insulin resistance and/or hyperinsulinemia become advanced, it may eventually cause lifestyle diseases such as obesity, type II diabetes, hyperlipidemia and hypertension. TZD differentiates adipocytes and improves insulin sensitivity. Likewise, metformin and/or improved lifestyle are also able to facilitate insulin sensitivity. Atrophic adipocyte has inadequate secretion of adiponectin and initiates insulin resistance and hyperinsulinemia. This figure was originally published in the Journal of Biological Chemistry and referred with permission from [73]. FFA free fatty acid, PAI‐1 plasminogen activator inhibitor 1, PPAR‐γ peroxisome proliferator‐activated receptor gamma, TNF‐α tumor necrosis factor alpha, TZD thiazolidinedione
Acknowledgments
We would like to express gratitude to Dr. Toshimasa Yamauchi for the support of the article. Also we thank Mr. Hayato Kaneshima, Ms. Eun‐Ju Choi, and Mr. Weston Aki Murai for proofreading of the manuscript.
References
- 1.The National Nutrition Survey in Japan (J‐NNS). Tokyo: DAI‐ICHI SHUPPAN; 2003.
- 2. Wilkin TJ, Voss LD. Metabolic syndrome: maladaptation to a modern world. J R Soc Med, 2004, 97, 511–520 10.1258/jrsm.97.11.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med, 1995, 122, 481–486 [DOI] [PubMed] [Google Scholar]
- 4. Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factor for clinical diabetes in men. Diabetes Care, 1994, 17, 961–969 10.2337/diacare.17.9.961 [DOI] [PubMed] [Google Scholar]
- 5. Enzi G, Busetto L, Carraro R et al. Ditshumheit H. et al. Association of multiple risk factors for cardiovascular disease and visceral obesity. A deadly quartet or sextet?. Obesity in Europe, 1994. London: Libbey; 411–418 [Google Scholar]
- 6. Sharpe RM, Franks S. Environment, lifestyle and infertility—an inter‐generational issue. Nat Cell Biol, 2002, 4 (Suppl) 33–40 10.1038/ncb‐nm‐fertilityS33 [DOI] [PubMed] [Google Scholar]
- 7. Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol, 1935, 29, 181–191 [Google Scholar]
- 8. Nestler JE, Stovall D, Akhter N, Juorno MJ, Jakubowics DJ. Strategies for the use of insulin‐sensitizing drugs to treat infertility in women with polycystic ovary syndrome. Fertil Steril, 2002, 77, 209–215 10.1016/S0015‐0282(01)02963‐6 [DOI] [PubMed] [Google Scholar]
- 9. Franks S, Gilling‐Smith C, Waston H, Willis D. Insulin action in the normal and polycystic ovary. Endocrinol Metab Clin N Am, 1999, 28, 361–378 10.1016/S0889‐8529(05)70074‐8 [DOI] [PubMed] [Google Scholar]
- 10. Wang JX, Daviies MJ, Norman RJ. Obesity increases the risk of spontaneous abortion during infertility treatment. Obes Res, 2002, 10, 551–554 10.1038/oby.2002.74 [DOI] [PubMed] [Google Scholar]
- 11. Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab, 2002, 13, 251–257 10.1016/S1043‐2760(02)00612‐4 [DOI] [PubMed] [Google Scholar]
- 12. Vrbikova J, Hill M, Starka L, Vondra K. Prediction of the effect of metformin treatment in patients with polycystic ovary syndrome. Gynecol Obstet Invest, 2002, 53, 100–104 10.1159/000053002 [DOI] [PubMed] [Google Scholar]
- 13. Baysal B, Batukan M, Batukan C. Biochemical and body weight changes with metformin in polycystic ovary syndrome. Clin Exp Obstet Gynecol, 2001, 28, 212–214 [PubMed] [Google Scholar]
- 14. Fleming R, Hopkinson ZE, Wallace AM, Greer IA, Sattar N. Ovarian function and metabolic factors in women with oligomenorrhea treated with metformin in randomized double blind placebo‐controlled trial. J Clin Endocrinol Metab, 2002, 87, 569–574 10.1210/jc.87.2.569 [DOI] [PubMed] [Google Scholar]
- 15. Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra‐abdominal fat accumulation to impairment of glucose and lipid metabolism. Metabolism, 1987, 36, 54–59 10.1016/0026‐0495(87)90063‐1 [DOI] [PubMed] [Google Scholar]
- 16.Matsuzawa Y, Fujioka S, Tokunaga K, Tarui S. A novel classification: visceral fat obesity and subcutaneous fat obesity. In: Berry EM, Blondheim SH, Shafrir EH, editors. Recent advances in obesity research V. London: John Libbey; 1987:92–6.
- 17. Kissenbah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev, 1994, 74, 761–811 [DOI] [PubMed] [Google Scholar]
- 18. Fujimoto WY, Abbate SL, Kahn SE, Hokanson FE, Burunzell JD. The visceral adiposity syndrome in Japanese‐American men. Obes Res, 1994, 2, 364–371 [DOI] [PubMed] [Google Scholar]
- 19. Depres JP, Nadeau A, Tremblay A. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes, 1989, 38, 304–309 10.2337/diabetes.38.3.304 [DOI] [PubMed] [Google Scholar]
- 20. Kissenbah AH, Peiris AN. Biology of regional body fat distribution: relationship to non‐insulin‐dependent diabetes mellitus. Diabetes Metab Res, 1989, 5, 83–109 10.1002/dmr.5610050202 [DOI] [PubMed] [Google Scholar]
- 21.Matsuzawa Y. Insulin resistance and atherosclerosis. In: Matsuzawa Y, Akanuma Y, editors. Diabetes mellitus, obesity and hyperlipidemia (proceedings of satellite symposium to 15th international diabetes federation congress). Tokyo: Axel Springer; 1995:1–6.
- 22. Dunaif A, Xia J, Book CB, Schenker E, Tang Z. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J Clin Invest, 1995, 96, 801–810 10.1172/JCI118126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non‐insulin‐dependent diabetes mellitus. J Clin Invest, 1995, 96, 520–527 10.1172/JCI118064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holte J, Bergh T, Berne C, Berglund L, Lithell H. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. J Clin Endocrinol Metab, 1994, 78, 1052–1058 10.1210/jc.78.5.1052 [DOI] [PubMed] [Google Scholar]
- 25.Holte J, Bergh T, Berne C, Lithell H. Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: relation to anthropometric, endocrine and metabolic variables. Clin Endocrinol (Oxf). 1994;41:463–71. [DOI] [PubMed]
- 26.Ciampelli M, Fulghesu AM, Cucunelli F, Pavone V, Caruso A, Mancuso S, Lanzone A. Heterogeneity in beta cell activity, hepatic insulin clearance and peripheral insulin sensitivity in women with polycystic ovary syndrome. Hum Reprod. 1997;12:1897–901. [DOI] [PubMed]
- 27. Matsuzawa Y. Pathophysiology and molecular mechanisms of visceral fat syndrome: the Japanese experience. Diabetes/Metab Rev, 1997, 13, 3–13 10.1002/(SICI)1099‐0895(199703)13:1<3::AID‐DMR178>3.0.CO;2‐N [DOI] [PubMed] [Google Scholar]
- 28. Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab, 1983, 57, 356–359 10.1210/jcem‐57‐2‐356 [DOI] [PubMed] [Google Scholar]
- 29. Dunaif A, Ginf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenemic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab, 1987, 65, 499–507 10.1210/jcem‐65‐3‐499 [DOI] [PubMed] [Google Scholar]
- 30. Dunaif A, Segal KR, Futterweil W, Dobrjansky A. Profound peripheral insulin resistance independent of obesity in polycystic syndrome. Diabetes, 1989, 38, 1165–1174 10.2337/diabetes.38.9.1165 [DOI] [PubMed] [Google Scholar]
- 31. Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholan T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes, 1992, 41, 1257–1266 10.2337/diabetes.41.10.1257 [DOI] [PubMed] [Google Scholar]
- 32. Rosenfield RL, Barnes RB, Cara JF, Licky AW. Dysregulation of cytochrome P450c17 alpha as the cause of polycystic ovarian syndrome. Fertil Steril, 1990, 53, 785–791 [PubMed] [Google Scholar]
- 33. Carmina E, Koyama T, Chang L, Stanczyk FZ, Lobo RA. Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome?. Am J Obstet Gynecol, 1992, 167, 1807–1812 [DOI] [PubMed] [Google Scholar]
- 34. Ehrmann DA, Rosenfield RL, Barnes RB, Brigell DF, Sheikh Z. Detection of functional ovarian hyperandrogenism in women with androgen excess. N Engl J Med, 1992, 327, 157–162 10.1056/NEJM199207163270304 [DOI] [PubMed] [Google Scholar]
- 35. Barbieri RL, Makris A, Randall RW. Insulin stimulates androgen accumulation on incubation of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab, 1986, 62, 904–910 10.1210/jcem‐62‐5‐904 [DOI] [PubMed] [Google Scholar]
- 36. Barbien RL, Smith S, Ryun KJ. The role of hyperinsulinemia in the pathogenesis of ovarian hyperandrogenism. Fertil Steril, 1988, 50, 197–212 [DOI] [PubMed] [Google Scholar]
- 37. Nahum R, Thong KJ, Hillier SG. Metabolic regulation of androgen production by human theca cells in vitro. Hum Reprod, 1995, 10, 75–81 10.1093/humrep/10.1.75 [DOI] [PubMed] [Google Scholar]
- 38. Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome relevance to mechanism anovulation. J Clin Endocrinol Metab, 1998, 83, 3984–3991 10.1210/jc.83.11.3984 [DOI] [PubMed] [Google Scholar]
- 39. Murray RD, Davison RM, Russell RC. Clinical presentation of PCOS following development of an insulinoma. Case report. Hum Reprod, 2000, 15, 86–88 10.1093/humrep/15.1.86 [DOI] [PubMed] [Google Scholar]
- 40. Suikkan AM, Koivisto VA, Rutanen EM. Insulin regulates the serum levels of low molecular weight insulin‐like growth factor‐binding protein. J Clin Endocrinol Metab, 1988, 66, 266–272 10.1210/jcem‐66‐2‐266 [DOI] [PubMed] [Google Scholar]
- 41. Suikkan AM, Koivisto VA, Korstinen R. Dose–response characteristics for suppression of low molecular weight plasma insulin‐like growth factor‐binding protein by insulin. J Clin Endocrinol Metab, 1989, 68, 135–140 10.1210/jcem‐68‐1‐135 [DOI] [PubMed] [Google Scholar]
- 42. Botwood N, Hamilton‐Fairly D, Kiddy D. Sex hormone‐binding globulin and female reproductive function. J Steroid Biochem Mol Biol, 1995, 53, 529–531 10.1016/0960‐0760(95)00108‐C [DOI] [PubMed] [Google Scholar]
- 43. Franks S, Robinson S, Willis D. Nutrition, insulin and polycystic ovary syndrome. Rev Reprod, 1996, 1, 47–53 10.1530/ror.0.0010047 [DOI] [PubMed] [Google Scholar]
- 44. Hillier SG. Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod, 1994, 9, 188–191 [DOI] [PubMed] [Google Scholar]
- 45. Clark AM, Ledger W, Galletly C, Tomlinson L, Blaney F, Wang X, Norman RJ. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod, 1995, 10, 2705–2712 [DOI] [PubMed] [Google Scholar]
- 46. Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta‐analysis. BMJ, 2003, 327, 951–953 10.1136/bmj.327.7421.951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Azziz R, Ehrmann D, Legro RS, Whitcomb RW, Hanley R, Fereshetian AG, O'keefe M, Ghazzi MN. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo‐controlled trial. J Clin Endocrinol Metab, 2001, 86, 1626–1632 10.1210/jc.86.4.1626 [DOI] [PubMed] [Google Scholar]
- 48. Mcyer F, Ipaktchi M, Clauser H. Specific inhibition of gluconeogenesis by biguanides. Nature, 1967, 213, 203–204 10.1038/213203a0 [DOI] [PubMed] [Google Scholar]
- 49. Wollen N, Bailey CJ. Inhibition of hepatic gluconeogenesis by metformin. Synergism with insulin. Biochem Pharmacol, 1988, 37, 4353–4358 10.1016/0006‐2952(88)90617‐X [DOI] [PubMed] [Google Scholar]
- 50. Metwally M, Amer S, Li TC, Ledger WL. An RCT of metformin versus orlistat for the management of obese anovulatory women. Hum Reprod, 2009, 24, 966–975 10.1093/humrep/den454 [DOI] [PubMed] [Google Scholar]
- 51. Attia GR, Rainey WE, Carr BR. Metformin directly inhibits androgen production in human thecal cells. Fertil Steril, 2001, 76, 517–524 10.1016/S0015‐0282(01)01975‐6 [DOI] [PubMed] [Google Scholar]
- 52. Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell, 1996, 87, 377–389 10.1016/S0092‐8674(00)81359‐8 [DOI] [PubMed] [Google Scholar]
- 53. Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev, 2005, 26, 439–451 10.1210/er.2005‐0005 [DOI] [PubMed] [Google Scholar]
- 54. Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond), 2008, 32 (Suppl 7) S13–S18 10.1038/ijo.2008.233 [DOI] [PubMed] [Google Scholar]
- 55. Ben‐Haroush A, Yogev Y, Fisch B. Insulin resistance and metformin in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol, 2004, 115, 125–133 10.1016/j.ejogrb.2003.11.027 [DOI] [PubMed] [Google Scholar]
- 56. Jakubowicz DJ, Seppala M, Jakubowicz S, Rodriguez‐Arms O, Rivas‐Santiago A, Koistinen H, Koistine R, Nestler JE. Insulin reduction with metformin increases luteal phase serum glycodelin and insulin‐like growth factor‐binding protein 1 concentrations and enhances uterine vascularity and blood flow in the polycystic ovary syndrome. J Clin Endocrinol Metab, 2001, 86, 1126–1133 10.1210/jc.86.3.1126 [DOI] [PubMed] [Google Scholar]
- 57. Seppala M, Riittinen L, Julkunen M, Koistinen R, Wahlstrom T, Iino K, Alfthan H, Stenman UH, Huhtala ML. Structural studies, localization in tissue and clinical aspect of human endometrial proteins. J Reprod Fertil Suppl, 1988, 36, 127–141 [PubMed] [Google Scholar]
- 58. Julkunen M, Koistinen R, Suikkari AM, Seppala M, Janne OA. Identification by hybridization histochemistry of human endometrial cells expressing mRNAs encoding a uterine β‐lactogloblin homologue and insulin‐like growth factor‐binding protein‐1. Mol Endocrinol, 1990, 4, 700–707 10.1210/mend‐4‐5‐700 [DOI] [PubMed] [Google Scholar]
- 59. Bolton AE, Pockley AG, Clough KJ, Mowles EA, Stoker RJ, Westwood OM, Chapman MG. Identification of placental protein 14 as an immunosuppressive factor in human reproduction. Lancet, 1987, 1, 593–595 10.1016/S0140‐6736(87)90235‐2 [DOI] [PubMed] [Google Scholar]
- 60. Julkunen M, Koiteinen R, Sjoberg J, Rutanen EM, Wahlstrom T, Seppala M. Secretary endometrium synthesizes placental protein 14. Endocrinology, 1986, 118, 1782–1786 10.1210/endo‐118‐5‐1782 [DOI] [PubMed] [Google Scholar]
- 61. Okamoto N, Uchida A, Takakura K, Karuya Y, Kanzaki H, Riittinen L, Koistinene R, Seppala M, Mori T. Suppression by human placental protein 14 of natural killer cell activity. Am J Reprod Immunol, 1991, 26, 137–142 [DOI] [PubMed] [Google Scholar]
- 62. Giudice LC, Mark SP, Irwin JC. Paracrine actions of insulin‐like growth factors and IGF binding protein‐1 in non‐pregnant human endometrium and at the decidual‐trophoblast interface. J Reprod Immunol, 1998, 39, 133–148 10.1016/S0165‐0378(98)00018‐7 [DOI] [PubMed] [Google Scholar]
- 63. Jones JI, Gockerman A, Busby WHJ, Wright G, Glemmons DR. Insulin‐like growth factor binding protein 1 stimulates cell migration and binds to the α5β1 integrin by means of its Arg‐Gly‐Asp sequence. Proc Natl Acad Sci USA, 1993, 90, 10553–10557 10.1073/pnas.90.22.10553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Regan L, Owen EJ, Jacobs HS. Hypersecretion of luteinising hormone, infertility, and miscarriage. Lancet, 1990, 336, 1141–1144 10.1016/0140‐6736(90)92765‐A [DOI] [PubMed] [Google Scholar]
- 65. Gray HR, Wu LY. Subfertility and risk of spontaneous abortion. Am J Public Health, 2000, 90, 1452–1454 10.2105/AJPH.90.9.1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liddell HS, Sowden K, Farquhar CM. Recurrent miscarriage: screening for polycystic ovaries and subsequent pregnancy outcome. Aust NZJ Obstet Gynaecol, 1997, 37, 402–406 10.1111/j.1479‐828X.1997.tb02447.x [DOI] [PubMed] [Google Scholar]
- 67. Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Roberts KA, Nestler JE. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. J Clin Endocrinol Metab, 2002, 87, 524–529 10.1210/jc.87.2.524 [DOI] [PubMed] [Google Scholar]
- 68. Glueck CJ, Wang P, Goldenberg N, Sieve‐Smith L. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum Reprod, 2002, 17, 2858–2864 10.1093/humrep/17.11.2858 [DOI] [PubMed] [Google Scholar]
- 69. Tanaka H, Shimabukuro T, Shimabukuro M. High prevalence of metabolic syndrome among men in Okinawa. J Atheroscler Thromb, 2005, 12, 284–288 [DOI] [PubMed] [Google Scholar]
- 70. Dunaif A. Drug insight: insulin‐sensitizing drugs in the treatment of polycycstic ovary syndrome—a reappraisal. Nat Clin Pract Endocrinol Metab, 2008, 4, 272–283 10.1038/ncpendmet0787 [DOI] [PubMed] [Google Scholar]
- 71. Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol, 2008, 199, 569–609 10.1016/j.ajog.2008.09.010 [DOI] [PubMed] [Google Scholar]
- 72. Norman RJ, Noaks M, Wu R, Davies MJ, Moran L, Wang JX. Improving reproductive performance in overweight/obese women with effective weight management. Hum Reprod, 2004, 10, 267–280 10.1093/humupd/dmh018 [DOI] [PubMed] [Google Scholar]
- 73.Yamauchi T, Kamon J, Waki H, Murakami K, Motojima K, Komeda K, Ide T, Kubota N, Terauchi Y, Tobe K, Miki H, Tsuchida A, Akanuma Y, Nagai R, Kimura S, Kadowaki T. The mechanisms by which both heterozygous peroxisome proliferator‐activated receptor γ (PPARγ) deficiency and PPARγ agonist improve insulin resistance. J Biol Chem. 2001;276(44):41245–54. [DOI] [PubMed]


