Abstract
Aim: The present study was undertaken to determine which fatty acids improve motility, viability, and increase acrosome reaction (AR) in boar spermatozoa.
Methods: Boar spermatozoa were washed, swum‐up and incubated at 37°C for 4 h in TALP medium supplemented with myristic, palmitic, stearic, lignoceric, oleic, linoleic, arachidonic, docosahexaenoic and palmitoleic acid. Sperm motility, viability and AR were evaluated during 4 h of incubation.
Results: Results show that oleic and linoleic acid significantly improved (P < 0.05) the motility and viability of boar spermatozoa. The AR was significantly improved (P < 0.05) by oleic and arachidonic acid in almost all incubation periods. When combinations of oleic, linoleic and arachidonic acid were studied for motility, viability and AR, it was found that oleic plus linoleic acid significantly increased (P < 0.05) motility, whereas arachidonic plus oleic acid significantly increased (P < 0.05) AR.
Conclusion: Unsaturated fatty acids, especially arachidonic acid, can improve boar sperm motility and AR. A combination of arachidonic and oleic acid is important for inducing boar sperm AR. (Reprod Med Biol 2007; 6: 235–239)
Keywords: acrosome reaction, boar spermatozoa, fatty acid, sperm motility, sperm viability
INTRODUCTION
MOST SPERM INCUBATION media contain bovine serum albumin (BSA) for supporting sperm viability and motility or capacitation in vitro. 1 These media contain as much as 15 µg of fatty acid per milligram of BSA. 2 Boar seminal plasma contains many more saturated and unsaturated fatty acids than the BSA fraction V (BSA‐V). 3 In our previous study, when fatty acids were added to polyvinyl alcohol (PVA), the motility and acrosome reaction (AR) was almost the same as that with BSA‐V. 4 It was found from this study that fatty acids play an important role in sperm movement and AR. Motility of rabbit 1 and hamster 5 spermatozoa was improved by fatty acids bound to BSA‐V; increasing of motility might have resulted from fatty acids bound to BSA. There is little information about the effect of fatty acids on boar sperm AR. In our previous study, fatty acids bound to BSA‐V enhanced boar sperm AR more than free fatty acids in the BSA in a time‐dependent manner. 4 With the addition of a fatty acids mixture to a fatty acid free BSA, the rate of AR again became similar with fatty acids bound to BSA‐V. This finding indicates the importance of fatty acids for boar sperm AR, but not all of the fatty acids in the fatty acid mixture may take part in the induction of AR. Exactly which fatty acids are responsible for boar sperm AR remains unclear. To date little attention has been given to the possible role of fatty acids on boar sperm AR.
Thus, the present study has been designed to investigate the effect of fatty acids on boar sperm AR, motility and viability.
MATERIALS AND METHODS
Chemicals
THE MYRISTIC, PALMITIC, stearic, lignoceric, oleic, linoleic, arachidonic, docosahexaenoic and palmitoleic acids, all approximately 99% pure, were obtained from Sigma Chemical Company (St Louis, MO, USA). All other chemicals were analytical grade and purchased from Nacalai Tesque (Kyoto, Japan).
Semen collection and preparation
Semen from Duroc boars aged between 2 and 3 years was collected using the gloved‐hand technique at Nagano Animal Industry Experiment Station, Shiojiri, Nagano, Japan. The semen was diluted according to the method of Johnson et al. 6 with a Modena extender giving a sperm concentration of 1 × 108/mL at room temperature. The vial of semen was carried to the laboratory within 30 min using a cork box to keep it at 21°C temperature. A detailed description of the procedure of semen preparation was provided in our previous report. 4 To evaluate motility and acrosome status, swim‐up spermatozoa were resuspended separately at a concentration of 5 × 106 and 20 × 106/mL, respectively, and incubated at 37°C temperature, 95% humidified air and 5% CO2 at pH 7.4 for 0–4 h.
Semen treatment
To evaluate the effect of each fatty acid on motility, viability and acrosome reaction, 1.25 mmol/L of palmitic, myristic, stearic, lignoceric, oleic, linoleic, arachidonic, docosahexaenoic or palmitoleic acid was added to a basic TALP 7 medium containing fatty acid free BSA according to our previous report. 8
Progressive motility
After completion of the specified incubation periods, sperm samples were subjected to motility and movement quality evaluations. The evaluation method and categories were the same as those previously described. 4 The percentage of spermatozoa with progressive motility was determined subjectively by scoring 400 individual spermatozoa using a phase‐contrast microscope.
Acrosome reaction and viability
The evaluation of AR and viability was carried out after a specific incubation period of spermatozoa by applying the triple‐staining technique. 9 The live, dead, acrosome reacted and unreacted spermatozoa were evaluated based on the staining pattern. Viability and AR of the spermatozoa were evaluated from randomly selected fields of the triple‐stained slides until 400 spermatozoa had been examined. Full details of the method and photography of triple‐stained slides were shown in our previous report. 4
Statistical analysis
The data were subjected to a Fisher's protected least significant difference test. The Number Crunchier Statistical System (NCSS statistical software; Kaysville, UT, USA) version 5.01 computer software package was used for all statistical analyses. Each experiment was repeated four times from two boars and differences were considered significant at P < 0.05.
RESULTS
THE EFFECT OF each fatty acid on progressive motility, viability and AR in boar spermatozoa during 0–4 h of incubation is shown in Figure 1. The percentage of progressive motility was significantly increased (P < 0.05) by oleic and linoleic acids from 1 to 4 h of incubation periods; oleic acid was higher than linoleic acid (Fig. 1a). Unsaturated fatty acids, namely arachidonic, docosahexaenoic or palmitoleic, and saturated fatty acids, palmitic, myristic, stearic or lignoceric, also slightly increased motility. Viability was significantly improved (P < 0.05) by oleic and linoleic acids only at 1 and 2 h of incubation (Fig. 1b). The induction of AR was significantly increased (P < 0.05) by oleic and arachidonic acids from 1 to 4 h of incubation; arachidonic was a higher inducer than oleic acid (Fig. 1c). Linoleic, docosahexaenoic and palmitoleic acids were also effective inducers of boar sperm AR. All the saturated fatty acids, namely myristic, palmitic, stearic and lignoceric, failed to induce AR, but myristic acid had an adverse effect. The oleic, linoleic and arachidonic acids showed good results individually; for this reason these three fatty acid combinations were used to study sperm functions in the following experiment. The effects of a combination of fatty acids, such as oleic acid (OA) + linoleic acid (LA), OA + arachidonic acid (AA), AA + LA, and OA + LA + AA, on progressive motility, viability and AR of boar spermatozoa are shown in Figure 2. Compared to the control and the combination of fatty acids, the percentage of progressive motility was significantly higher (P < 0.05) with OA + LA only at 2 h of incubation. Viability was not increased significantly by any of the treatments, although OA + LA showed a slightly higher value. The AR was significantly increased (P < 0.05) by OA + AA over 1–4 h incubation periods.
Figure 1.
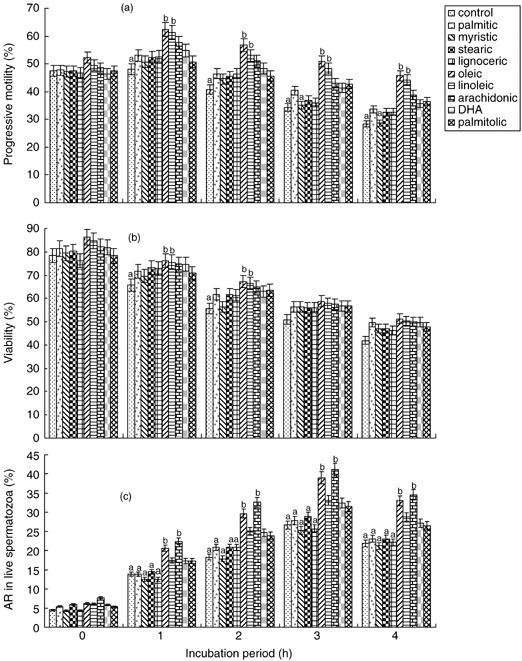
Mean effect of fatty acids on (a) the percentage of progressive motility, (b) viability and (c) acrosome‐reacted (AR) live spermatozoa from 0 to 4 h of incubation. Error bars represent standard error (n = 4). Data points without a common lowercase letter indicate differences (P < 0.05) among the treatments.
Figure 2.
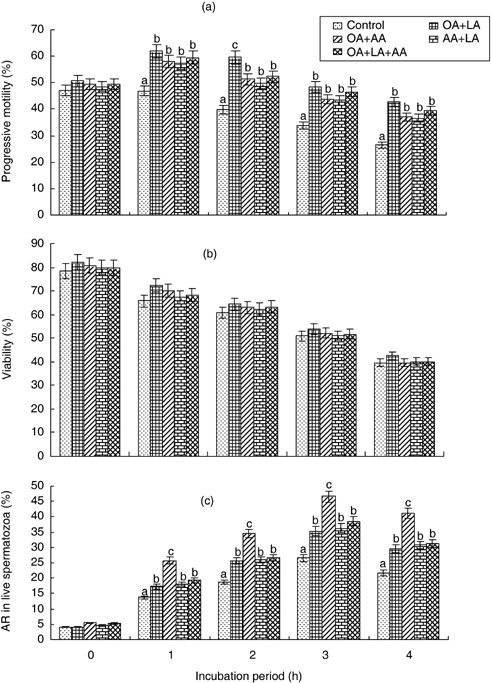
Mean effect of combinations of oleic acid (OA), linoleic acid (LA) and arachidonic acid (AA) on (a) the percentage of progressive motility, (b) viability and (c) acrosome‐reacted (AR) live spermatozoa from 0 to 4 h of incubation. Error bars represent standard error (n = 4). Data points without a common lowercase letter indicate differences (P < 0.05) among the treatments.
DISCUSSION
IN THIS STUDY, fatty acids played an important role in enhancing sperm movements and AR. Compared with the control, motility was increased by almost every fatty acid, and performance of the unsaturated fatty acids was higher than the saturated fatty acids. Fleming and Yanagimachi 10 observed that some unsaturated fatty acids were able to improve sperm motility in guinea pig spermatozoa. The present result is in agreement with this result. To some extent, unsaturated oleic and linoleic acids improved boar sperm viability more than the saturated fatty acids. In the present study, the performances of oleic, linoleic and arachidonic acids were higher for motility, viability and AR. In mammalian cells, oleic, linoleic and arachidonic acids are considered to have metabolic significance and most of the long‐chain, polyunsaturated fatty acids are derived from these fatty acids by chain elongation. 11 In the present study, nine fatty acids were used that are present in great amounts in BSA. These fatty acids resemble well the fatty acids of boar seminal plasma. 3 Peter 12 and Meizel 13 suggested that BSA releases a variety of small molecules, such as fatty acids and amines, which stimulate AR.
We postulate that fatty acids induce AR, an exocitotic event consisting of the fusion of the outer acrosomal membrane with the overlying plasma membrane, their fenestration, and the concomitant exposure, release or both of the acrosomal content. In general cell, when saturated fatty acids pass through the membrane they pack very tightly because saturated fatty acid chains have no gaps. In contrast, because of gaps in the chain, unsaturated fatty acids prevent the tight packing of fatty acids in the membrane and make it loose. The series of 18 and 20 carbon cis‐unsaturated fatty acids, oleic, linoleic, linolenic and arachidonic acids, are effective inducers of exocitosis in mammalian cells. 14 In this study, the highest induction of AR was carried out by arachidonic acid followed by oleic, linoleic, docosahexaenoic and palmitolic acids; whereas all of the saturated fatty acids, palmitic, stearic and lignoceric acids, failed to induce boar sperm AR. Myristic acid showed little adverse effect on AR. This result is in agreement with the result of Meizel and Turner, 15 who showed that arachidonic and oleic acids induced AR in hamster spermatozoa in vitro, whereas lauric, myristic and stearic acids were not effective. The cis‐unsaturated fatty acids enter a different lipid domain in sperm membranes than the trans‐unsaturated or saturated fatty acids. 16 In this study, compared to the control, the AR of live spermatozoa was increased twofold by arachidonic and oleic acids at 3 h of incubation. Dominguez et al. 17 also reported that arachidonic acid is the best inducer of AR in human spermatozoa. Together with oleic and arachidonic acids, linoleic acid is also an effective inducer of AR in guinea pig spermatozoa. 10 Thus, the present result is in agreement with this result. In this study, four combinations of oleic, linoleic and arachidonic acids were used to study the induction of AR, and a combination of arachidonic and oleic acids showed significantly higher induction than the other combinations. This might result from the combined effect of the two highest inducing fatty acids. However, many components, including unsaturated fatty acids of intracellular signaling systems, have been implicated in the induction of the AR. Breitbart and Spungin 18 found that arachidonic acid is converted to other metabolites to increase Ca2+ influx, which in turn induces AR. We believe that the unsaturated fatty acids supplemented in the medium produce ATP and may increase intracellular Ca2+ and cAMP concentrations, which in turn could be responsible for inducing AR in boar spermatozoa. Thus, the intracellular concentration of cAMP, Ca+ influx and protein tyrosine phosphorylation by unsaturated fatty acids in boar spermatozoa needs to be studied further for a complete understanding of this mechanism.
In conclusion, unsaturated fatty acids, especially arachidonic acid, can improve boar sperm motility and AR. A combination of arachidonic and oleic acids is important for inducing boar sperm AR.
REFERENCES
- 1. Harrison RAP, Dott HM, Foster GC. Bovine serum albumin, sperm motility and the dilution effect. J Exp Zool 1982; 222: 81–88. [DOI] [PubMed] [Google Scholar]
- 2. Chen FR. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem 1967; 242: 173–181. [PubMed] [Google Scholar]
- 3. Ahluwalia B, Holman RT. Fatty acid composition of lipids of bull, boar, rabbit and human semen. J Reprod Fert 1969; 18: 431–437. [DOI] [PubMed] [Google Scholar]
- 4. Hossain MS, Hyeong LJ, Miah AG, Tsujii H. Effect of fatty acids bound to bovine serum albumin‐V on acrosome reaction and utilization of glucose in boar spermatozoa. Reprod Med Biol 2007; 6: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uto N, Yamahama Y. The motility and fertility of golden hamster sperm cultured in BSA‐free medium. Biol Cell 1996; 88: 23–28. [DOI] [PubMed] [Google Scholar]
- 6. Johnson LA, Aalbers JG, Grooten HJG. Artificial insemination of swine: fecundity of boar semen stored in Beltsville TS (BTS), modified Modena (MM), or MR‐A and inseminated on one, three and four days after collection. Zuchthyg 1988; 23: 49–55. [Google Scholar]
- 7. Parrish JJ, Susko‐Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod 1988; 38: 1171–1180. [DOI] [PubMed] [Google Scholar]
- 8. Khandoker MAMY, Tsujii H. Effect of exogenous fatty acids on the in vitro development of rat embryos. Asian–Aus J Anim Sci 1999; 12: 169–173. [Google Scholar]
- 9. Ooba T, Sricharoen P, Areekijsree M, Kitiyanat Y, Pavasuthipaisit K. Evaluation of acrosome reaction in bovine sperm by a triple staining technique. J Physiol Sci 1990; 3: 91–104. [Google Scholar]
- 10. Fleming AD, Yanagimachi R. Evidence suggesting the importance of fatty acids and the fatty acid moieties of sperm membrane phospholipids in the acrosome reaction of guinea pig spermatozoa. J Exp Zool 1984; 229: 485–489. [DOI] [PubMed] [Google Scholar]
- 11. Mayes PA. Metabolism of unsaturated fatty acids and eicosanoids In: Barnes DA, ed. Harper's Biochemistry. New York: McGraw‐Hill, 2000; 250–258. [Google Scholar]
- 12. Peter TT. Serum albumin In: Putnam FW, ed. The Plasma Proteins: Structure, Function, and Genetic Control. New York: Academic Press, 1975: 1133–1181. [Google Scholar]
- 13. Meizel S. The mammalian sperm acrosome reaction, a biochemical approach In: Johnson MH, ed. Development in Mammals. Amsterdam: North‐Holland Publishing, 1979; 1–64. [Google Scholar]
- 14. Creutz CE. Cis‐unsaturated fatty acids induce the fusion of chromaffin granules aggregated by synexin. J Cell Biol 1981; 91: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meizel S, Turner KO. Stimulation of an exocytotic event, the hamster sperm acrosome reaction, by cis‐unsaturated fatty acids. FEBS Lett 1983; 161: 315–318. [DOI] [PubMed] [Google Scholar]
- 16. Klausner RD, Kleinfied AM, Hoover RL, Karnovsky MJ. Lipid domains in membranes: evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem 1980; 255: 1286–1295. [PubMed] [Google Scholar]
- 17. Dominguez L, Yunes RMF, Fornes MW, Burgos M, Mayorga LS. Calcium and phospholipase A2 are both required for the acrosome reaction mediated by G‐proteins stimulation in human spermatozoa. Mol Reprod Dev 1999; 52: 297–302. [DOI] [PubMed] [Google Scholar]
- 18. Breitbart H, Spungin B. The biochemistry of the acrosome reaction. Mol Hum Reprod 1997; 3: 195–202. [DOI] [PubMed] [Google Scholar]


