Abstract
Aim: The risk of chromosome aberrations in human spermatozoa with an abnormal head shape was examined using intracytoplasmic sperm injection into mouse oocytes.
Methods: Human spermatozoa with small, large, pointed or elongated head shape from a fertile donor were injected into mouse oocytes, and the hybrid oocytes were cytogenetically analyzed at the first cleavage metaphase.
Results: The oocyte activation rate was significantly lower in hybrid oocytes injected with pointed (90.2%) or elongated spermatozoa (94.1%) than with normal spermatozoa (99.0%). However, the frequency of intracytoplasmic sperm injection oocytes at the first cleavage metaphase did not differ among the sperm groups (71.8–77.2%). No difference in the incidences of aneuploidy (1.8, 2.6 and 1.4%), diploidy (0, 0 and 0%) and structural chromosome aberrations (6.3, 10.4 and 8.6%) was observed between small or pointed spermatozoa and normal spermatozoa. Only a small population of large spermatozoa (3.7%) was diploidy. Elongated spermatozoa showed significantly frequent structural chromosome aberrations (33.3%) as compared with normal spermatozoa.
Conclusion: The results reveal some interesting details of the mechanism of sperm nuclear condensation: small sperm size is attributed to the problem of nuclear condensation, not a decrease in chromosomes. Diploidy prevents sperm nucleus from condensing, resulting in a large sperm head. Elongation of sperm nucleus causes DNA lesions leading to structural chromosome aberrations. (Reprod Med Biol 2004; 3: 147–152)
Keywords: amorphous spermatozoa, chromosome aberrations, intracytoplasmic sperm injection, mouse oocytes
INTRODUCTION
FOLLOWING THE DEVELOPMENT of intracytoplasmic sperm injection (ICSI) technique, it became possible to obtain fertilized oocytes using spermatozoa that never penetrate into oocytes by in vitro fertilization. The ICSI technique has, accordingly, become applicable to the treatment of infertile patients showing severe oligozoospermia or teratozoospermia. However, sufficient information to understand the relationship between abnormal sperm heads and chromosomal abnormalities has not yet been obtained, because only a few cytogenetic studies have attempted this subject and have used small sperm numbers. 1 , 2 , 3 However, it was reported that in mice the incidence of structural chromosome aberrations was significantly higher in the spermatozoa with amorphous heads than the spermatozoa with normal heads, predicting an increasing risk of production of the chromosomally abnormal zygotes in ICSI of human amorphous spermatozoa. 4 In the present study, amorphous spermatozoa were classified into four types of head morphology (small, large, pointed and elongated) and were cytogenetically analyzed after injection into mouse oocytes to examine the relationship between sperm head morphology and chromosome aberrations.
MATERIALS AND METHODS
Collection of spermatozoa
HUMAN SEMEN SAMPLES used in the present study, showing normozoospermia according to the World Health Organization criteria 5 were obtained from a normal donor with proven fertility. The sample was liquefied for 30 min at 37°C in air, and was washed twice by centrifugation (700 g for 5 min) along with 6 mL HEPES‐Biggers Whitten Whittinham's medium. 6 , 7 Sperm pellets were suspended in 5% polyvinyl pyrrolidone (Sigma, Carlsbad, CA, USA) dissolved in Dulbecco's phosphate buffered saline and was then used for micromanipulation. 6 , 7
Oocyte collection
B6D2F1 female mice, 6–11 weeks old, were induced to superovulate by intraperitoneal injection of 7 IU pregnant mare's serum gonadotrophin (Teikoku‐zoki, Tokyo, Japan) followed by intraperitoneal injection of 7 IU of human chorionic gonadotropin (hCG) (Mochida Pharmac, Kyoto, Japan) 48 h later. Oocytes surrounded with cumulous cells were collected from oviducts 16 h after the hCG injection. The oocytes were freed from cumulous cells by 5 min treatment with 0.1% hyalronidase dissolved in HEPES‐Chatot, Ziomek and Bavister's (CZB) medium. 8 The oocytes were stored in CZB medium 9 , 10 at 37°C under 5% CO2 in air for up to 2 h prior to sperm injection.
Injection of abnormal spermatozoa into mouse oocytes
The target of the present study was spermatozoa with small, large and elongated heads determined by Menchveld et al. 11 and spermatozoa with pointed heads, which were classified by my own definition (Table 1, Fig. 1). The four types of head abnormalities were comparatively frequent (0.2–1.0%) in the sperm samples used, enabling to collect extensive data. Furthermore, as those types of abnormalities were easily identified under a microscope at a magnification of 200 (10 × 20), other researchers will be able to use data followed for treatment of infertility immediately. Injection of those spermatozoa into mouse oocytes was performed using a piezo‐driven micromanipulator (Prima‐ham, Tokyo, Japan) as described previously. 6 For injection of small spermatozoa (<3 µm in both length and width, Fig. 1b) and elongated spermatozoa (>6 µm in length and <3 µm in width, Fig. 1d), the diameter of injection needles was adjusted to 3 µm to prevent aspirating normal or large spermatozoa. For injection of large head spermatozoa (>5 µm in length and >3 µm in width, Fig. 1c), injection needles with 5 µm diameter were used. For normal head spermatozoa (3–5 µm in length and approximately 3 µm in width) or pointed head spermatozoa, the characteristics of which were within normal ranges except for having pointed acrosomes (Fig. 1d), injection needles with 3–4 µm diameter were used.
Table 1.
Activation and development of mouse oocytes after injection of human spermatozoa with abnormal heads
| Sperm head | Number of oocytes injected | Number of surviving oocytes (%) | Number of oocytes successfully prepared | Number of oocytes activated (%) | Number of activated oocytes with sperm transformed into (%)† | ||
|---|---|---|---|---|---|---|---|
| SH or PCC | PN | Mitotic chromosomes | |||||
| Normal | 1207 | 929 (77.0) | 900 | 891 (99.0) | 179 (20.1) | 59 (6.6) | 653 (73.3) |
| Small | 227 | 173 (76.2) | 166 | 159 (95.8) | 29 (18.2) | 10 (6.3) | 120 (75.5) |
| Large | 289 | 162 (56.1)* | 150 | 145 (96.7) | 24 (16.6) | 9 (6.2) | 112 (77.2) |
| Pointed | 160 | 128 (80.0) | 122 | 110 (90.2)* | 27 (24.5) | 4 (3.6) | 79 (71.8) |
| Elongated | 53 | 38 (71.6) | 34 | 32 (94.1)* | 6 (18.8) | 2 (6.3) | 24 (75.0) |
SH, swollen sperm head; PCC, premature chromosome condensation; PN, pronucleus.†Per number of oocytes activated × 100; *χ2 test, P < 0.05.
Figure 1.
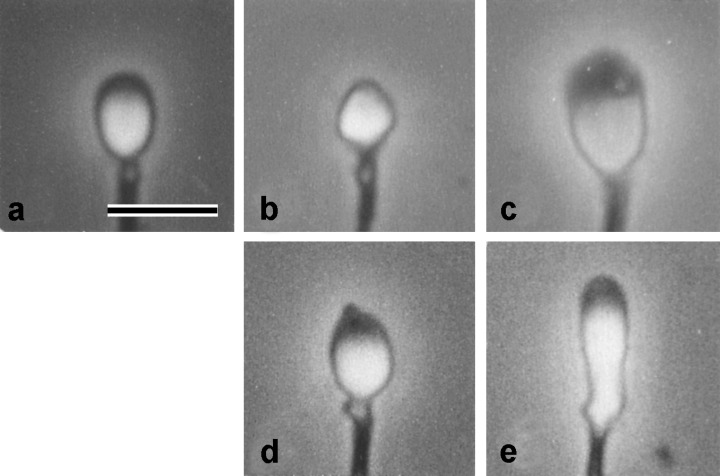
Photographs of spermatozoa with (a), normal; (b), small; (c), large; (d), pointed; (e), elongated heads. Bar = 5 µm.
Preparation of chromosome slides
The hybrid oocytes between human spermatozoa and mouse oocytes were incubated in CZB medium for 6 h and were then transferred into CZB medium containing 0.006 µg/mL vinblastin to block karyogamy and mitotic spindle formation. When the hybrid oocytes reached the first cleavage metaphase, 16–24 h after the microinjection, they were prepared for chromosome slides. After zona pellucida were removed by 5 min treatment with 0.5% actinase E (Kaken Pharmac, Tokyo, Japan), the hybrid oocytes were stored in hypotonic solution (0.5% sodium citrate containing 15% bovine serum albumin) for 10 min at room temperature. The oocytes were attached to glass slides and fixed using the gradual fixation‐air drying method. 12 Analysis of the chromosome slides was carried out twice after successive staining with 2% Giemsa and C‐banding 13 (Fig. 2).
Figure 2.
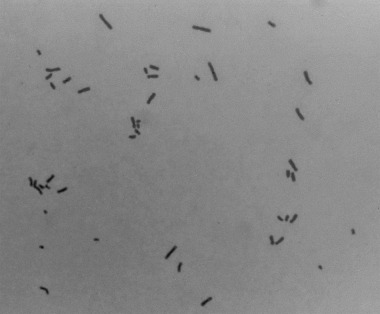
A metaphase plate of a diploid large head spermatozoon (2n = 46).
Statistical evaluation
The χ2 test was used and differences were considered significant at P < 0.05 level.
RESULTS
THE SUCCESS RATE of ICSI is shown in Table 1. The survival rates of the hybrid oocytes in small, pointed and elongated sperm groups did not significantly differ from the rate in the normal sperm group. In contrast, the large sperm group showed a significant decrease in the survival rate (56.1%) as compared with the normal sperm group (77.0%). The result must be attributed to the fact that the diameter of injection needles used for the large spermatozoa was longer (5 µm) than for other types of spermatozoa, because puncture of oocytes always happened within 30 min after large sperm‐injection. The rate of oocyte activation, which was assessed by the completion of the second meiosis resulting in the presence of mouse pronucleus or mitotic chromosomes, did not significantly differ among small (95.8%), large (96.7%) and normal sperm groups (99.0%). This result shows that the difference in diameter of injection needles did not affect oocyte activation rates. In the oocytes that were not activated, meiotic mouse chromosomes were observed along with swollen human sperm head or prematurely condensed human chromosomes. The rate of the hybrid oocytes at the first cleavage metaphase also did not differ among the three categories. In contrast, the oocyte activation rates were significantly lower in the pointed (90.2%) or elongated sperm groups (94.1%) than the normal sperm group (99.0%), although the hybrid oocytes activated frequently developed into the first cleavage metaphase.
The results of chromosome analysis in spermatozoa with normal or abnormal heads are shown in Table 2. Karyotypes of spermatozoa with structural chromosome aberrations found in each experimental group are summarized in Table 3. The incidences of aneuploidy, diploidy and structural chromosome aberrations in the small sperm group did not differ from those in the normal sperm group. In the large sperm group, the incidence of diploidy was significantly higher than that in the normal sperm group (3.7 vs 0.0%, Fig. 2), although the incidence of structural chromosome aberrations did not differ between these categories. In addition, no significant increase of aneuploidy was found in the large sperm group as compared with the normal sperm group. However, a hyperploid cell containing 31 chromosomes (Fig. 3) was observed in the large sperm group. No such type of hyperploidy was found in other sperm groups. In the pointed sperm group, no increase in numerical or structural chromosome aberrations was found. In the elongated sperm group, the incidence of structural chromosome aberrations (33.3%) was significantly higher than that (8.6%) in the normal sperm group, although the incidences of numerical aberrations did not differ between these categories.
Table 2.
Chromosome aberrations in human spermatozoa with morphologically abnormal head
| Sperm | Number of sperm analyzed | Number of aberrant spermatozoa (%) | Number of structural chromosome aberrations (per spermatozoon) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aneuploidy | Diploidy | Structural aberrations | ||||||||
| Hyper | Hypo | CHRB | CHRE | CHTB | CHTE | Total† | ||||
| Normal | 618 | 5 (0.8) | 4 (0.6) | 0 (0.0) | 53 (8.6) | 38 (0.061) | 4 (0.006) | 12 (0.019) | 9 (0.015) | 63 (0.102) |
| Small | 112 | 2 (1.8) | 0 (0.0) | 0 (0.0) | 7 (6.3) | 5 (0.045) | 0 (0.000) | 2 (0.018) | 0 (0.000) | 7 (0.063) |
| Large | 108 | 2 (1.9) | 1 (0.9) | 4* (3.7) | 9 (8.3) | 7 (0.065) | 1 (0.009) | 2 (0.019) | 0 (0.000) | 10 (0.093) |
| Pointed | 77 | 2 (2.6) | 0 (0.0) | 0 (0.0) | 8 (10.4) | 7 (0.091) | 0 (0.000) | 1 (0.013) | 0 (0.000) | 8 (0.104) |
| Elongated | 24 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8** (33.3) | 11 (0.458) | 0 (0.000) | 1 (0.042) | 0 (0.000) | 12 (0.500) |
CHRB, chromosome breakage; CHRE, chromosome exchange; CHTB, chromatid breakage; CHTE, chromatid exchange. *χ2‐test, P < 0.05; **χ2‐test, P < 0.01. †The higher total number of structural chromosome aberrations compared with the no. of the aberrant spermatozoa shows that some cells had multiple aberrations.
Table 3.
Karyotypes of amorphous human spermatozoa with structural chromosome aberrations
| Sperm | Karyotype × number of sperm |
|---|---|
| Small | 23, 1 CHRB × 5; 23, 1 CHTF × 2 |
| Large | 23, 1 CHRB × 5; 23, 2 CHRF × 1; 23, 1 CHTF × 1; 23, 1 DIC + 1 CHRF × 1 |
| Pointed | 23, 1 CHRB × 5; 23, 1 CHTF × 3 |
| Elongated | 23, 1 CHRB × 5; 23, 3 CHRF × 2; 23, 1 CHTF × 1 |
CHRB, chromosome breakage; CHRF, chromosome fragment; CHTF, chromatid fragment; DIC, dicentric chromosome.
Figure 3.
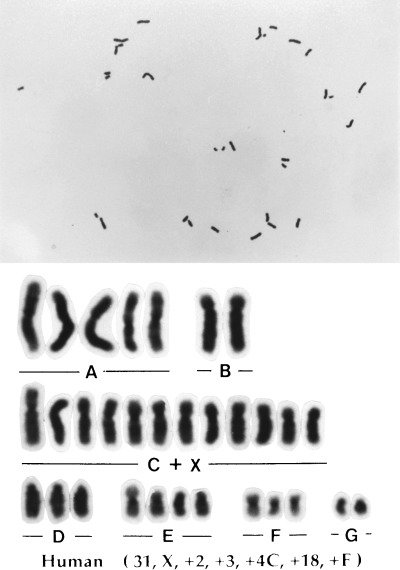
An aneuploid karyotype derived from a spermatozoon with a large head (n = 31).
DISCUSSION
IN THE SMALL spermatozoa, the incidences of chromosomally aberrant spermatozoa did not differ between small and normal sperm head groups, suggesting that small sperm heads were not involved in chromosome aberrations. Rybouchkin et al. has also reported no increase in chromosome aberrations in small round spermatozoa obtained from infertile patients. 3 It is therefore considered that small head spermatozoa do not result from a decreasing number of chromosomes but abnormal condensation of sperm nuclei during sperm maturation.
Most of the large head spermatozoa cytogenetically analyzed did not have chromosome aberrations in the present study, although there were some diploidy cells (Fig. 2) and a hyperploid cell with 31 chromosomes (Fig. 3). It is therefore considered that a main cause of large sperm heads is abnormal condensation of sperm nuclei. Haploid nuclei were actually predominant among large head spermatozoa in some cases of male infertility showing severe globozoosperma. 14 However, diploid spermatozoa were contained in only the large head sperm group in the current study (Table 2) and consequently, all diploid spermatozoa observed showed large headed morphology. Therefore, the diploid nucleus seems to be abnormally condensed, resulting in the large head. Correspondingly, Int’Veld et al. reported a case of male infertility in which almost all large spermatozoa were found to be diploidy or triploidy using sperm nuclear fluorescent in situ hybridization (FISH). 15 However, Martin et al. reported cases of infertile patients showing globozoospermia in which no significant increase of diploidy was found, although the incidences of large head spermatozoa in their ejaculates were not described in the report. 16 The variety of cytogenetic results in large head spermatozoa among the present and previous studies suggests that preliminary survey using sperm chromosome preparation or sperm head FISH are necessary when patients showing frequent large headed morphology in spermatozoa are treated using ICSI or in vitro fertilization.
No significant difference in chromosome aberrations was found between pointed and normal spermatozoa. The result seems to be explained by the observation that no morphological abnormality was found in pointed spermatozoa except for an acrosome region under a microscope. However, an unexpected decrease of oocyte activation rate was found in the pointed spermatozoa. This phenomenon was inconsistent with the localization of glucosamine‐6‐phosphate deaminase/oscillin, which is one of oocyte activation factors found in the equatorial and neck regions of human spermatozoa. Furthermore, it has been reported that this factor did not show the character expected as oocyte activating factors in mouse oocytes. 17 Therefore, the decreased rate of mouse oocyte activation in the current study may not exactly reflect a decrease in the capacity to activate human oocytes in human spermatozoa.
The incidence of structural chromosome aberrations was significantly higher in elongated spermatozoa than normal spermatozoa. The result suggests that the structural chromosome aberrations observed were a result of elongation of sperm nucleus accompanied by DNA lesions. Lee et al. has already found frequent structural chromosome aberrations in 24 abnormal spermatozoa which had amorphous, round or elongated heads. 1 However, Rybouchkin et al. reported no increased incidence of structural chromosome aberrations in round head spermatozoa from an infertile patient. 3 Therefore, almost all structural aberrations observed by Lee et al. 1 may be derived from elongated spermatozoa. It is interesting that DNA lesions were caused in elongated spermatozoa, the head of which extends to the head‐tail axis, although there was no increase of DNA lesions in the large head spermatozoa, the head of which extends to both the head‐tail axis and the perpendicular axis. The contrastive results may become a clue on understanding a very dense structure formed with sperm DNA and sperm specific nuclear protein in sperm nucleus, which is still in the stage of hypothesis.
Many investigators have attempted a cytogenetic analysis of human sperm nucleus using FISH to determine the relationship between sperm chromosome abnormality and infertility. 15 , 18 , 19 However, evaluation of the integrity of individual sperm nucleus recently became possible by comparing the images of sperm heads scanned before FISH with cytogenetic results from FISH. 20 Moreover, no efficient sperm FISH assay that detects structural chromosome aberrations in human spermatozoa has yet to be developed. These are reasons why relatively little information about the relationship between chromosome aberrations and sperm head morphology has been presented. The sperm chromosome assay with ICSI used in the current study is the only method allowing us to analyze structural chromosome aberrations in human spermatozoa and accordingly gives greater insight into the relationship between chromosome aberrations and sperm head morphology. Consequently, the present study is the first to demonstrate the risk of frequent structural chromosome aberrations in elongated head spermatozoa. This finding was obtained from a fertile donor and therefore it is necessary to confirm whether the result applies to infertile patients. However, an increase of structural chromosome aberrations in elongated head spermatozoa is highly probable in infertile patients, as the cytogenetic results in the small and the large head spermatozoa were very similar between the present and previous studies as described above. In practice, the types of abnormal spermatozoa examined in the current study are easily identified under a microscope and morphologically normal spermatozoa are applicable to the treatment for infertile patients. Therefore, the risk of fertilization of chromosomally abnormal spermatozoa does not increase. However, the present results seem to be beneficial on avoidance of fertilization by abnormal spermatozoa with a higher risk of chromosome aberrations when normal spermatozoa are not found in ejaculates of infertile patients.
ACKNOWLEDGMENTS
I WOULD LIKE to express my sincere appreciation to Professor Y. Kamiguchi (Department of Biological Sciences, Asahikawa Medical College) for guiding me during my work. Thanks are also due to Professor T. Kachi for giving me a chance to prepare this manuscript. I am grateful to Mr S.N. Bayley for his assistance in preparing this manuscript. This study was supported by Grant‐in‐Aid for Scientific Research (B), no. 10470339 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1. Lee JD, Kamiguchi Y, Yanagimachi R. Analysis of chromosome constitution of human spermatozoa with normal and aberrant head morphologies after injection into mouse oocytes. Hum Genet 1996; 11: 1942–6194. [DOI] [PubMed] [Google Scholar]
- 2. Rybouchkin AV, De Sutter P, Dhont M. Unprotected freezing of human sperm exerts a detrimental effect on their oocyte activating capacity and chromosome integrity. Zygote 1996; 4: 263–268. [DOI] [PubMed] [Google Scholar]
- 3. Rybouchkin A, Benijts J, De Sutter P, Dhont M. Disintegration of chromosomes in dead sperm cells as revealed by injection into mouse oocytes. Hum Reprod 1997; 12: 1693–1698. [DOI] [PubMed] [Google Scholar]
- 4. Kishikawa H, Tateno H, Yanagimachi R. Chromosome analysis of BALB/c mouse spermatozoa with normal and abnormal head morphology. Biol Reprod 1999; 61: 809–812. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm‐cervical Mucus Interaction. New York: Cambridge University Press, 1999. [Google Scholar]
- 6. Watanabe S. A detailed cytogenetic analysis of large numbers of fresh and frozen‐thawed human sperm after ICSI into mouse oocytes. Hum Reprod 2003; 18: 1150–1157. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe S. Frequent structural chromosome aberrations caused in immotile human sperm exposed to culture media. Hum Reprod 2004; 19: 940–947. [DOI] [PubMed] [Google Scholar]
- 8. Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod 1995; 52: 709–720. [DOI] [PubMed] [Google Scholar]
- 9. Chatot CL, Ziomek A, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random‐bred 1‐cell mouse embryos in vitro . J Reprod Fertil 1989; 86: 679–688. [DOI] [PubMed] [Google Scholar]
- 10. Chatot CL, Lewis L, Torres I, Ziomek CA. Development of 1‐cell mouse embryos from different strains of mice in CZB medium. Biol Reprod 1990; 42: 432–440. [DOI] [PubMed] [Google Scholar]
- 11. Menkveld R, Stander FS, Kotze TJ, Kruger TF, Van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod 1990; 5: 586–592. [DOI] [PubMed] [Google Scholar]
- 12. Mikamo K, Kamiguchi Y. A new assessment system for chromosomal mutagenicity using oocytes and early zygotes of Chinese hamster In: Ishihara T, Sasaki MS. (eds). Radiation‐Induced Chromosome Damage in Man. New York: Alan R. Liss, 1983; 411–432. [Google Scholar]
- 13. Watanabe S, Kamiguchi Y. Establishment of human sperm chromosome assay using intracytoplasmic sperm injection (ICSI) into mouse oocytes. Asahikawa Med Col Res Bull 2001; 2: 46–55. [Google Scholar]
- 14. Devillard F, Metzler‐Guillemain C, Pelletier R et al. Polyploidy in large‐headed sperm: FISH study of three cases. Hum Reprod 2002; 17: 1292–1298. [DOI] [PubMed] [Google Scholar]
- 15. Int’Veld PA, Broekmans FJK, De France HF, Peaarson PL, Pieters MHEC, Van Kooji RJ. Intracytoplasmic sperm injection (ICSI) and chromosomally abnormal spermatozoa. Hum Reprod 1997; 12: 752–754. [DOI] [PubMed] [Google Scholar]
- 16. Martin RH, Greene C, Rademaker AW. Sperm chromosome aneuploidy analysis in a man with globozoospermia. Fertil Steril 2003; 79: 1662–1664. [DOI] [PubMed] [Google Scholar]
- 17. Amireault P, Dube F. Cloning, sequencing, and expression analysis of mouse glucosamine‐6‐phosphate deaminase (GNPDA/oscillin). Mol Reprod Dev 2000; 56: 424–435. [DOI] [PubMed] [Google Scholar]
- 18. Miharu N, Best RG, Young SR. Numerical chromosome abnormalities in spermatozoa of fertile and infertile men detected by fluorescence in situ hybridization. Hum Genet 1994; 93: 502–506. [DOI] [PubMed] [Google Scholar]
- 19. Pang MG, Hoegerman SF, Cuticchia AJ et al. Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in‐situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Hum Reprod 1999;. 14: 1266–1273. [DOI] [PubMed] [Google Scholar]
- 20. Celik‐Ozenci C, Catalanotti J, Jakab A et al. Human sperm maintain their shape following decondensation and denaturation for fluorescent in situ hybridization: shape analysis and objective morphometry. Biol Reprod 2003; 69: 1347–1355. [DOI] [PubMed] [Google Scholar]


