Abstract
Background: Although recent technical advances have benefited infertile couples, inadequate embryo development as a result of poor quality oocytes still contributes to infertility. The purpose of the present study was to evaluate melatonin as a drug for improving oocyte quality in such cases.
Methods: Twenty‐seven women from whom fewer than three fertilized embryos were grown and who failed to fall pregnant in previous treatment cycles were enrolled in the current prospective clinical study. Subjects took 1 mg or 3 mg tablets of melatonin orally at 22:00 h from the fifth day of the previous menstrual cycle to the day they were injected with human chorionic gonadotropin. The numbers of mature follicles, retrieved oocytes, degenerate oocytes, and fertilized embryos were compared to their previous data without melatonin (the control cycle).
Results: Intrafollicular melatonin concentrations were significantly increased, and intrafollicular lipid peroxide concentrations showed a tendency towards lower levels in the 3 mg melatonin treatment cycles compared with the control cycles. The number of degenerate oocytes was significantly reduced, and the number of fertilized embryos showed a tendency towards an increase in the 3 mg cycle compared to the control cycle. Three women succeeded in falling pregnant.
Conclusion: Melatonin is likely to become the drug of choice for improving oocyte quality in women who cannot fall pregnant because of poor quality oocytes. (Reprod Med Biol 2003; 2: 139–144)
Keywords: assisted reproductive technology, embryo quality, intracytoplasmic sperm injection, melatonin, oocyte quality
INTRODUCTION
THE RECENT RAPID improvements in assisted reproductive technology (ART) have benefited numerous infertile couples; however, some couples, in particular older women, remain infertile because of only a few fertilized embryos as a result of poor quality oocytes. Although oocyte and embryo donations are provided to infertile couples in some countries, legal, ethical, and social prohibitions exist in countries such as Japan.
Defective oocyte cytoplasm as a result of injury by oxygen‐free radicals is likely to be the cause of poor oocyte quality, resulting in inadequate embryo development. 1 Oxygen radicals and their scavengers are produced in the follicles especially during ovulation, and the normal balance between them is important for healthy ova shed. 2 , 3 The radical scavenger glutathione can improve the in vitro embryo development from the bovine oocyte. 4
A higher concentration of melatonin is detected in the human follicular fluid than in serum, 5 melatonin is also a potent radical scavenger, 6 , 7 and its activity is stronger than that of glutathione. 8 Melatonin quenches oxygen radicals, in particular the hydroxyl radicals, and prevents the damage of biological membranes. 8 Melatonin administration to pregnant rats protects fetal brain damage by oxygen radicals, 9 and melatonin inhibits vasospastic action of hydrogen peroxide in the human umbilical artery. 10 Clinically, melatonin administration to septic newborns improves their clinical outcome. 11 Addition of melatonin may improve the quality of gathered oocytes by protecting them from this radical cytotoxicity.
In the present study, we examined whether melatonin administration would improve oocyte quality and result in pregnancy in women who failed to fall pregnant in previously repeated intracytoplasmic sperm injection (ICSI)‐embryo transfer (ET) treatment cycles as a result of poor oocyte quality.
MATERIALS AND METHODS
Patients and treatment
THE PRESENT STUDY was approved by the Ethics Committee on Human Experiments of Saiseikai‐Shimonoseki General Hospital according to the Declaration of Helsinki for Medical Research involving human subjects. Twenty‐seven women were enrolled in the present study. We had obtained fewer than three fertilized embryos from the women and they had failed to become pregnant in previously repeated ICSI‐ET cycles. The effects of melatonin on the results of ART were examined. The causes of infertility of the couples and/or women are shown in Table 1. All patients gave informed consent to participate in the current clinical study.
Table 1.
Causes of infertility in the present 27 subjects
| Causes of infertility | Number of subjects (%) |
|---|---|
| Male infertility | 20 (74.1) |
| Tubal infertility | 1 (3.7) |
| Ovarian poor responder† | 17 (63.0) |
| Unknown infertility | 8 (29.6) |
Patients with less than three matured follicles. Some patients have plural causes.
The mean age and mean sterile period were 34.0 ± 4.4 years (mean ± SD) and 6.2 ± 3.2 years, respectively. All women were treated according to a standardized ovarian stimulation protocol, that is, nasal administration of gonadotropin releasing hormone agonist (900 µg/day buserelin acetate, Suprecur; Aventis Pharma, Tokyo, Japan) from the previous mid‐luteal phase, intramuscular administration of 225 IU of follicle stimulating hormone (Fertinorm P; Serono‐Japan, Tokyo, Japan) on the third to fifth days, and intramuscular administration of 150 IU of human menopausal gonadotopin (Humegon; Organon Pharmaceuticals, Tokyo, Japan) on the sixth day and each day thereafter. When leading follicles reached at least 18 mm in diameter, an intramuscular injection of 10 000 IU human chorionic gonadotropin (hCG) (Profasi; Serono‐Japan, Tokyo, Japan) was given, and oocyte retrieval was carried out 35 h later guided by transvaginal ultrasonography. Patients took a 1 mg tablet of melatonin (KAL, Park City, UT, USA; 1 mg cycle: 13 patients) or a 3 mg tablet (3 mg cycle: 23 patients) orally at 22:00 h from the fifth day of the previous cycle to the day they were injected with hCG; nine of 13 patients in the 1 mg cycle were treated with 3 mg tablets in the next ICSI cycle (Fig. 1). The retrieved oocyte was denuded by exposure to hyaluronidase solution and was injected with sperm. The numbers of mature follicles, retrieved oocytes, degenerate oocytes, 12 and fertilized embryos in the melatonin treatment cycles were compared to their previous data without melatonin (control cycle data). The gonadotropin dosages consumed for follicle stimulation and serum estradiol levels immediately before hCG injection were also compared between the melatonin treatment and the control cycles. Follicular fluid was collected, and melatonin and lipid peroxide (LPO) concentrations were determined in nine subjects who took 1 mg and also 3 mg tablets of melatonin (Fig. 1). The mean value of the melatonin or LPO concentrations in mature follicles (more than 15 mm in diameter) was considered the follicular melatonin or LPO concentration in each patient.
Figure 1.
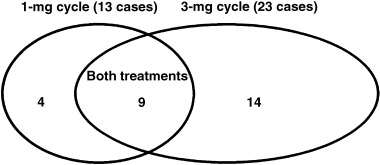
Number and relation of each treatment cycle.
Melatonin assay
Intrafollicular melatonin concentrations were determined by radioimmunoassay as reported previously. 13 Briefly, 2 mL chloroform was added to 0.5 mL serum, and 1.5 mL organic phase was aspirated, evaporated, and dissolved in the 250 µL of assay buffer (0.01 M sodium phosphate, 0.15 M NaCl, 0.1% BSA, 0.1% NaN3). Melatonin antiserum (100 µL, 1:30 000; HAC‐AA 92–03RBP86, Endocrinology Research Center, Gunma University, Tsukuba, Japan) and [3H]‐melatonin (5000 cpm/100 µL; Du Pont, Wilmington, DE, USA) were added to the tubes containing standards or samples, and they were incubated for 24 h at 4°C. The bound/free separation was carried out by the dextran‐charcoal method, and the radioactivity of supernatant was measured in a liquid scintillation counter (LSC5100, Aloka). The lower limit of the assay was 2.1 pg/tube, and the intra‐ and interassay coefficients of variation were less than 10%.
Lipid peroxide and estradiol assay
Commercially available assay kits were used for measuring LPO concentrations (LPO‐test; Wako‐Junyaku Industrial, Osaka, Japan) 14 in follicular fluid and serum estradiol concentrations (VIDAS Estradiol II, bioMérieux SA, Marcy‐l’Etoile, France). 15 Assay ranges of LPO and estradiol were 0–40 nmol/mL and 9–3000 pg/mL, respectively, and the coefficients of variations in both assays were less than 10%.
Statistical analysis
Intrafollicular melatonin or LPO concentrations were analyzed by analysis of variance and Duncan's new multiple range test. Paired values in the numbers of mature follicles, retrieved oocytes, degenerate oocytes, and fertilized embryos between the control and melatonin treatment cycles were analyzed by the Wilcoxon rank sum test. Gonadotropin dosages consumed for follicular stimulation and serum estradiol levels were analyzed by paired Student's t‐test. Statistical significance was defined as P < 0.05.
RESULTS
IN COMPARISON TO concentrations in the control cycle (130 ± 15 pg/mL, mean ± SEM), intrafollicular melatonin concentrations were slightly increased in the 1 mg cycle (237 ± 43 pg/mL), and concentrations were further (P < 0.01) increased in the 3 mg cycle (468 ± 109 pg/mL, Fig. 2). Intrafollicular LPO concentrations showed a tendency towards lower levels, but not significant, depending on the melatonin dosage (1.12 ± 0.16 nmol/mL in the control cycle, 0.91 ± 0.06 nmol/mL in the 1 mg cycle, and 0.88 ± 0.03 nmol/mL in the 3 mg cycle; mean ± SEM, Fig. 3). The number of degenerate oocytes was significantly (P < 0.05) reduced in the 3 mg cycle compared to those in the control cycle, which decreased in nine subjects, increased in only one subject, and did not change in 13 of 23 subjects (Fig. 4). The number of fertilized embryos showed a tendency towards an increase in the 3 mg cycle compared to those in the control cycle, which increased in 10 subjects, decreased in five subjects, and did not change in eight of 23 subjects (Fig. 5). The number of mature follicles and retrieved oocytes did not differ between the 3 mg cycle and the control cycle. The numbers of mature follicles, retrieved oocytes, degenerate oocytes, and fertilized embryos did not differ between the 1 mg cycle and the control cycle. Neither 1 mg nor 3 mg melatonin influenced the consumed gonadotropin dosages and serum estradiol levels immediately before the hCG injection (Table 2). From the present 27 patients, three succeeded in falling pregnant in the 3 mg cycle; one pregnancy resulted in abortion and two subjects gave birth to healthy newborns. The number of degenerate oocytes decreased in these three patients, but the number of fertilized embryos increased in only one of the three patients.
Figure 2.
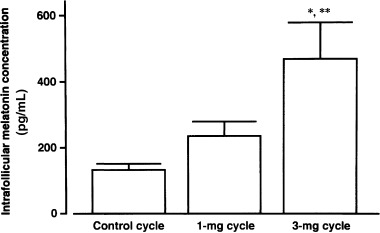
Intrafollicular melatonin concentrations in the control, the 1 mg, and the 3 mg cycles of nine subjects. Values are mean ± SEM of nine subjects. Data were analyzed by Duncan's new multiple range test. *P < 0.01, **P < 0.05, compared with the control cycle and the 1 mg cycle, respectively.
Figure 3.
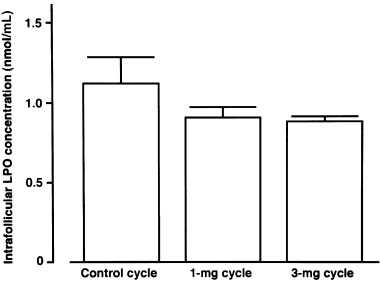
Intrafollicular lipid peroxide (LPO) concentrations in the control, the 1 mg, and the 3 mg cycles of nine subjects. Values are mean ± SEM of nine subjects. Data were analyzed by Duncan's new multiple range test.
Figure 4.
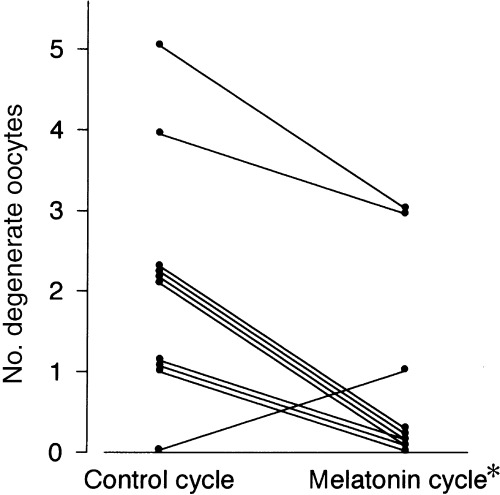
Effect of 3 mg melatonin on the number of degenerate oocytes. Data from 10 women are presented. The numbers in the other 13 patients were zero in the control and melatonin cycles. Paired data of all 23 patients were analyzed by the Wilcoxon rank sum test. *P < 0.05, compared with the control cycle.
Figure 5.
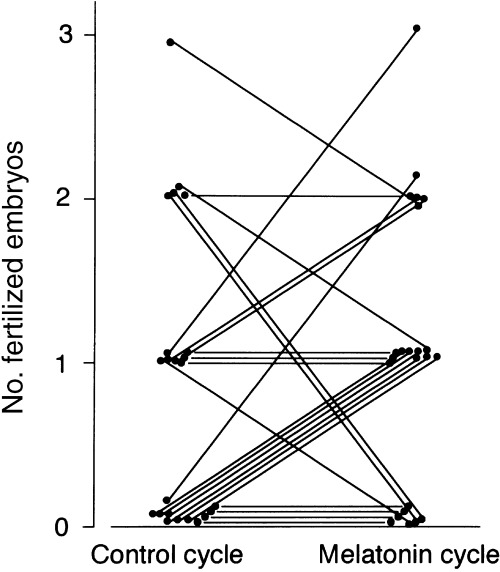
Effect of 3 mg melatonin on the number of fertilized embryos. Data from 23 women are presented. Paired data were analyzed by the Wilcoxon rank sum test.
Table 2.
Gonadotropin dosages consumed for follicle stimulation and serum estradiol levels immediately before human chorionic gonadotropin injection in the control, the 1 mg melatonin, and the 3 mg melatonin cycles
| Groups | Gonadotropin dosages (IU) | Estradiol levels (pg/mL) |
|---|---|---|
| Control cycle (n = 13) | 1933 ± 907 | 1210 ± 838 |
| 1 mg melatonin cycle (n = 13) | 1912 ± 953 | 1151 ± 756 |
| Control cycle (n = 23) | 1711 ± 740 | 1464 ± 937 |
| 3 mg melatonin cycle (n = 23) | 1747 ± 935 | 1291 ± 1053 |
Data are the mean ± SD of patient numbers in the parentheses. Data were analyzed by paired Student's t‐test.
DISCUSSION
THE PRESENT STUDY was the first clinical trial of melatonin use for infertile couples who fail to become pregnant through repeated ICSI‐ET treatments. Melatonin treatment with a 3 mg tablet, which increases intrafollicular melatonin concentration four times more than without treatment, is sufficient for improvement of oocyte and embryo quality. Melatonin can enter all tissues, however it concentrates only in the ovary, eye, and pineal gland when injected systemically. 16 The follicular cells are unable to produce melatonin, as suggested by the lack of N‐acetyltransferase (a key enzyme for melatonin production) messenger RNA in the ovary. 17 Active uptake by the ovary may explain why melatonin levels are higher in preovulatory follicles. How does melatonin improve oocyte quality?
Inadequate maturation of cytoplasm leads to defective oocyte, fertilization failure and impaired embryo viability, even with complete nuclear maturation. 18 The recent reproductive strategy of oocyte cytoplasm transfer has allowed such infertile couples to achieve pregnancy, 19 , 20 supporting the above theory; however, an unsolved problem of contaminating donor's mitochondrial DNA exists with this treatment. 21 In the present study, melatonin administration reduced the lipid peroxide concentrations in the human follicular fluid. Lipid peroxidation in biological membranes is implicated in free‐radical reactions, and radical scavengers can prevent the reaction. 22 , 23 Melatonin treatment likely prevents the injury in the biological membrane of oocytes, resulting in an increase in embryo viability. Mitochondrial DNA is a main target for oxygen radicals because of its location near the inner mitochondrial membrane sites where oxidants are formed and DNA repair activity is lacking. 24 Melatonin is known to prevent ischemia/reperfusion‐induced oxidative damage to mitochondria in fetal rat brain, 25 kainic acid inducing mitochondrial oxidative phosphorylation enzyme dysfunction in rat cerebellar granule neurons, 26 and hepatic mitochondrial dysfunction in senescence‐accelerated mice; 27 and melatonin is also known to stimulate electron transport and adenine triphosphate production in the inner‐mitochondrial membrane deferring age‐related degenerative conditions. 6 Melatonin may prevent mitochondrial damages in oocytes, resulting in improvement of oocyte quality.
Poor oocyte quality is the main cause of the age‐related decline in female fertility, 28 , 29 and this age‐related damage of oocyte is also induced by oxidative stress. 30 Melatonin production is well known to decrease with age. 31 However, the melatonin concentration in preovulatory follicles shows increasing tendency depending on age (Takasaki A, unpubl. data, 2002), which may be a compensatory phenomenon against oxidative stress increasing with aging. Melatonin treatment may improve oocytes made defective by aging. In the present study, eight of the 23 patients in the 3 mg melatonin group were over 37 years old. Although oocytes in five of the eight patients did not become fertilized in the control cycle, oocytes in six of these eight patients became fertilized; one woman then became pregnant in the 3 mg melatonin treatment cycle when the women underwent embryo transfer.
Intrafollicular melatonin concentrations are higher in large preovulatory follicles than in small atretic follicles. Furthermore, melatonin likely protects granulosa cells from attack by oxygen radicals during ovulation, increasing progesterone production. 32 Melatonin is therefore needed and plays an important role in healthy preovulatory follicles.
After the present study, four of the present 27 patients succeeded in falling pregnant by repeated 3 mg melatonin treatments: two women have given birth and the other two women are now pregnant without any complications. We also have used 3 mg melatonin tablets in 86 cycles of 61 additional women who were referred to our hospital because of poor results with previous ART; nine of these women became pregnant. Seven babies, including babies from the women in the current study, were born in association with the 3 mg melatonin treatment. No baby showed any abnormality. We have continued to evaluate the effects of melatonin, not only on the ART results but also on neonatal growth, although melatonin is reported to give no negative consequences. 33 , 34 Moreover, further basic studies are needed to reveal the mechanism of melatonin improving the pregnant ability in those couples. Melatonin is likely to become the drug of choice for improving oocyte quality for women who can not get pregnant because of poor quality oocytes. This new melatonin treatment strategy may benefit infertile women with poor quality oocytes or embryos.
ACKNOWLEDGMENT
WE ARE GRATEFUL to Dr Katsumi Wakabayashi (Endocrinology Research Center, Gunma University, Japan) for the ample supply of melatonin antiserum (HAC‐AA92–03RBP86).
REFERENCES
- 1. Van Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in‐vitro fertilisation and embryo transfer. Hum Reprod 1995; 10: 415–424. [DOI] [PubMed] [Google Scholar]
- 2. Miyazaki T, Sueoka K, Dharmarajan AM, Atlas SJ, Bulkley GB, Wallach EE. Effect of inhibition of oxygen free radical on ovulation and progesterone production by the in‐vitro perfused rabbit ovary. J Reprod Fertil 1991; 91: 207–212. [DOI] [PubMed] [Google Scholar]
- 3. Sato EF, Kobuchi H, Edashige K et al. Dynamic aspects of ovarian superoxide dismutase isozymes during the ovulatory process in the rat. FEBS Lett 1992; 303: 121–125. [DOI] [PubMed] [Google Scholar]
- 4. Luvoni GC, Keskintepe L, Brackett BG. Improvement in bovine embryo production in vitro by glutathione‐containing culture media. Mol Reprod Dev 1996; 43: 437–443. [DOI] [PubMed] [Google Scholar]
- 5. Brzezinski A, Seibel MM, Lynch HJ, Deng MH, Wurtman RJ. Melatoin in human preovulatory follicular fluid. J Clin Endocrinol Metab 1987; 64: 865–867. [DOI] [PubMed] [Google Scholar]
- 6. Reiter RJ, Tan DX, Manchester LCEI, Sawi M. Melatonin reduces oxidant damage and promotes mitochondrial respiration: implications for aging. Ann NY Acad Sci 2002; 959: 238–250. [DOI] [PubMed] [Google Scholar]
- 7. Tan DX, Reiter RJ, Manchester LC et al. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem 2002; 2: 181–197. [DOI] [PubMed] [Google Scholar]
- 8. Reiter RJ, Tan DX, Osuna C, Gitto E. Action of melatonin in the reduction of oxidative stress: a review. J Biomed Sci 2000; 7: 444–458. [DOI] [PubMed] [Google Scholar]
- 9. Wakatsuki A, Okatani Y, Izumiya C, Ikenoue N. Melatonin protects against ischemia and reperfusion induced oxidative lipid and DNA damage in fetal rat brain. J Pienal Res 1999; 26: 147–152. [DOI] [PubMed] [Google Scholar]
- 10. Okatani Y, Watanabe K, Hayashi K, Wakatsuki A, Sagara Y. Melatonin inhibits vasospastic action of hydrogen peroxide in human umbilical artery. J Pineal Res 1997; 22: 163–168. [DOI] [PubMed] [Google Scholar]
- 11. Gitto E, Karbownik M, Reiter RJ et al. Effects of melatonin treatment in septic newborns. Pediatr Res 2001; 50: 756–760. [DOI] [PubMed] [Google Scholar]
- 12. Veeck LL. Etracorporeal maturation: Norfolk, 1984. Ann NY Acad Sci 1985; 442: 357–367. [DOI] [PubMed] [Google Scholar]
- 13. Tamura H, Nakamura Y, Takiguchi S et al. Pinealectomy or melatonin implantation does not affect prolactin surge or luteal function in pseudopregnant rats. Endocr J 1998; 45: 377–383. [DOI] [PubMed] [Google Scholar]
- 14. Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med 1976; 15: 212–216. [DOI] [PubMed] [Google Scholar]
- 15. Dupont A, Dupont P, Cusan L et al. Comparative endocrinological and clinical effects of percutaneous estradiol and conjugated estrogens as replacement therapy in menopausal women. Maturitas 1991; 13: 297–311. [DOI] [PubMed] [Google Scholar]
- 16. Wurtman RJ, Axelrod J, Potter LT. The uptake of H3 melatonin in endocrine and nervous tissues and the effects of constant light exposure. J Pharmacol Exp Ther 1964; 143: 314–318. [PubMed] [Google Scholar]
- 17. Klein DC, Roseboom PH, Coon SL. New light is shining on the melatonin rhythm enzyme. Trends Endocrinol Metab 1996; 7: 106–112. [DOI] [PubMed] [Google Scholar]
- 18. Plachot M, Mandelbaum J. Oocyte maturation, fertilization and embryonic growth in vitro. Br Med Bull 1990; 46: 675–694. [DOI] [PubMed] [Google Scholar]
- 19. Cohen J, Scott R, Schimmel T, Levron J, Willadsen S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet 1997; 350: 186–187. [DOI] [PubMed] [Google Scholar]
- 20. Lanzendorf SE, Mayer JF, Toner J, Oehninger S, Saffan DS, Muasher S. Pregnancy following transfer of ooplasm from cryopreserved‐thawed donor oocytes into recipient oocytes. Fertil Steril 1997; 71: 575–577. [DOI] [PubMed] [Google Scholar]
- 21. St John JC, Barratt CL. Use of anucleate donor oocyte cytoplasm in recipient eggs. Lancet 1997; 350: 961–962. [DOI] [PubMed] [Google Scholar]
- 22. Hochstein P, Jain SK. Association of lipid peroxidation and polymerization of membrane proteins with erythrocyte aging. Federation Proc 1981; 40: 183–188. [PubMed] [Google Scholar]
- 23. Kato H, Sugino N, Takiguchi S, Kashida H, Nakamura Y. Roles of reactive oxygen species in the regulation of luteal function. Rev Reprod 1997; 67: 256–260. [DOI] [PubMed] [Google Scholar]
- 24. Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA 1994; 91: 10771–10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wakatsuki A, Okatani Y, Shinohara K, Ikenoue N, Fukaya T. Melatonin protects against ischemia/reperfusion‐induced oxidative damage to mitochondria in fetal rat brain. J Pienal Res 2001; 31: 167–172. [DOI] [PubMed] [Google Scholar]
- 26. Dabbeni‐Sala F, Floreani M, Franceschini D, Skaper SD, Giusti P. Kainic acid induces selective mitochondrial oxidative phosphorylation enzyme dysfunction in cerebellar granule neurons: protective effects of melatonin and GSH ethyl ester. FASEB J 2001; 15: 1786–1788. [DOI] [PubMed] [Google Scholar]
- 27. Okatani Y, Wakatsuki A, Reiter RJ, Miyahara Y. Hepatic mitochondrial dysfunction in senescence‐accelerated mice: correction by long‐term, orally administered physiological levels of melatonin. J Pineal Res 2002; 33: 127–133. [DOI] [PubMed] [Google Scholar]
- 28. Navot D, Bergh PA, Williams MA et al. Poor oocyte quality rather than implantation failure as a cause of age‐related decline in female fertility. Lancet 1991; 337: 1375–1377. [DOI] [PubMed] [Google Scholar]
- 29. Lim AST, Tsakok MFH. Age‐related decline in fertility: a link to degenerative oocytes? Fertil Steril 1997; 68: 265–271. [DOI] [PubMed] [Google Scholar]
- 30. Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC. Melatonin, hydroxyl radical‐mediated oxidative damage, and aging: a hypothesis. J Pineal Res 1993; 14: 151–168. [DOI] [PubMed] [Google Scholar]
- 31. Reiter RJ. The aging pineal gland and its physiological consequences. Bioessays 1992; 14: 169–175. [DOI] [PubMed] [Google Scholar]
- 32. Nakamura Y, Tamura H, Takayama H, Kato H. Increased endogenous level of melatonin in preovulatory human follicle does not directly influence progesterone production. Fertil Steril 2003;. 80: 1012–1016. [DOI] [PubMed] [Google Scholar]
- 33. Jahnke G, Marr M, Myers C, Wilson R, Travlos G, Price C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague‐Dawley rats. Toxicol Sci 1999; 50: 271–279. [DOI] [PubMed] [Google Scholar]
- 34. Reiter RJ, Tan DX, Sainz R, Mayo JC, Lopez‐Burillo S. Melatonin: reducing the toxicity and increasing the efficacy of drugs. J Pharma Pharmacol 2002; 54: 1299–1321. [DOI] [PubMed] [Google Scholar]


