Abstract
Introduction
Drugs have been shown to adversely affect male fertility and recently anti‐hypertensive drugs were added to the list. The anti‐fertility effects of nifedipine and similar calcium channel blockers are well‐illustrated in in vitro experiments but not in vivo.
Purpose
The present study was designed to experimentally elucidate the sub‐chronic effect of nifedipine, verapamil and diltiazem on sperm functions and reproductive hormone levels in vivo.
Methods
Male rats (150–200 g) were divided into four groups of ten rats each. Group 1 (control) received distilled water; Group 2 received nifedipine 0.57 mg/kg BW; Group 3 were given verapamil 3.40 mg/kg BW and Group 4 were given diltiazem 2.57 mg/kg BW. Each drug‐treated group had its own recovery group from which treatment was discontinued for 30 days before the animals were sacrificed. Blood samples were collected for hormonal assay of FSH, LH and testosterone. Semen evaluation was done and the testes, seminal vesicle, epididymis and prostate were removed, and weighed immediately.
Results
Nifedipine, verapamil and diltiazem significantly decreased (P < 0.05) sperm count and motility in drug treated groups. The weight of the epididymis was significantly reduced (P < 0.05) in the drug treated rats. Semen parameters and other associated changes were restored after 30 days of drug withdrawal.
Conclusion
Calcium channel blockers appear to have a reversible anti‐fertility effect on male rats which does not occur through inhibition of the pituitary‐gonadal axis.
Keywords: Nifedipine, Verapamil, Diltiazem, Sperm, Testosterone
Introduction
The development of normal, mature sperm is the key to male fertility, though once formed, this mature sperm, must also have the ability to induce conception in females [1]. In males, fertility depends upon adequate production, storage and transport of spermatozoa. An impairment of male fertility may arise because of germinal failure, leydig cell failure or failure of transport of normally‐formed spermatozoa [2]. Many other factors have equally been implicated in the pathology of infertility in males. These include age, substance abuse, genetic factors, environmental assaults, drugs, exposure to heavy metals, testicular exposure to overheating and radiation treatment.
Drugs have been previously shown to have deleterious effect on male fertility. For instance, hormonal drugs, androgenic drugs [3]; antimetabolites and other cytotoxic drugs such as colchian, cimetidine, nitrofuranotoxin [4]; anticonvulsants and opioids [5]; antimalarials such as chloroquine [6, 7], pyrimethanine [8], and artemether [9] have all been reported to have antifertility effects on male rats. These effects are mediated via inhibition of leydig cell steriodogenesis, depression of spermatogenesis, destruction of the germinal cell, inhibition of sperm function and metabolism.
Antihypertensive drugs such as calcium channel blockers (CCB) have also been implicated in infertility in males. There have been reports of reversible functional defects in sperm, and inability to fertilize eggs with standard semen analysis parameters in hypertensive patients taking CCB [10, 11]; however, a contrary report exists [12]. The anti‐fertility effects of nifedipine and similar CCB are well‐understood in in vitro experiments [13, 14, 15]. Juneja et al. [16] showed the ability of verapamil (a CCB) to cause partial arrest of spermatogenesis, marked decrease in sperm density, sperm motility and cellular energy content (ATP) in guinea pigs. Similarly, nifedipine has been reported to adversely impact spermatogenesis in peripubertal mice [17]. Amlodipine treatment has equally been demonstrated to have deleterious effects on the reproductive function of male rats evidenced by decreased follicle stimulating hormone (FSH), testosterone, and sperm density [18]. The present study was therefore designed to elucidate the individual sub‐chronic effect of nifedipine, verapamil and diltiazem on sperm functions and reproductive hormone levels in adult rats.
Materials and methods
Animals and drugs
Forty young male rats weighing 150–200 g obtained from the animal house of the College of Medicine, University of Lagos, were used for the study. The animals were randomly divided into four equal groups and treated as described in the dose regimen. They were kept in a well‐ventilated and photoperiod‐controlled (12 h light:12 h dark) animal house. The animals were housed in clear polypropylene cages lined with wood chip bedding. They were fed a standard pellet diet (Livestock Feeds, Lagos, Nigeria) and water ad libitum. The animals were allowed to acclimatize for a period of two weeks before the commencement of the study. Nifedipine, verapamil and diltiazem obtained from a local pharmacy were used in the study. The concentration of these drugs was prepared by dissolving known milligrams in known volumes of distilled water based on comparisons between human dosage and body weight of animals.
Study protocol
A total of forty male rats divided into four groups were treated as follows: Group 1 (control group) received distilled water which was the vehicle of the drug, Group 2 (nifedipine group) were given nifedipine (0.571 mg/kg BW) orally and Group 3 (Verapamil group) were given verapamil (3.40 mg/kg BW) and Group 4 (diltiazem group) were given diltiazem (2.57 mg/kg BW). Treatment was done intra‐gastrically via oral cannula for 30 days. Each drug treated group had its own recovery group from which treatment was discontinued for 30 days before the animals were sacrificed. Subsequently, the epididymis, seminal vesicle, prostate and testis were removed, cleared of adherent tissue and weighed immediately after sacrifice. Blood was collected from each animal via cardiac puncture and used for hormonal assay.
Sperm analysis
Sperm analysis was done on samples derived from the cauda epididymis by conventional methods [19]. Briefly, sperm motility was assessed immediately after autopsy and expressed as percent motility. Epididymal sperm counts were made using the haemocytometer and were expressed as million/ml of suspension. The sperm viability was also determined using eosin/nigrosin stain. Semen was smeared on microscopic slides and two drops of stain were added. Live (motile) sperm cells were stained while dead sperm cells remained unstained. Sperm cells were counted by hundreds from a well prepared uniform smear per slide in order to estimate the percentage live/dead ratio. Sperm morphology was determined from a count of 400 spermatozoa in sperm smear prepared with Wall and Ewas stain and examined under the microscope using X100 objective under oil immersion. The abnormal sperm cells were counted and expressed as percentages.
Hormonal assay
Blood samples were spun at 2500 rpm for 10 min in a tabletop centrifuge. Serum samples obtained were assayed for follicle stimulating hormone (FSH), luteinizing hormone (LH) and testosterone using the tube‐based enzyme immunoassay (EIA) method. The EIA kits were manufactured by Immunometrics (UK) Limited and meet the WHO standards in research programme for human reproduction. The intra and interassay variations were 10.02 and 10.05%, respectively, for FSH; 9.80 and 10.06% for LH; and 10.02 and 10.08% for testosterone. The optical density was read at wavelengths between 492 and 550 nm on a spectrophotometer.
Statistical analysis
Data are expressed as “mean ± SEM” and were analyzed using the Students t test. A P value of <0.05 was considered statistically significant.
Results
Effect of nifedipine, verapamil and diltiazem on sex organs weight in male rats
There was no significant change in the mean weights of the testis, seminal vesicle and prostate gland in the both the drug treated and recovery groups when compared with their respective control groups. However, the weight of the epididymis was significantly reduced (P < 0.05) in the drug treated groups (nifedipine, verapamil and diltiazem) compared with control. The epididymal weight was normal in all the recovery groups (Table 1).
Table 1.
Effect of nifedipine, verapamil and diltiazem on weight of sex organs
| Organ | Control | Nifedipine | Verapamil | Diltiazem |
|---|---|---|---|---|
| Testis | 1.13 ± 0.03 (1.29 ± 0.03) | 1.24 ± 0.06 (1.13 ± 0.02) | 1.17 ± 0.03 (1.28 ± 0.02) | 1.16 ± 0.02 (1.28 ± 0.01) |
| Prostate | 0.17 ± 0.01 (0.21 ± 0.01) | 0.18 ± 0.02 (0.20 ± 0.01) | 0.18 ± 0.01 (0.22 ± 0.01) | 0.19 ± 0.01 (0.21 ± 0.01) |
| Epididymis | 0.63 ± 0.02 (0.66 ± 0.02) | 0.55 ± 0.02* (0.68 ± 0.02) | 0.47 ± 0.03* (0.66 ± 0.03) | 0.48 ± 0.01* (0.67 ± 0.03) |
| Seminal vesicle | 0.76 ± 0.02 (0.79 ± 0.02) | 0.73 ± 0.02 (0.86 ± 0.02) | 0.68 ± 0.05 (0.72 ± 0.03) | 0.73 ± 0.01 (0.76 ± 0.02) |
Values are expressed as mean ± SEM; n = 5 per group. *P < 0.05 compared with control. Recovery values are in parenthesis. All units in grams
Effect of nifedipine, verapamil and diltiazem on sperm functions
There was a significant decrease (P < 0.05) in the sperm count in the drug treated rats compared with controls. However, sperm count in the recovery group was not significantly different when compared with the control group (Fig. 1). The sperm motility was significantly decreased (P < 0.05) in the nifedipine group (60%), verapamil group (69%) and diltiazem group (54%) compared with the control group (83%). The recovery group showed normal sperm motility with percentage motility of 83, 80, 81 and 78% for the control, nifedipine, verapamil and diltiazem groups, respectively (Fig. 2). The viability of the spermatozoa (i.e., percentage live) and normal morphology remained within control values in the drug treated groups. Similarly there was no significant difference (P > 0.05) in the viability and normal sperm morphology in the recovery groups when compared with the control group (Figs. 3, 4).
Figure 1.
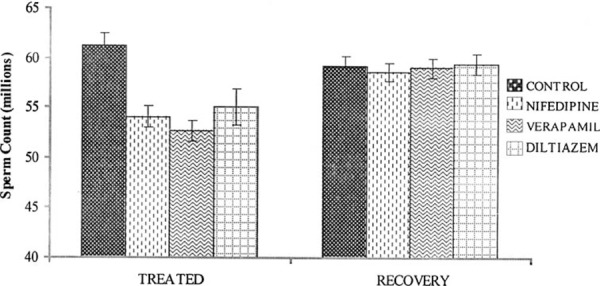
Effect of nifedipine, verapamil and diltiazem on sperm count in treated and recovered groups of rats. *P < 0.05 compared with controls
Figure 2.
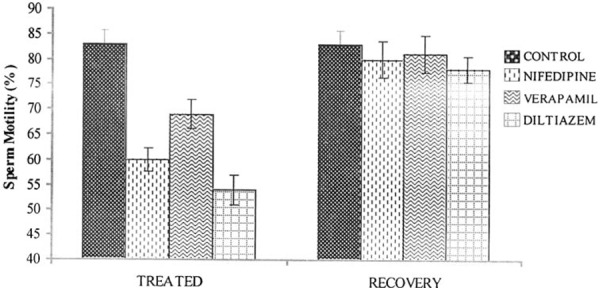
Effect of nifedipine, verapamil and diltiazem on sperm motility in treated and recovered groups of rats. *P < 0.05 compared with controls
Figure 3.
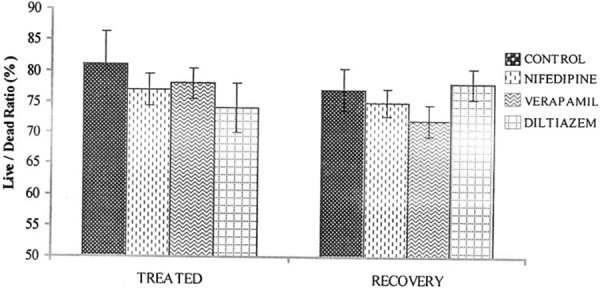
Effect of nifedipine, verapamil and diltiazem on sperm viability in treated and recovered groups of rats
Figure 4.
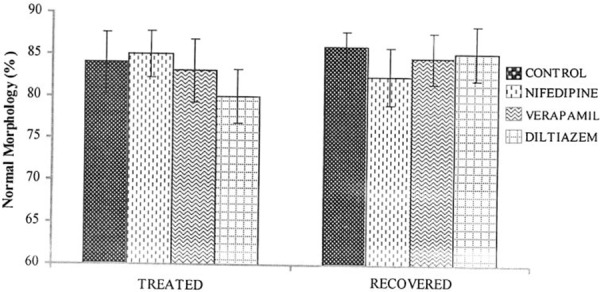
Effect of nifedipine, verapamil and diltiazem on sperm morphology in treated and recovered groups of rats
Effect of nifedipine, verapamil and diltiazem on serum hormonal level
There were no significant differences in the serum levels of FSH, LH and testosterone in drug treated rats compared with control rats. The serum concentration of FSH was 9.5 ± 0.37, 9.89 ± 0.24, 9.7 ± 0.22 and 9.48 ± 0.26 iu/l in the control, nifedipine, verapamil and diltiazem groups, respectively. Serum LH concentration for the control, nifedipine, verapamil and diltiazem groups were 2.61 ± 0.04, 2.56 ± 0.04, 2.48 ± 0.08 and 2.52 ± 0.09 iu/l, respectively. Serum testosterone concentration for the control was 0.45 ± 0.01 ng/ml; nifedipine 0.42 ± 0.01 ng/ml, verapamil 0.44 ± 0.01 ng/ml and diltiazem 0.43 ± 0.01 ng/ml. The recovery groups exhibited normal FSH, LH and testosterone hormone levels (Table 2).
Table 2.
Effect of nifedipine, verapamil and diltiazem on FSH, LH and testosterone hormone levels
| Hormones | Control | Nifedipine | Verapamil | Diltiazem |
|---|---|---|---|---|
| FSH (iu/l) | 9.50 ± 0.37 (9.83 ± 0.42) | 9.88 ± 0.24 (9.74 ± 0.31) | 9.70 ± 0.22 (10.13 ± 0.29) | 9.48 ± 0.26 (9.56 ± 0.34) |
| LH (iu/l) | 2.61 ± 0.06 (2.62 ± 0.06) | 2.56 ± 0.04 (2.63 ± 0.03) | 2.48 ± 0.08 (2.54 ± 0.05) | 2.52 ± 0.09 (2.59 ± 0.07) |
| Testosterone (ng/ml) | 0.45 ± 0.01 (0.44 ± 0.02) | 0.42 ± 0.01 (0.43 ± 0.02) | 0.44 ± 0.02 (0.45 ± 0.01) | 0.43 ± 0.01 (0.44 ± 0.01) |
Values are expressed as mean ± SEM; n = 5 per group. Recovery values are in parenthesis
Discussion
In this study it has been shown that nifedipine, verapamil and diltiazem considerably impair sperm motility and significantly reduce sperm count in vivo in rats. These effects are reversible. While the mechanisms of action of these drugs in terms of inhibition of calcium ion influx in the muscle cells of the heart and blood vessels are well documented [20, 21], it is highly likely that there are other non‐specific effects which are contributing to impairment of sperm motility and reduced sperm count.
Sperm functions such as count and motility are key indices of male fertility, as these are prime markers in testicular spermatogenesis and epididymal maturation. The reduction of these parameters by CCB treatment in our study is in agreement with the findings of others, where decreased sperm count and motility were observed [16, 18]. Motility of ejaculated spermatozoa has long been recognized as an extremely important functional characteristic that must be evaluated as an integral part of semen analysis [27] and poor motility had been correlated with infertility. Due to the presence in spermatozoa of high affinity binding sites for dihydropyridines [22, 23], drugs which block calcium entry in somatic tissues [24], and because the calcium ion is vital for the activation of sperm motility [25, 26], it appears there is inhibition of calcium entry into sperm membranes, thereby affecting the flagella motility of the sperm cell. Secondly, CCB may mimic gossypol, a binaphthalendialdehyde extracted from cotton seeds in its effects on sperm function. Gossypol as an anti‐fertility agent has an efficacy rate of 98.9% and does not affect production of testosterone and luteinizing hormones [11]. Because of the lipophilic nature of gossypol, it interacts directly with the phospholipid bilayer of biological membranes altering membrane structure, electrostatic charges and transmembrane ion fluxes. When studied in animal models, gossypol has been shown to inhibit calcium ion uptake, Ca2+Mg2+‐ATPase and Ca2+‐ATPase activities in ram and bull sperm plasma membrane vesicles [13]. In many respects, CCB effects changes on sperm membrane structure and functions similar to those of gossypol [10, 11]. These changes include blocking mannose lectins (an important binding protein on the sperm membrane) from moving to the surface of the cell membrane thereby leading to excess cholesterol on the sperm head and stiffening of the membrane, thus affecting the normal movement of the sperm [10, 11, 28].
Although in vivo drug administration resulted in reduced sperm count and motility in this study, reproductive hormones (LH, FSH and testosterone) are unaffected. LH and FSH are required for the initiation and maintenance of spermatogenesis in rats and for qualitatively normal spermatogenesis [29]. In males, LH stimulates the production of testosterone from the leydig cells and testosterone is absolutely required for spermatogenesis. It is known that potent anti‐fertility agents do not necessary affect production of testosterone and luteinizing hormones [11]. Therefore, it appears that the primary site of action of these calcium channel blockers is not on the hypothalamic‐pituitary‐testicular axis, but rather may be directly on the reproductive tissues and cells. In conclusion, nifedipine, verapamil and diltiazem have a deleterious effect on reproductive functions in male rats which is, however, reversible after drug withdrawal.
References
- 1. Hershlag A, Cooper GW, Benoff S. Pregnancy following discontinuation of a calcium channel blocker in the male partner. Hum Reprod, 1995, 10, 599–606 [DOI] [PubMed] [Google Scholar]
- 2. Aitken RJ. Sperm functions tests and fertility. Int J Androl, 2006, 29, 69–75 10.1111/j.1365‐2605.2005.00630.x [DOI] [PubMed] [Google Scholar]
- 3. Nkposong EO, Lawani J, Osanyintuji SO, Awojobi AO. Semen analysis in Ibadan. Niger Med J, 1982, 12, 181–186 [Google Scholar]
- 4. Nelson CM, Bunge RG. Semen analysis: evidence for changing parameters of male fertility potential. Fertil Steril, 1974, 25 (6) 503–507 [DOI] [PubMed] [Google Scholar]
- 5. Beeley L Whitfield HN. Hendry WF. The unwanted effects of drugs on the male reproductive system. Textbook of genito‐urinary surgery, 1985. Edinburgh: Churchill Livingstone; 1198–1203 [Google Scholar]
- 6. Okanlawon AO, Ashiru OA. Stereological estimation of seminiferous tubular dysfunction in chloroquine treated rats. Afr J Med Med Sci, 1998, 27, 101–106 [PubMed] [Google Scholar]
- 7. Adeeko AO, Dada OA. Chloroquine reduces the fertilizing capacity of epididymal sperm in rats. Afr J Med Med Sci, 1998, 27, 63–68 [PubMed] [Google Scholar]
- 8. Cosentino MJ, Pallyz RE, Fried J. Pyrimethamine: an approach to the development of a male contraceptive. Proc Natl Acad Sci USA, 1990, 87 (4) 1431–1435 10.1073/pnas.87.4.1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raji Y, Osonuga IO, Akinsomisoye OA, Osonuga OO, Mewoyeka OO. Gonadotoxicity evaluation of oral artemisinin derivative in male rats. J Med Sci, 2005, 5 (4) 303–306 10.3923/jms.2005.303.306 [Google Scholar]
- 10. Benoff S, Cooper GW, Hurley I, Mandel FS, Rosenfeld DL, Scholl GM et al. The effect of calcium ion channel blockers on sperm fertilization potential. Fertil Steril, 1994, 62 (3) 606–617 [PubMed] [Google Scholar]
- 11. Goodwin LO, Leeds NB, Hurley I, Mandel FS, Pergolizzi RG, Benoff S. Isolation and characterization of the primary structure of testis‐specific L‐type calcium channel: implications for contraception. Mol Hum Reprod, 1997, 3, 255–268 10.1093/molehr/3.3.255 [DOI] [PubMed] [Google Scholar]
- 12. Katsoff D, Check JH. A challenge to the concept that the use of calcium channel blockers causes reversible male infertility. Hum Reprod, 1997, 12 (7) 1480–1482 10.1093/humrep/12.7.1480 [DOI] [PubMed] [Google Scholar]
- 13. Kanwar U, Batla A, Sanyal S. Gossypol inhibition of Ca11‐uptake and Ca11‐ATPase in human ejaculated spermatozoal membrane vesicles. Contraception, 1989, 39, 431–445 10.1016/0010‐7824(89)90121‐2 [DOI] [PubMed] [Google Scholar]
- 14. Kirkman‐Brown JC, Barratt CL, Publicover SJ. Slow calcium oscillations in human spermatozoa. Biochem J, 2004, 378 (3) 827–832 10.1042/BJ20031368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saha L, Bhargava VK, Garg SK, Majumdar S. Effect of nimodipine on male reproductive functions in rats. Indian J Physiol Pharmacol, 2000, 44 (4) 449–455 [PubMed] [Google Scholar]
- 16. Juneja R, Gupta I, Wali A, Sanyal SN, Chakravarti RN, Majumda S. Verapamil stimulates Ca11‐uptake and Ca11‐ATPase in plasma membrane vesicles of guinea pig spermatozoa. Contraception, 1990, 41, 419–429 10.1016/0010‐7824(90)90041‐S [DOI] [PubMed] [Google Scholar]
- 17. Lee JH, Kim H, Kim DH, Gye MC. Effects of calcium channel blockers on the spermatogenesis and gene expression in peripubertal mouse testis. Arch Androl, 2006, 52 (4) 311–318 10.1080/01485010600664024 [DOI] [PubMed] [Google Scholar]
- 18. Almeida SA, Teofilo JM, Anselmo Franci JA, Brentegani LG, Lamano‐Carvalho TL. Antireproductive effects of the calcium channel blocker amlodipine in male rats. Exp Toxicol Pathol, 2000, 52 (4) 353–356 [DOI] [PubMed] [Google Scholar]
- 19. Raji Y, Oloyo AK, Morakinyo AO. Studies on the reproductive activities of methanol extract of Ricinus Communis Seed (Linn) on male albino rats. Asian J Androl, 2006, 8 (1) 115–121 10.1111/j.1745‐7262.2006.00055.x [DOI] [PubMed] [Google Scholar]
- 20. Wei XY, Perez‐Reyes E, Lacerda AE, Schuster G, Brown AM, Birnbaumer L. Heterologous regulation of the cardiac Ca2+ channel alpha 1 subunit by skeletal muscle beta and gamma subunits: Implications for the structure of cardiac L‐type Ca21 channels. J Biol Chem, 1991, 266, 21943–21947 [PubMed] [Google Scholar]
- 21. McAllister RG, Schloemer GL, Hamann SR. Kinetics and dynamics of calcium entry agents in systemic hypertension. Am J Cardiol, 1986, 57, 16D–21D 10.1016/0002‐9149(86)90800‐3 [DOI] [PubMed] [Google Scholar]
- 22. Babcock DF, Pfeiffer DR. Independent elevation of cytosolic [Ca2+] and pH of mammalian sperm by voltage‐dependent and pH‐sensitive mechanisms. J Biol Chem, 1987, 262, 15041–15047 [PubMed] [Google Scholar]
- 23. Cox T, Peterson RN. Identification of calcium conducting channels in isolated boar sperm plasma membranes. Biochem Biophys Res Commun, 1989, 161, 162–168 10.1016/0006‐291X(89)91575‐1 [DOI] [PubMed] [Google Scholar]
- 24. Vaghy PL, Williams JS, Schwartz A. Receptor pharmacology of calcium entry blocking agents. Am J Cardiol, 1987, 59, 9A–17A 10.1016/0002‐9149(87)90170‐6 [DOI] [PubMed] [Google Scholar]
- 25. Westenbroek RE, Babcock DF. Discrete regional distributions suggest diverse functional roles of calcium channel alpha1 subunits in sperm. Dev Biol, 1999, 207, 457–469 10.1006/dbio.1998.9172 [DOI] [PubMed] [Google Scholar]
- 26. Breitbart HS, Rubinstein S, Nass‐Arden L. The role of calcium and Ca2+‐ATPase in maintaining motility in ram spermatozoa. J Biol Chem, 1985, 260 (21) 11548–11553 [PubMed] [Google Scholar]
- 27. Mortimer D Keel BA. Webster BW. Objective analysis of sperm motility and kinematics. CRC handbook of the laboratory diagnosis and treatment of infertility, 1990. Boca Raton: CRC Press; 97–133 [Google Scholar]
- 28. Benoff S. Modelling human sperm–egg interactions in vitro: signal transduction pathways regulating the acrosome reaction. Mol Hum Reprod, 1998, 4 (5) 453–471 10.1093/molehr/4.5.453 [DOI] [PubMed] [Google Scholar]
- 29. Russell LD, Alger LF, Naquin LG. Hormonal control of pubertal spermatogenesis. Endocrinology, 1987, 120, 1615–1632 10.1210/endo‐120‐4‐1615 [DOI] [PubMed] [Google Scholar]


