Abstract
Aim: Although it is accepted that progesterone (P) induces acrosome reaction through non‐genomic regulation, it is not well known if P also affects hyperactivation of sperm.
Methods: Hamster spermatozoa were hyperactivated by incubation for 4 h on modified Tyrode's albumin lactate pyruvate medium and recorded on a DVD via a charge‐coupled device camera attached to a microscope with phase‐contrast illumination and a small CO2 incubator. Phosphorylation of proteins was detected by western blotting using antiphosphotyrosine antibodies.
Results: Sperm hyperactivation was significantly increased and accelerated by a non‐genomic signal of P. Although acceleration of motility of hyperactivated sperm occurred with 10, 20 and 40 ng/mL P, the most effective concentration was 20 ng/mL. Progesterone also significantly increased 80‐kDa tyrosine phosphorylation of sperm proteins. Both extracellular Ca2+ and albumin were essential for sperm hyperactivation, and the former was also essential for maintaining sperm flagellar movement. Moreover, phospholipase C (PLC) was associated with the regulation of hyperactivation by P.
Conclusion: It is likely that P regulates sperm hyperactivation by a non‐genomic signal in relation to tyrosine phosphorylation and PLC. (Reprod Med Biol 2008; 7: 63–74)
Keywords: capacitation, hyperactivation, non‐genomic regulation, progesterone, spermatozoa
INTRODUCTION
CAPACITATED SPERMATOZOA EXHIBIT acrosome reaction (AR) and hyperactivation and can fertilize an egg. The AR is a modified exocytotic event involving the acrosome, a large, secretory, granule‐like organelle in the sperm head, and the overlying sperm plasma membrane. 1 The AR is required for penetration of the zona pellucida (ZP) of an egg, which is a glycoprotein envelope, and for sperm–egg plasma membrane fusion. 2 Hyperactivation is a specialized movement of the sperm flagellum creating a propulsive force for passing through the ZP. Hyperactivated spermatozoa exhibit a large bend amplitude, whiplash and frenzied flagellar movements. 2 , 3 , 4
Albumin, calcium (Ca2+) and bicarbonate 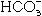 are essential molecules in the process of capacitation of mammalian spermatozoa. Albumin promotes capacitation by removing cholesterol from the sperm plasma membrane.
5
The fatty acids bound to albumin stimulate capacitation by increasing the incorporation and oxidation of glucose.
6
Ca2+ is involved in the intracellular signals
7
,
8
,
9
,
10
and
are essential molecules in the process of capacitation of mammalian spermatozoa. Albumin promotes capacitation by removing cholesterol from the sperm plasma membrane.
5
The fatty acids bound to albumin stimulate capacitation by increasing the incorporation and oxidation of glucose.
6
Ca2+ is involved in the intracellular signals
7
,
8
,
9
,
10
and 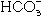 stimulates adenylate cyclase to increase cyclic adenosine monophosphate (cAMP).
11
During stimulation by essential components, many sperm proteins are phosphorylated during capacitation and it has been suggested that 80‐kDa tyrosine phosphorylation, in particular, is closely associated with the process.
12
,
13
,
14
,
15
Moreover, 80‐kDa tyrosine phosphorylation is regulated by Ca2+‐calmodulin‐dependent signals
16
and protein phosphatase 1/2A.
13
Spermatozoa are capacitated using the abovementioned signals and exhibit AR and hyperactivation.
stimulates adenylate cyclase to increase cyclic adenosine monophosphate (cAMP).
11
During stimulation by essential components, many sperm proteins are phosphorylated during capacitation and it has been suggested that 80‐kDa tyrosine phosphorylation, in particular, is closely associated with the process.
12
,
13
,
14
,
15
Moreover, 80‐kDa tyrosine phosphorylation is regulated by Ca2+‐calmodulin‐dependent signals
16
and protein phosphatase 1/2A.
13
Spermatozoa are capacitated using the abovementioned signals and exhibit AR and hyperactivation.
Progesterone (P), which is found in the follicular fluid, stimulates the AR of human spermatozoa, 17 and although P generally controls cell functions through genomic signals, it stimulates the AR by non‐genomic regulation. 18 , 19 , 20 In human spermatozoa, P stimulates Ca2+ influx, tyrosine phosphorylation, chloride efflux and cAMP increase. 19 , 20 , 21 It also stimulates ZP penetration and the AR in hamster spermatozoa. 22 , 23 Although the classic intracellular progesterone receptor (PR) does not exist in spermatozoa, a PR exists in the cell membrane. 18 , 19 , 20 , 24 , 25 Moreover, it has been suggested that P bound to the acrosome region and the PR are localized in the same region on human spermatozoa. 26 Phospholipase Cδ4 (PLCδ4) 27 and/or protein kinase A (PKA) 28 are involved in P‐induced AR. Progesterone also changes the parameters of motility 29 and enhances hyperactivation 30 on human spermatozoa. However, it is unclear whether P affects hyperactivation of rodent spermatozoa. Libersky and Boatman 22 suggested that only 20 ng/mL of P significantly increased hamster sperm penetration of the ZP, and they reported that the P concentration in hamster serum ranged from 5.56 to 12.85 ng/mL, the follicular fluid concentration was 4.2–7.4 µg/mL and the oviductal fluid concentration was 44.04–175.06 ng/mL 31 Although the effective concentration of P for inducing the AR is several micrograms per milliliter, concentrations at this level did not significantly increase hamster sperm penetration. 22 Moreover, 20 ng/mL of P did not induce the AR. 22
In the present study, we examined whether P affects hamster sperm hyperactivation and if it is regulated through a non‐genomic signal.
MATERIALS AND METHODS
Chemicals
ANTI‐PHOSPHOTYROSINE MONOCLONAL ANTI BODY (clone PT‐66), P, fluorescein isothiocyanate and bovine serum albumin conjugated progesterone (FITC/BAS‐P) and RU486 (mifepristone, 11b‐(4‐Dimethylamino)phenyl‐17b‐hydroxy‐17‐(1‐propynyl)estra‐4,9‐dien‐3‐one) were purchased from Sigma Chemical Company (St Louis, MO, USA). U73122 (1‐[6‐((17β‐3‐Methoxyestra‐1,3,5(10)‐trien‐17‐yl)amino)hexyl]‐1H‐pyrrole‐2,5‐dione), U73343 (1‐[6‐((17b‐3‐Methoxyestra‐1,3,5(10)‐trien‐17‐yl)amino)hexyl]‐2,5‐pyrrolidinedione), D609 potassium salt (Tricyclodecan‐9‐yl‐xanthogenate, K), ET‐18‐OCH3 (Edelfosine, 1‐O‐Octadecyl‐2‐O‐methyl‐rac‐glycero‐3‐phosphorylcholine), neomycine sulfate and spermine tetrahydrochloride were purchased from Merck KGaA (Darmstadt, Germany). Polyvinylidine difluoride (PVDF) membrane was purchased from Millipore (Bedford, MA, USA). An ECL Plus kit and films for electrochemoluminescence were purchased from Amersham Biosciences (Piscataway, NJ, USA). The molecular weight marker set was purchased from Invitrogen (Carlsbad, CA, USA). Bovine serum albumin (BSA), fraction V and other chemicals of reagent grade were purchased from Wako Pure Chemical Industries (Osaka, Japan).
Animals and preparation of hyperactivated spermatozoa
Spermatozoa were obtained from the caudal epididymis of sexually mature (12–20‐week‐old) male golden hamsters (Mesocricetus auratus).
Hyperactivated spermatozoa were prepared according to the method described by Si and Okuno 13 using modified Tyrode's albumin lactate pyruvate (mTALP) medium containing 101.02 mmol/L NaCl, 2.68 mmol/L KCl, 2 mmol/L CaCl2, 1.5 mmol/L MgCl2–6H2O, 0.36 mmol/L NaH2PO4–2H2O, 35.70 mmol/L NaHCO3, 4.5 mmol/L d‐glucose, 0.09 mmol/L sodium pyruvate, 9 mmol/L sodium lactate, 0.5 mmol/L hypotaurine, 0.05 mmol/L (–)epinephrine, 0.2 mmol/L sodium taurochoric acid, 5.26 mmol/L sodium metabisulfite, 0.05%(w/v) streptomycine sulfate, 0.05%(w/v) potassium penicillin G and 15 mg/mL BSA (pH 7.4 at 37°C under 5% CO2 in air). An aliquot of caudal epididymal spermatozoa was placed at the bottom of a test tube and several 1 mL of mTALP medium was carefully added before incubation for 5 min to allow the spermatozoa to swim up. The supernatant containing motile spermatozoa was collected, placed on a culture plate (35‐mm dish) and incubated for 4 h at 37°C under 5% CO2 in air to accomplish hyperactivation. Progesterone and inhibitors dissolved in ethanol (EtOH) were added to the mTALP medium after placing the motile spermatozoa on the culture plate. In all experiments the final concentration of EtOH was 0.1%.
Measurement of the motility and hyperactivation of spermatozoa
Motility and hyperactivation measurements were carried out according to the method described in our previous studies. 14 , 32 Hamster spermatozoa suspended in mTALP medium were diluted 10‐fold and placed on a culture plate (35‐mm dish). Sperm motility and hyperactivation were recorded on a DVD via a charge‐coupled device camera (Progressive 3CCD; Sony, Tokyo, Japan) attached to a microscope (IX70; Olympus, Tokyo, Japan) with phase‐contrast illumination and a small CO2 incubator (MI‐IBC; Olympus). Each observation was carried out at 37°C, recorded for 2 min, and analysis involved counting the numbers of total spermatozoa, motile spermatozoa and hyperactivated spermatozoa in 30 different fields. Motile spermatozoa (%) and hyperactivated spermatozoa (%) were defined as the number of motile spermatozoa/number of total spermatozoa) × 100 and the number of hyperactivated spermatozoa/numbers of motile spermatozoa) × 100, respectively. Statistical analysis was carried out using anova and post‐hoc tests.
Ligand assay of progesterone
The ligand assay of P was carried out using the FITC/BSA‐P as follows. An aliquot of caudal epididymal spermatozoa was placed at the bottom of a test tube and 1 mL of mTALP with FITC/BSA‐P was carefully added before incubation for 5 min to allow the spermatozoa to swim up. The supernatant containing motile spermatozoa was collected, placed on the culture dish and incubated for 5 min at 37°C under 5% CO2 in air. After incubation, 10 µL of the supernatant was placed on a glass slide without fluorescence and observed under a microscope (IX70; Olympus) with phase‐contrast illumination and a fluorescence unit.
Preparation of sperm protein extracts
Sperm proteins were extracted according to a previously described method. 32 In brief, spermatozoa were suspended at 30 mg/mL in a urea solution containing 7 mol/L urea and 10% 2‐mercaptoethanol. After pipetting, the suspension was incubated on ice for 10 min. Following centrifugation at 15 000 g for 10 min at 4°C, the supernatant was used as the urea extract. The precipitate was resuspended in the same volume of urea–thiourea solution containing 5 mol/L urea, 1 mol/L thiourea, 10% 2‐mercaptoethanol and 2% NP‐40 as the urea solution. After pipetting, the suspension was incubated on ice for 10 min, centrifuged at 15 000 g for 10 min at 4°C and the supernatant was used as the urea–thiourea extract.
Sodium dodecylsulfate‐polyacrylamide gel electrophoresis
Sodium dodecylsulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) was carried out according to the method of Laemmli 33 using a separating gel of 10% (w/v) polyacrylamide containing 0.1% (w/v) SDS.
Western blotting
Western blotting was carried out according to a previously described method. 14 , 32 The blotted membrane was blocked with 5% (w/v) skim milk in Tris buffered saline (TBS) containing 0.15 mol/L NaCl and 20 mmol/L Tris‐HCl (pH 7.5) for 1 h at 20°C, and incubated with primary antibody (1:1000 dilution) with 5% (w/v) skim milk in TBS for 1 h at 20°C. After a TBS wash, the membrane was incubated with secondary antibody conjugated peroxidase (1:5000 dilution) with 5% (w/v) skim milk in TBS. Color reaction was carried out using the ECL Plus kit. The reactivity of western blotting was measured using a densitometer (GS‐800 densitometer; Bio‐Rad Laboratories, Hercules, CA, USA) and analyzed using Quantity One Software version 4.6.1 (Bio‐Rad Laboratories) and anovas and post‐hoc tests were carried out.
RESULTS
Effects of progesterone on hamster sperm hyperactivation
ALTHOUGH 20 NG/ML OF P did not affect the rate of motile spermatozoa at all (data not shown), the number of hyperactivated spermatozoa significantly increased with 20 ng/mL P after incubation for 1.5, 2 or 2.5 h (Fig. 1a). Increased and accelerated sperm hyperactivation by P was significantly inhibited by the addition of RU486, which is a PR antagonist (Fig. 1a), but RU486 did not affect the rate of motile spermatozoa at all (data not shown).
Figure 1.
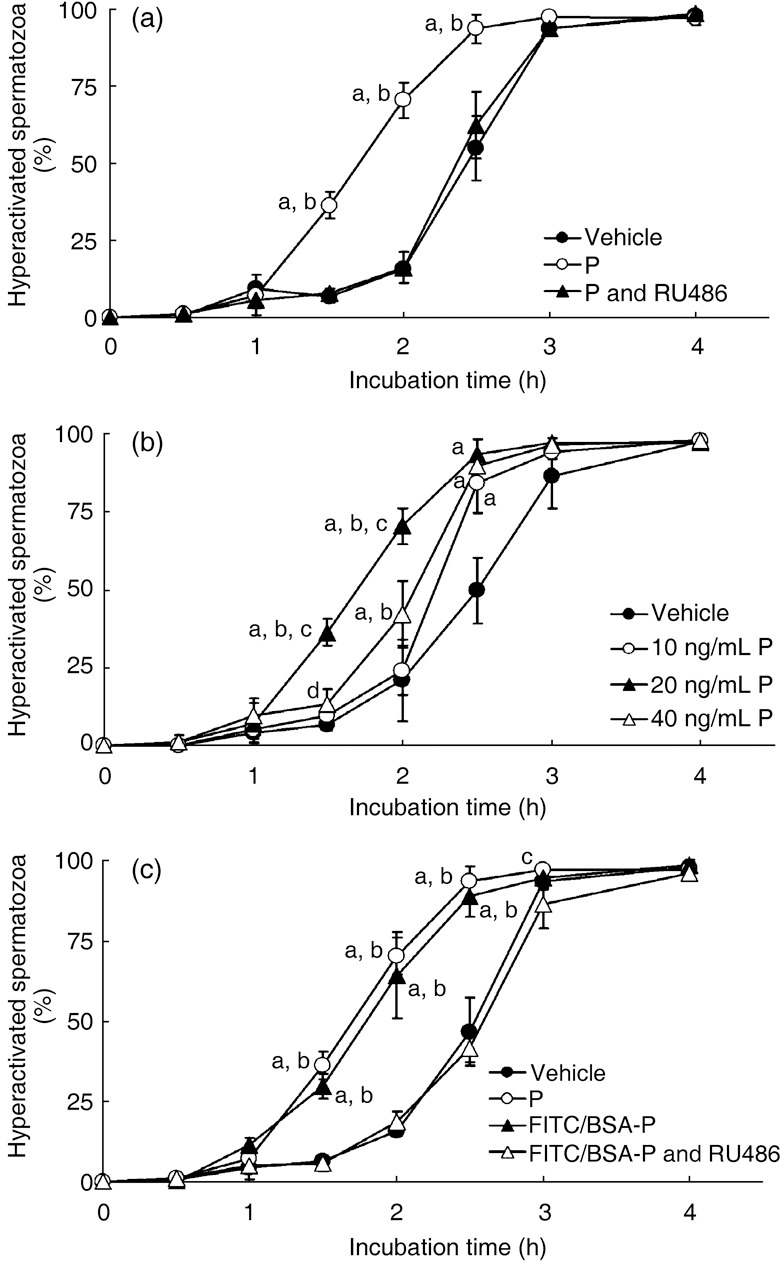
Effects of progesterone (P) on hamster sperm hyperactivation. (a) Significant increase with 20 ng/mL P. Values are means ± standard deviation. Modified Tyrode's albumin lactate pyruvate (mTALP) + 0.1% ethanol (EtOH) (vehicle,  ), mTALP + 20 ng/mL P + 0.1% EtOH (P;
), mTALP + 20 ng/mL P + 0.1% EtOH (P;  ) and mTALP + 20 ng/mL P + 23.4 µmol/L RU486 + 0.1% EtOH (P and RU486,
) and mTALP + 20 ng/mL P + 23.4 µmol/L RU486 + 0.1% EtOH (P and RU486,  ). aSignificantly different from vehicle (P < 0.01); bsignificantly different from P and RU486 (P < 0.01). (b) Sperm hyperactivation significantly increased by P in a concentration‐dependent manner. Values are means ± standard deviation. Vehicle (
). aSignificantly different from vehicle (P < 0.01); bsignificantly different from P and RU486 (P < 0.01). (b) Sperm hyperactivation significantly increased by P in a concentration‐dependent manner. Values are means ± standard deviation. Vehicle ( ), 10 ng/mL P (
), 10 ng/mL P ( ), 20 ng/mL P (
), 20 ng/mL P ( ) and 40 ng/mL P (
) and 40 ng/mL P ( ). aSignificantly different from vehicle (P < 0.01); bsignificantly different from 10 ng/mL P (P < 0.01); csignificantly different from 40 ng/mL P (P < 0.01); dsignificantly different from vehicle (P < 0.05). (c) Sperm hyperactivation increased by non‐genomic signals of P. Values are means ± standard deviation. Vehicle (
). aSignificantly different from vehicle (P < 0.01); bsignificantly different from 10 ng/mL P (P < 0.01); csignificantly different from 40 ng/mL P (P < 0.01); dsignificantly different from vehicle (P < 0.05). (c) Sperm hyperactivation increased by non‐genomic signals of P. Values are means ± standard deviation. Vehicle ( ), 20 ng/mL P (
), 20 ng/mL P ( ), mTALP + 7 nmol/L fluorescein isothiocyanate and bovine serum albumin conjugated progesterone + 0.1% EtOH (FITC/BSA‐P,
), mTALP + 7 nmol/L fluorescein isothiocyanate and bovine serum albumin conjugated progesterone + 0.1% EtOH (FITC/BSA‐P,  ) and mTALP + 7 nmol/L FITC/BSA‐P + 23.4 µmol/L RU486 + 0.1% EtOH (FITC/BSA‐P and RU486,
) and mTALP + 7 nmol/L FITC/BSA‐P + 23.4 µmol/L RU486 + 0.1% EtOH (FITC/BSA‐P and RU486,  ). aSignificantly different from vehicle (P < 0.01); bsignificantly different from FITC/BSA‐P and RU486 (P < 0.01); csignificantly different from FITC/BSA‐P and RU486 (P < 0.05). Experiments were carried out four times using four hamsters.
). aSignificantly different from vehicle (P < 0.01); bsignificantly different from FITC/BSA‐P and RU486 (P < 0.01); csignificantly different from FITC/BSA‐P and RU486 (P < 0.05). Experiments were carried out four times using four hamsters.
We examined the effects of differing P concentrations on hyperactivation. Although the rate of motile spermatozoa was unaffected (data not shown), the rate of hyperactivation of spermatozoa was affected by P in a concentration‐dependent manner (Fig. 1b). After incubation for 1.5 or 2 h, both 20 ng/mL and 40 ng/mL P significantly increased sperm hyperactivation compared with mTALP medium with 0.1% EtOH (Fig. 1b). In addition, 20 ng/mL P significantly increased hyperactivation compared with 10 ng/mL and 40 ng/mL P after incubation for 1.5 or 2 h (Fig. 1b), and 40 ng/mL P significantly increased hyperactivation compared with 10 ng/mL P after incubation for 2 h (Fig. 1b). In contrast, 10 ng/mL P was ineffective after incubation for 1.5 or 2 h. After incubation for 2.5 h, each concentration of P significantly increased sperm hyperactivation compared with the control (Fig. 1b) and after incubation for 3 or 4 h, there were no significant differences among each P concentration and the control.
Increase in sperm hyperactivation by non‐genomic regulation
Because P increased sperm hyperactivation (Fig. 1a), we used FITC/BSA‐P to determine whether hamster spermatozoa were hyperactivated through non‐genomic regulation by P. In general, P enters the cell, binds to the intracellular PR and induces gene expression. 18 , 19 , 20 However, FITC/BSA‐P cannot enter the cell because BSA blocks cellular entry of P. 34 Therefore, we consider that the effects of FITC/BSA‐P occurred through non‐genomic signals.
As for the rate of motile spermatozoa, 7 nmol/L FITC/BSA‐P, which is converted into approximately 20 ng/mL P, had no effect at all (data not shown). However, it significantly increased and accelerated hamster sperm hyperactivation compared with mTALP medium alone after incubation for 1.5, 2 or 2.5 h (Fig. 1c). There were no significant differences between 7 nmol/L FITC/BSA‐P and 20 ng/mL P. Increased and accelerated hyperactivation by FITC/BSA‐P was significantly inhibited by RU486 (Fig. 1c).
Binding of progesterone to hamster spermatozoa
Because it has been suggested that FITC/BSA‐P binds to the acrosome region of human spermatozoa, 26 we wanted to observe where in hamster spermatozoa the FITC/BSA‐P was bound (Fig. 2). Although fluorescence was observed in the acrosomal region of the sperm head and the middle piece of the sperm flagellum (Fig. 2), it appeared that the fluorescence of the flagellum was autofluorescence (Fig. 2h,j). As shown in Figure 2b,e, 7 nmol/L FITC/BSA‐P was bound to the acrosomal region of the sperm head. Binding of P to the sperm head was inhibited by RU486 (Fig. 2g,i). Moreover, both the site of binding of 3.5 nmol/L FITC/BSA‐P, which is converted into approximately 10 ng/mL P (Fig. 2a,d) and 14 nmol/L FITC/BSA‐P, which is converted into approximately 40 ng/mL P (Fig. 2c,f), was similar to the binding of 7 nmol/L FITC/BSA‐P (Fig. 2b,e).
Figure 2.
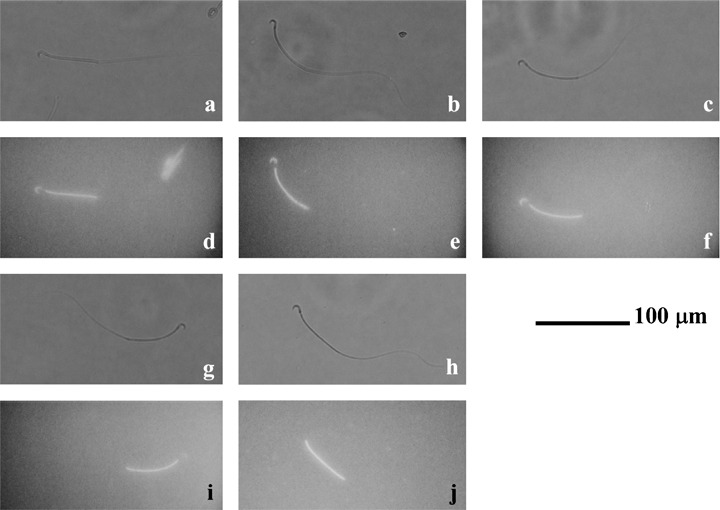
Binding of progesterone (P) to sperm heads. (a,d) Hamster spermatozoa incubated in modified Tyrode's albumin lactate pyruvate (mTALP) with 3.5 nmol/L fluorescein isothiocyanate and bovine serum albumin conjugated progesterone (FITC/BSA‐P), which converted into approximately 10 ng/mL P, and 0.1% ethanol (EtOH). (b,e) Hamster spermatozoa incubated in mTALP with 7 nmol/L FITC/BSA‐P and 0.1% EtOH. (c,f) Hamster spermatozoa incubated in mTALP with 14 nmol/L FITC/BSA‐P, which converted into approximately 40 ng/mL P, and 0.1% EtOH. (g,i) Hamster spermatozoa incubated in mTALP with 7 nmol/L FITC/BSA‐P, 23.4 µmol/L RU486 and 0.1% EtOH. (h,j) Hamster spermatozoa incubated in vehicle. (a–c,g,h) Observed under a light field; (d–f,i,j) observed under a fluorescent field. Fluorescence of the mitochondria sheath in the flagellum was autofluorescence. Bar represents 100 µm.
Effects of progesterone on tyrosine phosphorylation
As it is accepted that 80‐kDa tyrosine phosphorylation is associated with the regulation of sperm hyperactivation, 12 , 13 , 14 , 15 we examined whether 80‐kDa tyrosine phosphorylation was also increased when hyperactivation was increased by P. In our previous study, 32 four tyrosine phosphorylations were detected in two types of protein extracts obtained from hamster spermatozoa. As shown in Figure 3, in the present study four tyrosine phosphorylations were detected in two types of protein extracts, and were clearly increased by 20 ng/mL P during hyperactivation. The lower band of the two tyrosine phosphorylations, which was detected in the urea extracts, started to be detected after 1 h incubation in mTALP medium with P (Fig. 3c,f lanes a–f), although it was detected only after incubation for 4 h when P was not added to the medium (Fig. 3b,f lanes a–f). The upper band was slightly increased when P was supplied in the medium (Fig. 3b,c,e lanes a–f). The increase and acceleration of the phosphorylations were completely inhibited by RU486 (Fig. 3d–f lanes a–f). The other two tyrosine phosphorylations, which were detected in the urea–thiourea extracts, were also increased and accelerated by P (Fig. 3b,c lanes g–l). The lower band of those two tyrosine phosphorylations was accelerated and largely increased after incubation for 1 h when P was supplied in the medium (Fig. 3b,c,f lanes g–l). Tyrosine phosphorylation of the upper band was also largely increased by P (Fig. 3b,c,e lanes g–l). Those responses were partially inhibited by RU486 (Fig. 3d–f lanes g–l).
Figure 3.
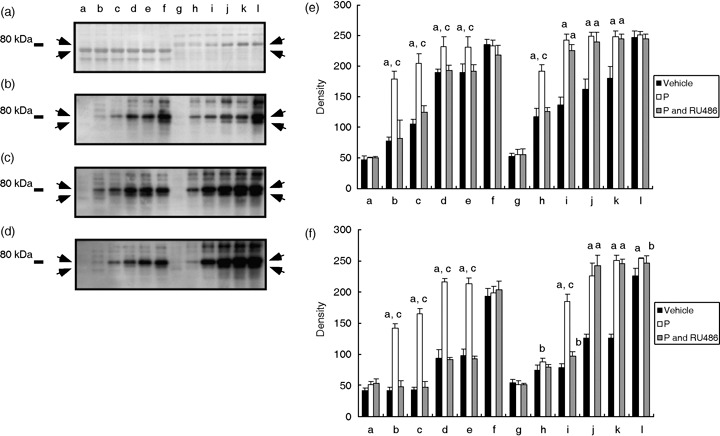
Acceleration and increasing of tyrosine phosphorylation of 80‐kDa sperm proteins by progesterone (P). (a) Typical Coomassie Brilliant Blue‐stained membrane after blotting. (b) Western blotting against proteins obtained from spermatozoa that were incubated in vehicle. (c) Western blotting against proteins obtained from spermatozoa that were incubated in 20 ng/mL P (P). (d) Western blotting against proteins obtained from spermatozoa that were incubated in 20 ng/mL P and RU486 (P and RU486). Arrows show tyrosine phosphorylation of 80‐kDa sperm proteins. Numbers on the left‐hand side indicate the molecular weight standard of 80 kDa. (e) Density of upper bands detected on (b–d). (F) Density of lower bands detected on (b–d). aSignificantly different than vehicle (P < 0.01); bsignificantly different than vehicle (P < 0.05); csignificantly different than P and RU486 (P < 0.01). Lanes a–f and g–l illustrate the results from urea extract and urea–thiourea extract, respectively. Lanes a and g were incubated for 0 h after supplying P. Lanes b and h were incubated for 0.5 h, lanes c and i were incubated for 1 h, lanes d and j were incubated for 2 h, lanes e and k were incubated for 3 h, and lanes f and l were incubated for 4 h.
Involvement of Ca2+ and albumin in the non‐genomic regulation of hyperactivation by progesterone
It is accepted that Ca2+ is an essential component for sperm function 2 , 3 , 4 , 10 and is a key molecule in non‐genomic regulation by P. 18 , 19 , 20 As shown in Figure 4a, the rate of motile hamster spermatozoa significantly decreased after incubation for 1.5 or 2 h when the spermatozoa were suspended in mTALP medium without Ca2+, even if 20 ng/mL P was added (Fig. 4a). After incubation for 2.5, 3 or 4 h, most of the spermatozoa could not move in the mTALP without Ca2+, even if 20 ng/mL P was added (Fig. 4a). As shown in Figure 4b, hamster spermatozoa could not be hyperactivated at all in mTALP without Ca2+ or in mTALP without Ca2+, but with 20 ng/mL P added.
Figure 4.
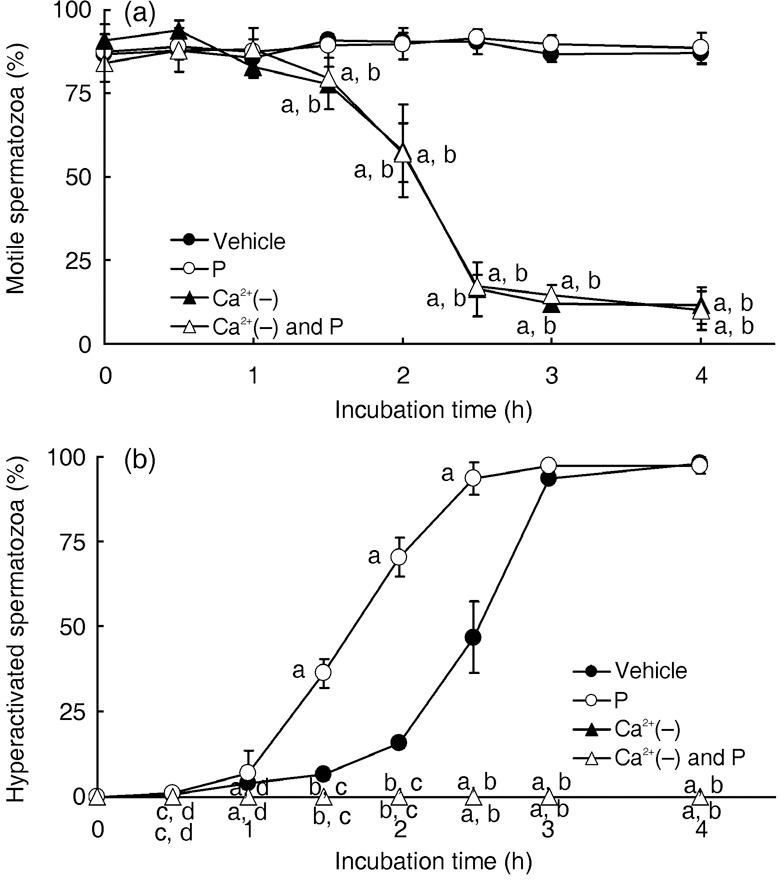
Effects of Ca2+ on sperm motility and hyperactivation. Rates of motile spermatozoa (a) and hyperactivated spermatozoa (b). Values are means ± standard deviation. Vehicle ( ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ), modified Tyrode's albumin lactate pyruvate (mTALP) without Ca2+ and with added 1 mmol/L ethyleneglycol bis(2‐aminoethyl ether)tetraacetic acid (EGTA) + 0.1% EtOH (Ca2+(–),
), modified Tyrode's albumin lactate pyruvate (mTALP) without Ca2+ and with added 1 mmol/L ethyleneglycol bis(2‐aminoethyl ether)tetraacetic acid (EGTA) + 0.1% EtOH (Ca2+(–),  ), mTALP without Ca2+ and with added 20 ng/mL P + 1 mmol/L EGTA + 0.1% EtOH (Ca2+(–) and P,
), mTALP without Ca2+ and with added 20 ng/mL P + 1 mmol/L EGTA + 0.1% EtOH (Ca2+(–) and P,  ). aSignificantly different from vehicle (P < 0.01); bsignificantly different from P (P < 0.01); csignificantly different from vehicle (P < 0.05); dsignificantly different from P (P < 0.05). Experiments were carried out four times using four hamsters.
). aSignificantly different from vehicle (P < 0.01); bsignificantly different from P (P < 0.01); csignificantly different from vehicle (P < 0.05); dsignificantly different from P (P < 0.05). Experiments were carried out four times using four hamsters.
It is also accepted that albumin is a key molecule in capacitation. 5 , 6 Although albumin did not affect the rate of motile spermatozoa at all in the present study (data not shown), hamster spermatozoa were not hyperactivated in mTALP medium without BSA, even if 20 ng/mL P was added (Fig. 5).
Figure 5.
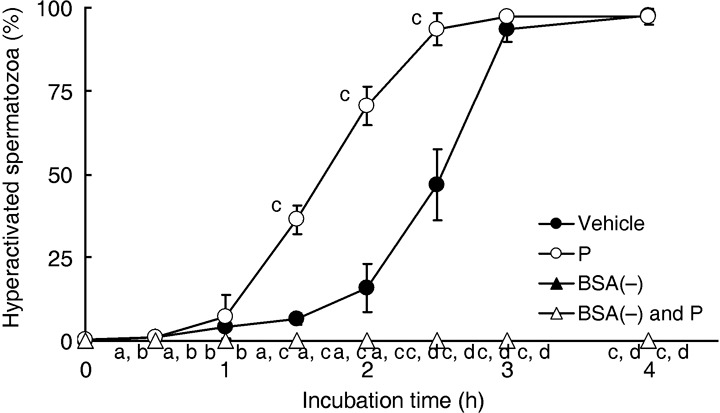
Effects of albumin on sperm hyperactivation. Values are means ± standard deviation. Vehicle ( ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ), modified Tyrode's albumin lactate pyruvate (mTALP) without bovine serum albumin (BSA) and with added 0.1% ethanol (EtOH) (BSA(–),
), modified Tyrode's albumin lactate pyruvate (mTALP) without bovine serum albumin (BSA) and with added 0.1% ethanol (EtOH) (BSA(–),  ) and mTALP without BSA and with added 20 ng/mL P + 0.1% EtOH (BSA(–) and P,
) and mTALP without BSA and with added 20 ng/mL P + 0.1% EtOH (BSA(–) and P,  ). aSignificantly different from vehicle (P < 0.05); bsignificantly different from P (P < 0.05); csignificantly different from vehicle (P < 0.01); dsignificantly different from P (P < 0.01). Experiments were carried out four times using four hamsters.
). aSignificantly different from vehicle (P < 0.05); bsignificantly different from P (P < 0.05); csignificantly different from vehicle (P < 0.01); dsignificantly different from P (P < 0.01). Experiments were carried out four times using four hamsters.
Involvement of phospholipase C in the non‐genomic regulation of hyperactivation by progesterone
When P induces the AR, it has been suggested that PLC is a key molecule. 18 , 19 , 20 , 27 Fukami et al. suggested that PLCδ4 is associated with the regulatory mechanism of P‐induced AR in mouse spermatozoa, 27 but in the present study U73122, which is an inhibitor of PLCδ, and U73343, which is a negative control of U73122, did not affect sperm hyperactivation itself or P‐induced sperm hyperactivation at all (Fig. 6a), nor did they affect sperm motility (data not shown). D609, which is a phosphatidylcholine‐PLC (PC‐PLC) specific inhibitor, did not inhibit the rate of motile spermatozoa, even if 20 ng/mL P was added to the mTALP medium (data not shown), nor did it inhibit sperm hyperactivation itself in mTALP medium without P (Fig. 6b). As shown in Figure 6b, however, D609 significantly inhibited only P‐induced sperm hyperactivation. ET‐18‐OCH3, which is a phosphatidylinositol‐PLC (PI‐PLC) specific inhibitor, also did not inhibit the rate of motile spermatozoa even when 20 ng/mL P was added to the mTALP medium (data not shown). In mTALP medium without P, sperm hyperactivation was not at all inhibited by ET‐18‐OCH3 (Fig. 6c). Progesterone‐induced hyperactivation, however, was significantly inhibited by ET‐18‐OCH3 (Fig. 6c). In addition, neomycine, which is a non‐specific inhibitor of PLC and a PC‐phospholipase D (PC‐PLD) inhibitor, did not inhibit the rate of motile spermatozoa at all (data not shown), nor did it inhibit sperm hyperactivation itself in mTALP medium without P (Fig. 6d). When 20 ng/mL P was added to the mTALP medium, neomycine significantly inhibited only P‐induced hyperactivation (Fig. 6d). Spermine, which is an inhibitor of PLCα and an activator of PLCδ, did not affect the rate of motile hamster spermatozoa at all, even if 20 ng/mL P was added to the mTALP medium (data not shown). Although spermine did not inhibit sperm hyperactivation in mTALP medium without P, it significantly inhibited P‐induced hyperactivation (Fig. 6e).
Figure 6.
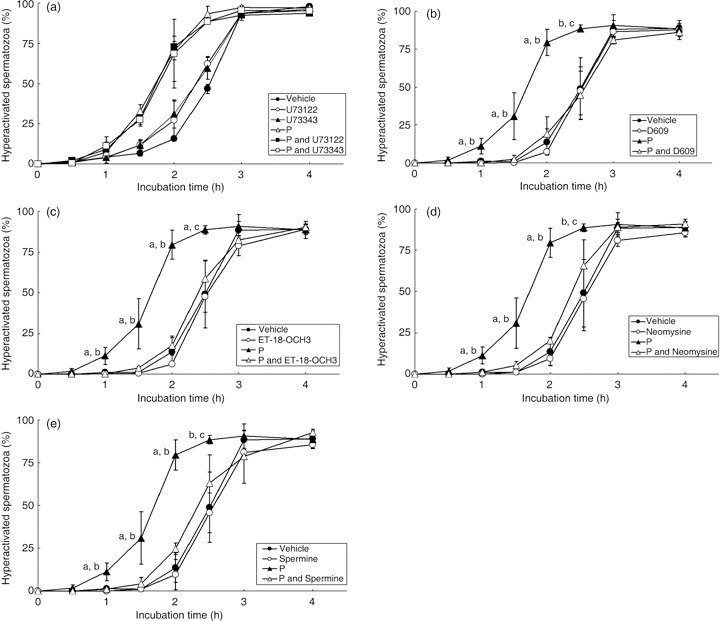
Effects of phospholipase C (PLC) inhibitors on sperm hyperactivation. (a) U73122, which is an inhibitor of PLCδ, and U73343, which is a negative control of U73122, were added to the modified Tyrode's albumin lactate pyruvate (mTALP) medium. Values are means ± standard deviation. Vehicle ( ), 1 µmol/L U73122 (U73122,
), 1 µmol/L U73122 (U73122,  ), 1 µmol/L U73343 (U73343,
), 1 µmol/L U73343 (U73343,  ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ), 20 ng/mL P + 1 µmol/L U73122 (P and U73122,
), 20 ng/mL P + 1 µmol/L U73122 (P and U73122,  ) and 20 ng/mL P + 1 µmol/L U73343 (P and U73343,
) and 20 ng/mL P + 1 µmol/L U73343 (P and U73343,  ). (b) D609, which is an inhibitor of phosphatidylcholine‐PLC (PC‐PLC), was added to the mTALP medium. Values are means ± standard deviation. Vehicle (
). (b) D609, which is an inhibitor of phosphatidylcholine‐PLC (PC‐PLC), was added to the mTALP medium. Values are means ± standard deviation. Vehicle ( ), 10 µmol/L D609 (D609,
), 10 µmol/L D609 (D609,  ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ), 20 ng/mL P + 10 µmol/L D609 (P and D609,
), 20 ng/mL P + 10 µmol/L D609 (P and D609,  ). (c) ET‐18‐OCH3, which is an inhibitor of phosphatidylinositol‐PLC (PI‐PLC), was added to the mTALP medium. Values are means ± standard deviation. Vehicle (
). (c) ET‐18‐OCH3, which is an inhibitor of phosphatidylinositol‐PLC (PI‐PLC), was added to the mTALP medium. Values are means ± standard deviation. Vehicle ( ), 15 µmol/L ET‐18‐OCH3 (ET‐18‐OCH3,
), 15 µmol/L ET‐18‐OCH3 (ET‐18‐OCH3,  ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ) and 20 ng/mL P + 15 µmol/L ET‐18‐OCH3 (P and ET‐18‐OCH3,
) and 20 ng/mL P + 15 µmol/L ET‐18‐OCH3 (P and ET‐18‐OCH3,  ). (d) Neomycine, which is a non‐specific inhibitor of PLC, was added to the mTALP medium. Values are means ± standard deviation. Vehicle (
). (d) Neomycine, which is a non‐specific inhibitor of PLC, was added to the mTALP medium. Values are means ± standard deviation. Vehicle ( ), 65 µmol/L neomycine (Neomycine,
), 65 µmol/L neomycine (Neomycine,  ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ) and 20 ng/mL P + 65 µmol/L neomycine (P and Neomycine,
) and 20 ng/mL P + 65 µmol/L neomycine (P and Neomycine,  ). (e) Spermine, which is an inhibitor of PLCα and activator of PLCδ, was added to the mTALP medium. Values are means ± standard deviation. Vehicle (
). (e) Spermine, which is an inhibitor of PLCα and activator of PLCδ, was added to the mTALP medium. Values are means ± standard deviation. Vehicle ( ), 1 mmol/L spermine (Spermine,
), 1 mmol/L spermine (Spermine,  ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ) and 20 ng/mL P + 1 mmol/L spermine (P and Spermine,
) and 20 ng/mL P + 1 mmol/L spermine (P and Spermine,  ). aSignificantly different from vehicle and inhibitor (P < 0.01); bsignificantly different from P and inhibitor (P < 0.01); csignificantly different from vehicle and inhibitor (P < 0.05); dsignificantly different from P and inhibitor (P < 0.05). Experiments were carried out four times using four hamsters.
). aSignificantly different from vehicle and inhibitor (P < 0.01); bsignificantly different from P and inhibitor (P < 0.01); csignificantly different from vehicle and inhibitor (P < 0.05); dsignificantly different from P and inhibitor (P < 0.05). Experiments were carried out four times using four hamsters.
DISCUSSION
IN HUMAN SPERMATOZOA, P induces the AR, penetration and hyperactivation; 17 , 18 , 19 , 20 , 21 , 29 , 30 however, in contrast, in rodent spermatozoa P only stimulates the AR and penetration. 22 , 23 , 27 In the spermatozoa of both these mammals, P stimulates Ca2+ influx, protein phosphorylation, chloride efflux and cAMP increase. 19 , 20 , 21 , 23 , 27 , 28 Moreover, it has been suggested that PLCδ is associated with the regulation of P‐induced AR, 27 but it is not known whether hyperactivation of rodent spermatozoa is also regulated by P.
Our results (Fig. 1) suggest that P significantly increases sperm hyperactivation and that the most effective concentration is 20 ng/mL. Libersky and Boatman 22 also found that only 20 ng/mL of P significantly increased sperm penetration, so it is likely that this is an important concentration for sperm function. The concentration of P in hamster serum ranges from 5.56 to 12.85 ng/mL and from 44.04 to 175.06 ng/mL in oviductal fluid, 31 so 20 ng/mL P is midway between the concentration in blood and oviductal fluid. Moreover, we report that FITC/BSA‐P significantly increased sperm hyperactivation and was bound to the acrosomal region of the sperm head (1, 2). Because FITC/BSA‐P cannot enter the cell, 34 this results suggests that the effects of P on spermatozoa occur via non‐genomic regulation, 18 , 19 , 20 probably through the membrane PR, 18 , 19 , 20 , 25 , 26 which we consider exists in the acrosomal region of the hamster sperm head.
Ca2+ is a key molecule in sperm functioning 7 , 8 , 9 , 10 and it has been suggested that sperm hyperactivation is regulated by Ca2+ because hyperactivated motility is induced by a Ca2+ ionophore. 35 We also observed that hamster spermatozoa needed extracellular Ca2+ to become hyperactivated and to maintain flagellar movement (Fig. 4). Ho et al. reported that intracellular Ca2+, which is released from Ca2+ stores, regulated hyperactivation, 8 , 36 , 37 and they also suggested that Ca2+ storage was an inositol 1,4,5‐trisphosphate receptor (IP3R)‐gated Ca2+ store located at the base of the sperm flagellum. 7 , 37 Moreover, it has been suggested that hyperactivation is regulated by calmodulin‐dependent protein kinase II (CAMK2). 38 Fukami et al. observed that Ca2+ oscillation induced by P occurred on the flagellum side of the sperm head and extended to the sperm head and flagellum. 27 They also demonstrated PLCδ4 was involved in the regulation of P‐induced AR by Ca2+ influx. 27 In addition, PLCδ4 is associated with ZP‐induced AR, 39 in which the Ca2+ influx occurs after the ZP binds with the spermatozoa. 27 Thus, Ca2+ influx and PLCδ4 play important roles when the AR is induced by P or the ZP. Thus, it seems likely that PLC is associated with the regulation of sperm hyperactivation because PLC is produced from PC/PI to IP3. 7 , 37 Is PLCδ4 involved in the non‐genomic signal regulated hyperactivation when sperm hyperactivation is increased by P? Inhibition of PLCδ by U73122 did not affect P‐induced sperm hyperactivation (Fig. 6a) and the activator of PLCδ, which is spermine, could not activate sperm hyperactivation (Fig. 6e). However, other inhibitors of PLC, such as D609, ET‐18‐OHC3, neomycine and spermine, inhibited only P‐induced sperm hyperactivation (Fig. 6b–e), and did not affect flagellar movement or sperm hyperactivation itself at all. Therefore, it is likely that PLC is involved in the regulation of P‐induced sperm hyperactivation, although PLCδ is not. Because spermine, which is an inhibitor of PLCα and an activator of PLCδ, could inhibit P‐induced sperm hyperactivation (Fig. 6e), it appears that PLCα, at least, is involved in the non‐genomic regulation of P‐induced sperm hyperactivation. It is also likely that IP3 is produced from both PC and PI because D609, which is a PC‐specific PLC inhibitor, and ET‐18‐OHC3, which is a PI‐specific PLC inhibitor, could inhibit P‐induced sperm hyperactivation (Fig. 6b,c).
Albumin is also a key molecule in sperm capacitation 2 because it removes cholesterol from the sperm plasma membrane. 5 When hamster spermatozoa were suspended in mTALP medium without BSA, they were not hyperactivated at all (Fig. 5), so it is likely that the removal of cholesterol by albumin is an important signal and an essential trigger for capacitation and hyperactivation. However, the downstream signals for the removal of cholesterol by albumin are not yet understood. 40
It is accepted that tyrosine phosphorylation of approximately 80‐kDa sperm proteins occurs during hyperactivation. 13 , 14 , 15 , 16 In our recent study, 32 four tyrosine phosphorylations of 80‐kDa proteins occurred during hyperactivation of hamster spermatozoa, and Jha and Shivaji 41 reported that 80‐kDa tyrosine‐phosphoprotein of hamster spermatozoa was AKAP. In addition, Si and Okuno 13 suggested that tyrosine phosphorylation of 80‐kDa proteins was accelerated, together with an increase in hyperactivation, by supplying inhibitors of protein phosphatase 1/2A. Other previous studies have suggested that hyperactivation and tyrosine phosphorylations are regulated by CAMK2, 16 , 38 and in the present study tyrosine phosphorylation of 80‐kDa proteins was accelerated in conjunction with P‐induced hyperactivation (Fig. 3). As both the regulation of tyrosine phosphorylation and the non‐genomic regulation by P involve Ca2+ signals, it is likely that the regulation of tyrosine phosphorylation interacts with the non‐genomic regulation of P.
To induce the AR by P, several concentrations of P have been used. 17 , 21 , 23 , 27 , 28 , 30 For hamster spermatozoa, Libersky and Boatman 22 suggested that only 20 ng/mL P significantly increased penetration and that a concentration of several micrograms per milliliter had little or no effect. Moreover, they suggested that 20 ng/mL P could not induce the AR. 22 Although in human spermatozoa hyperactivation is induced by P at several micrograms per milliliter, 29 , 30 only the AR is induced in rodent spermatozoa by P at the same concentration. 22 , 23 , 27 Because 20 ng/mL P could not induce the AR in hamster spermatozoa, 22 hamster spermatozoa may not exhibit AR in the oviductal fluid, in which the concentration of P ranges from 44.04 to 175.06 ng/mL. However, the ZP is also able to induce the AR in rodent spermatozoa, using a similar system to P. 27 , 39 Thus, it is likely that rodent spermatozoa are hyperactivated by stimulation from Ca2+ and albumin, modulated by P and the AR can be induced by the ZP.
Although we suggest that hyperactivation of hamster spermatozoa by non‐genomic regulation involves tyrosine phosphorylations and PLC, we believe that non‐genomic regulation by P is not the main system regulating hyperactivation. Basically, hamster spermatozoa could be hyperactivated in the mTALP medium without P and moreover, RU486 and inhibitors of PLC inhibited only P‐induced hyperactivation (1, 6). Hamster spermatozoa could not be hyperactivated at all in mTALP without Ca2+ or mTALP medium without albumin, even if P was included in the medium (4, 5). Therefore, it is likely that non‐genomic regulation by P modulates sperm hyperactivation under conditions in which hamster spermatozoa would be hyperactivated anyway.
In a previous study, sperm motility was classified into four types: pre‐initiation, initiation, activation and hyperactivation. 42 When sperm motility changed from pre‐initiation to activation via initiation, serine phosphorylations and cAMP‐dependent tyrosine phosphorylation were related to the regulation of sperm motility. 14 , 32 , 42 In contrast, tyrosine phosphorylations were related to the regulation of sperm motility when it changed from activation to hyperactivation. 12 , 32 Moreover, tyrosine phosphorylation and hyperactivation are regulated through Ca2+ signals via the IP3R‐gated Ca2+ store. 7 , 8 , 36 , 37 , 38 In addition, PLC is associated with the regulation of hyperactivation induced by P (Fig. 6), whereas the AR is regulated by Ca2+ signals via PLCδ. 27 , 39 Our proposed regulatory mechanism of the P‐induced AR and hyperactivation is that a high concentration (1–10 µg/mL) of P stimulates Ca2+ influx, activates PLCδ, releases Ca2+ from the Ca2+ store and induces the AR (Fig. 7). In contrast, it is likely that a low concentration (20 ng/mL) of P activates PLC and enhances tyrosine phosphorylation and hyperactivation.
Figure 7.
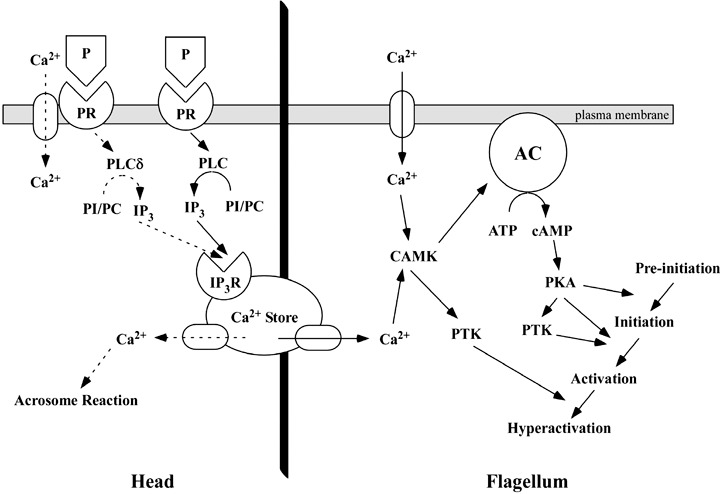
Regulatory mechanism of the acrosome reaction (AR) and hyperactivation induced by progesterone (P). Dotted arrows indicate the regulatory signals of the AR induced by P. Arrows indicate the regulatory signals of hyperactivation induced by P. A high concentration of P stimulates Phospholipase Cδ (PLCδ) and induces the AR. A low concentration of P stimulates PLC and induces hyperactivation. AC, adenylate cyclase; CAMK, calmodulin‐dependent protein kinase; cAMP, cyclic adenosine monophosphate; IP3R, inositol 1,4,5‐trisphosphate receptor; PC, phosphatidylcholine; PI, phosphatidylinositol; PKA, protein kinase A; PLC, phospholopase C; PR, progesterone receptor; PTK, protein tyrosine kinase.
ACKNOWLEDGMENTS
THIS WORK WAS supported by a Grant‐in‐Aid for Young Scientists (B) (No. 15790860 and No. 18791135) from the Ministry of Education, Culture, Sports, Science and Technology of Japan to M. Fujinoki.
REFERENCES
- 1. Yudin AI, Gottlieb W, Meizel S. Ultrastructural studies of the early events of the human sperm acrosome reaction as initiated by human follicular fluid. Gamete Res 1988; 20: 11–24. [DOI] [PubMed] [Google Scholar]
- 2. Yanagimachi R. Mammalian fertilization In: Neill K, Pfaff GM, eds. The Physiology of Reproduction, Vol. 1, 2nd edn. New York: Raven Press, 1994; 189–317. [Google Scholar]
- 3. Morisawa M. Cell signaling mechanisms for sperm motility. Zool Sci 1994; 11: 647–662. [PubMed] [Google Scholar]
- 4. Suarez SS, Ho HC. Hyperactivated motility in sperm. Reprod Dom Anim 2003; 38: 119–124. [DOI] [PubMed] [Google Scholar]
- 5. Langlais J, Roberts KD. A molecular membrane model of sperm capacitation and the acrosome reaction of mammalian spermatozoa. Gamete Res 1985; 13: 183–224. [Google Scholar]
- 6. Hossain MDS, Hyeong LJ, Miah AG, Tsujii H. Effect of fatty acids bound to bovine serum albumin‐V on acrosome reaction and utilization of glucose in boar spermatozoa. Reprod Med Biol 2007; 6: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ho HC, Suarez SS. An inositol 1,4,5‐trisphoshate receptor‐gated intracellular Ca2+ store is involved in regulating sperm hyperactivated motility. Biol Reprod 2001; 65: 1606–1616. [DOI] [PubMed] [Google Scholar]
- 8. Ho HC, Granish KA, Suarez SS. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev Biol 2002; 250: 208–217. [DOI] [PubMed] [Google Scholar]
- 9. Visconti PE, Galantino‐Homer H, Ning X et al The molecular basis of capacitation. J Androl 1998; 19: 242–248. [PubMed] [Google Scholar]
- 10. Visconti PE, Kopf GS. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod 1998; 59: 1–6. [DOI] [PubMed] [Google Scholar]
- 11. Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through the direct activation of adenylate cyclase. J Biol Chem 1985; 260: 9699–9705. [PubMed] [Google Scholar]
- 12. Visconti PE, Bailey JL, Moore GD, Pan D, Old‐Clarke P, Kopf GS. Capacitation of mouse spermatozoa I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995; 121: 1129–1137. [DOI] [PubMed] [Google Scholar]
- 13. Si Y, Okuno M. Role of tyrosine phosphorylation of flagellar proteins in hamster sperm hyperactivation. Biol Reprod 1999; 61: 240–246. [DOI] [PubMed] [Google Scholar]
- 14. Fujinoki M, Ohtake H, Okuno M. Tyrosine phosphorylation and dephosphorylation associated with motility of hamster spermatozoa. Biomed Res 2001; 22: 147–155. [Google Scholar]
- 15. Jha K, Shivaji S. Capacitation‐associated changes in protein tyrosine phosphorylation, hyperactivation and acrosome reaction in hamster spermatozoa. Andorologia 2001; 33: 95–104. [DOI] [PubMed] [Google Scholar]
- 16. Carrera A, Moos J, Ning XP et al Regulation of protein tyrosine phosphorylation in human sperm by a calcium/calmodulin‐dependent mechanism. Identification of A kinase anchoring proteins as major substrates for tyrosine phosphorylation. Dev Biol 1996; 180: 284–296. [DOI] [PubMed] [Google Scholar]
- 17. Osman RA, Andria ML, Jones AD, Meizel S. Steroid induced exocytosis: the human sperm acrosome reaction. Biochem Biophys Res Commun 1989; 160: 828–833. [DOI] [PubMed] [Google Scholar]
- 18. Baldi E, Luconi M, Bonaccorsi L, Forti G. Nongenomic effects of progesterone on spermatozoa: mechanisms of signal transduction and clinical implications. Front Biosci 1998; 3: 1051–1059. [DOI] [PubMed] [Google Scholar]
- 19. Lösel R, Wehling M. Nongenomic actions of steroid hormones. Nature Rev Mol Cell Biol 2003; 4: 46–56. [DOI] [PubMed] [Google Scholar]
- 20. Luconi M, Francavilla F, Porazzi I, Macerola B, Forti G, Baldi E. Human spermatozoa as a model for studying membrane receptors mediating rapid nongenomic effects of progesterone and estrogens. Steroids 2004; 69: 553–559. [DOI] [PubMed] [Google Scholar]
- 21. Harper CV, Barratt CLR, Publicover SJ. Stimulation of human spermatozoa with progesterone gradients to stimulate approach to the oocyte. J Biol Chem 2004; 279: 46315–46325. [DOI] [PubMed] [Google Scholar]
- 22. Libersky EA, Boatman DE. Effects of progesterone on in vitro sperm capacitation and egg penetration in the golden hamster. Biol Reprod 1995; 53: 483–487. [DOI] [PubMed] [Google Scholar]
- 23. Llanos MN, Anabalon MC. Studies related to progesterone‐induced hamster sperm acrosome reaction. Mol Reprod Dev 1996; 45: 313–319. [DOI] [PubMed] [Google Scholar]
- 24. Sabeur K, Edwards DP, Meizel S. Human sperm plasma membrane progesterone receptor(s) and the acrosome reaction. Biol Reprod 1996; 54: 993–1001. [DOI] [PubMed] [Google Scholar]
- 25. Jang S, Yi LSH. Identification of a 71 kDa protein as a putative non‐genomic membrane progesterone receptor in boar spermatozoa. J Endocrinol 2005; 184: 417–425. [DOI] [PubMed] [Google Scholar]
- 26. Gadkar S, Shah CA, Sachdeva G, Samant U, Puri CP. Progesterone receptor as an indicator of sperm function. Biol Reprod 2002; 67: 1327–1336. [DOI] [PubMed] [Google Scholar]
- 27. Fukami K, Yoshida M, Inoue T et al Phospholipase Cδ4 is required for Ca2+ mobilization essential for acrosome reaction in sperm. J Cell Biol 2003; 161: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harrison DA, Carr DW, Meizel S. Involvement of protein kinase A and a kinase anchoring protein in the progesterone‐initiated human sperm acrosome reaction. Biol Reprod 2000; 62: 811–820. [DOI] [PubMed] [Google Scholar]
- 29. Yang J, Serres C, Philibert D, Robel P, Baulieu EE, Jouannet P. Progesterone and RU486: opposing effects on human sperm. Proc Natl Acad Sci USA 1994; 91: 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sueldo CE, Alexander NJ, Oehninger S et al Effect of progesterone on human zona pellucida sperm binding and oocyte penetrationg capacity. Fertil Steril 1993; 60: 137–140. [DOI] [PubMed] [Google Scholar]
- 31. Libersky EA, Boatman DE. Progesterone concentration in serum, follicular fluid, and oviductal fluid of the golden hamster during the periovulatory period. Biol Reprod 1995; 53: 477–482. [DOI] [PubMed] [Google Scholar]
- 32. Fujinoki M, Suzuki T, Takayama T, Shibahara H, Ohtake H. Profiling of proteins phosphorylated or dephosphorylated during hyperactivation on hamster spermatozoa. Reprod Med Biol 2006; 5: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 34. Baldi E, Luconi M, Bonaccorsi L et al Nongenomic progesterone receptor on human spermatozoa: biochemical aspects and clinical implications. Steroids 1999; 64: 143–148. [DOI] [PubMed] [Google Scholar]
- 35. Suarez SS, Vincenti L, Ceglia MW. Hyperactivated motility induced in mouse sperm by calcium ionophore A23187 is reversible. J Exp Zool 1987; 244: 331–336. [DOI] [PubMed] [Google Scholar]
- 36. Ho HC, Suarez SS. Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction 2001; 122: 519–526. [DOI] [PubMed] [Google Scholar]
- 37. Ho HC, Suarez SS. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol Reprod 2003; 68: 1590–1596. [DOI] [PubMed] [Google Scholar]
- 38. Ignotz GG, Suarez SS. Calcium/calmodulin and calmodulin kinase II stimulate hyperactivation in demembranated bovine sperm. Biol Reprod 2005; 73: 519–526. [DOI] [PubMed] [Google Scholar]
- 39. Fukami K, Nakano K, Inoue T et al Requirement of phospholipase Cδ4 for the zona pellucida‐induced acrosome reaction. Science, 2001; 292: 920–923. [DOI] [PubMed] [Google Scholar]
- 40. Salicioni AM, Platt MD, Wertheimer EV et al Sigmalling pathways involved in sperm capacitation In: Roldan ERS, Gomendio M, eds. Spermatology. Nottingham: Nottingham University Press, 2007; 245–259. [PubMed] [Google Scholar]
- 41. Jha K, Shivaji S. Identification of the major tyrosine phosphorylated protein of capacitated hamster spermatozoa as a homologue of mammalian sperm A kinase anchoring protein. Mol Reprod Dev 2002; 61: 258–270. [DOI] [PubMed] [Google Scholar]
- 42. Fujinoki M, Ohtake H, Okuno M. Serine phosphorylation of flagellar proteins associated with the motility activation of hamster spermatozoa. Biomed Res 2001; 22: 45–58. [Google Scholar]


