Abstract
In most mammalian species including cattle, heat stress has deleterious effects on nutritional, physiological and reproductive functions. Exposure of animals to a hot environment causes an increase in body temperature in mammals, including domestic animals. High ambient temperature also causes a decrease in the length and intensity of estrus by disturbing ovarian function as well as decreasing pregnancy rate after artificial insemination. Therefore, it is important to understand the effects of heat stress on reproductive function in order to improve the production of domestic animals. Heat stress decreases appetite, weight gain, and milk yield in dairy cattle. It also adversely affects the reproductive performance of both sexes. In males, it reduces spermatogenic activity, while in females it adversely impacts oogenesis, oocyte maturation, fertilization development and implantation rate. Detection and evaluation of the deteriorating effects of heat stress on reproductive organs and cells can help to design measures to prevent them and improve reproductive functions. In this review, we discuss the impacts of heat stress on reproductive functions.
Keywords: Heat stress, Livestock animal, Mammal, Oxidative stress, Reproductive function
Introduction
There are many different climatic zones around the world which are highly affected by latitude, altitude, water area, oceans, winds or evaporative conditions. Mammals, including humans and livestock animals, are living in such variable environmental conditions. Most mammals have body temperatures of 35–39°C [1]. These temperatures are maintained above environmental temperatures through the generation of metabolic heat. Body temperatures are normally maintained in a narrow range by heat production and loss, although disease, poor nutrition and extreme environmental temperatures can upset the balance.
Heat stress caused by high ambient temperature in summer can result in increased body temperatures and can decrease growth, milk production and fertility in livestock. Many studies have examined the effects of ambient temperature and humidity on the physiology of livestock. Berman et al. [2] suggested that the upper limit of ambient temperatures at which high milk‐yielding dairy cows may maintain a stable body temperature (38.5°C) is 25–26°C, and that at environmental temperatures above 25°C, practices should be instituted to minimize the rise in body temperature. High environmental temperature increases the body temperature in lactating cows to near 40°C [3]; however, this drastic elevation of body temperature is not observed in heifers even under high environmental temperature [3]. This may be because of the extra heat produced in association with milk production and less heat loss as a result of the smaller difference of temperature between body and environment. Milk production significantly decreases with increasing body temperature [4, 5]. In addition, heat stress decreases food intake and body weight in pigs [6, 7]. As well as productivity, heat stress widely affects the reproductive functions in mammals with a reduction of pregnancy rate in cattle [8] and pigs [9]. The objective of this review is to describe the effect of heat stress on reproductive functions in male and female mammals including livestock animals.
Heat stress on male reproduction
In males, the testis is suspended in a scrotum outside the body in order to keep the temperature lower than core body temperature, which is required for normal spermatogenesis. The testis temperature is between 2 and 8°C below core body temperature in mice [10], humans [11] and bulls [12]. In bulls, bovine testicular temperature must not exceed 33–34.5°C for normal spermatogenesis [13, 14]. Hyperthermia has a detrimental effect on testicular functions such as inhibiting spermatogenesis in mice [15], rats [16], pigs [17, 18], sheep [19], cows [20] and horses [21]. For effective production of livestock animals, high fertility of semen is necessary for obtaining fertility after artificial insemination or natural mating; however, high summer temperatures have been shown to decrease semen quality in bulls, rams [19, 22, 23, 24] and boars [18, 25]. Heat stress to testis with acute scrotal heating decreases not only semen quality, but also decreases embryo quality after fertilization with normal female mice [15, 26, 27, 28] as well as fetal growth [26]. Heat stress has several adverse effects on reproductive tissues in mice and cows, including germ cell loss, poor morphology, low sperm quality, and abnormal DNA and chromatin structure [29, 30, 31]. Heat stress to the testis increased the number of apoptotic germ cells in mice [27, 32] and rats [16, 33] and disturbed gene expression in mice [10, 30, 34, 35]. Some evidence showed that X and Y spermatozoa are differentially affected by heat stress. The sex ratio of embryos shifted towards female when female mice were crossed with male mice treated with heat stress on the day of mating [30].
Heat stress also affects the endocrine and biochemical condition of male animals. Summer heat increased the level of thiobarbituric acid reactive substances (TBARs) which is an oxidative marker, and decreased glutathione peroxidase (GPx) level which is an antioxidant enzyme in bovine seminal plasma [36, 37]. Similar changes were observed in rams [38, 39]. Heat stress also has endocrine effects, reducing the plasma luteinizing hormone (LH) level in bulls [40, 41] and increasing the plasma testosterone level in boars [18].
Effects of heat stress on female reproduction
Estrus and endocrine status
Heat stress reduces the duration and intensity of estrus in dairy cows [42, 43, 44], increases anestrus and silent ovulation [43], and reduces the number of mounts in hot weather than in cold weather [45]. These changes make it difficult to detect estrus, so that artificial inseminations are less successful and the number of pregnancies is reduced. Heat stress also affects reproductive functions in beef cows. Pedometer measurements showed a decrease in the number of steps, which reflects the intensity of estrous behavior, at the day of estrus with an increase in ambient temperature in summer (Fig. 1). In another case, a clear increase in the number of steps was observed in an individual cow when ambient temperature decreased (Fig. 1).
Figure 1.
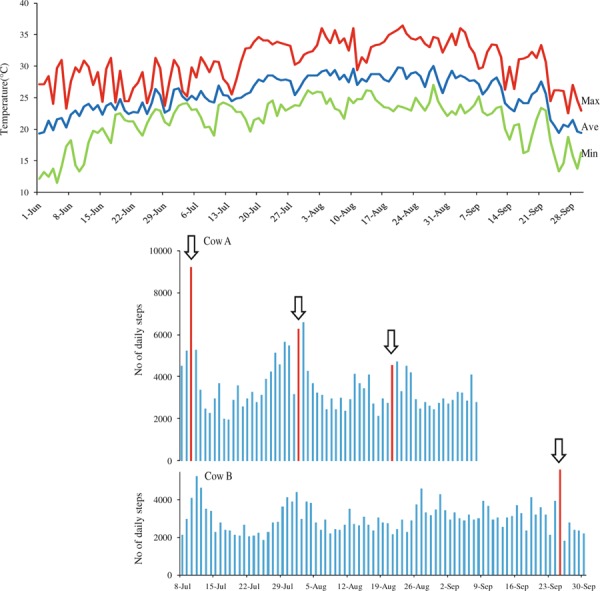
Disturbance of estrus behavior of cows in summer season (June–September, 2010). Upper Temperature and humidity at Kumamoto Japan in summer. Lower Number of daily steps of individual cow. Red bars show the number of daily steps on the day in which the cows showed standing estrus (arrows)
Heat stress affects many reproductive functions including endocrine activities in females. High temperature disturbs hormone secretion such as decreasing LH, follicle‐stimulating hormone (FSH) in cows [46, 47], progesterone in cows [48, 49], and estradiol (E2) in goats [50]. Heat stress also reduces the level of progesterone and causes a loss of LH surge in sheep [51]. Heat stress also changes the luteal phase and ovulation in humans [52], and reduces the levels of estradiol and follicular estradiol concentration, aromatase activity and level of LH receptor associated with delayed ovulation [50] in goats. Heat stress also lowers the levels of gonadotropin receptors and aromatase activity of granulosa cells and the follicular fluid concentrations of estradiol collected from rat follicle [53]. An in vitro study revealed a decrease of follicular steroidogenesis, androstenedione and estradiol of follicle wall exposed to heat stress [54]. On the other hand, less effects on insulin‐like growth factor binding protein, E2 and progesterone levels in dominant follicles have also been reported after heat stress exposure to dairy cows despite the elevation of rectal temperature [55]. These different responses need to be considered with regard to exposure time, estrous cycle, nutritional status [56], and other environmental conditions such as wind and humidity. Ovarian function in lactating cows is different from that in dry cows and heifers, because lactating cows generate more heat as a result of milk production [3].
Effect of heat stress on follicular development and oocyte quality
Heat stress negatively affects ovaries by inhibiting follicular growth and oocyte quality. Heat stress reduces inhibin levels by hastening the decrease in size of the first‐wave dominant follicle and the emergence of the second dominant follicle [57, 58]. Intrafollicular condition is important for oocyte growth and quality. High ambient temperatures significantly decrease the number of oocytes and developmental competence following in vitro fertilization in dairy cows but have less effect on beef cows [59]. Exposing dairy cows to heat stress decreased estradiol production and viability of granulosa cells and also decreased androstenedione production by thecal cells [60]. Some metabolic markers such as blood level of glucose and non‐esterified fatty acid (NEFA) affect the condition of the follicles under heat stress conditions. It is reported that the glucose level of bovine follicular fluid is about 85% of the plasma glucose level in the cool season and that the follicular glucose level significantly decreases in summer with a similar decrease in blood glucose level [61]. In contrast, heat stress did not affect the level of NEFA in spite of a significant increase in plasma level [61]. Taken together, these results indicate that the condition of follicles is affected by body blood nutrition or biochemical components which vary in the summer season. However, oxygen is probably not a factor because its concentration in the follicular fluid does not vary in heat and non‐heat stressed conditions [62].
Although rectal temperatures are often considered representative of core body (and hence tissue) temperatures, ovarian temperatures are kept 1–1.5°C cooler than rectal temperatures in several species, including cattle, pigs, rabbits and humans [63, 64, 65, 66]. Maternal heat stress did not affect the blood oxygen pressure in the ovarian vein of swine [67]. On the contrary, ovarian, cervical and oviductal blood flows decreased by 20–30% by heat‐stressed rabbit while vulval blood flow rose by 40%, irrespective of pregnancy and/or lactation status [68]. These studies indicate that it is necessary to study the effect of body temperature and local blood flow associated with local temperature and distribution of nutrition to follicles for oocyte growth. However, it is unclear how follicular temperature is affected in heat‐stressed ovaries. Further studies are needed to determine how heat stress affects local reproductive organs to clarify the follicular and oocyte growth.
Effects of heat stress on oocyte growth, fertilization and early embryonic development
Many in vitro and in vivo studies have examined the effects of heat stress on maturation and developmental competence of oocytes. Exposing females to heat stress after fertilization caused decreases in the quality and quantity of embryos in cows after superovulation [69, 70] and mice [71, 72, 73, 74, 75], and caused decreases in fetal growth in pigs [67], mice [74] and beef cows [76]. In vitro studies also revealed the effect of heat stress on oocyte maturation. Exposing GV stage oocytes to high temperature inhibits the rate of MII stage oocytes in mice [77, 78] and cows [79, 80, 81, 82]. Although, experimental heat stress coincident with ovulation and oocyte maturation may or may not have an effect on the capacity of oocytes to be fertilized, the resultant embryos are more likely to develop slowly or abnormally. Exposure of oocytes to heat stress during in vitro maturation caused nuclear and cytoskeletal alterations in mice [77], pigs [83] and cows [79, 84]. Heat stress also induces cumulus–oocyte complexes (COCs) to undergo apoptosis. Figure 2 shows the increase in the number of TUNEL‐positive cells in cumulus cells surrounding bovine oocytes when COCs were exposed to heat stress during in vitro maturation. Heat stress also induced apoptosis in bovine oocytes [82, 85, 86] and an increase in phosphatidylserine, an indicator of apoptosis in porcine oocytes [87]. On the other hand, short exposures to heat stress seem to have less effect on oocyte maturation in vitro [88, 89, 90]. Careful analysis is necessary to clarify the opposite results of heat stress on oocyte maturation in in vitro or in vivo conditions. Heat stress at the time of fertilization also decreased subsequent embryo development, which suggests that heat stress has detrimental effects on both on oocytes and sperm [81]. In males, heat stress reduces the number of sperm with intact acrosomes at the time of ejaculation [18].
Figure 2.
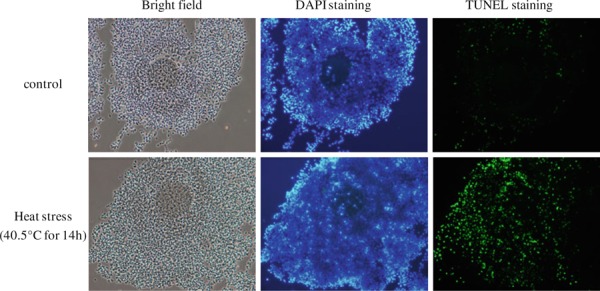
Heat stress‐induced apoptosis in bovine matured cumulus–oocyte complexes (COCs). After COCs were collected from follicles, they were matured for 20 h in maturation medium. COCs were exposed to 40.5°C for the latter 14 h followed by fixation and TUNEL staining
In addition to oocyte maturation, development of preimplantation embryos after fertilization is also affected by the surrounding environment. Exposing preimplantation embryos to heat stress decreased their development in mice [91] and cows [92, 93] and decreased the total cell number of blastocysts [93].
Developing embryos during the short period (4–8 days) between fertilization and implantation undergo dynamic growth, cell proliferation, cell differentiation, and many changes in gene expression. Therefore, if the maternal body is exposed to heat stress during this period, it is likely that the preimplantation development is severely affected directly by heat stress itself or indirectly by the deleterious change of reproductive tracts. The stage at which embryos become susceptible to heat stress has been studied. In vivo maternal heat stress inhibited embryo development at an early stage in mice [71, 75] and cows [70]. In vitro studies have clearly shown that the sensitivity of bovine embryos to heat stress is stage‐specific [93, 94, 95] as well as in in vivo studies [70]. In cows, in vivo and in vitro experiments showed that embryo development is significantly inhibited by heat stress approximately 48–72 h after fertilization, which corresponds to the 8–16 cell stage [93]. After this stage, heat stress exposure has less effect on the rate of development and cell proliferation [93]. In mouse and cow embryos, the stage that is most sensitive to heat stress is approximately the time of zygotic genome activation (ZGA), which occurs at the 2‐cell stage in mice [96] and at the 4‐ to 8‐cell stage in cows [97, 98]. Both during and after ZGA, heat stress can also change the chromatin structure of embryonic cells [99], which might disturb gene expression. In addition to inducing apoptosis in maturing oocytes, heat stress also induces apoptosis in embryonic cells in cows [86, 100, 101], pigs [102, 103] and rabbits [104].
Knowledge of when an embryo is most sensitive to heat stress can be used to select the best time for embryo transfer by preventing the early embryonic loss after artificial insemination in cows [105, 106].
Effects of heat stress on post‐hatching development and placentation
Maternal heat exposure after fertilization decreases the pregnancy rate and causes embryonic death before implantation [70, 71]. Such conditions might disturb the intrauterine environment both for embryos and uterine tissue. We recently found that the viability of uterine epithelial cells recovered from uterine flushing at the time of embryo collection in beef cows decreased in summer (Fig. 3). At this time, embryo quality also tended to decline.
Figure 3.
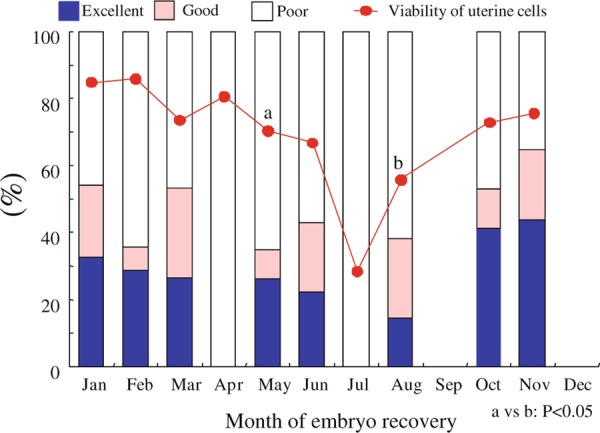
Seasonal changes of embryo quality and viability of uterine epithelial cells recovered on day 7 from Japanese black cows. Uterine epithelial cell clots were recovered from uterine flushing at the time of embryo collection in beef cows. After enzymatic dispersion, cell viability was determined by trypan blue staining
A high level of prostaglandin F2α (PGF2α) inhibited implantation, altered embryo development and induced luteal regression [107]. Maternal recognition of pregnancy is an initial step for embryo implantation and placentation. The corpus luteum secretes progesterone, which has roles in follicular growth and the establishment and maintenance of pregnancy. Luteal function is inhibited by summer heat, causing decreases in progesterone levels in luteinized granulosa cells, theca cells and plasma [108] in both dairy cows [48] and beef cows [49]. One of the many factors controlling luteal function is PGF2α which is mainly secreted by the uterus. On the other hand, elevated uterine luminal concentrations of PGF2α have been negatively associated with embryo quality and pregnancy rates [109] and have been shown to have a toxic effect on in vitro development of embryos in rabbits [110], rats [111] and cows [112, 113]. Administration of PGF2α to pregnant mice on day 4 reduced the decidual reaction around the implantation chamber [114]. Secretion of PGF2α is affected by heat stress in endometrial tissues of pregnant and non‐pregnant bovine uterus [115]. Also, maternal heat stress increases placental PGF2α and PGFM levels [116].
Heat stress also has detrimental effects on placentation and fetal growth. Maternal heat stress decreases growth retardation [117] and size of placenta in rats [118], and placental weight [119, 120] and placentome size [121] in sheep, but has less effect on humans [122]. Maternal heat stress in cow reduces the levels of placental hormones, which disturbs placental function and slows fetal development [123]. Maternal heat stress and nutritional status during gestation also have strong effects on fetal development [124]. Heating the scrota for extended periods also results in decreased fetal weight [125] which indicates that the impact of heat stress on paternal gamates at the earlier stage highly affects fetal growth.
Oxidative stress in heat stressed reproductive functions
Heat stress not only affects animals by reducing the reproductive functions but it also has physiological and nutritional effects followed by retardation and reduced milk production in cattle. In summer, an increase of body temperature significantly decreased milk production [4, 5]. Increase of body temperature by heat stress also caused decrease of food intake and body weight in pigs [6, 7]. As well as reducing productivity, heat stress widely affects the reproductive functions in mammals with a reduction of pregnancy rate in cattle.
Oxidative stress is one of the many parameters used to indicate the physiological status in cells and tissues of an animals’ body. Heat stress affects the oxidative stress‐related physiological status in females as well as males. Heat stress caused increases in oxidative markers, such as the levels of TBARS, superoxide dismutase (SOD) and catalase in plasma and erythrocytes in humans [126], cows [127], goats [128] and mice [129], and in the liver in rats [130]. Maternal heat stress changes the redox status in the oviduct in mice [75, 129]. Several antioxidant enzymes that are expressed in the oviduct vary during the estrous cycle in cows [131]. We also found seasonal differences in the redox status of oviductal fluid collected from dairy and beef cows (Fig. 4). Together, these studies referring to [75, 129, 131] and Fig. 4 indicate that decreased redox status and/or increased oxidative stress lead to deterioration of intraoviductal conditions with adverse effects on ovulated oocytes, ejaculated sperm and fertilized embryos.
Figure 4.
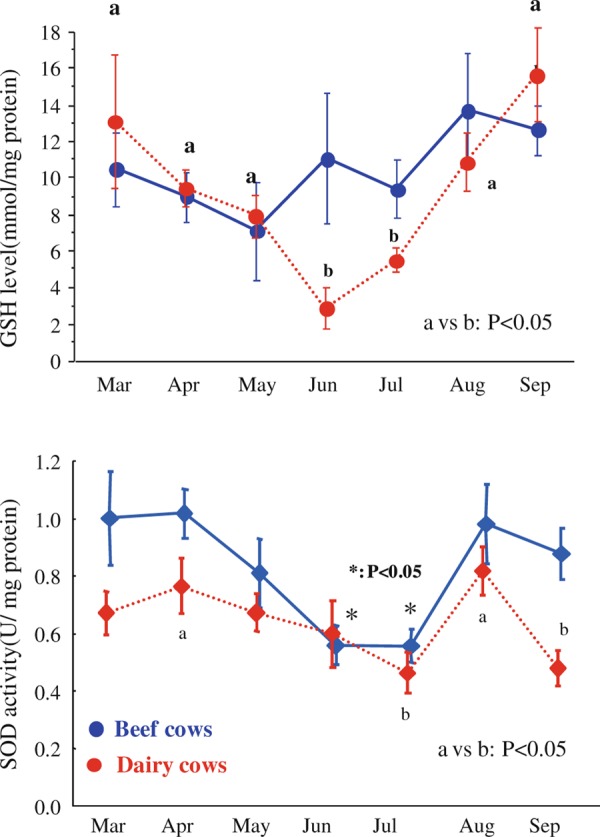
Seasonal changes of GSH levels and SOD activity in oviductal fluids collected from beef and dairy cows. Oviducts were collected from slaughtered dairy and beef cows from March to September. After trimming, oviducts were flushed with 0.5 ml PBS. Collected flushing was centrifuged to remove the cell clots. GSH levels and SOD activities were then measured. Measured GSH and SOD levels were normalized to total protein concentration in the oviductal flushing
Early embryonic development is also affected by heat stress‐induced oxidative stress. Heat stress increased intracellular reactive oxygen species (ROS) in embryos of mice [71] and cows [93]. Interestingly, at the time when bovine embryo development is critically inhibited by heat stress (days 0–2), ROS generation in heat‐stressed embryos significantly increases [93]. After day 2, heat stress has less effect on embryo development as well as less effect on ROS generation or accumulation. Administration of antioxidative polyphenol [74], vitamin E [132] or melatonin [133] to heat‐stressed female mice improved the development of mouse embryos. β‐Carotene had a similar effect on the pregnancy rate of heat‐stressed dairy cows [134, 135]. In vitro administration of antioxidants such as anthocyanin [136], astaxanthin [137] or 2‐mercaptoethanol [138] improved embryo development of heat‐stressed bovine embryos associated with intracellular ROS and glutathione (GSH) synthesis [138]. Furthermore, in vivo heat stress also caused a decrease in GSH of oocytes and embryos, and elevated ROS levels associated with DNA damage in mice [71].
These findings provide both direct and indirect evidence of a close relationship between heat and oxidative stress in embryo development. GSH maintains the intracellular redox status of embryos and is associated with their development and quality in many species including mice [139], rats [140], rabbits [141], pigs [142] and cows [143, 144]. GSH can improve the thermotolerance of mice [145], which suggests that redox status is an important determinant of thermotolerance. Therefore, using antioxidants to control the intracellular or extracellular redox status both in vivo and in vitro may be a way to reduce heat stress‐related oxidative stress.
Impact of heat stress on human live food production and reproduction in future
Global temperatures have risen about 0.7°C since the beginning of the industrial revolution in the 18th century, causing climate change all over the world by possible greenhouse gasses. Recent weather reports show that the incidences of summer heat waves, heavy rains or drought have been increasing with rising temperature. It is likely that global warming will have severe impacts on the physiology and reproduction of mammals of both sexes. The impact of elevated temperatures on reproductive functions in males and females is summarized in Fig. 5.
Figure 5.
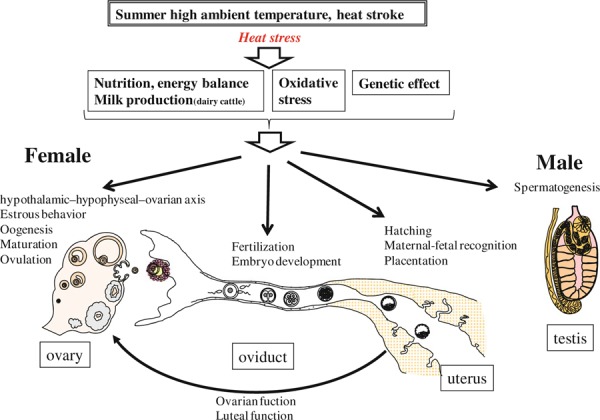
Schematic diagram of the effects of heat stress on reproductive functions in male and female mammals
Mammals including livestock animals have adapted to variable environments all over the world which typically include high ambient temperatures. In these environments, mammals have acquired genetic variation and improved mechanisms for controlling body temperature and managing heat stress. On the other hand, genetic selection of livestock by humans has made them more susceptible to heat stress. This is especially the case with dairy cows which generate large amounts of metabolic heat for milk production. In dairy cows selected for high milk production, the conception rate decreases dramatically in summer than in winter [146]. However, the effects of heat stress on milk production and body temperature vary among breeds [147], and are less in beef cows than in dairy cows. This decline of reproductive performance is thought to be due to an imbalance of heat production and loss [148]. Such breed differences in heat stress are attributed not only to biological body but also to cellular response of immune cells [149], embryos [150, 151, 152] as well as a combination of semen [124, 153, 154]. Therefore, further studies of genes involved in cellular and physiological responses to heat stress are needed to control and improve mammalian reproduction.
References
- 1. Prosser CL, Heath JE Prosser CL. Temperature. Comparative animal physiology, environmental and metabolic animal physiology, 1991. 4 New York: Wiley; 109–166 [Google Scholar]
- 2. Berman A, Folman Y, Kaim M, Mamen M, Herz Z, Wolfenson D, Arieli A, Graber Y. Upper critical temperatures and forced ventilation effects for high‐yielding dairy cows in a subtropical climate. J Dairy Sci, 1985, 68, 1488–1495 10.3168/jds.S0022‐0302(85)80987‐5 [DOI] [PubMed] [Google Scholar]
- 3. Sartori R, Sartor‐Bergfelt R, Mertens SA, Guenther JN, Parrish JJ, Wiltbank MC. Fertilization and early embryonic development in heifers and lactating cows in summer and lactating and dry cows in winter. J Dairy Sci, 2002, 85, 2803–2812 10.3168/jds.S0022‐0302(02)74367‐1 [DOI] [PubMed] [Google Scholar]
- 4. Bohmanova J, Misztal I, Cole JB. Temperature‐humidity indices as indicators of milk production losses due to heat stress. J Dairy Sci, 2007, 90, 1947–1956 10.3168/jds.2006‐513 [DOI] [PubMed] [Google Scholar]
- 5. Ravagnolo O, Misztal I, Hoogenboom G. Genetic component of heat stress in dairy cattle, development of heat index function. J Dairy Sci, 2000, 83, 2120–2125 10.3168/jds.S0022‐0302(00)75094‐6 [DOI] [PubMed] [Google Scholar]
- 6. Renaudeau D, Noblet J. Effects of exposure to high ambient temperature and dietary protein level on sow milk production and performance of piglets. J Anim Sci, 2001, 79, 1540–1548 [DOI] [PubMed] [Google Scholar]
- 7. Huynh TT, Aarnink AJ, Verstegen MW, Gerrits WJ, Heetkamp MJ, Kemp B, Canh TT. Effects of increasing temperatures on physiological changes in pigs at different relative humidities. J Anim Sci, 2005, 83, 1385–1396 [DOI] [PubMed] [Google Scholar]
- 8. Garcia‐Ispierto I, Lopez‐Gatius F, Santolaria P, Yaniz JL, Nogareda C, Lopez‐Bejar M, Rensis F. Relationship between heat stress during the peri‐implantation period and early fetal loss in dairy cattle. Theriogenology, 2006, 65, 799–807 10.1016/j.theriogenology.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 9. Wettemann RP, Bazer FW. Influence of environmental temperature on prolificacy of pigs. J Reprod Fertil Suppl, 1985, 33, 199–208 [PubMed] [Google Scholar]
- 10. Banks S, King SA, Irvine DS, Saunders PT. Impact of a mild scrotal heat stress on DNA integrity in murine spermatozoa. Reproduction, 2005, 129, 505–514 10.1530/rep.1.00531 [DOI] [PubMed] [Google Scholar]
- 11. Ivell R. Lifestyle impact and the biology of the human scrotum. Reprod Biol Endocrinol, 2007, 5, 15 10.1186/1477‐7827‐5‐15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waites GMH. Temperature regulation and the testis. In: Johnson, editors. The testis. 1970. p. 241–79.
- 13. Wildeus S, Entwistle KW. Spermiogram and sperm reserves in hybrid Bos indicus × Bos taurus bulls after scrotal insulation. J Reprod Fertil, 1983, 69, 711–716 10.1530/jrf.0.0690711 [DOI] [PubMed] [Google Scholar]
- 14. Barth AD, Bowman PA. The sequential appearance of sperm abnormalities after scrotal insulation or dexamethasone treatment in bulls. Can Vet J, 1994, 35, 93–102 [PMC free article] [PubMed] [Google Scholar]
- 15. Yaeram J, Setchell BP, Maddocks S. Effect of heat stress on the fertility of male mice in vivo and in vitro. Reprod Fertil Dev, 2006, 18, 647–653 10.1071/RD05022 [DOI] [PubMed] [Google Scholar]
- 16. Lue Y, Hikim AP, Wang C, Im M, Leung A, Swerdloff RS. Testicular heat exposure enhances the suppression of spermatogenesis by testosterone in rats: the “two‐hit” approach to male contraceptive development. Endocrinology, 2000, 141, 1414–1424 10.1210/en.141.4.1414 [DOI] [PubMed] [Google Scholar]
- 17. Wettemann RP, Wells ME, Omtvedt IT, Pope CE, Turman EJ. Influence of elevated ambient temperature on reproductive performance of boars. J Anim Sci, 1976, 42, 664–669 [DOI] [PubMed] [Google Scholar]
- 18. Murase T, Imaeda N, Yamada H, Miyazawa K. Seasonal changes in semen characteristics, composition of seminal plasma and frequency of acrosome reaction induced by calcium and calcium ionophore A23187 in Large White boars. J Reprod Dev, 2007, 53, 853–865 10.1262/jrd.19026 [DOI] [PubMed] [Google Scholar]
- 19. Mieusset R, Quintana Casares PI, Sanchez‐Partida LG, Sowerbutts SF, Zupp JL, Setchell BP. The effects of moderate heating of the testes and epididymides of rams by scrotal insulation on body temperature, respiratory rate, spermatozoa output and motility, and on fertility and embryonic survival in ewes inseminated with frozen semen. Ann N Y Acad Sci, 1991, 637, 445–458 10.1111/j.1749‐6632.1991.tb27329.x [DOI] [PubMed] [Google Scholar]
- 20. Skinner JD, Louw GN. Heat stress and spermatogenesis in Bos indicus and Bos taurus cattle. J Appl Physiol, 1966, 21, 1784–1790 [DOI] [PubMed] [Google Scholar]
- 21. Love CC, Kenney RM. Scrotal heat stress induces altered sperm chromatin structure associated with a decrease in protamine disulfide bonding in the stallion. Biol Reprod, 1999, 60, 615–620 10.1095/biolreprod60.3.615 [DOI] [PubMed] [Google Scholar]
- 22. Meyerhoeffer DC, Wettemann RP, Coleman SW, Wells ME. Reproductive criteria of beef bulls during and after exposure to increased ambient temperature. J Anim Sci, 1985, 60, 352–357 [DOI] [PubMed] [Google Scholar]
- 23. Kastelic JP, Cook RB, Coulter GH. Contribution of the scrotum and testes to scrotal and testicular thermoregulation in bulls and rams. J Reprod Fertil, 1996, 108, 81–85 10.1530/jrf.0.1080081 [DOI] [PubMed] [Google Scholar]
- 24. Kastelic JP, Cook RB, Coulter GH. Effects of ambient temperature and scrotal fleece cover on scrotal and testicular temperatures in rams. Can J Vet Res, 1999, 63, 157–160 [PMC free article] [PubMed] [Google Scholar]
- 25. Kunavongkrit A, Suriyasomboon A, Lundeheim N, Heard TW, Einarsson S. Management and sperm production of boars under differing environmental conditions. Theriogenology, 2005, 63, 657–667 10.1016/j.theriogenology.2004.09.039 [DOI] [PubMed] [Google Scholar]
- 26. Jannes P, Spiessens C, Auwera I, D'Hooghe T, Verhoeven G, Vanderschueren D. Male subfertility induced by acute scrotal heating affects embryo quality in normal female mice. Hum Reprod, 1998, 13, 372–375 10.1093/humrep/13.2.372 [DOI] [PubMed] [Google Scholar]
- 27. Paul C, Murray AA, Spears N, Saunders PT. A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction, 2008, 136, 73–84 10.1530/REP‐08‐0036 [DOI] [PubMed] [Google Scholar]
- 28. Setchell BP, Ekpe G, Zupp JL, Surani MA. Transient retardation in embryo growth in normal female mice made pregnant by males whose testes had been heated. Hum Reprod, 1998, 13, 342–347 10.1093/humrep/13.2.342 [DOI] [PubMed] [Google Scholar]
- 29. Sailer BL, Sarkar LJ, Bjordahl JA, Jost LK, Evenson DP. Effects of heat stress on mouse testicular cells and sperm chromatin structure. J Androl, 1997, 18, 294–301 [PubMed] [Google Scholar]
- 30. Perez‐Crespo M, Pintado B, Gutierrez‐Adan A. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol Reprod Dev, 2008, 75, 40–47 10.1002/mrd.20759 [DOI] [PubMed] [Google Scholar]
- 31. Ax RL, Gilbert GR, Shook GE. Sperm in poor quality semen from bulls during heat stress have a lower affinity for binding hydrogen‐3 heparin. J Dairy Sci, 1987, 70, 195–200 10.3168/jds.S0022‐0302(87)79994‐9 [DOI] [PubMed] [Google Scholar]
- 32. Shiraishi K, Takihara H, Matsuyama H. Elevated scrotal temperature, but not varicocele grade, reflects testicular oxidative stress‐mediated apoptosis. World J Urol, 2010, 28, 359–364 10.1007/s00345‐009‐0462‐5 [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto CM, Sinha Hikim AP, Huynh PN, Shapiro B, Lue Y, Salameh WA, Wang C, Swerdloff RS. Redistribution of Bax is an early step in an apoptotic pathway leading to germ cell death in rats, triggered by mild testicular hyperthermia. Biol Reprod, 2000, 63, 1683–1690 10.1095/biolreprod63.6.1683 [DOI] [PubMed] [Google Scholar]
- 34. Li Y, Zhou Q, Hively R, Yang L, Small C, Griswold MD. Differential gene expression in the testes of different murine strains under normal and hyperthermic conditions. J Androl, 2009, 30, 325–337 10.2164/jandrol.108.005934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iuchi Y, Kaneko T, Matsuki S, Sasagawa I, Fujii J. Concerted changes in the YB2/RYB‐a protein and protamine 2 messenger RNA in the mouse testis under heat stress. Biol Reprod, 2003, 68, 129–135 10.1095/biolreprod.102.005124 [DOI] [PubMed] [Google Scholar]
- 36. Nichi M, Bols PE, Zuge RM, Barnabe VH, Goovaerts IG, Barnabe RC, Cortada CN. Seasonal variation in semen quality in Bos indicus and Bos taurus bulls raised under tropical conditions. Theriogenology, 2006, 66, 822–828 10.1016/j.theriogenology.2006.01.056 [DOI] [PubMed] [Google Scholar]
- 37. Kowalowka M, Wysocki P, Fraser L, Strzezek J. Extracellular superoxide dismutase of boar seminal plasma. Reprod Domest Anim, 2008, 43, 490–496 10.1111/j.1439‐0531.2007.00943.x [DOI] [PubMed] [Google Scholar]
- 38.Casao A, Cebrian I, Asumpcao ME, Perez‐Pe R, Abecia JA, Forcada F, Cebrian‐Perez JA, Muino‐Blanco T. Seasonal variations of melatonin in ram seminal plasma are correlated to those of testosterone and antioxidant enzymes. Reprod Biol Endocrinol. 8:59. [DOI] [PMC free article] [PubMed]
- 39. Marti E, Mara L, Marti JI, Muino‐Blanco T, Cebrian‐Perez JA. Seasonal variations in antioxidant enzyme activity in ram seminal plasma. Theriogenology, 2007, 67, 1446–1454 10.1016/j.theriogenology.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 40. Rhynes WE, Ewing LL. Testicular endocrine function in Hereford bulls exposed to high ambient temperature. Endocrinology, 1973, 92, 509–515 10.1210/endo‐92‐2‐509 [DOI] [PubMed] [Google Scholar]
- 41. Minton JE, Wettemann RP, Meyerhoeffer DC, Hintz RL, Turman EJ. Serum luteinizing hormone and testosterone in bulls during exposure to elevated ambient temperature. J Anim Sci, 1981, 53, 1551–1558 [DOI] [PubMed] [Google Scholar]
- 42. Younas M, Fuquay JW, Smith AE, Moore AB. Estrous and endocrine responses of lactating Holsteins to forced ventilation during summer. J Dairy Sci, 1993, 76, 430–436 10.3168/jds.S0022‐0302(93)77363‐4 [DOI] [PubMed] [Google Scholar]
- 43. Gwazdauskas FC, Thatcher WW, Kiddy CA, Paape MJ, Wilcox CJ. Hormonal patterns during heat stress following PGF(2)alpha‐tham salt induced luteal regression in heifers. Theriogenology, 1981, 16, 271–285 10.1016/0093‐691X(81)90012‐1 [DOI] [PubMed] [Google Scholar]
- 44. Trout JP, McDowell LR, Hansen PJ. Characteristics of the estrous cycle and antioxidant status of lactating Holstein cows exposed to heat stress. J Dairy Sci, 1998, 81, 1244–1250 10.3168/jds.S0022‐0302(98)75685‐1 [DOI] [PubMed] [Google Scholar]
- 45. Pennington JA, Albright JL, Diekman MA, Callahan CJ. Sexual activity of Holstein cows: seasonal effects. J Dairy Sci, 1985, 68, 3023–3030 10.3168/jds.S0022‐0302(85)81197‐8 [DOI] [PubMed] [Google Scholar]
- 46. Wise ME, Armstrong DV, Huber JT, Hunter R, Wiersma F. Hormonal alterations in the lactating dairy cow in response to thermal stress. J Dairy Sci, 1988, 71, 2480–2485 10.3168/jds.S0022‐0302(88)79834‐3 [DOI] [PubMed] [Google Scholar]
- 47. Gilad E, Meidan R, Berman A, Graber Y, Wolfenson D. Effect of heat stress on tonic and GnRH‐induced gonadotrophin secretion in relation to concentration of oestradiol in plasma of cyclic cows. J Reprod Fertil, 1993, 99, 315–321 10.1530/jrf.0.0990315 [DOI] [PubMed] [Google Scholar]
- 48. Howell JL, Fuquay JW, Smith AE. Corpus luteum growth and function in lactating Holstein cows during spring and summer. J Dairy Sci, 1994, 77, 735–739 10.3168/jds.S0022‐0302(94)77007‐7 [DOI] [PubMed] [Google Scholar]
- 49. Burke JM, Spiers DE, Kojima FN, Perry GA, Salfen BE, Wood SL, Patterson DJ, Smith MF, Lucy MC, Jackson WG, Piper EL. Interaction of endophyte‐infected fescue and heat stress on ovarian function in the beef heifer. Biol Reprod, 2001, 65, 260–268 10.1095/biolreprod65.1.260 [DOI] [PubMed] [Google Scholar]
- 50. Ozawa M, Tabayashi D, Latief TA, Shimizu T, Oshima I, Kanai Y. Alterations in follicular dynamics and steroidogenic abilities induced by heat stress during follicular recruitment in goats. Reproduction, 2005, 129, 621–630 10.1530/rep.1.00456 [DOI] [PubMed] [Google Scholar]
- 51. Hill TG, Alliston CW. Effects of thermal stress on plasma concentrations of luteinizing hormone, progesterone, prolactin and testosterone in the cycling ewe. Theriogenology, 1981, 15, 201–209 10.1016/S0093‐691X(81)80008‐8 [DOI] [PubMed] [Google Scholar]
- 52. Carpenter AJ, Nunneley SA. Endogenous hormones subtly alter women's response to heat stress. J Appl Physiol, 1988, 65, 2313–2317 [DOI] [PubMed] [Google Scholar]
- 53. Shimizu T, Ohshima I, Ozawa M, Takahashi S, Tajima A, Shiota M, Miyazaki H, Kanai Y. Heat stress diminishes gonadotropin receptor expression and enhances susceptibility to apoptosis of rat granulosa cells. Reproduction, 2005, 129, 463–472 10.1530/rep.1.00502 [DOI] [PubMed] [Google Scholar]
- 54. Bridges PJ, Brusie MA, Fortune JE. Elevated temperature (heat stress) in vitro reduces androstenedione and estradiol and increases progesterone secretion by follicular cells from bovine dominant follicles. Domest Anim Endocrinol, 2005, 29, 508–522 10.1016/j.domaniend.2005.02.017 [DOI] [PubMed] [Google Scholar]
- 55. Guzeloglu A, Ambrose JD, Kassa T, Diaz T, Thatcher MJ, Thatcher WW. Long‐term follicular dynamics and biochemical characteristics of dominant follicles in dairy cows subjected to acute heat stress. Anim Reprod Sci, 2001, 66, 15–34 10.1016/S0378‐4320(01)00082‐3 [DOI] [PubMed] [Google Scholar]
- 56. Rensis F, Scaramuzzi RJ. Heat stress and seasonal effects on reproduction in the dairy cow—a review. Theriogenology, 2003, 60, 1139–1151 10.1016/S0093‐691X(03)00126‐2 [DOI] [PubMed] [Google Scholar]
- 57. Wolfenson D, Thatcher WW, Badinga L, Savio JD, Meidan R, Lew BJ, Braw‐Tal R, Berman A. Effect of heat stress on follicular development during the estrous cycle in lactating dairy cattle. Biol Reprod, 1995, 52, 1106–1113 10.1095/biolreprod52.5.1106 [DOI] [PubMed] [Google Scholar]
- 58. Roth Z, Meidan R, Braw‐Tal R, Wolfenson D. Immediate and delayed effects of heat stress on follicular development and its association with plasma FSH and inhibin concentration in cows. J Reprod Fertil, 2000, 120, 83–90 10.1530/reprod/120.1.83 [PubMed] [Google Scholar]
- 59. Hansen PJ, Drost M, Rivera RM, Paula‐Lopes FF, al‐Katanani YM, Krininger CE 3rd, Chase CC Jr. Adverse impact of heat stress on embryo production: causes and strategies for mitigation. Theriogenology, 2001, 55, 91–103 10.1016/S0093‐691X(00)00448‐9 [DOI] [PubMed] [Google Scholar]
- 60. Roth Z, Meidan R, Shaham‐Albalancy A, Braw‐Tal R, Wolfenson D. Delayed effect of heat stress on steroid production in medium‐sized and preovulatory bovine follicles. Reproduction, 2001, 121, 745–751 10.1530/rep.0.1210745 [PubMed] [Google Scholar]
- 61. Shehab‐El‐Deen MA, Leroy JL, Fadel MS, Saleh SY, Maes D, Soom A. Biochemical changes in the follicular fluid of the dominant follicle of high producing dairy cows exposed to heat stress early post‐partum. Anim Reprod Sci, 2010, 117, 189–200 10.1016/j.anireprosci.2009.04.013 [DOI] [PubMed] [Google Scholar]
- 62. Castro EPLA, Andrzejewski J, Julian D, Spicer LJ, Hansen PJ. Oxygen and steroid concentrations in preovulatory follicles of lactating dairy cows exposed to acute heat stress. Theriogenology, 2008, 69, 805–813 10.1016/j.theriogenology.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 63.Grøndahl C, Greve T, Schmidt M, Hunter RHF. Bovine pre‐ovulatory follicles are cooler than ovarian stroma and deep rectal temperature. Theriogenology. 1996;45 abstract:289.
- 64. Hunter RH, Bogh IB, Einer‐Jensen N, Muller S, Greve T. Pre‐ovulatory graafian follicles are cooler than neighbouring stroma in pig ovaries. Hum Reprod, 2000, 15, 273–283 10.1093/humrep/15.2.273 [DOI] [PubMed] [Google Scholar]
- 65. Grinsted J, Blendstrup K, Andreasen AG, Byskov MP. Temperature measurements of rabbit antral follicles. J Reprod Fertil, 1980, 60, 149–155 10.1530/jrf.0.0600149 [DOI] [PubMed] [Google Scholar]
- 66. Grinsted J, Kjer JJ, Blendstrup K, Pedersen JF. Is low temperature of the follicular fluid prior to ovulation necessary for normal oocyte development?. Fertil Steril, 1985, 43, 34–39 [DOI] [PubMed] [Google Scholar]
- 67. Wettemann RP, Bazer FW, Thatcher WW, Caton D, Roberts RM. Conceptus development, uterine response, blood gases and endocrine function of gilts exposed to increased ambient temperature during early pregnancy. Theriogenology, 1988, 30, 57–74 10.1016/0093‐691X(88)90263‐4 [DOI] [PubMed] [Google Scholar]
- 68. Lublin A, Wolfenson D. Lactation and pregnancy effects on blood flow to mammary and reproductive systems in heat‐stressed rabbits. Comp Biochem Physiol A Physiol, 1996, 115, 277–285 10.1016/S0300‐9629(96)00060‐6 [DOI] [PubMed] [Google Scholar]
- 69. Putney DJ, Drost M, Thatcher WW. Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between days 1 to 7 post insemination. Theriogenology, 1988, 30, 195–209 10.1016/0093‐691X(88)90169‐0 [DOI] [PubMed] [Google Scholar]
- 70. Ealy AD, Drost M, Hansen PJ. Developmental changes in embryonic resistance to adverse effects of maternal heat stress in cows. J Dairy Sci, 1993, 76, 2899–2905 10.3168/jds.S0022‐0302(93)77629‐8 [DOI] [PubMed] [Google Scholar]
- 71. Ozawa M, Hirabayashi M, Kanai Y. Developmental competence and oxidative state of mouse zygotes heat‐stressed maternally or in vitro. Reproduction, 2002, 124, 683–689 10.1530/rep.0.1240683 [DOI] [PubMed] [Google Scholar]
- 72. Aroyo A, Yavin S, Arav A, Roth Z. Maternal hyperthermia disrupts developmental competence of follicle‐enclosed oocytes: in vivo and ex vivo studies in mice. Theriogenology, 2007, 67, 1013–1021 10.1016/j.theriogenology.2006.12.001 [DOI] [PubMed] [Google Scholar]
- 73. Baumgartner AP, Chrisman CL. Analysis of post‐implantation mouse embryos after maternal heat stress during meiotic maturation. J Reprod Fertil, 1988, 84, 469–474 10.1530/jrf.0.0840469 [DOI] [PubMed] [Google Scholar]
- 74. Roth Z, Aroyo A, Yavin S, Arav A. The antioxidant epigallocatechin gallate (EGCG) moderates the deleterious effects of maternal hyperthermia on follicle‐enclosed oocytes in mice. Theriogenology, 2008, 70, 887–897 10.1016/j.theriogenology.2008.05.053 [DOI] [PubMed] [Google Scholar]
- 75. Ozawa M, Matsuzuka T, Hirabayashi M, Kanai Y. Redox status of the oviduct and CDC2 activity in 2‐cell stage embryos in heat‐stressed mice. Biol Reprod, 2004, 71, 291–296 10.1095/biolreprod.103.022152 [DOI] [PubMed] [Google Scholar]
- 76. Biggers BG, Geisert RD, Wetteman RP, Buchanan DS. Effect of heat stress on early embryonic development in the beef cow. J Anim Sci, 1987, 64, 1512–1518 [DOI] [PubMed] [Google Scholar]
- 77. Wang JZ, Sui HS, Miao DQ, Liu N, Zhou P, Ge L, Tan JH. Effects of heat stress during in vitro maturation on cytoplasmic versus nuclear components of mouse oocytes. Reproduction, 2009, 137, 181–189 10.1530/REP‐08‐0339 [DOI] [PubMed] [Google Scholar]
- 78. LaRosa C, Downs SM. Meiotic induction by heat stress in mouse oocytes: involvement of AMP‐activated protein kinase and MAPK family members. Biol Reprod, 2007, 76, 476–486 10.1095/biolreprod.106.057422 [DOI] [PubMed] [Google Scholar]
- 79. Payton RR, Romar R, Coy P, Saxton AM, Lawrence JL, Edwards JL. Susceptibility of bovine germinal vesicle‐stage oocytes from antral follicles to direct effects of heat stress in vitro. Biol Reprod, 2004, 71, 1303–1308 10.1095/biolreprod.104.029892 [DOI] [PubMed] [Google Scholar]
- 80.Andreu‐Vazquez C, Lopez‐Gatius F, Garcia‐Ispierto I, Maya‐Soriano MJ, Hunter RH, Lopez‐Bejar M. Does heat stress provoke the loss of a continuous layer of cortical granules beneath the plasma membrane during oocyte maturation? Zygote. 18:293–9. [DOI] [PubMed]
- 81. Sugiyama S, McGowan M, Phillips N, Kafi M, Young M. Effects of increased ambient temperature during IVM and/or IVF on the in vitro development of bovine zygotes. Reprod Domest Anim, 2007, 42, 271–274 10.1111/j.1439‐0531.2006.00776.x [DOI] [PubMed] [Google Scholar]
- 82. Zhandi M, Towhidi A, Nasr‐Esfahani MH, Eftekhari‐Yazdi P, Zare‐Shahneh A. Unexpected detrimental effect of Insulin like growth factor‐1 on bovine oocyte developmental competence under heat stress. J Assist Reprod Genet, 2009, 26, 605–611 10.1007/s10815‐009‐9364‐0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ju JC, Tseng JK. Nuclear and cytoskeletal alterations of in vitro matured porcine oocytes under hyperthermia. Mol Reprod Dev, 2004, 68, 125–133 10.1002/mrd.20054 [DOI] [PubMed] [Google Scholar]
- 84. Roth Z, Hansen PJ. Disruption of nuclear maturation and rearrangement of cytoskeletal elements in bovine oocytes exposed to heat shock during maturation. Reproduction, 2005, 129, 235–244 10.1530/rep.1.00394 [DOI] [PubMed] [Google Scholar]
- 85. Roth Z, Hansen PJ. Involvement of apoptosis in disruption of developmental competence of bovine oocytes by heat shock during maturation. Biol Reprod, 2004, 71, 1898–1906 10.1095/biolreprod.104.031690 [DOI] [PubMed] [Google Scholar]
- 86. Soto P, Smith LC. BH4 peptide derived from Bcl‐xL and Bax‐inhibitor peptide suppresses apoptotic mitochondrial changes in heat stressed bovine oocytes. Mol Reprod Dev, 2009, 76, 637–646 10.1002/mrd.20986 [DOI] [PubMed] [Google Scholar]
- 87. Tseng JK, Tang PC, Ju JC. In vitro thermal stress induces apoptosis and reduces development of porcine parthenotes. Theriogenology, 2006, 66, 1073–1082 10.1016/j.theriogenology.2006.03.003 [DOI] [PubMed] [Google Scholar]
- 88. Schrock GE, Saxton AM, Schrick FN, Edwards JL. Early in vitro fertilization improves development of bovine ova heat stressed during in vitro maturation. J Dairy Sci, 2007, 90, 4297–4303 10.3168/jds.2007‐0002 [DOI] [PubMed] [Google Scholar]
- 89. Kim M, Geum D, Khang I, Park YM, Kang BM, Lee KA, Kim K. Expression pattern of HSP25 in mouse preimplantation embryo: heat shock responses during oocyte maturation. Mol Reprod Dev, 2002, 61, 3–13 10.1002/mrd.1125 [DOI] [PubMed] [Google Scholar]
- 90. Edwards JL, Bogart AN, Rispoli LA, Saxton AM, Schrick FN. Developmental competence of bovine embryos from heat‐stressed ova. J Dairy Sci, 2009, 92, 563–570 10.3168/jds.2008‐1495 [DOI] [PubMed] [Google Scholar]
- 91. Arechiga CF, Hansen PJ. Response of preimplantation murine embryos to heat shock as modified by developmental stage and glutathione status. In Vitro Cell Dev Biol Anim, 1998, 34, 655–659 10.1007/s11626‐996‐0016‐8 [DOI] [PubMed] [Google Scholar]
- 92. Ealy AD, Howell JL, Monterroso VH, Arechiga CF, Hansen PJ. Developmental changes in sensitivity of bovine embryos to heat shock and use of antioxidants as thermoprotectants. J Anim Sci, 1995, 73, 1401–1407 [DOI] [PubMed] [Google Scholar]
- 93. Sakatani M, Kobayashi S, Takahashi M. Effects of heat shock on in vitro development and intracellular oxidative state of bovine preimplantation embryos. Mol Reprod Dev, 2004, 67, 77–82 10.1002/mrd.20014 [DOI] [PubMed] [Google Scholar]
- 94. Edwards JL, Hansen PJ. Differential responses of bovine oocytes and preimplantation embryos to heat shock. Mol Reprod Dev, 1997, 46, 138–145 10.1002/(SICI)1098‐2795(199702)46:2<138::AID‐MRD4>3.0.CO;2‐R [DOI] [PubMed] [Google Scholar]
- 95. Krininger CE 3rd, Stephens SH, Hansen PJ. Developmental changes in inhibitory effects of arsenic and heat shock on growth of pre‐implantation bovine embryos. Mol Reprod Dev, 2002, 63, 335–340 10.1002/mrd.90017 [DOI] [PubMed] [Google Scholar]
- 96. Schultz RM. Regulation of zygotic gene activation in the mouse. Bioessays, 1993, 15, 531–538 10.1002/bies.950150806 [DOI] [PubMed] [Google Scholar]
- 97. Telford NA, Watson AJ, Schultz GA. Transition from maternal to embryonic control in early mammalian development: a comparison of several species. Mol Reprod Dev, 1990, 26, 90–100 10.1002/mrd.1080260113 [DOI] [PubMed] [Google Scholar]
- 98. Sousa PA, Caveney A, Westhusin ME, Watson AJ. Temporal patterns of embryonic gene expression and their dependence on oogenetic factors. Theriogenology, 1998, 49, 115–128 10.1016/S0093‐691X(97)00406‐8 [DOI] [PubMed] [Google Scholar]
- 99. Edwards MJ. Apoptosis, the heat shock response, hyperthermia, birth defects, disease and cancer. Where are the common links?. Cell Stress Chaperones, 1998, 3, 213–220 10.1379/1466‐1268(1998)003<0213:ATHSRH>2.3.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Paula‐Lopes FF, Hansen PJ. Apoptosis is an adaptive response in bovine preimplantation embryos that facilitates survival after heat shock. Biochem Biophys Res Commun, 2002, 295, 37–42 10.1016/S0006‐291X(02)00619‐8 [DOI] [PubMed] [Google Scholar]
- 101. Paula‐Lopes FF, Hansen PJ. Heat shock‐induced apoptosis in preimplantation bovine embryos is a developmentally regulated phenomenon. Biol Reprod, 2002, 66, 1169–1177 [DOI] [PubMed] [Google Scholar]
- 102. Isom SC, Prather RS, Rucker EB 3rd. Heat stress‐induced apoptosis in porcine in vitro fertilized and parthenogenetic preimplantation‐stage embryos. Mol Reprod Dev, 2007, 74, 574–581 10.1002/mrd.20620 [DOI] [PubMed] [Google Scholar]
- 103. Jin YX, Lee JY, Choi SH, Kim T, Cui XS, Kim NH. Heat shock induces apoptosis related gene expression and apoptosis in porcine parthenotes developing in vitro. Anim Reprod Sci, 2007, 100, 118–127 10.1016/j.anireprosci.2006.06.017 [DOI] [PubMed] [Google Scholar]
- 104. Makarevich AV, Olexikova L, Chrenek P, Kubovicova E, Freharova K, Pivko J. The effect of hyperthermia in vitro on vitality of rabbit preimplantation embryos. Physiol Res, 2007, 56, 789–796 [DOI] [PubMed] [Google Scholar]
- 105. Drost M, Ambrose JD, Thatcher MJ, Cantrell CK, Wolfsdorf KE, Hasler JF, Thatcher WW. Conception rates after artificial insemination or embryo transfer in lactating dairy cows during summer in Florida. Theriogenology, 1999, 52, 1161–1167 10.1016/S0093‐691X(99)00208‐3 [DOI] [PubMed] [Google Scholar]
- 106. Putney DJ, Drost M, Thatcher WW. Influence of summer heat stress on pregnancy rates of lactating dairy cattle following embryo transfer or artificial insemination. Theriogenology, 1989, 31, 765–778 10.1016/0093‐691X(89)90022‐8 [DOI] [PubMed] [Google Scholar]
- 107. Stocco CO, Deis RP. Participation of intraluteal progesterone and prostaglandin F2 alpha in LH‐induced luteolysis in pregnant rat. J Endocrinol, 1998, 156, 253–259 10.1677/joe.0.1560253 [DOI] [PubMed] [Google Scholar]
- 108. Wolfenson D, Sonego H, Bloch A, Shaham‐Albalancy A, Kaim M, Folman Y, Meidan R. Seasonal differences in progesterone production by luteinized bovine thecal and granulosa cells. Domest Anim Endocrinol, 2002, 22, 81–90 10.1016/S0739‐7240(01)00127‐8 [DOI] [PubMed] [Google Scholar]
- 109. Schrick FN, Inskeep EK, Butcher RL. Pregnancy rates for embryos transferred from early postpartum beef cows into recipients with normal estrous cycles. Biol Reprod, 1993, 49, 617–621 10.1095/biolreprod49.3.617 [DOI] [PubMed] [Google Scholar]
- 110. Maurer RR, Beier HM. Uterine proteins and development in vitro of rabbit preimplantation embryos. J Reprod Fertil, 1976, 48, 33–41 10.1530/jrf.0.0480033 [DOI] [PubMed] [Google Scholar]
- 111. Breuel KF, Fukuda A, Schrick FN. Effect of prostaglandin F2 on development of 8‐cell rat embryos in vitro. Biol Reprod, 1993, 48 (Suppl. 1) 173 [Google Scholar]
- 112. Scenna FN, Edwards JL, Rohrbach NR, Hockett ME, Saxton AM, Schrick FN. Detrimental effects of prostaglandin F2alpha on preimplantation bovine embryos. Prostaglandins Other Lipid Mediat, 2004, 73, 215–226 10.1016/j.prostaglandins.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 113. Soto P, Natzke RP, Hansen PJ. Identification of possible mediators of embryonic mortality caused by mastitis: actions of lipopolysaccharide, prostaglandin F2alpha, and the nitric oxide generator, sodium nitroprusside dihydrate, on oocyte maturation and embryonic development in cattle. Am J Reprod Immunol, 2003, 50, 263–272 10.1034/j.1600‐0897.2003.00085.x [DOI] [PubMed] [Google Scholar]
- 114. Scott JE, Persaud TV. Prostaglandin F2alpha conceptus–endometrial interaction during early gestation in the mouse. Prostaglandins Med, 1981, 7, 133–147 10.1016/0161‐4630(81)90057‐4 [DOI] [PubMed] [Google Scholar]
- 115. Putney DJ, Malayer JR, Gross TS, Thatcher WW, Hansen PJ, Drost M. Heat stress‐induced alterations in the synthesis and secretion of proteins and prostaglandins by cultured bovine conceptuses and uterine endometrium. Biol Reprod, 1988, 39, 717–728 10.1095/biolreprod39.3.717 [DOI] [PubMed] [Google Scholar]
- 116. Nakamura H, Matsuzaki I, Hatta K, Ogino K. Physiological involvement of placental endothelin‐1 and prostaglandin F2alpha in uteroplacental circulatory disturbance in pregnant rats exposed to heat stress. Can J Physiol Pharmacol, 2004, 82, 225–230 10.1139/y04‐011 [DOI] [PubMed] [Google Scholar]
- 117. Hensleigh PA, Johnson DC. Heat stress effects during pregnancy. I. Retardation of fetal rat growth. Fertil Steril, 1971, 22, 522–527 [DOI] [PubMed] [Google Scholar]
- 118. Padmanabhan R, Al‐Menhali NM, Ahmed I, Kataya HH, Ayoub MA. Histological, histochemical and electron microscopic changes of the placenta induced by maternal exposure to hyperthermia in the rat. Int J Hyperth, 2005, 21, 29–44 10.1080/02656730410001716614 [DOI] [PubMed] [Google Scholar]
- 119. Bell AW, McBride BW, Slepetis R, Early RJ, Currie WB. Chronic heat stress and prenatal development in sheep: I. Conceptus growth and maternal plasma hormones and metabolites. J Anim Sci, 1989, 67, 3289–3299 [DOI] [PubMed] [Google Scholar]
- 120. Vatnick I, Ignotz G, McBride BW, Bell AW. Effect of heat stress on ovine placental growth in early pregnancy. J Dev Physiol, 1991, 16, 163–166 [PubMed] [Google Scholar]
- 121. Early RJ, McBride BW, Vatnick I, Bell AW. Chronic heat stress and prenatal development in sheep: II. Placental cellularity and metabolism. J Anim Sci, 1991, 69, 3610–3616 [DOI] [PubMed] [Google Scholar]
- 122. Gericke GS, Hofmeyr GJ, Laburn H, Isaacs H. Does heat damage fetuses?. Med Hypotheses, 1989, 29, 275–278 10.1016/0306‐9877(89)90111‐4 [DOI] [PubMed] [Google Scholar]
- 123. Collier RJ, Doelger SG, Head HH, Thatcher WW, Wilcox CJ. Effects of heat stress during pregnancy on maternal hormone concentrations, calf birth weight and postpartum milk yield of Holstein cows. J Anim Sci, 1982, 54, 309–319 [DOI] [PubMed] [Google Scholar]
- 124. Hansen PJ. Effects of heat stress on mammalian reproduction. Philos Trans R Soc Lond B Biol Sci, 2009, 364, 3341–3350 10.1098/rstb.2009.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ghasemi N, Babaei H, Azizallahi S, Kheradmand A. Effect of long‐term administration of zinc after scrotal heating on mice spermatozoa and subsequent offspring quality. Andrologia, 2009, 41, 222–228 10.1111/j.1439‐0272.2009.00920.x [DOI] [PubMed] [Google Scholar]
- 126. Balog T, Sobocanec S, Sverko V, Krolo I, Rocic B, Marotti M, Marotti T. The influence of season on oxidant‐antioxidant status in trained and sedentary subjects. Life Sci, 2006, 78, 1441–1447 10.1016/j.lfs.2005.07.039 [DOI] [PubMed] [Google Scholar]
- 127. Bernabucci U, Ronchi B, Lacetera N, Nardone A. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J Dairy Sci, 2002, 85, 2173–2179 10.3168/jds.S0022‐0302(02)74296‐3 [DOI] [PubMed] [Google Scholar]
- 128.Wang L, Xue B, Wang K, Li S, Li Z. Effect of heat stress on endotoxin flux across mesenteric‐drained and portal‐drained viscera of dairy goat. J Anim Physiol Anim Nutr (Berl). 2010. doi:10.1111/j.1439‐0396. [DOI] [PubMed]
- 129. Matsuzuka T, Ozawa M, Nakamura A, Ushitani A, Hirabayashi M, Kanai Y. Effects of heat stress on the redox status in the oviduct and early embryonic development in mice. J Reprod Dev, 2005, 51, 281–287 10.1262/jrd.16089 [DOI] [PubMed] [Google Scholar]
- 130. Ando M, Katagiri K, Yamamoto S, Asanuma S, Usuda M, Kawahara I, Wakamatsu K. Effect of hyperthermia on glutathione peroxidase and lipid peroxidative damage in river. J Therm Biol, 1994, 19, 177–185 10.1016/0306‐4565(94)90029‐9 [Google Scholar]
- 131. Lapointe J, Bilodeau JF. Antioxidant defenses are modulated in the cow oviduct during the estrous cycle. Biol Reprod, 2003, 68, 1157–1164 10.1095/biolreprod.102.007476 [DOI] [PubMed] [Google Scholar]
- 132. Sakamoto N, Ozawa M, Yokotani‐Tomita K, Morimoto A, Matsuzuka T, Ijiri D, Hirabayashi M, Ushitani A, Kanai Y. DL‐alpha‐tocopherol acetate mitigates maternal hyperthermia‐induced pre‐implantation embryonic death accompanied by a reduction of physiological oxidative stress in mice. Reproduction, 2008, 135, 489–496 10.1530/REP‐07‐0379 [DOI] [PubMed] [Google Scholar]
- 133. Matsuzuka T, Sakamoto N, Ozawa M, Ushitani A, Hirabayashi M, Kanai Y. Alleviation of maternal hyperthermia‐induced early embryonic death by administration of melatonin to mice. J Pineal Res, 2005, 39, 217–223 10.1111/j.1600‐079X.2005.00260.x [DOI] [PubMed] [Google Scholar]
- 134. Arechiga CF, Staples CR, McDowell PJ, Hansen LR. Effects of timed insemination and supplemental beta‐carotene on reproduction and milk yield of dairy cows under heat stress. J Dairy Sci, 1998, 81, 390–402 10.3168/jds.S0022‐0302(98)75589‐4 [DOI] [PubMed] [Google Scholar]
- 135. Arechiga CF, Vazquez‐Flores S, Ortiz O, Hernandez‐Ceron J, Porras A, McDowell LR, Hansen PJ. Effect of injection of beta‐carotene or vitamin E and selenium on fertility of lactating dairy cows. Theriogenology, 1998, 50, 65–76 10.1016/S0093‐691X(98)00114‐9 [DOI] [PubMed] [Google Scholar]
- 136. Sakatani M, Suda I, Oki T, Kobayashi S, Kobayashi S, Takahashi M. Effects of purple sweet potato anthocyanins on development and intracellular redox status of bovine preimplantation embryos exposed to heat shock. J Reprod Dev, 2007, 53, 605–614 10.1262/jrd.18124 [DOI] [PubMed] [Google Scholar]
- 137. Namekawa T, Ikeda S, Sugimoto M, Kume S. Effects of astaxanthin‐containing oil on development and stress‐related gene expression of bovine embryos exposed to heat stress. Reprod Domest Anim, 2010, 45, 387–391 10.1111/j.1439‐0531.2010.01584.x [DOI] [PubMed] [Google Scholar]
- 138. Sakatani M, Yamanaka K, Kobayashi S, Takahashi M. Heat‐shock‐derived reactive oxygen species induce embryonic mortality in in vitro early stage bovine embryos. J Reprod Dev, 2008, 54, 496–501 10.1262/jrd.20017 [DOI] [PubMed] [Google Scholar]
- 139. Gardiner CS, Reed DJ. Status of glutathione during oxidant‐induced oxidative stress in the preimplantation mouse embryo. Biol Reprod, 1994, 51, 1307–1314 10.1095/biolreprod51.6.1307 [DOI] [PubMed] [Google Scholar]
- 140. Ishibashi M, Akazawa S, Sakamaki H, Matsumoto K, Yamasaki H, Yamaguchi Y, Goto S, Urata Y, Kondo T, Nagataki S. Oxygen‐induced embryopathy and the significance of glutathione‐dependent antioxidant system in the rat embryo during early organogenesis. Free Radic Biol Med, 1997, 22, 447–454 10.1016/S0891‐5849(96)00338‐3 [DOI] [PubMed] [Google Scholar]
- 141. Wells PG, Kim PM, Laposa RR, Nicol CJ, Parman T, Winn LM. Oxidative damage in chemical teratogenesis. Mutat Res, 1997, 396, 65–78 10.1016/S0027‐5107(97)00175‐9 [DOI] [PubMed] [Google Scholar]
- 142. Whitaker BD, Knight JW. Mechanisms of oxidative stress in porcine oocytes and the role of anti‐oxidants. Reprod Fertil Dev, 2008, 20, 694–702 10.1071/RD08037 [DOI] [PubMed] [Google Scholar]
- 143. Takahashi M, Nagai T, Hamano S, Kuwayama M, Okamura N, Okano A. Effect of thiol compounds on in vitro development and intracellular glutathione content of bovine embryos. Biol Reprod, 1993, 49, 228–232 10.1095/biolreprod49.2.228 [DOI] [PubMed] [Google Scholar]
- 144. Takahashi M, Nagai T, Okamura N, Takahashi H, Okano A. Promoting effect of beta‐mercaptoethanol on in vitro development under oxidative stress and cystine uptake of bovine embryos. Biol Reprod, 2002, 66, 562–567 10.1095/biolreprod66.3.562 [DOI] [PubMed] [Google Scholar]
- 145. Arechiga CF, Ealy AD, Hansen PJ. Evidence that glutathione is involved in thermotolerance of preimplantation murine embryos. Biol Reprod, 1995, 52, 1296–1301 10.1095/biolreprod52.6.1296 [DOI] [PubMed] [Google Scholar]
- 146. Lopez‐Gatius F. Is fertility declining in dairy cattle? A retrospective study in northeastern Spain. Theriogenology, 2003, 60, 89–99 10.1016/S0093‐691X(02)01359‐6 [DOI] [PubMed] [Google Scholar]
- 147. Srikandakumar A, Johnson EH. Effect of heat stress on milk production, rectal temperature, respiratory rate and blood chemistry in Holstein, Jersey and Australian Milking Zebu cows. Trop Anim Health Prod, 2004, 36, 685–692 10.1023/B:TROP.0000042868.76914.a9 [DOI] [PubMed] [Google Scholar]
- 148. Al‐Katanani YM, Webb DW, Hansen PJ. Factors affecting seasonal variation in 90‐day nonreturn rate to first service in lactating Holstein cows in a hot climate. J Dairy Sci, 1999, 82, 2611–2616 10.3168/jds.S0022‐0302(99)75516‐5 [DOI] [PubMed] [Google Scholar]
- 149. Lacetera N, Bernabucci U, Scalia D, Basirico L, Morera P, Nardone A. Heat stress elicits different responses in peripheral blood mononuclear cells from Brown Swiss and Holstein cows. J Dairy Sci, 2006, 89, 4606–4612 10.3168/jds.S0022‐0302(06)72510‐3 [DOI] [PubMed] [Google Scholar]
- 150. Hernandez‐Ceron J, Chase CC Jr, Hansen PJ. Differences in heat tolerance between preimplantation embryos from Brahman, Romosinuano, and Angus breeds. J Dairy Sci, 2004, 87, 53–58 10.3168/jds.S0022‐0302(04)73141‐0 [DOI] [PubMed] [Google Scholar]
- 151. Paula‐Lopes FF, Chase CC Jr, Al‐Katanani YM, Krininger CE 3rd, Rivera RM, Tekin S, Majewski AC, Ocon OM, Olson TA, Hansen PJ. Genetic divergence in cellular resistance to heat shock in cattle: differences between breeds developed in temperate versus hot climates in responses of preimplantation embryos, reproductive tract tissues and lymphocytes to increased culture temperatures. Reproduction, 2003, 125, 285–294 10.1530/rep.0.1250285 [DOI] [PubMed] [Google Scholar]
- 152. Hansen PJ. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim Reprod Sci, 2004, 82–83, 349–360 10.1016/j.anireprosci.2004.04.011 [DOI] [PubMed] [Google Scholar]
- 153. Barros CM, Pegorer MF, Vasconcelos JL, Eberhardt BG, Monteiro FM. Importance of sperm genotype (indicus versus taurus) for fertility and embryonic development at elevated temperatures. Theriogenology, 2006, 65, 210–218 10.1016/j.theriogenology.2005.09.024 [DOI] [PubMed] [Google Scholar]
- 154.Pegorer MF, Vasconcelos JL, Trinca LA, Hansen PJ, Barros CM. Influence of sire and sire breed (Gyr versus Holstein) on establishment of pregnancy and embryonic loss in lactating Holstein cows during summer heat stress. Theriogenology. 2007;67:692–7. [DOI] [PubMed]


