Abstract
Purpose
The viability of mammalian eggs after ovulation is reported to be improved by the presence of ascorbic acid in the culture medium. However, the pro‐survival mechanisms of ascorbic acid are poorly understood. The molecular pathways of apoptosis are evolutionarily conserved among animal species, and Xenopus eggs are technically and ethically more suitable for biochemical analyses than mammalian eggs. We used Xenopus egg cytoplasmic extracts to examine the direct intracellular effects of ascorbic acid.
Methods
Incubation of egg extracts for more than 4 h induces the spontaneous release of cytochrome c from mitochondria. This event triggers the activation of caspases, cleavage of substrate proteins, and execution of apoptosis. Multiple signal transduction pathways including proteolysis and protein phosphorylation are also involved in this process. We examined whether any of these events might be inhibited by the addition of ascorbic acid.
Results
Ascorbic acid showed no effect against cytochrome c release, but prevented caspase activation and substrate cleavage. Ascorbic acid also blocked the proteolysis of apoptosis inhibitor proteins and the dephosphorylation of p42 MAP kinase. However, dehydroascorbic acid (oxidized form of ascorbic acid) and acetate (unrelated acid) were equally effective, indicating that these effects were primarily due to their acidity. In addition, dehydroascorbic acid inhibited caspase activities directly in vitro.
Conclusions
The anti‐apoptotic effect of ascorbic acid in Xenopus egg extracts is mainly due to cytoplasmic acidification rather than its intracellular antioxidant activity. Instead, oxidative conversion of ascorbic acid into dehydroascorbic acid may inhibit apoptosis through the inhibition of caspases.
Keywords: Ascorbic acid, Apoptosis, Caspases, Dehydroascorbic acid, Xenopus egg extracts
Introduction
Aged eggs long after ovulation spontaneously degenerate and die mainly by apoptosis. It is poorly understood how the fate of ovulated eggs is switched from survival to death. Many reports suggest that the viability of isolated oocytes, eggs and early embryos from various animals is improved by the addition of antioxidants into culture medium. For example, ascorbic acid (AA) and/or α‐tocopherol prevented the maternal aging and apoptosis of mouse oocytes [1, 2, 3, 4]. Compromised oocyte function of cat oocytes was overridden by AA or cysteine [5]. AA and α‐tocopherol also enhanced the developmental competence of porcine oocytes [6, 7], but not of bovine oocytes [8]. It is generally believed that antioxidants protect cells from reactive oxygen generated by intracellular metabolism and extracellular environments. However, many water‐soluble antioxidants such as AA may not penetrate into eggs without specific transporters. Therefore, it is not clear whether antioxidants exert their pro‐survival effects intracellularly, extracellularly, or both.
Xenopus egg cytoplasmic extracts are a suitable tool for biochemical analyses of apoptosis regulation [9, 10, 11]. The use of this cell‐free system allows us to evaluate the direct intracellular effects of membrane‐impermeable, water‐soluble molecules. Incubation of egg extracts for more than 4 h induces the spontaneous release of cytochrome c from mitochondria, followed by the activation of caspases. Subsequently, caspase‐mediated cleavages of substrate proteins including poly (ADP‐ribose) polymerase (PARP) execute apoptosis. This phenomenon physiologically reflects the aging‐induced apoptosis of oocytes and eggs in vivo.
The molecular mechanisms of apoptosis are evolutionarily conserved between mammals and Xenopus. We previously reported that xXIAP, a Xenopus homolog of mammalian X‐linked inhibitor of apoptosis protein (XIAP), was a physiological apoptosis inhibitor in egg extracts [12, 13]. xEIAP/XLX, another structurally related protein, might be also involved in the inhibition of apoptosis. xXIAP was abruptly degraded at the onset of apoptosis, whereas xEIAP/XLX was highly unstable in egg extracts. Therefore, the proteolytic regulation of xXIAP and xEIAP/XLX may determine the timing for apoptotic execution, and apoptosis may be inhibited when the proteolysis of xXIAP and xEIAP/XLX is blocked. For both xXIAP and xEIAP/XLX, proteolysis was not inhibited by Z‐VAD‐FMK (a pan‐caspase inhibitor) or MG‐132 (a proteasome inhibitor), suggesting the involvement of hitherto unidentified proteolytic pathways.
We and others also reported that cytostatic factor (CSF)‐arrested metaphase egg extracts required longer incubation time than interphase egg extracts to execute spontaneous apoptosis. This delay was dependent on the phosphorylation and activation of p42 mitogen‐activated protein kinase (p42MAPK) in CSF‐arrested egg extracts [12, 14]. p42MAPK is normally dephosphorylated and inactivated upon fertilization in eggs in vivo, and the addition of 0.4 mM CaCl2 into CSF‐arrested egg extracts also dephosphorylates and inactivates p42MAPK in vitro. When p42MAPK dephosphorylation is blocked, sustained activity of p42MAPK may delay apoptosis in egg extracts.
In this study, we examined whether any of these events might be inhibited by the addition of AA into egg extracts. We also compared the cytoplasmic anti‐apoptotic functions of AA with those of oxidized form of AA (dehydroascorbic acid, DHA) and unrelated acid (acetate, Ac).
Materials and methods
Preparation of Xenopus egg extracts and reagents
Animal care and use of female frogs (Xenopus laevis) is approved by the Animal Research Committee for Animal Experimentation of Toho University. Preparations of fresh CSF‐arrested, interphase, and apoptotic egg extracts were previously described [12, 13]. AA (Wako), DHA (Wako or Sigma), and Ac (Wako) were dissolved in water as 0.5 M stock solutions and added to egg extracts at 10 mM. The pH values of the extracts were routinely checked using pH indicator paper (Spezial Indikatorpapier pH 6.4–8.0, Macherey‐Nagel). Where indicated, a pan‐caspase inhibitor Z‐VAD‐FMK (Peptide Institute) or a proteasome inhibitor MG‐132 (Peptide Institute) was added to the egg extracts at 100 μM.
SDS‐PAGE, antibodies and western blot
SDS‐PAGE was carried out using 12% separating gels for PARP, p42MAPK, and actin, or 15% separating gels for cytochrome c. Resolved samples were electrically transferred to Immobilon‐P PVDF membranes (Millipore). Mouse monoclonal antibodies against PARP, MAPK, and phosphorylated MAPK (pT202/pY204) were purchased from BD Biosciences. Mouse monoclonal antibody against cytochrome c and rabbit polyclonal antibody against actin were from Lab Vision and Sigma, respectively. All antibodies were used at 1:1,000 dilutions for western blot as previously described [12, 13]. Alkaline phosphatase‐linked anti‐mouse and anti‐rabbit secondary antibodies (Santa Cruz) were used, and signals were visualized using 5‐bromo‐4‐chloro‐3‐indolyl phosphate (Sigma) and nitroblue tetrazolium (Wako).
Quantification of cytoplasmic cytochrome c
Egg extracts after incubation for indicated hours were filtered through 0.1 µm pore using Ultrafree‐MC (Millipore) at 10,000×g for 30 min according to the method by Tashker et al. [14]. The protein concentration of each filtrate was determined using Bio‐Rad Protein Assay kit (Bio‐Rad). Equal amounts of proteins were subjected to SDS‐PAGE and western blot for cytochrome c and actin.
Quantification of caspase activities
To measure the caspase activity in Xenopus egg extracts, 1.5 µl of extracts was taken out at indicated times after incubation and rapidly frozen. Each sample was then thawed and mixed with 150 µl of reaction buffer (50 mM HEPES pH 7.4, 0.1 M NaCl, 1 mM EDTA, 10% glycerol, 0.1% CHAPS, 10 mM DTT) containing 200 µM Ac‐DEVD‐pNA (BIOMOL). The increase of absorbance at 405 nm during incubation for 1 h at room temperature was measured. For the assay of purified caspases in vitro, recombinant active human caspases and corresponding substrates (Ac‐DEVD‐pNA for caspases‐3/7, Ac‐LEHD‐pNA for caspases‐2/9, and Ac‐VEID‐pNA for caspase‐6) were used (BIOMOL or Calbiochem). Caspase activities in the presence of various concentrations of AA, DHA, and Ac were determined according to manufacture's instruction, except that optimal DTT concentration was experimentally determined for each caspase.
Protein stability assay using 35S‐radiolabeled recombinant proteins
Open reading frames of Xenopus caspases (xCasp‐2/3/6/7/9) were amplified by RT‐PCR from Xenopus egg or stomach mRNA and cloned into either Nde I‐Bam HI site or Xho I‐Eco RI site of pET‐15b (Novagen) [15, 16]. In vitro translations of 35S‐radiolabeled, N‐terminally 6XHis‐tagged Xenopus caspases, xEIAP/XLX, and xXIAP in rabbit reticulocyte lysates (TnT Quick T7, Promega) were carried out as previously described [12, 13]. Rabbit reticulocyte lysates containing recombinant proteins were mixed with egg extracts at 1:9 and incubated at room temperature for indicated hours. After incubation, samples were resolved by SDS‐PAGE using 12% separating gels for xXIAP and xEIAP/XLX, or 15% separating gels for Xenopus caspases, and detected by a BAS‐5000 image analyzer (Fuji Film).
Results
In control Xenopus interphase egg extracts, the spontaneous cleavage of endogenous PARP, a typical apoptotic substrate, was observed after 4 h incubation (Fig. 1a, Ctrl). Hydrolysis of Ac‐DEVD‐pNA, a typical synthetic substrate for caspases, also increased in a time‐dependent manner (Fig. 1b, Ctrl). We added several antioxidants to egg extracts and examined whether they prevented these apoptotic changes. Frequently used antioxidants such as α‐tocopherol at 10 mM did not inhibit apoptosis (data not shown). In contrast, 10 mM AA effectively inhibited both PARP cleavage and caspase activation (Fig. 1a, b, AA). To examine whether the antioxidant activity of AA was responsible for these effects, we tested DHA, the oxidized form of AA without antioxidant activity. However, 10 mM DHA was equally effective in preventing apoptotic changes (Fig. 1a, b, DHA). It should be noted that AA and DHA are unstable at neutral pH, so that both reagents were dissolved in water and added to egg extracts as acidic solutions [17]. To determine whether the apoptosis‐inhibiting activity is specific to AA and DHA or simply due to their acidity, we also used 10 mM Ac as an unrelated acidic solution without antioxidant activity. Unexpectedly, Ac also inhibited apoptosis as strongly as AA and DHA (Fig. 1a, b, Ac). Phosphate and HCl also inhibited apoptosis at 10 mM (data not shown), suggesting that the inhibition of apoptosis observed above was dependent on their acidity but not their antioxidant activity. The addition of these three acidic solutions slightly reduced the pH of egg extracts, as measured directly using pH indicator papers (~0.2 pH unit, Fig. 1a, lower). Therefore, the addition of acidic solution at 10 mM slightly reduced cytoplasmic pH and completely prevented apoptosis in egg extracts. We then tested whether spontaneous cytochrome c release from mitochondria was inhibited by the addition of acidic solutions, using a method reported by Tashker et al. [14]. In control extracts, cytoplasmic cytochrome c began to appear at 4 h and increased at 6 h (Fig. 1c, Ctrl, Cyt. c). This time‐dependent cytoplasmic release of cytochrome c was not significantly inhibited by Ac, or even accelerated by AA and DHA (Fig. 1c, AA, DHA, and Ac, Cyt. c). The amounts of cytoplasmic actin as a loading control were approximately the same in all samples (Fig. 1c, Actin). These data suggest that the downstream events after the cytoplasmic release of cytochrome c may be affected by the addition of acidic solutions.
Figure 1.
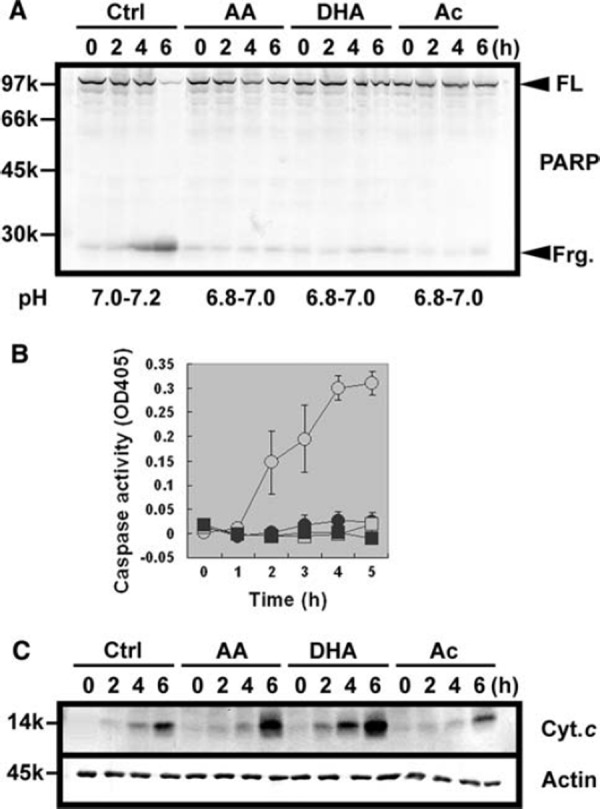
a Apoptotic PARP cleavage was inhibited by acidic solutions in Xenopus egg extracts. Interphase egg extracts in the absence or presence of 10 mM AA, 10 mM DHA, and 10 mM Ac were incubated at room temperature for indicated hours. Apoptotic cleavage of endogenous PARP was detected by western blot. Full length form (FL) and N‐terminal fragment (Frg.) are indicated by arrowheads. The pH values of egg extracts are also indicated at the bottom. b Apoptotic activation of endogenous caspases was inhibited by acidic solutions in Xenopus egg extracts. Interphase egg extracts were similarly treated as in a, and caspase activities hydrolyzing Ac‐DEVD‐pNA were measured. Data are presented as means ± SEM (N = 4). Open circle control, closed circle AA, open square DHA, and closed square Ac. c Spontaneous release of cytochrome c from mitochondria was not inhibited by acidic solutions in Xenopus egg extracts. Interphase egg extracts were similarly treated as in a, followed by centrifugation through a filter with 0.1 µm pore size. Equal amounts of protein were loaded for each lane and subjected to western blot against cytochrome c (Cyt. c) and actin (Actin)
We next analyzed whether the processing of caspases was prevented by the addition of acidic solutions. As specific antibodies for endogenous Xenopus caspases are not yet available, recombinant 35S‐radiolabeled Xenopus caspases translated in rabbit reticulocyte lysates were mixed with egg extracts, and their apoptotic changes were examined. Our preliminary analysis suggested that five recombinant Xenopus caspases (xCasps‐2/3/6/7/9) were processed during apoptosis in egg extracts (data not shown). In control egg extracts, all of these five caspases were fully processed 4 h after incubation (Fig. 2, Ctrl, compare 0 and 4 h). In contrast, processing was completely inhibited in the presence of AA, DHA, and Ac for all caspases tested (Fig. 2, AA, DHA, and Ac). Thus, processing and resultant activation of caspases was inhibited by the addition of acidic solutions. Interestingly, treatment with acidic solutions produced a modified, slow‐migrating form only in 6XHis‐xCasp‐3. The nature of this modification remains to be characterized.
Figure 2.
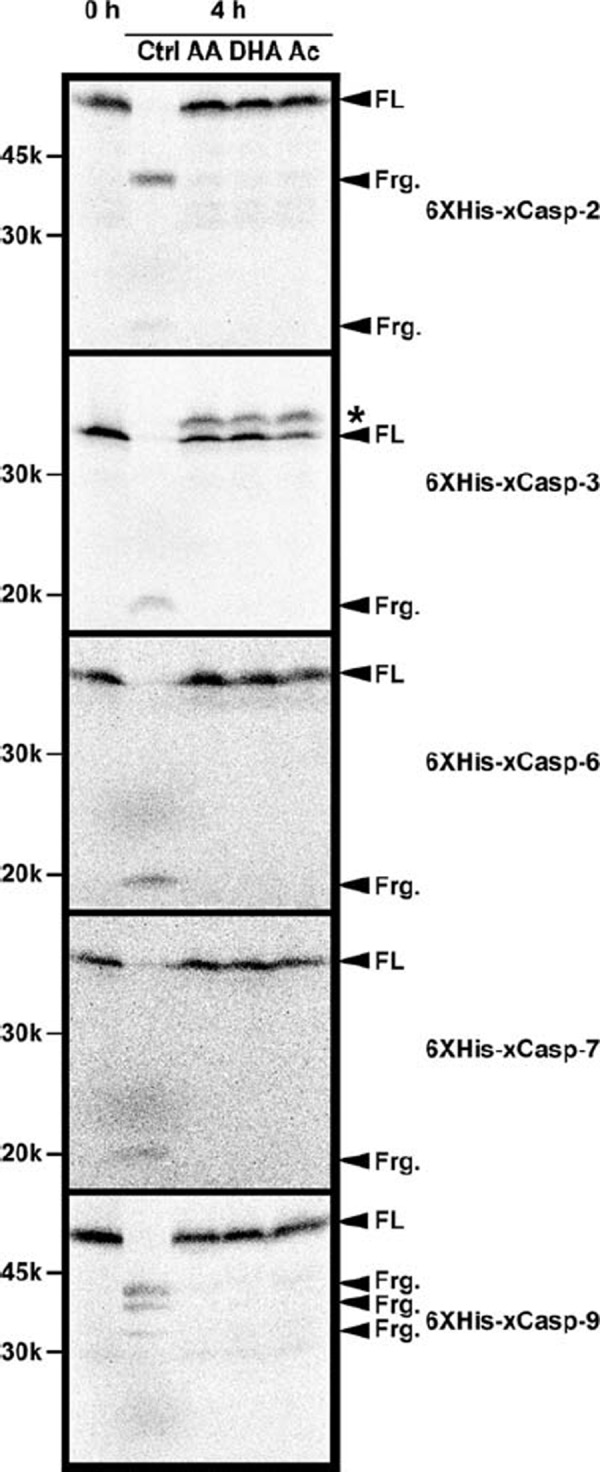
Apoptotic processing of recombinant Xenopus caspases were inhibited by acidic solutions in Xenopus egg extracts. Interphase egg extracts in the absence or presence of 10 mM AA, 10 mM DHA, and 10 mM Ac were mixed with rabbit reticulocyte lysates containing 35S‐radiolabeled recombinant Xenopus caspases and incubated at room temperature for 0 or 4 h. Samples were resolved by SDS‐PAGE and detected by image analyzer. For each caspase, full length form (FL) and processed fragments (Frg.) are indicated. In the case of xCasp‐3, a slow‐migrating form is indicated by an asterisk
To determine whether AA, DHA and Ac were direct inhibitors of caspase activity, we next turned to in vitro assay system using recombinant active human caspases. Acidic solutions were neutralized by reaction buffer in this assay, and pH‐dependent effects might be minimized. For caspases‐2/3/7, DHA showed dose‐dependent inhibition of enzymatic activities, whereas caspases‐6/9 were less sensitive to DHA (Fig. 3). In contrast, AA and Ac did not inhibit the activities of caspases significantly. The inhibition by DHA was canceled by increasing the concentration of reducing reagent (DTT) in the buffer, suggesting that DHA might inhibit caspases in a redox‐sensitive manner (data not shown).
Figure 3.
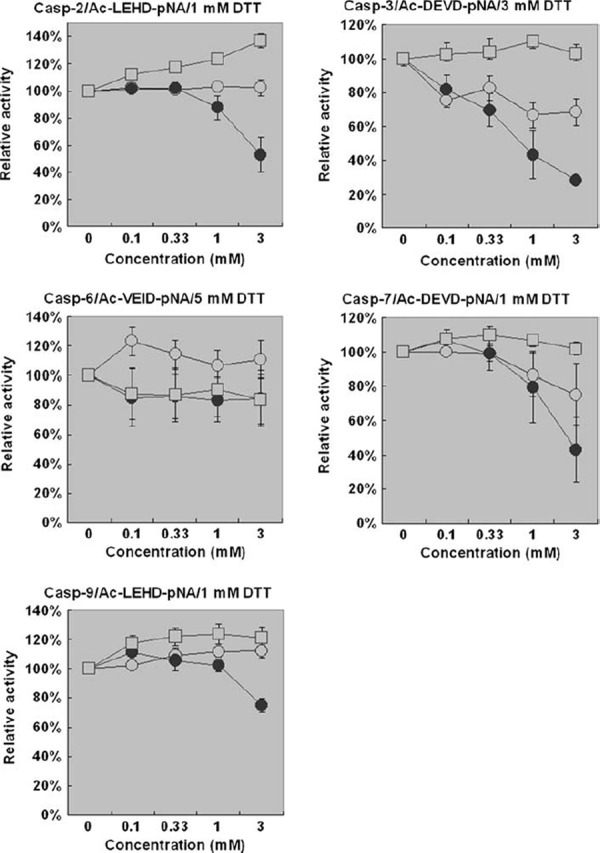
Caspase activities were directly inhibited by DHA in vitro. Activities of recombinant active human caspases were measured in the presence of AA, DHA, and Ac at various concentrations. Data are presented as means ± SEM (N = 3). Open circle AA, closed circle DHA, open square Ac
Finally, we examined whether other apoptosis‐regulating pathways were modified by the addition of acidic solutions. We noticed that the dephosphorylation of p42MAPK after the addition of 0.4 mM CaCl2 into CSF‐arrested metaphase egg extracts was inhibited by AA, DHA, and Ac, but not by a caspase inhibitor Z‐VAD‐FMK or a proteasome inhibitor MG‐132 (Fig. 4, phospho‐MAPK). The total amount of p42MAPK did not change within 4 h in all samples (Fig. 4, total MAPK). This result suggests that the sustained phosphorylation and activation of p42MAPK may be involved in the inhibition of apoptosis by acidic solutions in egg extracts.
Figure 4.

Multiple anti‐apoptotic pathways were affected by acidic solutions in Xenopus egg extracts. Interphase egg extracts in the absence or presence of 10 mM AA, 10 mM DHA, 10 mM Ac, 100 µM Z‐VAD‐FMK, and 100 µM MG‐132 were incubated at room temperature for indicated hours. Samples were resolved by SDS‐PAGE and subjected to western blot against p42MAPK (Total MAPK) and Thr/Tyr‐phosphorylated p42MAPK (Phospho‐MAPK), or detected by image analyzer (6XHis‐xXIAP and 6XHis‐xEIAP)
As previously reported, recombinant 35S‐radiolabeled 6XHis‐xXIAP produced in rabbit reticulocyte lysates was rather stable in healthy egg extracts, but was rapidly degraded at the onset of apoptosis after 4 h incubation [12, 13]. In the presence of AA, DHA, and Ac, degradation of 6XHis‐xXIAP at 4 h was completely inhibited (Fig. 4, 6XHis‐xXIAP). In addition, upward electrophoretic mobility shift of 6XHis‐xXIAP was observed in these three samples, suggesting that 6XHis‐xXIAP was modified in acid‐treated egg extracts. In contrast, neither Z‐VAD‐FMK nor MG‐132 was able to inhibit 6XHis‐xXIAP degradation at 4 h, and upward electrophoretic mobility shift was not observed in the egg extracts containing these inhibitors. Further studies will be required to identify the molecular basis of this electrophoretic mobility shift. The degradation of recombinant 35S‐radiolabeled 6XHis‐xEIAP produced in rabbit reticulocyte lysates was also blocked in egg extracts containing AA, DHA, and Ac, but not in egg extracts treated with Z‐VAD‐FMK or MG‐132 (Fig. 4, 6XHis‐xEIAP). These results indicate that the stabilized xXIAP and xEIAP/XLX could also contribute to the inhibition of apoptosis in acid‐treated egg extracts [18, 19]. Altogether, multiple apoptosis‐regulating pathways were affected by the addition of acidic solutions to egg extracts.
Discussion
We showed that the anti‐apoptotic effects of AA, DHA, and Ac in egg extracts observed in this study were primarily due to their acidifying function. Other studies indicated that the artificial modifications of cytoplasmic pH changed the timing of maturation in Xenopus oocytes [20, 21]. Therefore, cytoplasmic pH is critical for various physiological events in Xenopus oocytes and eggs. In contrast, the antioxidant activity of AA did not show pro‐survival roles in Xenopus egg extracts. Other reports using Xenopus egg extracts also failed to show the anti‐apoptotic effects of reducing reagents such as reduced glutathione (GSH) and N‐acetyl cysteine [9, 22]. Moreover, pretreatment of Xenopus oocytes with GSH‐ethyl ester partially prevented sphingomyelinase‐induced caspase‐3 activation and oocyte death, but did not affect the spontaneous caspase‐3 activation [23]. These results indicate that increasing cytoplasmic antioxidant activity antagonizes artificial generation of reactive oxygen species, but does not prevent spontaneous apoptosis in this system. This may be because sufficient amounts of antioxidant activity are present and maintained in egg cytoplasm. Otherwise, antioxidant activity may be dispensable for the inhibition of spontaneous apoptosis in egg extracts. Rather, our data suggest that the oxidant activity of DHA can inhibit caspase activities directly in vitro. One possibility is that DHA generated by the oxidation of AA, rather than AA itself, may exert anti‐apoptotic effects in cytoplasm. In the case of NF‐κB signaling, AA quenches reactive oxygen whereas DHA inhibits IκBα kinases, and both reactions prevent NF‐κB activation together [24]. Further studies will be required to establish the unified mechanisms of cytoplasmic AA‐DHA conversion and cellular survival.
In a number of reports suggesting the pro‐survival roles of AA, the reagents are added to culture medium or administrated orally in cellular or animal experiments, respectively [1, 2, 3, 4, 5, 6, 7, 8]. Intracellular uptake of AA requires the function of specific Na+‐ascorbate co‐transporter, and glucose transporters are necessary for the intracellular transport of DHA [25]. Xenopus oocytes incorporate neither AA nor DHA significantly without exogenous expression of corresponding transporters [26]. In our cellular assay, the presence of AA and DHA in the extracellular buffer did not improve the viability of Xenopus eggs (data not shown). It is not known whether mammalian oocytes and eggs express these transporters to transport AA and DHA into the cytoplasm. The pro‐survival activity of AA in culture medium may be to prevent the oxidation of extracellular materials or medium components, and this extracellular effect may be specific to mammalian oocytes and eggs. In contrast, our study using cytoplasmic extracts is aimed to clarify the intracellular roles of AA and DHA. The metabolic and apoptotic pathways are evolutionarily conserved, and our study will complement the previous cellular studies to elucidate the physiological functions of AA for reproductive biology.
Acknowledgments
We thank the members of our laboratories for discussion. This study was supported by Project Research Grant 18‐10 from Toho University School of Medicine and in part by Grants‐in Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1. Tarín, JJ , Vendrell, FJ , Ten, J , Cano, A . Antioxidant therapy counteracts the disturbing effects of diamide and maternal ageing on meiotic division and chromosomal segregation in mouse oocytes. Mol Hum Reprod, 1998, 4, 281–8 10.1093/molehr/4.3.281 [DOI] [PubMed] [Google Scholar]
- 2. Eppig, JJ , Hosoe, M , O'Brien, MJ , Pendola, FM , Requena, A , Watanabe, S . Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose and ascorbic acid. Mol Cell Endocrinol, 2000, 163, 109–16 10.1016/S0303‐7207(99)00247‐6 [DOI] [PubMed] [Google Scholar]
- 3. Guérin, P , El Mouatassim, S , Ménézo, Y . Oxidative stress and protection against reactive oxygen species in the pre‐implantation embryo and its surroundings. Hum Reprod Update, 2001, 7, 175–89 10.1093/humupd/7.2.175 [DOI] [PubMed] [Google Scholar]
- 4. Tarín, JJ , Pérez‐Albalá, S , Cano, A . Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Mol Reprod Dev, 2002, 61, 385–97 10.1002/mrd.10041 [DOI] [PubMed] [Google Scholar]
- 5. Comizzoli, P , Wildt, DE , Pukazhenthi, BS . Overcoming poor in vitro nuclear maturation and developmental competence of domestic cat oocytes during the non‐breeding season. Reproduction, 2003, 126, 809–16 10.1530/rep.0.1260809 [PubMed] [Google Scholar]
- 6. Tatemoto, H , Ootaki, K , Shigeta, K , Muto, N . Enhancement of developmental competence after in vitro fertilization of porcine oocytes by treatment with ascorbic acid 2‐O‐α‐glucoside during in vitro maturation. Biol Reprod, 2001, 65, 1800–6 10.1095/biolreprod65.6.1800 [DOI] [PubMed] [Google Scholar]
- 7. Tao, Y , Zhou, B , Xia, G , Wang, F , Wu, Z , Fu, M . Exposure to l‐ascorbic acid or α‐tocopherol facilitates the development of porcine denuded oocytes from metaphase I to metaphase II and prevents cumulus cells from fragmentation. Reprod Domest Anim, 2004, 39, 52–7 10.1046/j.1439‐0531.2003.00478.x [DOI] [PubMed] [Google Scholar]
- 8. Dalvit, G , Llanes, SP , Descalzo, A , Insani, M , Beconi, M , Cetica, P . Effect of α‐tocopherol and ascorbic acid on bovine oocyte in vitro maturation. Reprod Domest Anim, 2005, 40, 93–7 10.1111/j.1439‐0531.2004.00522.x [DOI] [PubMed] [Google Scholar]
- 9. Newmeyer, DD , Farschon, DM , Reed, JC . Cell‐free apoptosis in Xenopus egg extracts: inhibition by Bcl‐2 and requirement for an organelle fraction enriched in mitochondria. Cell, 1994, 79, 353–64 10.1016/0092‐8674(94)90203‐8 [DOI] [PubMed] [Google Scholar]
- 10. Ahsen, O , Newmeyer, DD . Cell‐free apoptosis in Xenopus laevis egg extracts. Methods Enzymol, 2000, 322, 183–98 10.1016/S0076‐6879(00)22018‐1 [DOI] [PubMed] [Google Scholar]
- 11. Deming, P , Kornbluth, S . Study of apoptosis in vitro using the Xenopus egg extract reconstitution system. Methods Mol Biol, 2006, 322, 379–93 10.1007/978‐1‐59745‐000‐3_27 [DOI] [PubMed] [Google Scholar]
- 12. Tsuchiya, Y , Murai, S , Yamashita, S . Apoptosis‐inhibiting activities of BIR family proteins in Xenopus egg extracts. FEBS J, 2005, 272, 2237–50 10.1111/j.1742‐4658.2005.04648.x [DOI] [PubMed] [Google Scholar]
- 13. Tsuchiya, Y , Yamashita, S . p42MAPK‐mediated phosphorylation of xEIAP/XLX in Xenopus cytostatic factor‐arrested egg extracts. BMC Biochem, 2007, 8, 5 10.1186/1471‐2091‐8‐5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tashker, JS , Olson, M , Kornbluth, S . Post‐cytochrome c protection from apoptosis conferred by a MAPK pathway in Xenopus egg extracts. Mol Biol Cell, 2002, 13, 393–401 10.1091/mbc.01‐06‐0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yaoita, Y , Nakajima, K . Induction of apoptosis and CPP32 expression by thyroid hormone in a myoblastic cell line derived from tadpole tail. J Biol Chem, 1997, 272, 5122–7 10.1074/jbc.272.8.5122 [DOI] [PubMed] [Google Scholar]
- 16. Nakajima, K , Takahashi, A , Yaoita, Y . Structure, expression, and function of the Xenopus laevis caspase family. J Biol Chem, 2000, 275, 10484–91 10.1074/jbc.275.14.10484 [DOI] [PubMed] [Google Scholar]
- 17. Bode, AM , Cunningham, L , Rose, RC . Spontaneous decay of oxidized ascorbic acid (dehydro‐l‐ascorbic acid) evaluated by high‐pressure liquid chromatography. Clin Chem, 1990, 36, 1807–9 [PubMed] [Google Scholar]
- 18. Holley, CL , Olson, MR , Colón‐Ramos, DA , Kornbluth, S . Reaper eliminates IAP proteins through stimulated IAP degradation and generalized translational inhibition. Nat Cell Biol, 2002, 4, 439–44 10.1038/ncb798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greenwood, J , Gautier, J . XLX is an IAP family member regulated by phosphorylation during meiosis. Cell Death Differ, 2007, 14, 559–67 10.1038/sj.cdd.4402031 [DOI] [PubMed] [Google Scholar]
- 20. Flament, S , Browaeys, E , Rodeau, J‐L , Bertout, M , Vilain, J‐P . Xenopus oocyte maturation: cytoplasm alkalization is involved in germinal vesicle migration. Int J Dev Biol, 1996, 40, 471–6 [PubMed] [Google Scholar]
- 21. Sellier, C , Bodart, J‐F , Flament, S , Baert, F , Gannon, J , Vilain, J‐P . Intracellular acidification delays hormonal G2/M transition and inhibits G2/M transition triggered by thiophosphorylated MAPK in Xenopus oocytes. J Cell Biochem, 2006, 98, 287–300 10.1002/jcb.20764 [DOI] [PubMed] [Google Scholar]
- 22. Nutt, LK , Margolis, SS , Jensen, M , Herman, CE , Dunphy, WG , Rathmell, JC , Kornbluth, S . Metabolic regulation of oocyte cell death through the CaMKII‐mediated phosphorylation of caspase‐2. Cell, 2005, 123, 89–103 10.1016/j.cell.2005.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coll, O , Morales, A , Fernández‐Checa, JC , Garcia‐Ruiz, C . Neutral sphingomyelinase‐induced ceramide triggers germinal vesicle breakdown and oxidant‐dependent apoptosis in Xenopus laevis oocytes. J Lipid Res, 2007, 48, 1924–35 10.1194/jlr.M700069‐JLR200 [DOI] [PubMed] [Google Scholar]
- 24. Cárcamo, JM , Pedraza, A , Bórquez‐Ojeda, O , Zhang, B , Sanchez, R , Golde, DW . Vitamin C is a kinase inhibitor: dehydroascorbic acid inhibits IκBα kinase β. Mol Cell Biol, 2004, 24, 6645–52 10.1128/MCB.24.15.6645‐6652.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tullio, MC , Arrigoni, O . Hopes, disillusions and more hopes from vitamin C. Cell Mol Life Sci, 2004, 61, 209–19 10.1007/s00018‐003‐3203‐8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daruwala, R , Song, J , Koh, WS , Rumsey, SC , Levine, M . Cloning and functional characterization of the human sodium‐dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett, 1999, 460, 480–84 10.1016/S0014‐5793(99)01393‐9 [DOI] [PubMed] [Google Scholar]


