Abstract
In most mammals, oocyte maturation is the final process of oogenesis, from the prophase of the first meiosis (germinal vesicle stage) to the metaphase of the second meiosis (MII), during which the oocyte acquires fertilizable competence as well as post‐fertilization development competence. The nuclear and cytoplasmic maturation processes occur in synchrony but independently. Cytoplasmic maturation entails biochemical and structural changes in the cytoplasm, which give rise to oocytes capable of being fertilized and developing into embryos. Herein we review the literature and results from our own experiments on the structural and molecular events regulating cytoplasmic maturation in oocytes, concentrating on (1) the appropriate reorganization of active mitochondria and the endoplasmic reticulum, a structural and functional feature of cytoplasmic maturation, and (2) factors involved in regulatory mechanisms such as cumulus cell–oocyte gap junctional signaling, cumulus cell–oocyte bidirectional paracrine signaling, and the complex interactions of these signaling processes and follicular fluid constituents in the follicle environment.
Keywords: Active mitochondria, Cumulus cell–oocyte interaction, Cytoplasmic maturation, Endoplasmic reticulum, Midkine
Introduction
Oocyte maturation is the final stage of oogenesis during which the fully grown oocyte becomes fertilizable. In mammals, the fully grown oocytes enclosed by cumulus cells in large antral ovarian follicles are kept arrested at prophase of the first meiosis, or the germinal vesicle (the prophase nucleus, GV) stage. The meiotic arrest is induced by inhibitory factors provided by interaction with surrounding cumulus cells and substances in follicular fluid, such as cyclic adenosine 3′‐5′ monophosphate (cAMP) [1, 2] and purine hypoxanthine (Hx) [3, 4, 5]. The resumption of meiosis is triggered by a preovulatory luteinizing hormone (LH) surge in vivo [6]. The meiotic process entails GV breakdown (GVBD, lysis of the nuclear envelope), chromatin condensation, the formation of the spindle, migration of the spindle in the metaphase of the first meiosis (MI) to the oocyte cortex, extrusion of the first polar body to complete the first meiotic division, and then establishment of the spindle in metaphase of the second meiosis (MII). The oocytes are arrested again at the MII stage. In addition to nuclear maturation exemplified by chromosome‐related events, oocytes also undergo cytoplasmic maturation. This entails biochemical and morphological changes, which give rise to oocytes able to support fertilization and the subsequent development of preimplantation embryos [7]. Although nuclear and cytoplasmic maturation usually occur in synchrony, cytoplasmic maturation can occur even when nuclear maturation becomes arrested at the MI stage [8, 9], indicating that they are independent phenomena.
In most mammals, both the nuclear and cytoplasmic maturation of preovulatory oocytes are completed in large antral follicles, and so mainly regulated by microenvironmental factors in the follicles, including (1) follicular fluid containing hormones, growth factors, and other substances such as sterols, and (2) intercellular communication between oocytes and follicular cells. Meiotic progression involves dramatic structural rearrangements and changes in the metabolic activity of organelles within the cytoplasm of oocytes. Such cytoplasmic remodeling is important for the completion of cytoplasmic maturation [10].
In the present paper, we review the literature on the spatial and temporal reorganization of mitochondria and the endoplasmic reticulum (ER) in mammalian oocytes during maturation, which is a structural and functional feature of cytoplasmic maturation, and on cytoplasmic maturation‐promoting factors such as cAMP‐elevating agents, Hx and follicle stimulating hormone (FSH), growth factors in ovarian follicular fluid, and, moreover, molecules involved in intercellular communication between oocytes and surrounding cumulus cells via gap junctional and paracrine signaling. We will also describe our own experimental data on midkine (MK) as a cytoplasmic maturation‐promoting factor.
Energy‐generating activity and distribution of mitochondria in oocytes during cytoplasmic maturation
Mitochondria are the main generators of energy, in the form of ATP, within the oocyte cytoplasm. Increased oxidative activity of mitochondria leading to an increase in ATP content has been observed in oocytes from the beginning (GV stage) to end (MII stage) of the maturation process [11, 12, 13, 14]. In bovine species, it was reported that the morphology of oocytes exhibiting lower levels of ATP progressively worsened during the maturation period [12]. Because oocyte morphology is linked to the ability to develop to the blastocyst stage after fertilization, a relationship between ATP content and developmental competence may exist. However, such a relationship is not evident in other species. It was reported that intracellular levels of ATP do not appear to be related to the difference in developmental competence in porcine oocytes [15].
A direct relationship between the distribution of active mitochondria within the blastomeres of early cleavage embryos and the ability to develop to the blastocyst stage normally in vitro has been demonstrated in rodents [16, 17]. The reorganization of active mitochondria is required for various events involved in cytoplasmic maturation [18]. In bovine oocytes, active mitochondria mainly have a cortical distribution at the GV stage, and then relocate toward the center during the maturation process, resulting in a diffuse distribution in the cytoplasm of MII oocytes. However, the relocation of active mitochondria is not observed in oocytes with poor developmental competence, in which the mitochondria remain confined to the cortex [12]. A similar relocation of active mitochondria during maturation has been observed in porcine oocytes [11, 13, 19, 20, 21]. The relocation occurs in porcine oocytes with high developmental competence, whereas in oocytes with low developmental competence, the mitochondria persisted in the cortex region [11, 21]. Furthermore, the distribution of active mitochondria throughout the cytoplasm of oocytes at the end of maturation is known to be required for triggering Ca2+ signaling during egg activation [22]. These results indicate that the relocation of active mitochondria during the oocyte maturation process is critical to cytoplasmic maturation, and consequently to the acquisition of fertilizable and developmental competence (Fig. 1). Therefore, a correct reorganization of the active mitochondria seems likely to be vital, and is possibly related to the need for an appropriate distribution of energy within the cytoplasm of oocytes.
Figure 1.
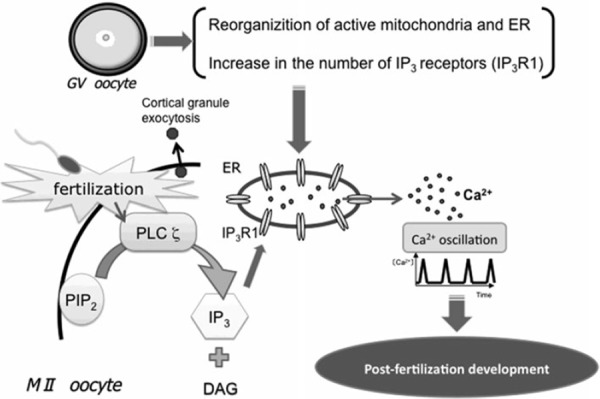
Structural events involved in the cytoplasmic maturation of oocytes, especially the reorganization of active mitochondria and the endoplasmic reticulum (ER), and the increase in the number of IP3 receptors, which occur during the maturation period from the GV to MII stages. These events are important for sperm‐triggered long‐lasting Ca2+ oscillations and development after fertilization
Development of the ability to trigger sperm‐induced Ca2+ oscillations at egg activation and cytoplasmic maturation
In most mammals, fertilization occurs in oocytes at the MII stage, the end of the maturation process. After sperm enter, the oocyte is activated by an oocyte‐activating factor, phospholipase C ζ (PLC ζ) [23, 24, 25]. PLC ζ introduced by a fertilizing spermatozoon, cleaves plasma membrane‐bound phosphatidylinositol 4,5‐bisphosphate to produce 1,4,5‐inositol trisphosphate (IP3) and diacylglycerol. IP3 binds to its receptor on the membrane of the ER and Ca2+ is released from the ER lumen into the cytoplasm. This initiates the oscillatory release of Ca2+ in the oocyte cytoplasm, which terminates after a few hours, at the time of pronuclear formation (Fig. 1) [26, 27, 28, 29].
The sperm‐induced Ca2+ oscillations are essential for the activation of embryonic development [28]. They trigger fertilization processes including resumption and completion of the second meiosis, exocytosis of cortical granules to the perivitelline space, and early embryonic development [30, 31]. When the natural regime of Ca2+ oscillations is precociously interrupted, embryo implantation is compromised, whereas an excess of Ca2+ transients compromises post‐implantation development [32, 33]. The ability of oocytes to generate Ca2+ oscillations increases during the maturation period. When oocytes are fertilized at the GV stage (prophase I), fewer Ca2+ transients are generated, compared to those in oocytes fertilized at the MII stage [34, 35]. Interestingly, the first peak of Ca2+ oscillations has a higher amplitude and the oscillations last longer in mature than in immature oocytes [35, 36, 37]. Moreover, oocytes spontaneously arrested in the MI stage, unable to achieve the MII stage even after a prolonged culture, are able to produce Ca2+ oscillations similar to those generated in fertilized MII oocytes [35]. It is therefore suggested that the ability to generate Ca2+ oscillations is closely related to cytoplasmic maturation. The cytoplasmic changes occurring during maturation, which play important roles in the accurate generation of Ca2+ oscillations initiated by the fertilizing sperm, mainly involve reorganization of the ER, calcium stores in oocytes, and an increasing number of IP3 receptors on the ER (Fig. 1).
Reorganization of the ER and increasing numbers of IP3 receptors in oocytes during cytoplasmic maturation
During the process of oocyte maturation, the ER undergoes a dynamic reorganization, from cytoplasmic accumulation in the GV oocytes to distinctive cortical clustering in the MII oocytes, which can be visualized using dicarbocyanine dyes and confocal microscopy [38]. In mouse and hamster oocytes at the GV stage, the ER forms a fine network with patch‐like accumulation within the inner cytoplasm and in the cortex area [39, 40, 41]. Following GVBD, the ER accumulates in the form of a dense ring in the center of the oocyte around the meiotic apparatus [39, 40, 41]. This reorganization of the ER does not occur in GV‐arrested oocytes and is prevented by nocodazole, a microtubule‐depolymerizing agent, and by the inhibition of cytoplasmic dynein, a microtubule‐associated motor protein [39, 42]. Therefore, it is suggested that the formation of the ER ring at GVBD is dynein‐driven and cell cycle‐dependent. After GVBD, the ER ring surrounding the prometaphase I spindle moves toward the oocyte cortex. Around the time of the first polar body's extrusion, the dense ER ring disappears, and thereafter, cortical clusters of ER are formed close to when the MI to MII transition occurs. However, ER clusters are absent above the MII spindle [39, 40, 41, 43]. Formation of the characteristic ER clusters is prevented by the depolymerization of microfilaments with latrunculin A, an actin‐depolymerising agent, but not of microtubules [39]. Taken together, the ER's reorganization during oocyte maturation is thought to be a complex process involving microtubule‐ and microfilament‐dependent phases. Moreover, it is indicated that the generation of cortical ER clusters, which normally occurs at the time of the MI–MII transition, is independent of meiotic progression towards the MII stage, that is, even when oocytes spontaneously arrest in the MI stage due to a failure to extrude the first polar body, they are capable of taking part in cortical ER clusters [39]. The reorganization of the ER is thus a structural feature of cytoplasmic maturation, which especially allows oocytes to acquire fertilizable and developmental competence (Fig. 1).
As described above, Ca2+ oscillations are essential for the activation of embryonic development and the repetitive release of Ca2+ from the ER lumen occurs through the type 1 IP3 receptor (IP3R1), the isoform predominantly expressed in oocytes [44, 45]. The number of IP3R1 increases during oocyte maturation. Western blot analyses have indicated that MII oocytes contain almost two times more IP3R1 than GV oocytes [44, 45, 46]. This increase plays a crucial role in the ability to generate Ca2+ oscillations.
The distribution of IP3R1 changes during oocyte maturation, as well. In GV oocytes, IP3R1 are present throughout the cytoplasm, but mainly located in the cortex, where in rodents they form a thin layer or show a patch‐like distribution [41, 44, 46]. In human oocytes, the distribution is different, that is, IP3R1 form patch‐like structures in the inner cytoplasm and are less abundant in peripheral areas [47]. On the other hand, the IP3R1 in rodent and human MII oocytes are ubiquitous in the cytoplasm, but accumulate in the cortex, where they form well‐defined clusters [41, 46, 47, 48]. Immunofluorescent study reveals that IP3R1 are located predominantly in ER clusters [48]. Thus, the clustering of the ER and IP3R1 in the cortex in MII oocytes may facilitate the initiation of Ca2+ oscillations (Fig. 1).
Signaling molecules promoting cytoplasmic maturation
Both the nuclear and cytoplasmic maturation of preovulatory oocytes proceed in synchrony in large antral follicles after the LH surge in vivo. The in vitro maturation (IVM) of GV oocytes is now widespread, producing MII oocytes with a fertilizable and post‐fertilization developmental competence. However, IVM fundamentally differs from maturation in vivo. Fully grown GV oocytes surrounded by a multilayer of cumulus cells (cumulus cell‐enclosed oocytes, CEOs) are usually collected from antral follicles for IVM. CEOs, however, spontaneously undergo nuclear maturation before reaching optimal cytoplasmic maturity, and are not necessarily competent to support normal fertilization and further embryonic development. The inhibitory influences on the nuclear maturation of oocytes in the follicular environment provide an optimal balance between nuclear and cytoplasmic maturation processes in vivo. In IVM, CEOs removed from the follicles therefore result in loss of the natural inhibiting environment, leading to spontaneous maturation of the oocytes, which can cause an imbalance in the maturation processes. The poor developmental capacity of CEOs matured in vitro is inherent to the heterogeneity of the starting population. Furthermore, our understanding of what regulates the cytoplasmic maturation of oocytes, including the factors secreted from CEOs and other follicular cells, is still limited. Therefore, in an attempt to develop an IVM system that confers optimal developmental competence to a heterogeneous population of oocytes, greater efforts are now directed at trying to understand the molecular mechanisms by which oocyte maturation, especially cytoplasmic maturation, is regulated with substances included in follicular fluids as well as the complex interplay between oocytes and their surrounding follicular cells through gap junctional complexes and paracrine factors.
Roles of cAMP‐elevating agents and intercellular communication between cumulus cells and oocytes via gap junctional complexes
The follicle is the basic structural and functional unit of the mammalian ovary that provides the environment necessary for oocyte growth and maturation. When GV‐arrested CEOs are removed from follicles, they can spontaneously resume meiosis. Thus, the fully grown CEOs in antral follicles are kept arrested at the GV stage due to inhibitory factors provided by interaction with the follicular somatic cells and to substances present in the follicular fluids, such as Hx [3, 4, 5, 49]. Hx is a phosphodiesterase inhibitor, which is capable of increasing intracellular concentrations of cAMP by preventing its degradation [4]. Using IVM, abundant evidence showing that the intracellular cAMP plays an important role in controlling oocyte maturation in several species, in particular rodents, has been revealed [2, 50, 51, 52, 53]. Adding cAMP‐elevating agents, such as the cAMP analogue dibutyryl cAMP, adenylate cyclase activator forskolin, and phosphodiesterase inhibitors Hx and 3‐isobutyl‐1‐methylxanthine, to IVM cultures prevents spontaneous meiotic resumption of CEOs temporarily or for long periods [2, 50, 51, 52, 53, 54, 55, 56, 57]. Relatively high levels of cAMP within the oocyte are essential to maintaining meiotic arrest at the GV stage, and a drop in the intraoocyte concentration of cAMP is required for resumption of meiosis. However, the source of the inhibitory cAMP has not yet been determined. One suggestion is that the oocytes themselves generate it through the activation of a membrane‐bound Gs protein, which in turn stimulates an adenylate cyclase [58, 59, 60]. Alternatively, the inhibitory cAMP produced in the surrounding granulosa and cumulus cells may be transferred into the oocytes via gap junctional complexes, thereby maintaining meiotic arrest [2, 61, 62]. This hypothesis suggests that an LH‐induced resumption of oocyte maturation occurs following the interruption of gap junctional complexes caused by this gonadotropin in the ovarian follicle [61, 63, 64], and is strongly supported by the finding that maturation resumes spontaneously when CEOs are recovered from the ovarian follicle and placed in IVM medium, and that the interruption of gap junctional complexes within ovarian follicles with carbenoxolone, a selective blocker of gap junctional complexes, that takes place in organ cultures, effectively promotes the resumption of meiosis, which is accompanied by a substantial decrease in intraoocyte concentrations of cAMP [65].
The treatment with cAMP‐elevating agents, such as dibutyryl cAMP, Hx, and FSH, of CEOs during IVM improves developmental competence after in vitro fertilization (IVF) [66, 67, 68, 69, 70, 71, 72]. How this positive effect on cytoplasmic maturation is exerted remains unclear. However, when CEOs at the GV stage immediately after removal from ovarian follicles are cultured in IVM medium with such agents, GVBD is delayed, and the duration of oocyte–cumulus cell gap junctional communication during the period of meiotic resumption is simultaneously extended for several hours, compared to untreated CEOs [57, 73, 74]. It is therefore suggested that a prolongation of the cross‐talk between oocytes and their surrounding cumulus cells via gap junctions, resulting in an increase in intraoocyte cAMP levels, leads to the acquisition of high developmental competence after fertilization (Fig. 2).
Figure 2.
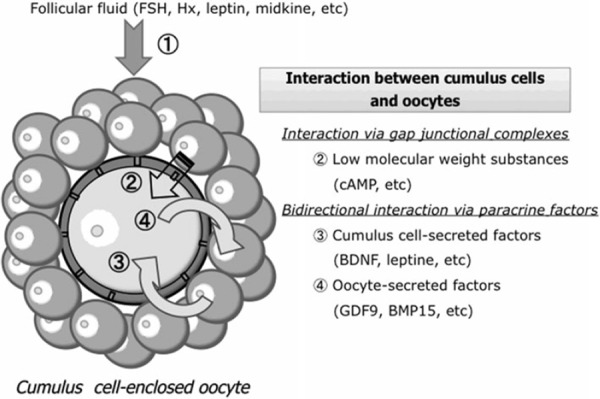
Regulation of the cytoplasmic maturation of cumulus cell‐enclosed oocytes (CEOs) in the follicular microenvironment. The cytoplasmic maturation of CEOs is promoted by (1) substances contained within follicular fluid such as FSH, purine hypoxanthine (Hx), leptin and midkine, and (2) bidirectional communication between cumulus cells and their surrounding oocyte via gap junctional and paracrine signaling. cAMP transported from cumulus cells to the oocyte via gap junctional complexes has the ability to promote cytoplasmic maturation. In a paracrine fashion, BDNF and leptin secreted by cumulus cells, and GDF9 and BMP15 secreted by the oocyte itself, can promote the cytoplasmic maturation of oocytes
Follicular fluid and its effective components other than cAMP‐elevating agents
Follicular fluid is a complex mixture of transudates obtained from serum through the blood–follicle barrier and components secreted from follicular cells, such as granulosa cells and cumulus cells [75], and contains high levels of nutrients, gonadotropins, and growth factors, some of which have been suggested as playing a key role in nuclear and cytoplasmic maturation [76, 77]. Intrinsic factors such as the size of the follicle of origin could also influence the cytoplasmic maturation of oocytes, because larger antral follicles contain oocytes more likely to develop into blastocysts after fertilization than oocytes from smaller antral follicles [78, 79, 80]. However, there are reports that follicular fluid is not always influenced by follicle size but may be affected by the quality of follicles from which it is obtained [79, 81]. Furthermore, it was also reported that exposure of CEOs to follicular fluid collected from females at different times after the LH surge profoundly affected the rate of development to the blastocyst stage after IVF [82]. Therefore, it is generally considered that a beneficial effect on development is had by follicular fluid in dominant healthy follicles but not in small growing or atretic follicles [80]. However, there have been reports suggesting inhibitory effects of follicular fluid on the cytoplasmic maturation of oocytes [83, 84, 85]. We also found that when pooled bovine follicular fluid was partitioned into heparin‐adsorbed and non‐adsorbed fractions by affinity chromatography and effects on IVM of bovine CEOs were examined, the non‐adsorbed fraction had an inhibitory effect and the adsorbed fraction, a stimulatory effect [86].
Thus, if those factors in follicular fluid that are beneficial to oocyte maturation competence could be isolated and determined, they might help to improve the IVM system and to clarify the mechanism underlying cytoplasmic maturation. So far, several cytoplasmic maturation‐promoting factors in follicular fluid, such as EGF [87, 88, 89, 90, 91], TGF‐α [89], activin [92, 93], inhibin [93], follicular fluid meiosis‐activating sterol (FF‐MAS) [94], and leptin [95, 96], have been reported. Recently, we also identified MK isolated from bovine follicular fluid using heparin affinity chromatography as a cytoplasmic maturation‐promoting factor (Fig. 2) [97].
MK is a member of a new family of heparin‐binding growth factors, and was first identified as a product of a retinoic acid‐inducible gene in a teratocarcinoma cell line [98, 99]. MK is a basic, non‐glycosylated protein with a molecular mass of about 13 kDa [100], and mainly composed of two domains held by disulfide linkages [101]. The expression of MK is spatially and temporally regulated in mouse embryogenesis [98] and widespread in adult tissues [102]. In the ovary, MK is present in follicular fluid at high concentrations [103, 104]. MK mRNA is expressed exclusively in granulosa cells of healthy follicles and increased by the administration of gonadotropins, pregnant mare serum gonadotrophin, and FSH [105, 106]. In view of its pleiotropic functions, such as the promotion of growth [102], survival [107], migration [108] and gene expression [109] in various target cells, and of its presence in the ovary, it seems likely that MK is involved in the development of ovarian follicles and growth and maturation of oocytes. In fact, double knockout mice lacking MK and pleiotrophin, which have 50% amino acid sequence homology and many redundant functions, and whose receptors and signaling systems are closely related [110, 111], exhibit female infertility, especially defects in follicular maturation and ovulation [112]. To clarify the role of MK in bovine oocyte maturation, we first cloned bovine MK (bMK) and produced a recombinant bMK protein using a baculovirus expression system [97]. As shown in Fig. 3, the nucleotide sequence of bMK cDNA and predicted amino acid sequence are highly homologous to those of mouse (87 and 84%, respectively) and human (92 and 93%, respectively). We then examined the effects of the recombinant bMK at physiological concentrations during IVM on nuclear maturation and developmental competence after the IVF of bovine CEOs at the GV stage (Table 1) [97]. Although nuclear maturation from the GV to MII stage was not affected, the efficiency with which CEOs developed to the blastocyst stage after IVF was increased by adding the recombinant bMK to the IVM culture medium, suggesting that bMK has the ability to promote the cytoplasmic maturation of bovine CEOs. The promoting effect of bMK was inhibited by heparitinase treatment of CEOs, suggesting that heparan sulfate chains on the surface of cumulus cells and/or oocytes are required for MK to exert its effect [97]. Since the promoting effect was not exerted in oocytes freed from CEOs (denuded oocyte; DO), but was exerted in DOs co‐cultured with cumulus cells isolated from CEOs, it seems to be mediated by some soluble factor(s) secreted from cumulus cells surrounding oocytes [113]. Furthermore, a ligation‐mediated PCR analysis of internucleosomal DNA fragmentation and the TUNEL assay revealed that bMK suppresses the apoptosis that occurs spontaneously in bovine CEOs during IVM (Fig. 4) [113]. Thus, MK acts on cumulus cells to help them survive and secrete factors acting directly on oocytes. The substances involved in the MK‐promoted cytoplasmic maturation of CEOs are yet to be clarified. It was recently found that leptin [95, 96] and brain‐derived neurotrophic factor (BDNF) [114, 115, 116, 117] promoted developmental competence in mammalian oocytes during IVM culture (Fig. 2). Whether these factors are involved in the MK‐regulated cytoplasmic maturation of oocytes remains to be determined.
Figure 3.
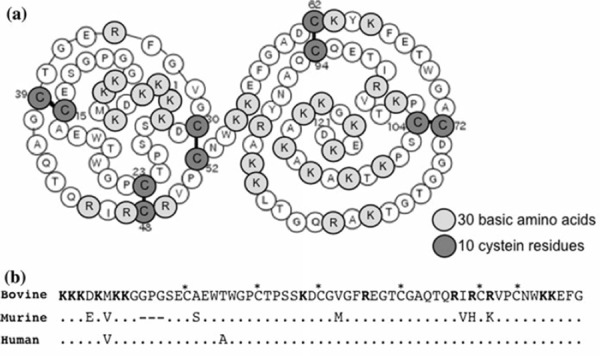
Primary structure of bovine midkine (bMK). a bMK is composed of two domains, the N‐terminally located domain, bMK (15–52) and C‐terminally located domain, bMK (62–104), and of 121 amino acids in which 30 basic amino acids and 10 cysteine residues are included. b Alignment of the amino acid sequences of bovine, mouse and human MK. Amino acids in the mouse and human MK that differ from the bovine sequence are indicated. Basic amino acids are marked in bold. Asterisks (*) denote cysteine residues. All the disulfide bonds are conserved, from Ref. [97]
Table 1.
Effect of bMK during IVM on post‐fertilization development of bovine oocytes
| BMK (ng/ml) A | Number of IVM/IVF oocytes | % cleavage embryos | % blastocysts |
|---|---|---|---|
| 0 | 56 | 68.5 A | 31.0 A |
| 200 | 59 | 76.5 A | 65.2b |
Values in the same column with different superscripts (a, b) differ significantly (P < 0.05), from Ref. [97]
ACEOs were matured in serum‐free modified synthetic oviduct fluid (mSOF) medium containing essential and non‐essential amino acids, polyvinyl alcohol, estradiol 17 β and human chorionic gonadotropin, supplemented with or without bMK
Figure 4.
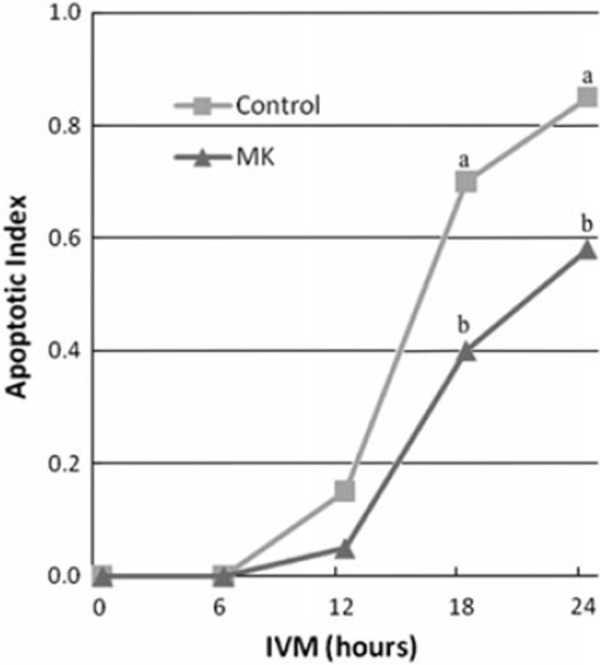
LM‐PCR analysis of internucleosomal DNA fragmentation in bovine CEOs during IVM culture with or without bMK. The intensity of ladder‐like bands derived from the apoptotic DNA fragments (1–3 nucleosomal units of DNA fragments) relative to the intensity of the band for β‐actin was measured. The relative intensity for the onset of IVM (0 h) was subtracted from that for each time point of IVM and the difference was designated as an apoptotic index. Different letters (a and b) depict significant differences between the treatments at the same time point (P < 0.05), from Ref. [113]
Intercellular communication between cumulus cells and oocytes via paracrine signaling molecules
As described above, intercellular communication between cumulus cells and oocytes via gap junctional complexes, which permits the transfer of small molecules such as cAMP, is required for post‐fertilization developmental competence (Fig. 2). Furthermore, it has been suggested that the developmental potential of oocytes is regulated by some soluble factor(s) secreted from cumulus cells surrounding the oocyte, because the co‐culture of DOs with isolated cumulus cells is needed for the developmental competence of DOs during IVM culture [113, 118]. On the other hand, in the intercellular communication between cumulus cells and oocytes via paracrine factors, the concept that the oocyte secretes paracrine factors involved in the regulation of cumulus cell function to produce paracrine factors that promote oocyte developmental competence, is now accepted [119]. The molecular basis of the paracrine communication axis from oocytes to cumulus cells is now indicated and two key growth factors, growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15), have been identified as secreted substances (Fig. 2) [119]. These growth factors are essentially unique to gametes and belong to the TGF‐β superfamily. Mice deficient in GDF9 and/or BMP15 exhibit abnormalities including female sterility, indicating critical importance to oocyte function and fertility [120]. In experiments in which bovine CEOs were matured in IVM medium supplemented with recombinant GDF9 and/or BMP15, developmental potential to the blastocyst stage after IVF was substantially improved by these growth factors [119]. Interestingly, BMP15 has the ability to prevent apoptosis, which occurs in cumulus cells of CEOs during IVM, whereas GDF9 has no significant effect [121]. Thus, secreted factors, especially GDF9 and BMP15, have the ability to promote the cytoplasmic maturation of CEOs and act by regulating the viability and function of cumulus cells of CEOs. Taken together, bidirectional communication via paracrine factors between cumulus cells and oocytes is important for promotion of the cytoplasmic maturation of CEOs (Fig. 2).
Conclusions
Although the nuclear and cytoplasmic maturation of oocytes proceed in synchrony, they are independent phenomena. Notably, cytoplasmic maturation is important for oocytes to acquire fertilizable and post‐fertilization developmental competence during maturation, and is mainly regulated by microenvironmental factors in healthy antral ovarian follicles.
A growing body of evidence shows that structural rearrangements of organelles, especially active mitochondria and the ER, in the cytoplasm of oocytes, take place during maturation, and it seems likely that the accurate relocation of active mitochondria and the ER plays important roles in the appropriate distribution of energy and generation of repetitive Ca2+ oscillations within the oocyte cytoplasm, respectively, which are essential for fertilization and post‐fertilization development. Thus, both phenomena are profoundly involved in the cytoplasmic maturation of oocytes.
Cytoplasmic maturation is regulated by the follicular microenvironment. Several constituents in follicular fluid that promote the cytoplasmic maturation of CEOs have already been identified, such as EGF, TGF‐α, activin, inhibin, FF‐MAS and leptin, as well as the cAMP‐elevating agents Hx and FSH. Recently, we identified MK isolated from follicular fluid as a cytoplasmic maturation‐promoting factor. Our studies have shown that MK acts on cumulus cells to help them survive and secrete soluble factors to promote oocyte cytoplasmic maturation.
The cytoplasmic maturation of CEOs is regulated by intercommunication between oocytes and surrounding cumulus cells via gap junctional and paracrine signaling. FSH and Hx added to the IVM culture medium are capable of improving the developmental competence of CEOs. They are also known to delay the interruption of gap junctional communication between cumulus cells and oocytes in CEOs, resulting in an increase in the accumulation of cAMP in the oocyte. This seems to lead to greater developmental competence after fertilization. On the other hand, it has been shown that paracrine factors derived from cumulus cells promote cytoplasmic maturation of CEOs, and moreover, that the oocyte is a fundamental regulator of its own developmental competence. The oocyte‐regulated effects are achieved by increasing the viability and functions of cumulus cells with oocyte‐secreted factors. Some of the factors involved in oocyte‐cumulus cell signaling have been identified; leptin and BDNF from cumulus cells act on oocytes, and GDF9 and BMP15 from oocytes act on cumulus cells. However, a comprehensive understanding of the molecular mechanisms by which cytoplasmic maturation is regulated by cumulus cell–oocyte gap junctional signaling, cumulus cell–oocyte bidirectional paracrine signaling, and the complex interactions of these signaling processes and components of follicular fluid, still remains to be achieved.
References
- 1. Cho WK, Stern S, Biggers JD. Inhibitory effect of dibutyryl cAMP on mouse oocyte maturation in vitro. J Exp Zool, 1974, 187, 383–386 10.1002/jez.1401870307 [DOI] [PubMed] [Google Scholar]
- 2. Dekel N, Beers WH. Rat oocyte maturation in vitro: relief of cyclic AMP inhibition by gonadotropins. Proc Natl Acad Sci USA, 1978, 75, 4369–4373 10.1073/pnas.75.9.4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Downs SM, Eppig JJ. Induction of mouse oocyte maturation in vivo by perturbants of purine metabolism. Biol Reprod, 1987, 36, 431–437 10.1095/biolreprod36.2.431 [DOI] [PubMed] [Google Scholar]
- 4. Downs SM. Purine control of mouse oocyte maturation: evidence that nonmetabolized hypoxanthine maintains meiotic arrest. Mol Reprod Dev, 1993, 35, 82–94 10.1002/mrd.1080350114 [DOI] [PubMed] [Google Scholar]
- 5. Eppig JJ, Ward‐Bailey PF, Coleman DL. Hypoxanthine and adenosine in murine ovarian follicular fluid: concentrations and activity in maintaining oocyte meiotic arrest. Biol Reprod, 1985, 33, 1041–1049 10.1095/biolreprod33.5.1041 [DOI] [PubMed] [Google Scholar]
- 6. Lindner HR, Tsafriri A, Lieberman ME, Zor U, Koch Y, Bauminger S et al. Gonadotropin action on cultured Graafian follicles: induction of maturation division of the mammalian oocyte and differentiation of the luteal cell. Recent Prog Horm Res, 1974, 30, 79–138 [DOI] [PubMed] [Google Scholar]
- 7. Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev, 1996, 8, 485–489 10.1071/RD9960485 [DOI] [PubMed] [Google Scholar]
- 8. Eppig JJ, Schultz RM, O'Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol, 1994, 164, 1–9 10.1006/dbio.1994.1175 [DOI] [PubMed] [Google Scholar]
- 9. Kikuchi K, Nagai T, Ding J, Yamauchi N, Noguchi J, Izaike Y. Cytoplasmic maturation for activation of pig follicular oocytes cultured and arrested at metaphase I. J Reprod Fertil, 1999, 116, 143–156 10.1530/jrf.0.1160143 [DOI] [PubMed] [Google Scholar]
- 10. Combelles CM, Albertini DF. Microtubule patterning during meiotic maturation in mouse oocytes is determined by cell cycle‐specific sorting and redistribution of gamma‐tubulin. Dev Biol, 2001, 239, 281–294 10.1006/dbio.2001.0444 [DOI] [PubMed] [Google Scholar]
- 11. Brevini TA, Vassena R, Francisci C, Gandolfi F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes. Biol Reprod, 2005, 72, 1218–1223 10.1095/biolreprod.104.038141 [DOI] [PubMed] [Google Scholar]
- 12. Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod, 2001, 64, 904–909 10.1095/biolreprod64.3.904 [DOI] [PubMed] [Google Scholar]
- 13. Torner H, Brussow KP, Alm H, Ratky J, Pohland R, Tuchscherer A et al. Mitochondrial aggregation patterns and activity in porcine oocytes and apoptosis in surrounding cumulus cells depends on the stage of pre‐ovulatory maturation. Theriogenology, 2004, 61, 1675–1689 10.1016/j.theriogenology.2003.09.013 [DOI] [PubMed] [Google Scholar]
- 14. Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in vitro fertilization and embryo transfer. Hum Reprod, 1995, 10, 415–424 [DOI] [PubMed] [Google Scholar]
- 15. Brad AM, Bormann CL, Swain JE, Durkin RE, Johnson AE, Clifford AL et al. Glutathione and adenosine triphosphate content of in vivo and in vitro matured porcine oocytes. Mol Reprod Dev, 2003, 64, 492–498 10.1002/mrd.10254 [DOI] [PubMed] [Google Scholar]
- 16. Bavister BD, Squirrell JM. Mitochondrial distribution and function in oocytes and early embryos. Hum Reprod, 2000, 15 (Suppl 2) 189–198 [DOI] [PubMed] [Google Scholar]
- 17. Muggleton‐Harris AL, Brown JJ. Cytoplasmic factors influence mitochondrial reorganization and resumption of cleavage during culture of early mouse embryos. Hum Reprod, 1988, 3, 1020–1028 [DOI] [PubMed] [Google Scholar]
- 18. Blerkom J, Runner MN. Mitochondrial reorganization during resumption of arrested meiosis in the mouse oocyte. Am J Anat, 1984, 171, 335–355 10.1002/aja.1001710309 [DOI] [PubMed] [Google Scholar]
- 19. Sun QY, Lai L, Wu GM, Park KW, Day BN, Prather RS et al. Microtubule assembly after treatment of pig oocytes with taxol: correlation with chromosomes, gamma‐tubulin, and MAP kinase. Mol Reprod Dev, 2001, 60, 481–490 10.1002/mrd.1113 [DOI] [PubMed] [Google Scholar]
- 20. Sun QY, Wu GM, Lai L, Park KW, Cabot R, Cheong HT et al. Translocation of active mitochondria during pig oocyte maturation, fertilization and early embryo development in vitro. Reproduction, 2001, 122, 155–163 10.1530/rep.0.1220155 [PubMed] [Google Scholar]
- 21. Brevini TA, Vassena R, Paffoni A, Francisci C, Fascio U, Gandolfi F. Exposure of pig oocytes to PCBs during in vitro maturation: effects on developmental competence, cytoplasmic remodelling and communications with cumulus cells. Eur J Histochem, 2004, 48, 347–356 [PubMed] [Google Scholar]
- 22. Liu L, Hammar K, Smith PJ, Inoue S, Keefe DL. Mitochondrial modulation of calcium signaling at the initiation of development. Cell Calcium, 2001, 30, 423–433 10.1054/ceca.2001.0251 [DOI] [PubMed] [Google Scholar]
- 23. Knott JG, Kurokawa M, Fissore RA, Schultz RM, Williams CJ. Transgenic RNA interference reveals role for mouse sperm phospholipase Czeta in triggering Ca2+ oscillations during fertilization. Biol Reprod, 2005, 72, 992–996 10.1095/biolreprod.104.036244 [DOI] [PubMed] [Google Scholar]
- 24. Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM et al. PLC zeta: a sperm‐specific trigger of Ca2+ oscillations in eggs and embryo development. Development, 2002, 129, 3533–3544 [DOI] [PubMed] [Google Scholar]
- 25. Swann K, Larman MG, Saunders CM, Lai FA. The cytosolic sperm factor that triggers Ca2+ oscillations and egg activation in mammals is a novel phospholipase C: PLCzeta. Reproduction, 2004, 127, 431–439 10.1530/rep.1.00169 [DOI] [PubMed] [Google Scholar]
- 26. Larman MG, Saunders CM, Carroll J, Lai FA, Swann K. Cell cycle‐dependent Ca2+ oscillations in mouse embryos are regulated by nuclear targeting of PLCzeta. J Cell Sci, 2004, 117, 2513–2521 10.1242/jcs.01109 [DOI] [PubMed] [Google Scholar]
- 27. Lee B, Yoon SY, Fissore RA. Regulation of fertilization‐initiated [Ca2+]i oscillations in mammalian eggs: a multi‐pronged approach. Semin Cell Dev Biol, 2006, 17, 274–284 10.1016/j.semcdb.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 28. Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S et al. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5‐trisphosphate receptor in fertilized hamster eggs. Science, 1992, 257, 251–255 10.1126/science.1321497 [DOI] [PubMed] [Google Scholar]
- 29. Yoda A, Oda S, Shikano T, Kouchi Z, Awaji T, Shirakawa H et al. Ca2+ oscillation‐inducing phospholipase C zeta expressed in mouse eggs is accumulated to the pronucleus during egg activation. Dev Biol, 2004, 268, 245–257 10.1016/j.ydbio.2003.12.028 [DOI] [PubMed] [Google Scholar]
- 30. Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol, 2006, 17, 324–332 10.1016/j.semcdb.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 31. Xu Z, Kopf GS, Schultz RM. Involvement of inositol 1,4,5‐trisphosphate‐mediated Ca2+ release in early and late events of mouse egg activation. Development, 1994, 120, 1851–1859 [DOI] [PubMed] [Google Scholar]
- 32. Ozil JP, Banrezes B, Toth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol, 2006, 300, 534–544 10.1016/j.ydbio.2006.08.041 [DOI] [PubMed] [Google Scholar]
- 33. Toth S, Huneau D, Banrezes B, Ozil JP. Egg activation is the result of calcium signal summation in the mouse. Reproduction, 2006, 131, 27–34 10.1530/rep.1.00764 [DOI] [PubMed] [Google Scholar]
- 34. Cheung A, Swann K, Carroll J. The ability to generate normal Ca2+ transients in response to spermatozoa develops during the final stages of oocyte growth and maturation. Hum Reprod, 2000, 15, 1389–1395 10.1093/humrep/15.6.1389 [DOI] [PubMed] [Google Scholar]
- 35. Jones KT, Carroll J, Whittingham DG. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J Biol Chem, 1995, 270, 6671–6677 10.1074/jbc.270.12.6671 [DOI] [PubMed] [Google Scholar]
- 36. Carroll J, Swann K, Whittingham D, Whitaker M. Spatiotemporal dynamics of intracellular [Ca2+]i oscillations during the growth and meiotic maturation of mouse oocytes. Development, 1994, 120, 3507–3517 [DOI] [PubMed] [Google Scholar]
- 37. Mehlmann LM, Kline D. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod, 1994, 51, 1088–1098 10.1095/biolreprod51.6.1088 [DOI] [PubMed] [Google Scholar]
- 38. Kline D. Attributes and dynamics of the endoplasmic reticulum in mammalian eggs. Curr Top Dev Biol, 2000, 50, 125–154 10.1016/S0070‐2153(00)50007‐6 [DOI] [PubMed] [Google Scholar]
- 39. FitzHarris G, Marangos P, Carroll J. Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev Biol, 2007, 305, 133–144 10.1016/j.ydbio.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 40. Mehlmann LM, Terasaki M, Jaffe LA, Kline D. Reorganization of the endoplasmic reticulum during meiotic maturation of the mouse oocyte. Dev Biol, 1995, 170, 607–615 10.1006/dbio.1995.1240 [DOI] [PubMed] [Google Scholar]
- 41. Shiraishi K, Okada A, Shirakawa H, Nakanishi S, Mikoshiba K, Miyazaki S. Developmental changes in the distribution of the endoplasmic reticulum and inositol 1,4,5‐trisphosphate receptors and the spatial pattern of Ca2+ release during maturation of hamster oocytes. Dev Biol, 1995, 170, 594–606 10.1006/dbio.1995.1239 [DOI] [PubMed] [Google Scholar]
- 42. Mallik R, Gross SP. Molecular motors: strategies to get along. Curr Biol, 2004, 14, R971–R982 10.1016/j.cub.2004.10.046 [DOI] [PubMed] [Google Scholar]
- 43. FitzHarris G, Marangos P, Carroll J. Cell cycle‐dependent regulation of structure of endoplasmic reticulum and inositol 1,4,5‐trisphosphate‐induced Ca2+ release in mouse oocytes and embryos. Mol Biol Cell, 2003, 14, 288–301 10.1091/mbc.E02‐07‐0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fissore RA, Longo FJ, Anderson E, Parys JB, Ducibella T. Differential distribution of inositol trisphosphate receptor isoforms in mouse oocytes. Biol Reprod, 1999, 60, 49–57 10.1095/biolreprod60.1.49 [DOI] [PubMed] [Google Scholar]
- 45. Parrington J, Brind S, Smedt H, Gangeswaran R, Lai FA, Wojcikiewicz R et al. Expression of inositol 1,4,5‐trisphosphate receptors in mouse oocytes and early embryos: the type I isoform is upregulated in oocytes and downregulated after fertilization. Dev Biol, 1998, 203, 451–461 10.1006/dbio.1998.9071 [DOI] [PubMed] [Google Scholar]
- 46. Mehlmann LM, Mikoshiba K, Kline D. Redistribution and increase in cortical inositol 1,4,5‐trisphosphate receptors after meiotic maturation of the mouse oocyte. Dev Biol, 1996, 180, 489–498 10.1006/dbio.1996.0322 [DOI] [PubMed] [Google Scholar]
- 47. Goud PT, Goud AP, Oostveldt P, Dhont M. Presence and dynamic redistribution of type I inositol 1,4,5‐trisphosphate receptors in human oocytes and embryos during in‐vitro maturation, fertilization and early cleavage divisions. Mol Hum Reprod, 1999, 5, 441–451 10.1093/molehr/5.5.441 [DOI] [PubMed] [Google Scholar]
- 48. Kline D, Mehlmann L, Fox C, Terasaki M. The cortical endoplasmic reticulum (ER) of the mouse egg: localization of ER clusters in relation to the generation of repetitive calcium waves. Dev Biol, 1999, 215, 431–442 10.1006/dbio.1999.9445 [DOI] [PubMed] [Google Scholar]
- 49. Dekel N, Beers WH. Development of the rat oocyte in vitro: inhibition and induction of maturation in the presence or absence of the cumulus oophorus. Dev Biol, 1980, 75, 247–254 10.1016/0012‐1606(80)90160‐8 [DOI] [PubMed] [Google Scholar]
- 50. Dekel N. Regulation of oocyte maturation. The role of cAMP. Ann N Y Acad Sci, 1988, 541, 211–216 10.1111/j.1749‐6632.1988.tb22258.x [DOI] [PubMed] [Google Scholar]
- 51. Eppig JJ. The participation of cyclic adenosine monophosphate (cAMP) in the regulation of meiotic maturation of oocytes in the laboratory mouse. J Reprod Fertil Suppl, 1989, 38, 3–8 [PubMed] [Google Scholar]
- 52. Eppig JJ. Maintenance of meiotic arrest and the induction of oocyte maturation in mouse oocyte–granulosa cell complexes developed in vitro from preantral follicles. Biol Reprod, 1991, 45, 824–830 10.1095/biolreprod45.6.824 [DOI] [PubMed] [Google Scholar]
- 53. Eppig JJ, Freter RR, Ward‐Bailey PF, Schultz RM. Inhibition of oocyte maturation in the mouse: participation of cAMP, steroid hormones, and a putative maturation‐inhibitory factor. Dev Biol, 1983, 100, 39–49 10.1016/0012‐1606(83)90198‐7 [DOI] [PubMed] [Google Scholar]
- 54. Aktas H, Wheeler MB, Rosenkrans CF Jr, First NL, Leibfried‐Rutledge ML. Maintenance of bovine oocytes in prophase of meiosis I by high [cAMP]i. J Reprod Fertil, 1995, 105, 227–235 10.1530/jrf.0.1050227 [DOI] [PubMed] [Google Scholar]
- 55. Sirard MA, First NL. In vitro inhibition of oocyte nuclear maturation in the bovine. Biol Reprod, 1988, 39, 229–234 10.1095/biolreprod39.2.229 [DOI] [PubMed] [Google Scholar]
- 56. Thomas RE, Armstrong DT, Gilchrist RB. Differential effects of specific phosphodiesterase isoenzyme inhibitors on bovine oocyte meiotic maturation. Dev Biol, 2002, 244, 215–225 10.1006/dbio.2002.0609 [DOI] [PubMed] [Google Scholar]
- 57. Thomas RE, Armstrong DT, Gilchrist RB. Bovine cumulus cell–oocyte gap junctional communication during in vitro maturation in response to manipulation of cell‐specific cyclic adenosine 3′,5′‐monophosphate levels. Biol Reprod, 2004, 70, 548–556 10.1095/biolreprod.103.021204 [DOI] [PubMed] [Google Scholar]
- 58. Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol, 2003, 258, 385–396 10.1016/S0012‐1606(03)00134‐9 [DOI] [PubMed] [Google Scholar]
- 59. Mehlmann LM, Jones TL, Jaffe LA. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science, 2002, 297, 1343–1345 10.1126/science.1073978 [DOI] [PubMed] [Google Scholar]
- 60. Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL et al. The Gs‐linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science, 2004, 306, 1947–1950 10.1126/science.1103974 [DOI] [PubMed] [Google Scholar]
- 61. Dekel N, Lawrence TS, Gilula NB, Beers WH. Modulation of cell‐to‐cell communication in the cumulus–oocyte complex and the regulation of oocyte maturation by LH. Dev Biol, 1981, 86, 356–362 10.1016/0012‐1606(81)90193‐7 [DOI] [PubMed] [Google Scholar]
- 62. Sherizly I, Galiani D, Dekel N. Regulation of oocyte maturation: communication in the rat cumulus–oocyte complex. Hum Reprod, 1988, 3, 761–766 [DOI] [PubMed] [Google Scholar]
- 63. Gilula NB, Epstein ML, Beers WH. Cell‐to‐cell communication and ovulation. A study of the cumulus‐oocyte complex. J Cell Biol, 1978, 78, 58–75 10.1083/jcb.78.1.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sela‐Abramovich S, Chorev E, Galiani D, Dekel N. Mitogen‐activated protein kinase mediates luteinizing hormone‐induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology, 2005, 146, 1236–1244 10.1210/en.2004‐1006 [DOI] [PubMed] [Google Scholar]
- 65. Sela‐Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology, 2006, 147, 2280–2286 10.1210/en.2005‐1011 [DOI] [PubMed] [Google Scholar]
- 66. Bagg MA, Nottle MB, Grupen CG, Armstrong DT. Effect of dibutyryl cAMP on the cAMP content, meiotic progression, and developmental potential of in vitro matured pre‐pubertal and adult pig oocytes. Mol Reprod Dev, 2006, 73, 1326–1332 10.1002/mrd.20555 [DOI] [PubMed] [Google Scholar]
- 67. Funahashi H, Cantley TC, Day BN. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol Reprod, 1997, 57, 49–53 10.1095/biolreprod57.1.49 [DOI] [PubMed] [Google Scholar]
- 68. Luciano AM, Pocar P, Milanesi E, Modina S, Rieger D, Lauria A et al. Effect of different levels of intracellular cAMP on the in vitro maturation of cattle oocytes and their subsequent development following in vitro fertilization. Mol Reprod Dev, 1999, 54, 86–91 10.1002/(SICI)1098‐2795(199909)54:1<86::AID‐MRD13>3.0.CO;2‐C [DOI] [PubMed] [Google Scholar]
- 69. Merriman JA, Whittingham DG, Carroll J. The effect of follicle stimulating hormone and epidermal growth factor on the developmental capacity of in vitro matured mouse oocytes. Hum Reprod, 1998, 13, 690–695 10.1093/humrep/13.3.690 [DOI] [PubMed] [Google Scholar]
- 70. Nogueira D, Cortvrindt R, Matos DG, Vanhoutte L, Smitz J. Effect of phosphodiesterase type 3 inhibitor on developmental competence of immature mouse oocytes in vitro. Biol Reprod, 2003, 69, 2045–2052 10.1095/biolreprod.103.021105 [DOI] [PubMed] [Google Scholar]
- 71. Schoevers EJ, Kidson A, Verheijden JH, Bevers MM. Effect of follicle‐stimulating hormone on nuclear and cytoplasmic maturation of sow oocytes in vitro. Theriogenology, 2003, 59, 2017–2028 10.1016/S0093‐691X(02)01288‐8 [DOI] [PubMed] [Google Scholar]
- 72. Thomas RE, Thompson JG, Armstrong DT, Gilchrist RB. Effect of specific phosphodiesterase isoenzyme inhibitors during in vitro maturation of bovine oocytes on meiotic and developmental capacity. Biol Reprod, 2004, 71, 1142–1149 10.1095/biolreprod.103.024828 [DOI] [PubMed] [Google Scholar]
- 73. Luciano AM, Modina S, Vassena R, Milanesi E, Lauria A, Gandolfi F. Role of intracellular cyclic adenosine 3′,5′‐monophosphate concentration and oocyte–cumulus cells communications on the acquisition of the developmental competence during in vitro maturation of bovine oocyte. Biol Reprod, 2004, 70, 465–472 10.1095/biolreprod.103.020644 [DOI] [PubMed] [Google Scholar]
- 74. Salustri A, Siracusa G. Metabolic coupling, cumulus expansion and meiotic resumption in mouse cumuli oophori cultured in vitro in the presence of FSH or dcAMP, or stimulated in vivo by hCG. J Reprod Fertil, 1983, 68, 335–341 10.1530/jrf.0.0680335 [DOI] [PubMed] [Google Scholar]
- 75. Edwards RG. Follicular fluid. J Reprod Fertil, 1974, 37, 189–219 10.1530/jrf.0.0370189 [DOI] [PubMed] [Google Scholar]
- 76. Artini PG, Battaglia C, D'Ambrogio G, Barreca A, Droghini F, Volpe A et al. Relationship between human oocyte maturity, fertilization and follicular fluid growth factors. Hum Reprod, 1994, 9, 902–906 [DOI] [PubMed] [Google Scholar]
- 77. Driancourt MA, Thuel B. Control of oocyte growth and maturation by follicular cells and molecules present in follicular fluid. A review. Reprod Nutr Dev, 1998, 38, 345–362 10.1051/rnd:19980401 [DOI] [PubMed] [Google Scholar]
- 78. Ali A, Coenen K, Bousquet D, Sirard MA. Origin of bovine follicular fluid and its effect during in vitro maturation on the developmental competence of bovine oocytes. Theriogenology, 2004, 62, 1596–1606 10.1016/j.theriogenology.2004.03.011 [DOI] [PubMed] [Google Scholar]
- 79. Lonergan P, Monaghan P, Rizos D, Boland MP, Gordon I. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Mol Reprod Dev, 1994, 37, 48–53 10.1002/mrd.1080370107 [DOI] [PubMed] [Google Scholar]
- 80. Sirard MA, Roy B, Patrick P, Mermillod P, Gullbault LA. Origin of the follicular fluid added to the media during bovine IVM influences embryonic development. Theriogenology, 1995, 44, 85–94 10.1016/0093‐691X(95)00150‐7 [Google Scholar]
- 81. Carolan C, Lonergan P, Monget P, Monniaux D, Mermillod P. Effect of follicle size and quality on the ability of follicular fluid to support cytoplasmic maturation of bovine oocytes. Mol Reprod Dev, 1996, 43, 477–483 10.1002/(SICI)1098‐2795(199604)43:4<477::AID‐MRD10>3.0.CO;2‐X [DOI] [PubMed] [Google Scholar]
- 82. Romero‐Arredondo A, Seidel GE Jr. Effects of follicular fluid during in vitro maturation of bovine oocytes on in vitro fertilization and early embryonic development. Biol Reprod, 1996, 55, 1012–1016 10.1095/biolreprod55.5.1012 [DOI] [PubMed] [Google Scholar]
- 83. Ayoub MA, Hunter AG. Inhibitory effect of bovine follicular fluid on in vitro maturation of bovine oocytes. J Dairy Sci, 1993, 76, 95–100 10.3168/jds.S0022‐0302(93)77327‐0 [DOI] [PubMed] [Google Scholar]
- 84. Choi YH, Takagi M, Kamishita H, Wijayagunawardane MP, Acosta TJ, Miyazawa K et al. Developmental capacity of bovine oocytes matured in two kinds of follicular fluid and fertilized in vitro. Anim Reprod Sci, 1998, 50, 27–33 10.1016/S0378‐4320(97)00087‐0 [DOI] [PubMed] [Google Scholar]
- 85. Kim K, Mitsumizo N, Fujita K, Utsumi K. The effects of follicular fluid on in vitro maturation, oocyte fertilization and the development of bovine embryos. Theriogenology, 1996, 45, 787–799 10.1016/0093‐691X(96)00008‐8 [DOI] [PubMed] [Google Scholar]
- 86. Ikeda S, Azuma T, Hashimoto S, Yamada M. In vitro maturation of bovine oocytes with fractions of bovine follicular fluid separated by heparin affinity chromatography. J Reprod Dev, 1999, 45, 397–404 10.1262/jrd.45.397 [Google Scholar]
- 87. Das K, Stout LE, Hensleigh HC, Tagatz GE, Phipps WR, Leung BS. Direct positive effect of epidermal growth factor on the cytoplasmic maturation of mouse and human oocytes. Fertil Steril, 1991, 55, 1000–1004 [DOI] [PubMed] [Google Scholar]
- 88. La Fuente R, O'Brien MJ, Eppig JJ. Epidermal growth factor enhances preimplantation developmental competence of maturing mouse oocytes. Hum Reprod, 1999, 14, 3060–3068 10.1093/humrep/14.12.3060 [DOI] [PubMed] [Google Scholar]
- 89. Kobayashi K, Yamashita S, Hoshi H. Influence of epidermal growth factor and transforming growth factor‐alpha on in vitro maturation of cumulus cell‐enclosed bovine oocytes in a defined medium. J Reprod Fertil, 1994, 100, 439–446 10.1530/jrf.0.1000439 [DOI] [PubMed] [Google Scholar]
- 90. Lonergan P, Carolan C, Langendonckt A, Donnay I, Khatir H, Mermillod P. Role of epidermal growth factor in bovine oocyte maturation and preimplantation embryo development in vitro. Biol Reprod, 1996, 54, 1420–1429 10.1095/biolreprod54.6.1420 [DOI] [PubMed] [Google Scholar]
- 91. Park KW, Iga K, Niwa K. Exposure of bovine oocytes to EGF during maturation allows them to develop to blastocysts in a chemically‐defined medium. Theriogenology, 1997, 48, 1127–1135 10.1016/S0093‐691X(97)00345‐2 [DOI] [PubMed] [Google Scholar]
- 92. Silva CC, Knight PG. Modulatory actions of activin‐A and follistatin on the developmental competence of in vitro‐matured bovine oocytes. Biol Reprod, 1998, 58, 558–565 10.1095/biolreprod58.2.558 [DOI] [PubMed] [Google Scholar]
- 93. Stock AE, Woodruff TK, Smith LC. Effects of inhibin A and activin A during in vitro maturation of bovine oocytes in hormone‐ and serum‐free medium. Biol Reprod, 1997, 56, 1559–1564 10.1095/biolreprod56.6.1559 [DOI] [PubMed] [Google Scholar]
- 94. Marin Bivens CL, Grondahl C, Murray A, Blume T, Su YQ, Eppig JJ. Meiosis‐activating sterol promotes the metaphase I to metaphase II transition and preimplantation developmental competence of mouse oocytes maturing in vitro. Biol Reprod, 2004, 70, 1458–1464 10.1095/biolreprod.103.026351 [DOI] [PubMed] [Google Scholar]
- 95. Cioffi JA, Blerkom J, Antczak M, Shafer A, Wittmer S, Snodgrass HR. The expression of leptin and its receptors in pre‐ovulatory human follicles. Mol Hum Reprod, 1997, 3, 467–472 10.1093/molehr/3.6.467 [DOI] [PubMed] [Google Scholar]
- 96. Craig J, Zhu H, Dyce PW, Petrik J, Li J. Leptin enhances oocyte nuclear and cytoplasmic maturation via the mitogen‐activated protein kinase pathway. Endocrinology, 2004, 145, 5355–5363 10.1210/en.2004‐0783 [DOI] [PubMed] [Google Scholar]
- 97. Ikeda S, Nishikimi A, Ichihara‐Tanaka K, Muramatsu T, Yamada M. cDNA cloning of bovine midkine and production of the recombinant protein, which affects in vitro maturation of bovine oocytes. Mol Reprod Dev, 2000, 57, 99–107 10.1002/1098‐2795(200009)57:1<99::AID‐MRD13>3.0.CO;2‐2 [DOI] [PubMed] [Google Scholar]
- 98. Kadomatsu K, Huang RP, Suganuma T, Murata F, Muramatsu T. A retinoic acid responsive gene MK found in the teratocarcinoma system is expressed in spatially and temporally controlled manner during mouse embryogenesis. J Cell Biol, 1990, 110, 607–616 10.1083/jcb.110.3.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kadomatsu K, Tomomura M, Muramatsu T. cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid‐gestation period of mouse embryogenesis. Biochem Biophys Res Commun, 1988, 151, 1312–1318 10.1016/S0006‐291X(88)80505‐9 [DOI] [PubMed] [Google Scholar]
- 100. Tomomura M, Kadomatsu K, Matsubara S, Muramatsu T. A retinoic acid‐responsive gene, MK, found in the teratocarcinoma system. Heterogeneity of the transcript and the nature of the translation product. J Biol Chem, 1990, 265, 10765–10770 [PubMed] [Google Scholar]
- 101. Fabri L, Maruta H, Muramatsu H, Muramatsu T, Simpson RJ, Burgess AW et al. Structural characterisation of native and recombinant forms of the neurotrophic cytokine MK. J Chromatogr, 1993, 646, 213–225 10.1016/S0021‐9673(99)87023‐X [DOI] [PubMed] [Google Scholar]
- 102. Muramatsu H, Shirahama H, Yonezawa S, Maruta H, Muramatsu T. Midkine, a retinoic acid‐inducible growth/differentiation factor: immunochemical evidence for the function and distribution. Dev Biol, 1993, 159, 392–402 10.1006/dbio.1993.1250 [DOI] [PubMed] [Google Scholar]
- 103. Hirota Y, Osuga Y, Nose E, Koga K, Yoshino O, Hirata T et al. The presence of midkine and its possible implication in human ovarian follicles. Am J Reprod Immunol, 2007, 58, 367–373 10.1111/j.1600‐0897.2007.00522.x [DOI] [PubMed] [Google Scholar]
- 104. Ohyama Y, Miyamoto K, Minamino N, Matsuo H. Isolation and identification of midkine and pleiotrophin in bovine follicular fluid. Mol Cell Endocrinol, 1994, 105, 203–208 10.1016/0303‐7207(94)90171‐6 [DOI] [PubMed] [Google Scholar]
- 105. Karino S, Minegishi T, Ohyama Y, Tano M, Nakamura K, Miyamoto K et al. Regulation and localization of midkine in rat ovary. FEBS Lett, 1995, 362, 147–150 10.1016/0014‐5793(95)00231‐W [DOI] [PubMed] [Google Scholar]
- 106. Minegishi T, Karino S, Tano M, Ibuki Y, Miyamoto K. Regulation of midkine messenger ribonucleic acid levels in cultured rat granulosa cells. Biochem Biophys Res Commun, 1996, 229, 799–805 10.1006/bbrc.1996.1883 [DOI] [PubMed] [Google Scholar]
- 107. Owada K, Sanjo N, Kobayashi T, Mizusawa H, Muramatsu H, Muramatsu T et al. Midkine inhibits caspase‐dependent apoptosis via the activation of mitogen‐activated protein kinase and phosphatidylinositol 3‐kinase in cultured neurons. J Neurochem, 1999, 73, 2084–2092 [PubMed] [Google Scholar]
- 108. Maeda N, Ichihara‐Tanaka K, Kimura T, Kadomatsu K, Muramatsu T, Noda M. A receptor‐like protein‐tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin‐binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J Biol Chem, 1999, 274, 12474–12479 10.1074/jbc.274.18.12474 [DOI] [PubMed] [Google Scholar]
- 109. Sato W, Kadomatsu K, Yuzawa Y, Muramatsu H, Hotta N, Matsuo S et al. Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J Immunol, 2001, 167, 3463–3469 [DOI] [PubMed] [Google Scholar]
- 110. Muramatsu T. Midkine (MK), the product of a retinoic acid responsive gene, and pleiotrophin constitute a new protein family regulating growth and differentiation. Int J Dev Biol, 1993, 37, 183–188 [PubMed] [Google Scholar]
- 111. Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem, 2002, 132, 359–371 [DOI] [PubMed] [Google Scholar]
- 112. Muramatsu H, Zou P, Kurosawa N, Ichihara‐Tanaka K, Maruyama K, Inoh K et al. Female infertility in mice deficient in midkine and pleiotrophin, which form a distinct family of growth factors. Genes Cells, 2006, 11, 1405–1417 10.1111/j.1365‐2443.2006.01028.x [DOI] [PubMed] [Google Scholar]
- 113. Ikeda S, Saeki K, Imai H, Yamada M. Abilities of cumulus and granulosa cells to enhance the developmental competence of bovine oocytes during in vitro maturation period are promoted by midkine; a possible implication of its apoptosis suppressing effects. Reproduction, 2006, 132, 549–557 10.1530/rep.1.01066 [DOI] [PubMed] [Google Scholar]
- 114. Anderson RA, Bayne RA, Gardner J, Sousa PA. Brain‐derived neurotrophic factor is a regulator of human oocyte maturation and early embryo development. Fertil Steril, 2010, 93, 1394–1406 10.1016/j.fertnstert.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 115. Kawamura K, Kawamura N, Mulders SM, Sollewijn Gelpke MD, Hsueh AJ. Ovarian brain‐derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. Proc Natl Acad Sci USA, 2005, 102, 9206–9211 10.1073/pnas.0502442102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lee E, Jeong YI, Park SM, Lee JY, Kim JH, Park SW et al. Beneficial effects of brain‐derived neurotropic factor on in vitro maturation of porcine oocytes. Reproduction, 2007, 134, 405–414 10.1530/REP‐06‐0288 [DOI] [PubMed] [Google Scholar]
- 117. Martins da Silva SJ, Gardner JO, Taylor JE, Springbett A, Sousa PA, Anderson RA. Brain‐derived neurotrophic factor promotes bovine oocyte cytoplasmic competence for embryo development. Reproduction, 2005, 129, 423–434 10.1530/rep.1.00471 [DOI] [PubMed] [Google Scholar]
- 118. Ge L, Han D, Lan GC, Zhou P, Liu Y, Zhang X et al. Factors affecting the in vitro action of cumulus cells on the maturing mouse oocytes. Mol Reprod Dev, 2008, 75, 136–142 10.1002/mrd.20753 [DOI] [PubMed] [Google Scholar]
- 119. Hussein TS, Thompson JG, Gilchrist RB. Oocyte‐secreted factors enhance oocyte developmental competence. Dev Biol, 2006, 296, 514–521 10.1016/j.ydbio.2006.06.026 [DOI] [PubMed] [Google Scholar]
- 120. McNatty KP, Moore LG, Hudson NL, Quirke LD, Lawrence SB, Reader K et al. The oocyte and its role in regulating ovulation rate: a new paradigm in reproductive biology. Reproduction, 2004, 128, 379–386 10.1530/rep.1.00280 [DOI] [PubMed] [Google Scholar]
- 121. Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci, 2005, 118, 5257–5268 10.1242/jcs.02644 [DOI] [PubMed] [Google Scholar]


