Summary
Humanized mice are increasingly appreciated as an incredibly powerful platform for infectious disease research. The often very narrow species tropism of many viral infections, coupled with the sometimes misleading results from preclinical studies in animal models further emphasize the need for more predictive model systems based on human cells rather than surrogates. Humanized mice represent such a model and have been greatly enhanced with regards to their immune system reconstitution as well as immune functionality in the past years, resulting in their recommendation as a preclinical model by the US Food and Drug Administration. This review aims to give a detailed summary of the generation of human peripheral blood lymphocyte‐, CD34+ haematopoietic stem cell‐ and bone marrow/liver/thymus‐reconstituted mice and available improved models (e.g. myeloid‐ or T‐cell‐only mice, MISTRG, NSG‐SGM3). Additionally, we summarize human‐tropic viral infections, for which humanized mice offer a novel approach for the study of disease pathogenesis as well as future perspectives for their use in biomedical, drug and vaccine research.
Keywords: Haematopoiesis, humanized mice, immune system, stem cell, viral
Abbreviations
- AAV
adeno‐associated virus
- AdV
adenovirus
- BLT
bone marrow/liver/thymus
- CMV
cytomegalovirus
- DENV
dengue virus
- EBV
Epstein–Barr virus
- HIS
human immune system
- HIV
human immunodeficiency virus
- HLA
human leucocyte antigen
- HSC
haematopoietic stem cell
- HTLV
human T‐lymphotrophic virus
- huPBL
human peripheral blood leucocyte
- KSHV
Kaposi's sarcoma‐associated herpesvirus
- MHC
major histocompatibility complex
- SIRPα
signal regulatory protein α
- ZIKV
Zika virus
Humanized mouse models
Humanized mice have become an essential tool in validating infectious disease research in recent years. As affordable small animal models for studying basic research and translational medicine, the field is rapidly expanding and is accompanied by the demand for improved models with increased humanization and efficacy.
The study of many viruses in vivo requires the use of surrogate models (e.g. simian immunodeficiency virus and non‐human primates) or pathogen adaptation to non‐human systems (e.g. Ebola virus in mice1). Yet, the variation in viral species and host requirements makes these alternative models less suitable for studying virus–host interactions. The development of humanized mice allows the study of pathogens within their natural host cells, offering the affordability, accessibility and flexibility that other models cannot, making them a powerful tool for cutting‐edge biomedical and preclinical research.
However, despite the promising improvements observed in recent years, numerous aspects of immune system development within these models are still under‐represented or underdeveloped and the goal remains to create a completely physiological human immune response comprising all haematopoietic lineages, encompassing the functionality and correct proportions observed in a human. Hence, there is still much need for the advancement and development of current and novel humanized mouse models.
HuPBL mice
The first humanized mouse developed in 1983 was the human peripheral blood lymphocyte (huPBL) mouse model, created via intraperitoneal injection of human peripheral blood lymphocytes into an immunodeficient mouse that may be exposed to a sub‐lethal dose of irradiation2 (Fig. 1). The lack of a fully functioning murine immune system facilitates the temporary circulation of human cells, particularly high levels of the completely functional, educated T‐cell populations in all major organs.
Figure 1.
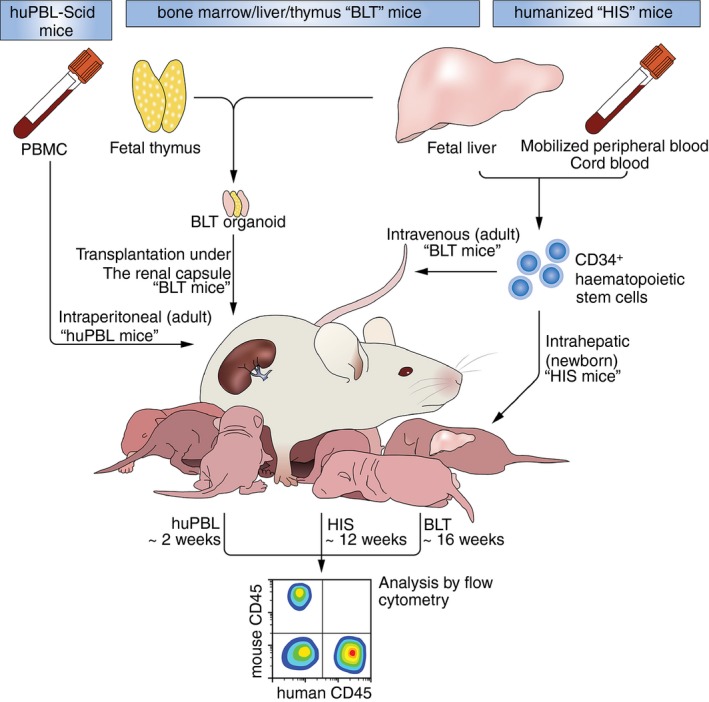
Generation of humanized mouse models, cellular origin, delivery routes and reconstitution times of huPBL, HIS and BLT mice.
The huPBL model also facilitates engraftment of low levels of B cells and some antibody production can be observed, with immune memory maintained from the donor. Engraftment of other important haematopoietic lineages (e.g. myeloid derived cells) is not supported in this model because of the rapid expansion of T cells. Furthermore, the injected T cells maintain their education from the donor, resulting in their rapid activation as they recognize the murine cells, ultimately leading to the development of graft‐versus‐host disease making this model only suitable for short‐term studies.3
HIS mice
The human immune system (HIS) mice use immunodeficient mouse strains injected with human CD34+ haematopoietic stem cells (HSC).4 most commonly derived from cord blood,5 fetal livers or granulocyte–macrophage colony‐stimulating factor (GM‐CSF) ‐mobilized peripheral blood (Fig. 1).6 Engraftment depends heavily on stem cell origin, injection route, HSC donor, background murine strain, irradiation status and engraftment age. Most haematopoietic lineages are engrafted, including several myeloid subsets. However, the major functionality of the T‐cell population is limited by the absence of human primary lymphoid organs, hence T cells are educated via murine major histocompatibility complex (MHC) class I and II molecules, stunting T‐cell development.
Bone marrow, Liver and Thymus (BLT) mice
Co‐xenotransplantation of autologous human fetal liver and thymic tissue under the murine renal capsule, alongside an intravenous CD34+ HSC injection, subverts many issues seen in HIS mice (Fig. 1). Primarily the human fetal thymic tissue allows the formation of a ‘BLT organoid’, which supports functional and educated T‐cell populations and boasts superior engraftment of all other major haematopoietic lineages.7 The engraftment of the HSC and the thymic transplant is aided by the addition of the fetal liver tissue. However, this liver tissue is not maintained in the mature organoid. Unlike previous models, which demonstrate a lack of human immune cell engraftment at mucosal surfaces, the BLT mouse model improves not only the cellular localization, dissemination and lymphoid nodule development accompanied by an increased colonization of lymphoid organs but also enhances reconstitution of the gastrointestinal and mucosal tracts.7 In addition to this, gut‐associated lymphoid tissues contain aggregates of human cells, functional IgA‐producing plasma cells and detectable levels of human IgG and IgM.7, 8 The BLT mouse model is a widely used tool for dissecting pathogen transmission, dissemination and pathogenesis, alongside analysis of vaccine candidates and microbicides.9, 10, 11
Background strains
Small animal models are an affordable tool for answering a variety of biological questions regarding infectious diseases, genetic conditions, therapeutic testing and vaccine candidates. Humanized mouse models can be developed by using a highly immunodeficient background strain e.g. severe combined immunodeficiency (scid)−/−, NOD.Cg‐scid −/− (NS), NOD.Cg‐scid −/− IL2Rγ −/− (NSG), NOD.CgRag1 −/− IL2Rγ −/− (NRG) and NOD/Shi‐scid IL2rg −/− (NOG) (Table 1). The use of protein kinase, DNA‐activated, catalytic polypeptide (Prkdc) knockout leads to defective adaptive immune cell development, yielding the scid phenotype.2 Similarly, the use of recombination activating gene (Rag1/2) knockouts severely impairs B‐cell and T‐cell receptor recombination and natural killer (NK) ‐cell development12 (Table 1). However, complications still arise with the residual murine myeloid cell compartment, which can still phagocytose non‐self human cells. To overcome this, many models include the NOD background, whose human signal regulatory protein‐α (SIRPα) allele has been shown to restrict murine macrophages from phagocytosing human cells through SIRPα/CD47 interaction.13 Alternatively, human SIRPα can also be expressed transgenically14, 15 (Table 2).
Table 1.
Basic immunodeficient murine background strains for xenotransplantation
| Name abbreviation | Strain name | Details | Reference |
|---|---|---|---|
| scid | Prkdc scid−/− | Lacks B and T cells due to Prkdc deficiency | 16 |
| NOD.scid | NOD.CB17‐Prkdc scid | Lacks B and T cells due to Prkdc deficiency | 4, 17, 18 |
| NOD SIRPa gene aids in preventing murine phagocytosis of donor HSC | |||
| NSG | NOD.Cg‐Prkdcscid Il2rgtm1WjI | NOD SIRPa gene aids in preventing murine phagocytosis of donor HSC | 19, 22 |
| Lacks B and T cells due to Prkdc deficiency | |||
| IL2rg deficiency prevents NK cell development | |||
| NRG | NOD.Cg‐Rag1 tm1Mom Il2rg tm1Wjl | NOD SIRPa gene aids in preventing murine phagocytosis of donor HSC | 23 |
| Rag1 deficiency prevents recombination and thus maturation of B and T cells | |||
| IL2rg−/−deficiency prevents NK cell development | |||
| NOG | NOD/Shi‐scid IL2rg −/− | NOD SIRPa gene aids in preventing murine phagocytosis of donor HSC | 8, 24 |
| Contains non‐functional truncated IL2rg, resulting in stunted maturation of B and T cells |
HSC, haematopoietic stem cell; IL‐2, interleukin‐2; SIRPa, signal regulatory protein α gene.
Table 2.
Cell subsets availability and functionality in humanized mice
| Cell subset | Model | Present/absent | Reported improvements to engraftment and subset development | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|
| B cells | huPBL | + | – | Donor humoral repertoire transferred | Low levels of human B cells | 19 |
| No de novo immune responses | ||||||
| Rapid development of GvHD | ||||||
| HIS | + | – | – | Low serum human immunoglobulin | 25, 26 | |
| Limited class‐switching and SHM | ||||||
| + | IL‐4, GM‐CSF | Class‐switching and increased serum IgG | ||||
| + | IL‐6 (BR6) | Class‐switching and increased serum IgG | ||||
| BLT | + | – | – | Low serum human immunoglobulin | 27 | |
| + | IL3, SCF, GM‐CSF (NSG‐SGM3) | Class‐switching and increased serum IgG | Limited class‐switching and SHM | |||
| T cells | huPBL | + | – | Donor immune repertoire transfer | Uniform T‐cell activation due to MHC mismatch | 19, 28 |
| Rapid development of GvHD | ||||||
| + | b2m−/−, HLA‐KbDb (MHC class I), H2‐Ab1−/− (MHC class II) | Donor immune repertoire transfer | Uniform T‐cell activation due to MHC mismatch | |||
| Delayed onset of GvHD | Haemochromatosis | |||||
| HIS | + | – | Enables long‐term studies | T‐cell education of murine H2‐restricted T cells | 29, 30, 31, 32 | |
| T cells predominantly Th2 polarized | ||||||
| + | HLA‐A2.1tg or HLA‐A2/HHD (NSG‐A2), HLA‐DR1tg or HLA‐DR4tg (NSG‐DR1 or NSG‐DR4), NSG‐A2/DR1 | Enables study of MHC‐restricted T‐cell responses | ||||
| + | IL12 | Improves Th1/Th2 ratio | ||||
| TOM | + | – | T‐cell‐only model for long‐term studies | – | 33 | |
| BLT | + | – | Fully functional thymic education, improved mucosal engraftment | – | 7, 34 | |
| NK cells | huPBL | − | – | – | Absent | |
| HIS | − | – | – | Largely absent and impaired functionality | 14, 35 | |
| + | IL15 (SRG15) | Improved frequency and functionality | ||||
| + | Flt3L | Improved frequency and functionality | ||||
| BLT | – | – | Largely absent and impaired functionality | |||
| Macrophages | huPBL | − | – | – | Absent | |
| HIS | + | – | – | Low numbers of human macrophages | 15, 36, 37, 38 | |
| + | M‐CSF, GM‐CSF, IL3 (NSG‐SGM3) | Functional macrophage repopulation | Unphysiological number of Treg cells | |||
| + | IL3, GM‐CSF, M‐CSF, TPO (MISTRG/MITRG) | Functional macrophage repopulation | Availability, anaemia and short life‐span | |||
| MOM | + | – | Macrophage‐only model for long‐term studies | B cells still present | 39 | |
| BLT | + | – | Increased stability and frequency of myeloid cells | 38 | ||
| Dendritic cells | huPBL | − | – | – | Absent | |
| HIS | + | – | – | Low numbers of human dendritic cells | 35, 40 | |
| + | Flt3L | Functional dendritic cell responses and higher frequencies | – | |||
| BLT | + | – | Functional dendritic cell responses and higher frequencies | – | ||
| Neutrophils | huPBL | − | – | Unknown | ||
| HIS | − | – | – | – | 15, 41 | |
| + | IL3, GM‐CSF, M‐CSF, TPO (MISTRG/MITRG) | – | Availability, anaemia and short life‐span | |||
| BLT | + | – | Increased stability and frequency of myeloid cells | – | 38 | |
| Eosinophils | huPBL | − | – | – | Unknown | |
| HIS | + | – | – | – | 37 | |
| + | IL3, GM‐CSF, M‐CSF, TPO (MISTRG/MITRG) | – | Availability, anaemia and short life‐span | 15 | ||
| BLT | ? | – | – | Unknown | ||
| Mast cells | huPBL | − | – | – | Unknown | |
| HIS | ? | – | – | Unknown | 24, 42, 43, 44 | |
| + | SCF | Increased stability and frequency of myeloid cells | – | |||
| + | IL3, SCF, GM‐CSF (NSG‐SGM3) | Increased stability and frequency of myeloid cells | Unphysiological frequency of Treg cells | |||
| BLT | + | – | – | Unknown | 24 | |
| + | IL3, SCF, GM‐CSF (NSG‐SGM3) | Increased stability and frequency of myeloid cells | Unphysiological frequency of Treg cells | |||
| Erythrocytes | huPBL | − | – | – | Absent | |
| HIS | − | – | – | Absent | 17 | |
| + | IL15, Flt3L, Epo, IL3 | Low numbers reported | – | 45 | ||
| BLT | − | – | – | Absent |
BLT, bone marrow, liver, thymus mouse; GM‐CSF, granulocyte–macrophage colony‐stimulating factor; GvHD, graft‐versus‐host disease; huPBL, human peripheral blood leucocyte mouse; HIS, human immune system mouse; IL‐4, interleukin‐4; SCF, stem cell factor; SHM, somatic hypermutation; Th1, T helper type 1; Treg, regulatory T cell.
Further developments have shown increased humanization of particular cell lineages among the previously mentioned background strains due to additional cytokine knock‐in, transient protein expression through hydrodynamic delivery and vectors, recombinant protein injection or the expression of additional transgenes. For example, the transient expression of Epo and Il3 displayed increased engraftment levels of human erythrocytes and the transient expression of Il15 and Flt3 ligand increases NK cell reconstitution.45 Similarly, previous reports demonstrated that depletion of murine macrophages increases engraftment and maturation of human erythrocytes (Table 2).
The SGM3 model developed on the NSG background including additional knock‐ins of human Il3, GM‐CSF and stem cell factor, boasts superior as well as stable engraftment of myeloid derived cells and improves B‐cell development, antigen‐specific antibody responses and T regulatory cell development.38
Overall, within all humanized mouse models, the degree of chimerism, defined by the proportion of human CD45+ cells in the total leucocyte population, varies among background strains, chosen model, HSC donor and between mice. Recently, more advanced models with the goal of dissecting cell‐specific responses to infections have been developed, e.g. myeloid‐only or T‐cell‐only responses.33, 39, 46
Innate immunity in humanized mice
Myeloid progenitor cells require external stimulatory factors to efficiently differentiate to their subsequent lineages. The main difficulty for this process in humanized mice is the lack of cross reactivity among these factors, rendering the differentiation and maturation of the majority of myeloid derived cells very inefficient in vivo (Table 2).
To overcome this, essential cytokines must be added either transiently or through gene knock‐in to establish differentiation of myeloid subsets. This has been a main focus of humanized mouse model development in recent years and led to the development of background strains (SGM3, MISTRG, SRG‐1514, 15, 36) that include human cytokine knock‐in [e.g. interleukin‐3 (IL‐3), IL‐15, GM‐CSF, macrophage colony stimulating factor (M‐CSF)] and so support the engraftment of a wider variety of human cells, particularly myeloid derived cells.
The innate immune cell reconstitution of most humanized mouse models remains substantially lower and does not reflect human immune cell composition. More basic background strains have been shown to support engraftment of classical CD14high CD16– monocyte‐like phenotype in lung tissue of humanized BLT NOD.Cgscid −/− and NSG mice. They also display low levels of CD68+ macrophage cell engraftment in the lymphoid and splenic tissue of engrafted mice.7 Furthermore, these models are also able to engraft with low levels of different dendritic cell subsets (e.g. CD141+, CD303+ or CD1c+), which exhibit the appropriate maturation status and gene expression profiles following priming of T cells and are sufficiently able to respond to endogenous and exogenous stimulation with Toll‐like receptor ligands and interferon treatment.47 Both plasmacytoid dendritic cells and myeloid dendritic cells are able to engraft, with the former creating the majority of the dendritic cell population in peripheral blood, similar to what is observed in humans.7, 47
The newly developed MITRG and MISTRG models allow for the superior development and more physiological levels of haematopoietic cells essential in innate immune responses, specifically myeloid derived cell subsets such as monocytes and macrophages, but also NK cells.15 Both models, MITRG and MISTRG, use immunodeficient Rag2 −/− Il2rg −/− mouse strains and contain gene knock‐ins of Il3, Thpo, Csf1 and Csf2 encoding M‐CSF and GM‐CSF, respectively. In addition to these, the MISTRG model also provides a bacterial artificial chromosome transgene encoding human SIRPα, so facilitating the improved engraftment of myeloid subsets15 (Table 2).
Furthermore, it has recently been shown that IL‐15 poses a species barrier restricting the development of NK cells.48 To allow a more physiological level of engraftment of human NK cells, the SRG‐15 mice were generated. The SRG‐15 incorporates the Rag2 −/− Il2rg −/− background with additional knock‐ins of human IL‐15 and human SIRPα driven by the endogenous murine promoter.14
Although these improved models display superior engraftment of innate immune cells, their impact on the development and functionality of the subsequent adaptive immune responses is still unknown. Furthermore, many of the gene knock‐ins result in superior humanization. which leads to detrimental effects for the murine host (e.g. rapid development of anaemia14).
Despite the presence of myeloid derived cell subsets within many humanized mouse models, the proportion of these cell subsets still remains very unphysiological and so there is still need for more development within all of the models to create an immune cell composition similar to that observed in humans (Table 2).
Adaptive immunity in humanized mice
One of the main advantages of using humanized mice for in vivo research has been the quality of the adaptive immune responses. Each model has its benefits and drawbacks and the choice should be made according to the question to be answered. However, most of the models show both CD4+ and CD8+ T‐cell engraftment and multi‐organ dissemination.49, 50
The huPBL model retains the immune memory and antigen specificity from the donor, which may be an advantage; however, this ultimately means that the engrafted human immune system, which consists predominantly of T cells, recognizes and targets the murine cells and ultimately leads to xenogeneic graft‐versus‐host disease.19
Despite some major drawbacks in several models due to the lack of human MHC/HLA molecules and the subsequent reactivity against murine MHC molecules, there have been several ways to overcome this, e.g. by introducing human MHC class I and II transgenes or by using the more advanced models (e.g. BLT;29, 51 Table 2).
BLT mice also exhibit functional antigen‐specific CD4+ and CD8+ T‐cell responses, but despite this, these cell populations are often biased to display a more naive phenotype, expressing high levels of CD45RA, CD27 and CCR7.7, 52
The introduction of both the heavy‐chain and light‐chain human HLA class I transgenes (NSG‐HLA‐A2/HHD) induces the development of both CD4+ and CD8+ cells expressing T‐cell receptor‐αβ or CD8+ cells expressing T‐cell receptor‐γδ. The maturation status of CD8+ cells is also depicted through their ability to degranulate, production of interferon‐γ (IFN‐γ) and their expression of either CD45RO or CD45RA, distinguishing memory from naive CD8+ cells.29 In addition, NSG‐HLA‐A2/DR1 mice engraft with not only functional T‐cell populations displaying T helper type 1 (Th1), Th2 and Th17 phenotypes, but also 15–20% of CD4+ and CD8+ exhibited a T effector cell phenotype (CD62L– CCR7– HLA‐DR+). These cells display high polyfunctionality with the ability to produce IFN‐γ, tumour necrosis factor‐α, IL‐4 and IL‐17 alongside both granzyme and low levels of perforin.30 It has also been demonstrated that transgenic mice co‐expressing human HLA‐A2 and HLA‐DR4 did not significantly alter the maturation or development of T‐cell populations; but the HLA‐DR4 single knock‐in mice have an improved polyfunctionality and antigen specificity of effector CD8+ cells over the HLA‐A2 mice.31 Furthermore, HLA‐DR4 mice have been reported to engraft with high levels of CD4+ CXCR5+ PD1++ T follicular helper cells and their subsequent precursors, which are essential in coordinating downstream B‐cell responses. These cells are disseminated efficiently to mucosal surfaces within these mice, including gut‐associated lymphoid tissue and vaginal mucosa within this model.53
Despite the engraftment and functionality of the adaptive immune responses in different humanized mouse models, the inability of human cells to cross react with murine cytokines leads to differentiation problems. It was demonstrated that an increase in IL‐4 production, driving GATA3+ expression and double‐positive GATA3+ T‐bet+‐expressing cells among CD4+ T‐cell populations is common to all humanized mouse models. This indicates that T‐cell differentiation is driven towards a Th2‐like status, which is not representative of human CD4 or CD8 T‐cell populations. However, the administration of recombinant human IL‐12 can revert this phenotype and down‐regulate GATA3 and increase T‐bet expression in both CD4+ and CD8+ T cells accompanied by increased IFN‐γ production, indicative of a Th1 phenotype.32 Furthermore, there is evidence to suggest that there is active CD8+ antigen‐specific T‐cell responses, as studies using human immunodeficiency virus (HIV) have shown that the virus accumulates mutations, actively evolving the dominant T‐cell epitopes, indicating that the CD8+ cell responses are dynamically working to suppress viral replication in BLT mice54 (Table 2).
Furthermore, adeno‐associated virus delivery of human IL‐2 has been demonstrated to increase the engraftment of regulatory T cells in NSG‐BLT mice.55 Notably, however, in certain background strains there is already an unphysiologically high proportion of T regulatory cells, which can be undesirable when investigating responses to infections (Table 2).
Humoral immunity in humanized mice
Key issues arise in the development of humoral immune responses in humanized mice due to many background strains containing the IL‐2 common γ‐chain deficiency. This leads to dramatically under‐developed secondary lymphoid tissues (e.g. lymphoid follicles and gut‐associated lymphoid tissues).56 Despite many models exhibiting improved B‐cell development or antibody production, there is still a need for more physiological antibody levels, improved B‐cell development, somatic hypermutation and class switching abilities, before humanized mice can be used for vaccine studies (Table 2).
Previous reports show that the engrafted B‐cell population, even in the more advanced models, displays an immature phenotype with high levels of CD10 and CD5 expression.34 Certain models demonstrate an improved diversity of B‐cell subsets, displaying both the B1 and B2 phenotype.57 It has, however, been demonstrated that class switching in these models is limited but that B1 phenotyped B cells are able to display a VH‐DH‐JH composition similar to that of the CD34+ donor cord blood from which the mice engrafted.57 Similarly, plasma cell differentiation and the production of IgM, IgA and IgG antibody subtypes is observed, displaying a similar proportion to that observed in humans, but at lower levels than are required for productive antibody responses.7, 58 Furthermore, it has been reported that HLA‐DR4 mice display increased B‐cell antibody class switching and IgG secretion.31 Similarly, injection of recombinant human IL‐4 and GM‐CSF before tetanus toxoid immunization stimulates memory B‐cell clonal expansion, induces improved B‐cell class switching capacity and provokes increased total IgG, IgM and tetanus‐specific IgG responses25 (Table 2). Additionally, humanized mouse models have been used for the study of antigen‐specific antibody responses to various infections.15, 59
The addition of human Il6 gene knock‐in has demonstrated improved haematopoiesis, class switching and a high frequency of somatic hypermutation.26
Due to this, a further advancement of the MISTRG mouse was created, with the addition of the human Il6 by gene knock‐in, resulting in the new MISTRG‐6 strain.26 This new model shows improved B‐cell and malignant plasma cell development, to investigate their effects on lytic bone disease, however, this strain is yet to be used in the context of infectious disease.60
Viral infections in humanized mice
The development of humanized mice has dramatically improved the quality of in vivo research for lymphotropic viral infections. The availability of these small animal models for widespread use in infectious disease research allows an inexpensive, convenient alternative model for dissecting human immune responses and host–pathogen interactions to viral infections, eliminating the need for using chimeric viruses or alternative species as models. Recently, their use has also been expanded to other non‐lymphotropic viral infections.
Human immunodeficiency virus
As one of the most researched viral infection of the past 30 years, HIV remains a key target for vaccine development, anti‐retroviral therapies and basic research to fully elucidate the mechanisms of infection. Due to the specific CD4+ T‐cell tropism, the development of humanized mouse models has had a particular impact on HIV research, especially the BLT model. It has been demonstrated that many characteristic indicators of HIV infection are recapitulated in humanized mice including viraemia, CD4+ T‐cell depletion,61, 62 CD8+ T‐cell exhaustion and observed up‐regulation of programmed cell death protein 1,7, 63 rectal and vaginal transmission and gastrointestinal colonization,8, 9, 10, 11, 62 development of viral latency64 and also evaluation of novel antiretroviral treatments and regimens.64, 65, 66 The use of humanized mice within HIV research has emphasized the significance of varying cell subsets in HIV infection and persistence. The use of novel myeloid‐only mice and T‐cell only mice to dissect cell‐specific immune responses has highlighted the relevance of both macrophages and T cells in sustaining HIV infection.33, 39 The significance of HIV replication in a model with completely absent T‐cell populations allowed the description of HIV persistence in macrophages throughout antiretroviral treatment.33, 39, 46 Furthermore, reactivating persistent HIV in the perivascular macrophages, astrocytes and microglia in the brain of infected humanized mice further highlights the importance of atypical cell subsets in HIV viral persistence.67
Epstein–Barr virus
Epstein–Barr virus (EBV) infection in humanized mice can recapitulate both the lytic and latent phases of infection, stimulate EBV‐induced lymphomas,68 create specific CD8+ T‐cell response and expansion,69, 70 NK‐cell‐mediated EBV restriction, activation and plasmacytoid dendritic cell depletion71, 72 and both latent and lytic EBV antigens can be detected.73 Tumorigenesis from EBV infection can develop in both the peripheral blood injection model (e.g. huPBLscid) and in the CD34+ HSC injection models upon depletion of human cell subsets controlling oncogenesis.5, 27
Kaposi's sarcoma‐associated herpes virus
Kaposi's sarcoma‐associated herpes virus (KSHV) transmission studies have been advanced by the BLT humanized mice, due to the increased immune cell presence in mucosal tissues, supporting natural KSHV transmission routes (e.g. oral and vaginal). Both latent and lytic phases of infection are supported and viral transcripts can be detected in tissues.74 However, it has also been shown that simpler models, such as the CD34+ injected mice, are susceptible to co‐infection with EBV and KSHV – producing genetic expression profiles similar to that observed in primary effusion lymphoma, which is linked to KSHV infection.75
Human cytomegalovirus
As another potent lymphotropic pathogen, aggressively targeting immunocompromised individuals, accompanied by a lack of appropriate in vivo models means that human cytomegalovirus (hCMV) research has welcomed humanized mice as an infection model. It was first demonstrated that it is possible to infect humanized mice with hCMV and recapitulate major steps in the viral disease progression, testing novel antiviral treatments, detection of both early and late transcripts following G‐CSF treatment of humanized mice and reactivation of latent hCMV in multiple organs.76 Furthermore, as a common nosocomial infection, transmission of hCMV through peripheral blood stem cell transplantation has been replicated in humanized mouse models and viral DNA was detected in bone marrow, spleen and liver.77
Dengue virus
Humanized mice have been validated as a tool to study dengue virus (DENV) infection due to the appearance of a similar symptomatic infection to those seen in patients, including a rash, thrombocytopenia and fever.78 Similarly, the presence of anti‐DENV specific antibodies was observed as early as 2 weeks after infection.79 Furthermore, the mice can be infected through mosquito bites, simulating natural transmission.4 In addition, studies using NSG mice with transient IL‐15 and Flt3 ligand expression via hydrodynamic injection highlight the importance of NK cells during DENV infection and display infected dendritic cells, which bind directly to, and activate NK cells and subsequently prevent DENV infection in monocytes.80
Human T‐lymphotropic virus
Human T‐lymphotropic virus (HTLV) research, much like HIV research, has also benefitted from the development of humanized models, despite the availability of rabbit, rat and non‐human primate models.81 Using many background strains including NODscid −/−, SCID−/− or NOG engrafted with HSC or peripheral blood mononuclear cells or using the BLT models, it has been demonstrated that many aspects of HTLV pathogenesis are recapitulated in humanized mice, such as viraemia,82 observing both single and multiple HTLV‐1 integrations,83 lobulated lymphocyte nuclei, presence of CD4+ Tac+ leukaemic cells and neoplastic proliferation, depletion of peripheral CD4+ T cells, adult T‐cell leukaemia development84 and even neuropathological signs such as myelin breakdown.82 It was also described that these models are efficient for the testing and development of drugs, e.g. reverse transcriptase inhibitors.85
Adenovirus
Adenovirus infection is also incredibly limited by the lack of in vivo models available to study infection. Adenovirus infection in humanized mice has recently been reported to mimic both the adenovirus acute and latent phases of infection.86 Humanized NSG‐A2 mice demonstrate asymptomatic infection in 66% of infected mice, yielding only the expression of E1A, which can be detected throughout major organs within humanized mice including lymph nodes, spleen and bone marrow.86 Asymptomatic, latent phase infection produces a productive CD8+ antigen‐specific T‐cell response and are able to produce IFN‐γ upon ex vivo stimulation.86
In contrast, the acute infection shows an increase in gene expression driven by the late promoter and displays fibrotic liver histology, and increased monocyte and macrophage cell infiltrates alongside haemorrhagic spots in 34% of infected mice. The acute infection generates increased total IgM levels when compared with the latent infection.86 This novel infection model creates a much‐needed method of evaluating adenovirus infection course, potential antiviral candidates and treatment regimens.
Viral haemorrhagic fevers
The recent use of humanized mice for the study of viral haemorrhagic fevers has improved the quality of research for several haemorrhagic fever viruses including Ebola virus, Hantavirus and Crimean–Congo haemorrhagic fever virus. The distinct pathogenesis of these infections can be recapitulated in humanized mice, which can display features such as Ebola virus antigen detection and liver‐specific histological changes and inflammatory cell infiltrates1, 87 and species‐specific infection with Ebola virus or Reston virus.88 In humanized HLA‐A2 mice, Hantavirus infection induces a dramatic decrease in total platelet count and infiltration of lymphocytes into the lungs, and increases weight loss.89 Similarly, in Crimean–Congo haemorrhagic fever infection, there is distinct strain variability observed, liver and brain pathological changes, increased polyfunctionality of CD8+ T cells and viral RNA observed in the blood and tissues of infected mice.90
Zika virus
Recent publications from Yi et al.91 described the first humanized mouse infections with Zika virus. NSG‐HLA‐DR4 mice engrafted with a human immune system from an allogeneic DR4+ HSC donor are reportedly susceptible to Zika virus infection. These data elucidate that within this model, the main reservoir of the virus focuses primarily on B cells and myeloid‐derived cells, particularly monocytes. In addition to this, a Zika virus prM and E protein encoding vaccine has also been demonstrated to show efficacy in humanized mice and to elicit protein‐E‐specific antibody production alongside neutralizing antibodies, following 6 weeks of immunization.91
Influenza virus
Regardless of the abundance of small animal models available for influenza virus research, vaccine protection, efficacy testing and screening have particularly benefitted from the use of humanized mouse models. It has been described that both the huPBL model and the HIS models are susceptible to influenza virus infection.92, 93
Humanized NODscid −/− β2m −/− mice display CD8+ T‐cell expansion in both peripheral blood and tissues, and display antigen‐specific reactivity towards the matrix M1 protein, with high‐affinity epitope binding upon vaccination with a trivalent attenuated live vaccine candidate.28 However, this effect appears largely dependent on the myeloid cell engraftment, namely for the interplay with antigen‐presenting cells, particularly dendritic cells.28 In addition, reports of humanized HLA‐DR1 mice, transgenic for human MHC class II, created productive CD4+ T‐cell responses specific to H5N1 strains of influenza A virus following priming with H1N1 influenza A virus strains, accompanied by recognition from conserved viral epitopes.92 It is also important to note that murine cells (e.g. epithelial cells) are also susceptible to influenza virus infection and wild‐type mice are commonly used as a model for influenza. Hence, it is difficult to define where the response originates in the humanized mice due to the cross reactivity with human and murine chemokines, cytokines and cell–cell interactions.94
Respiratory syncytial virus
As for influenza virus, using humanized mice for respiratory syncytial virus (RSV) infections is advantageous due to researching the virus exploiting its natural tropism; however, the virus itself naturally also infects murine cells, so characterizing the correct response can be difficult.95 Despite this, humanized mice are still an encouraging tool for the study of RSV infection and potential treatments. It has been demonstrated that, using adeno‐associated viral vectors, humanized NSG mice that transiently express several human cytokines, including IL‐3, IL‐4, IL‐7, IL‐15, GM‐CSF, M‐CSF and B‐cell‐activating factor were susceptible to RSV infection. Furthermore, this model displayed the expected lung pathologies such as peribronchiolar inflammation, neutrophil infiltration and increased mucus production.41 These HIS mice displayed lower levels of infection over NSG controls with increased levels of human IL‐1β and CCL‐3.41 Furthermore, the human IgA production in the sera was elevated and RSV epitope‐specific CD8+ splenocytes were isolated following infection and capable of producing IFN‐γ.41
The future for humanized mice
In the past 35 years, the progression and development of humanized mouse models has been astounding. The availability of humanized mice has revolutionized the research of many infectious diseases, especially those that were previously restricted because of their lymphotropic nature. However, there is still much need for improved responses and a correct representation of the heterologous human immune cell populations. Main concerns are that the total human cell count is substantially lower than murine cell counts in wild‐type mice, the deficiency in lymphoid structures, species restrictions with Fc receptor identification and the incompatibility of human and murine MHC molecule presentation and recognition. This subsequently affects the immune response elicited, e.g. total antibody or cytokine serum concentrations, positive and negative selection of T cells.
Ultimately, several areas of human immunity are significantly under‐represented (Fig. 2), primarily humoral responses and serum antibody diversity. Additionally, the lack of productive VDJ recombination and total serum immunoglobulin concentration preventing efficient execution of the complement pathways and an increased somatic hypermutation.
Figure 2.
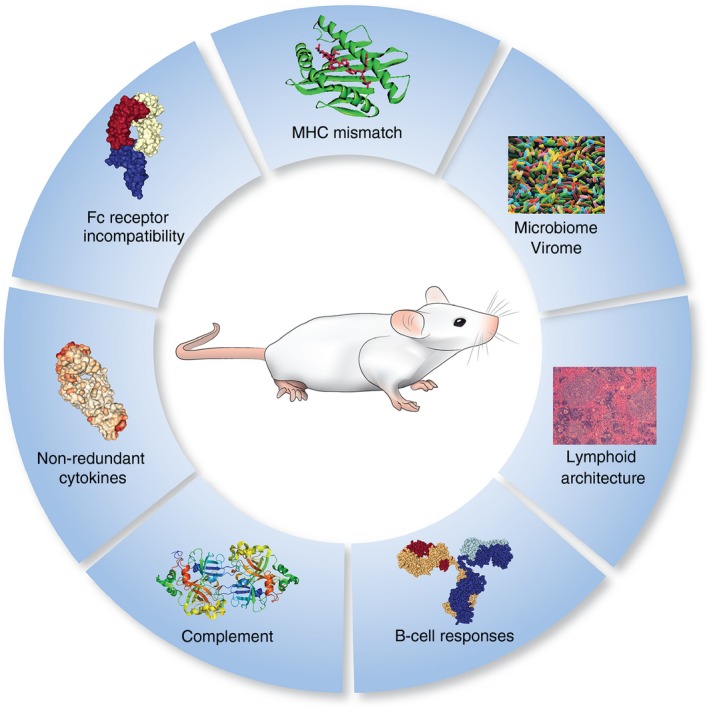
Remaining challenges in the optimization of humanized mice.
However, there were substantial improvements in these models in recent years, with the improved engraftment and proficiency of human cells. One of the most notable is the introduction of non‐redundant cytokines (e.g. GM‐CSF, IL‐3), which has increased the efficiency and functionality of the innate immune cells and their response to infection. However, the transgenic mice expressing several of these factors (e.g. MISTRG) have been shown to develop anaemia and only allow short experimental timeframes and so ways of subverting these issues need to be addressed.
Finally, it has also been shown that the microbiome and virome are becoming increasingly relevant for immune responses and can play crucial roles in the course of many infectious diseases, e.g. bacterial translocation from the gut (Fig. 2). Hence, the incorporation of these variables would be an incredibly exciting development in the field.96, 97
Humanized mouse development is a rapidly evolving field and having a model that fully recapitulates human responses is highly desirable. With a plethora of current models available, optimization of humanization, comparison of different model systems and facilitation of easy access to these models is paramount to their widespread use.
Disclosure
The authors declare no competing interests.
References
- 1. Bird BH, Spengler JR, Chakrabarti AK, Khristova ML, Sealy TK, Coleman‐McCray JD et al Humanized mouse model of ebola virus disease mimics the immune responses in human disease. J Infect Dis 2016; 213:703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature 1983; 301:527–30. [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann‐Fezer G, Gall C, Zengerle U, Kranz B, Thierfelder S. Immunohistology and immunocytology of human T‐cell chimerism and graft‐versus‐host disease in SCID mice. Blood 1993; 81:3440–8. [PubMed] [Google Scholar]
- 4. Bente DA, Melkus MW, Garcia JV, Rico‐Hesse R. Dengue fever in humanized NOD/SCID mice. J Virol 2005; 79:13797–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cocco M, Bellan C, Tussiwand R, Corti D, Traggiai E, Lazzi S et al CD34+ cord blood cell‐transplanted Rag2–/– γ(c)–/– mice as a model for Epstein–Barr virus infection. Am J Pathol 2008; 173:1369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antsiferova O, Müller A, Rämer PC, Chijioke O, Chatterjee B, Raykova A et al Adoptive transfer of EBV specific CD8+ T cell clones can transiently control EBV infection in humanized mice. PLoS Pathog 2014; 10:e1004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brainard DM, Seung E, Frahm N, Cariappa A, Bailey CC, Hart WK et al Induction of robust cellular and humoral virus‐specific adaptive immune responses in human immunodeficiency virus‐infected humanized BLT mice. J Virol 2009; 83:7305–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wahl A, Victor Garcia J. The use of BLT humanized mice to investigate the immune reconstitution of the gastrointestinal tract. J Immunol Methods 2014; 410:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deruaz M, Luster AD. BLT humanized mice as model to study HIV vaginal transmission. J Infect Dis 2013; 208(Suppl. 2):S131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olesen R, Wahl A, Denton PW, Garcia JV. Immune reconstitution of the female reproductive tract of humanized BLT mice and their susceptibility to human immunodeficiency virus infection. J Reprod Immunol 2011; 88:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Denton PW, Estes JD, Sun Z, Othieno FA, Wei BL, Wege AK et al Antiretroviral pre‐exposure prophylaxis prevents vaginal transmission of HIV‐1 in humanized BLT mice. PLoS Med 2008; 5:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG‐1‐deficient mice have no mature B and T lymphocytes. Cell 1992; 68:869–77. [DOI] [PubMed] [Google Scholar]
- 13. Strowig T, Rongvaux A, Rathinam C, Takizawa H, Borsotti C, Philbrick W et al Transgenic expression of human signal regulatory protein α in Rag2–/– γ(c)–/– mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A 2011; 108:13218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herndler‐Brandstetter D, Shan L, Yao Y, Stecher C, Plajer V, Lietzenmayer M et al Humanized mouse model supports development, function, and tissue residency of human natural killer cells. Proc Natl Acad Sci U S A 2017; 114:E9626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL et al Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol 2014; 32:364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kern ER, Rybak RJ, Hartline CB, Bidanset DJ. Predictive efficacy of SCID‐hu mouse models for treatment of human cytomegalovirus infections. Antivir Chem Chemother 2001; 12(Suppl. 1):149–56. [PubMed] [Google Scholar]
- 17. Hayakawa J, Hsieh MM, Anderson DE, Phang O, Uchida N, Washington K et al The assessment of human erythroid output in NOD/SCID mice reconstituted with human hematopoietic stem cells. Cell Transplant 2010; 19:1465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Banerjee P, Tripp A, Lairmore MD, Crawford L, Sieburg M, Ramos JC et al Adult T‐cell leukemia/lymphoma development in HTLV‐1‐infected humanized SCID mice. Blood 2010; 115:2640–8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Ali N, Flutter B, Rodriguez RS, Sharif‐Paghaleh E, Barber LD, Lombardi G et al Xenogeneic graft‐versus‐host‐disease in NOD‐scid IL‐2Rγnull mice display a T‐effector memory phenotype. PLoS ONE 2012; 7:e44219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Audigé A, Rochat MA, Li D, Ivic S, Fahrny A, Muller CK et al Long‐term leukocyte reconstitution in NSG mice transplanted with human cord blood hematopoietic stem and progenitor cells. BMC Immunol 2017; 18:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chung YS, Son JK, Choi B, Joo SY, Lee YS, Park JB et al Co‐transplantation of human fetal thymus, bone and CD34+ cells into young adult immunodeficient NOD/SCID IL2Rγ null mice optimizes humanized mice that mount adaptive antibody responses. Clin Immunol 2015; 157:156–65. [DOI] [PubMed] [Google Scholar]
- 22. Covassin L, Jangalwe S, Jouvet N, Laning J, Burzenski L, Shultz LD et al Human immune system development and survival of non‐obese diabetic (NOD)‐scid IL2rγ null (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin Exp Immunol 2013; 174:372–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris DT, Badowski M, Balamurugan A, Yang OO. Long‐term human immune system reconstitution in non‐obese diabetic (NOD)‐Rag–‐γ chain– (NRG) mice is similar but not identical to the original stem cell donor. Clin Exp Immunol 2013; 174:402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito R, Takahashi T, Katano I, Kawai K, Kamisako T, Ogura T et al Establishment of a human allergy model using human IL‐3/GM‐CSF‐transgenic NOG mice. J Immunol 2013; 191:2890–9. [DOI] [PubMed] [Google Scholar]
- 25. Chen Q, He F, Kwang J, Chan JK, Chen J. GM‐CSF and IL‐4 stimulate antibody responses in humanized mice by promoting T, B, and dendritic cell maturation. J Immunol 2012; 189:5223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu H, Borsotti C, Schickel JN, Zhu S, Strowig T, Eynon EE et al A novel humanized mouse model with significant improvement of class‐switched, antigen‐specific antibody production. Blood 2017; 129:959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma SD, Hegde S, Young KH, Sullivan R, Rajesh D, Zhou Y et al A new model of Epstein–Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J Virol 2011; 85:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chun IY, Gallegos M, Marches F, Zurawski G, Ramilo O, García‐Sastre A et al Broad influenza‐specific CD8+ T‐cell responses in humanized mice vaccinated with influenza virus vaccines. Blood 2008; 112:3671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M et al Generation of functional human T‐cell subsets with HLA‐restricted immune responses in HLA class I expressing NOD/SCID/IL2r γ null humanized mice. Proc Natl Acad Sci U S A 2010; 107:13022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Billerbeck E, Horwitz JA, Labitt RN, Donovan BM, Vega K, Budell WC et al Characterization of human antiviral adaptive immune responses during hepatotropic virus infection in HLA‐transgenic human immune system mice. J Immunol 2013; 191:1753–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Majji S, Wijayalath W, Shashikumar S, Pow‐Sang L, Villasante E, Brumeanu TD et al Differential effect of HLA class‐I versus class‐II transgenes on human T and B cell reconstitution and function in NRG mice. Sci Rep 2016; 6:28093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Billerbeck E, Labitt RN, Vega K, Frias‐Staheli N, Dorner M, Xiao JW et al Insufficient interleukin‐12 signalling favours differentiation of human CD4+ and CD8+ T cells into GATA‐3+ and GATA‐3+ T‐bet+ subsets in humanized mice. Immunology 2014; 143:202–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Honeycutt JB, Wahl A, Archin N, Choudhary S, Margolis D, Garcia JV. HIV‐1 infection, response to treatment and establishment of viral latency in a novel humanized T cell‐only mouse (TOM) model. Retrovirology 2013; 10:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Biswas S, Chang H, Sarkis PT, Fikrig E, Zhu Q, Marasco WA. Humoral immune responses in humanized BLT mice immunized with West Nile virus and HIV‐1 envelope proteins are largely mediated via human CD5+ B cells. Immunology 2011; 134:419–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ding Y, Wilkinson A, Idris A, Fancke B, O'Keeffe M, Khalil D et al FLT3‐ligand treatment of humanized mice results in the generation of large numbers of CD141+ and CD1c+ dendritic cells in vivo . J Immunol 2014; 192:1982–9. [DOI] [PubMed] [Google Scholar]
- 36. Billerbeck E, Barry WT, Mu K, Dorner M, Rice CM, Ploss A. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor‐, granulocyte–macrophage colony‐stimulating factor‐, and interleukin‐3‐expressing NOD‐SCID IL2Rγ null humanized mice. Blood 2011; 117:3076–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coughlan AM, Harmon C, Whelan S, O'Brien EC, O'Reilly VP, Crotty P et al Myeloid engraftment in humanized mice: impact of granulocyte‐colony stimulating factor treatment and transgenic mouse strain. Stem Cells Dev 2016; 25:530–41. [DOI] [PubMed] [Google Scholar]
- 38. Jangalwe S, Shultz LD, Mathew A, Brehm MA. Improved B cell development in humanized NOD‐scid IL2Rγnull mice transgenically expressing human stem cell factor, granulocyte‐macrophage colony‐stimulating factor and interleukin‐3. Immun Inflamm Dis 2016; 4:427–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Honeycutt JB, Wahl A, Baker C, Spagnuolo RA, Foster J, Zakharova O et al Macrophages sustain HIV replication in vivo independently of T cells. J Clin Invest 2016; 126:1353–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Y, Mention JJ, Court N, Masse‐Ranson G, Toubert A, Spits H et al A novel Flt3‐deficient HIS mouse model with selective enhancement of human DC development. Eur J Immunol 2016; 46:1291–9. [DOI] [PubMed] [Google Scholar]
- 41. Sharma A, Wu W, Sung B, Huang J, Tsao T, Li X et al RSV pulmonary infection in humanized mice induces human anti‐RSV immune responses and pathology. J Virol 2016; 90:5068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bryce PJ, Falahati R, Kenney LL, Leung J, Bebbington C, Tomasevic N et al Humanized mouse model of mast cell‐mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis. J Allergy Clin Immunol 2016; 138:769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burton OT, Stranks AJ, Tamayo JM, Koleoglou KJ, Schwartz LB, Oettgen HC. A humanized mouse model of anaphylactic peanut allergy. J Allergy Clin Immunol 2016; 139:314–22.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tanaka S, Saito Y, Kunisawa J, Kurashima Y, Wake T, Suzuki N et al Development of mature and functional human myeloid subsets in hematopoietic stem cell‐engrafted NOD/SCID/IL2rγKO mice. J Immunol 2012; 188:6145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Amaladoss A, Chen Q, Liu M, Dummler SK, Dao M, Suresh S et al De novo generated human red blood cells in humanized mice support Plasmodium falciparum infection. PLoS ONE 2015; 10:e0129825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Honeycutt JB, Thayer WO, Baker CE, Ribeiro RM, Lada SM, Cao Y et al HIV persistence in tissue macrophages of humanized myeloid‐only mice during antiretroviral therapy. Nat Med 2017; 23:638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Minoda Y, Virshup I, Rojas IL, Haigh O, Wong Y, Miles JJ et al Human CD141+ dendritic cell and CD1c+ dendritic cell undergo concordant early genetic programming after activation in humanized mice in vivo . Front Immunol 2017; 8:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pek EA, Chan T, Reid S, Ashkar AA. Characterization and IL‐15 dependence of NK cells in humanized mice. Immunobiology 2011; 216:218–24. [DOI] [PubMed] [Google Scholar]
- 49. Ladel CH, Kaufmann SH, Bamberger U. Localisation of human peripheral blood leukocytes after transfer to C.B‐17 scid/scid mice. Immunol Lett 1993; 38:63–8. [DOI] [PubMed] [Google Scholar]
- 50. Hiramatsu H, Nishikomori R, Heike T, Ito M, Kobayashi K, Katamura K et al Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/γcnull mice model. Blood 2003; 102:873–80. [DOI] [PubMed] [Google Scholar]
- 51. Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 2006; 108:487–92. [DOI] [PubMed] [Google Scholar]
- 52. Long BR, Stoddart CA. α Interferon and HIV infection cause activation of human T cells in NSG‐BLT mice. J Virol 2012; 86:3327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Allam A, Majji S, Peachman K, Jagodzinski L, Kim J, Ratto‐Kim S et al TFH cells accumulate in mucosal tissues of humanized‐DRAG mice and are highly permissive to HIV‐1. Sci Rep 2015; 5:10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dudek TE, Allen TM. HIV‐specific CD8⁺ T‐cell immunity in humanized bone marrow‐liver‐thymus mice. J Infect Dis 2013; 208(Suppl. 2):S150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Durost PA, Aryee KE, Manzoor F, Tisch RM, Mueller C, Jurczyk A et al Gene therapy with an adeno‐associated viral vector expressing human interleukin‐2 alters immune system homeostasis in humanized mice. Hum Gene Ther 2017; https://doi.org/10.1089/hum.2017.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nochi T, Denton PW, Wahl A, Garcia JV. Cryptopatches are essential for the development of human GALT. Cell Rep 2013; 3:1874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Quách TD, Hopkins TJ, Holodick NE, Vuyyuru R, Manser T, Bayer RL et al Human B‐1 and B‐2 B cells develop from Lin–CD34+CD38lo stem cells. J Immunol 2016; 197:3950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tonomura N, Habiro K, Shimizu A, Sykes M, Yang YG. Antigen‐specific human T‐cell responses and T cell‐dependent production of human antibodies in a humanized mouse model. Blood 2008; 111:4293–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Crawford LB, Tempel R, Streblow DN, Kreklywich C, Smith P, Picker LJ et al Human cytomegalovirus induces cellular and humoral virus‐specific immune responses in humanized BLT mice. Sci Rep 2017; 7:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Das R, Strowig T, Verma R, Koduru S, Hafemann A, Hopf S et al Microenvironment‐dependent growth of preneoplastic and malignant plasma cells in humanized mice. Nat Med 2016; 22:1351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zou W, Denton PW, Watkins RL, Krisko JF, Nochi T, Foster JL et al Nef functions in BLT mice to enhance HIV‐1 replication and deplete CD4+CD8+ thymocytes. Retrovirology 2012; 9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Berges BK, Akkina SR, Folkvord JM, Connick E, Akkina R. Mucosal transmission of R5 and X4 tropic HIV‐1 via vaginal and rectal routes in humanized Rag2–/– γc–/– (RAG‐hu) mice. Virology 2008; 373:342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Seung E, Dudek TE, Allen TM, Freeman GJ, Luster AD, Tager AM. PD‐1 blockade in chronically HIV‐1‐infected humanized mice suppresses viral loads. PLoS ONE 2013; 8:e77780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Denton PW, Krisko JF, Powell DA, Mathias M, Kwak YT, Martinez‐Torres F et al Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV‐1 transmission in humanized BLT mice. PLoS ONE 2010; 5:e8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Denton PW, Othieno F, Martinez‐Torres F, Zou W, Krisko JF, Fleming E et al One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 2011; 85:7582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wahl A, Swanson MD, Nochi T, Olesen R, Denton PW, Chateau M et al Human breast milk and antiretrovirals dramatically reduce oral HIV‐1 transmission in BLT humanized mice. PLoS Pathog 2012; 8:e1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Llewellyn GN, Alvarez‐Carbonell D, Chateau M, Karn J, Cannon PM. HIV‐1 infection of microglial cells in a reconstituted humanized mouse model and identification of compounds that selectively reverse HIV latency. J Neurovirol 2017; https://doi.org/10.1007/s13365-017-0604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yajima M, Imadome KI, Nakagawa A, Watanabe S, Terashima K, Nakamura H et al A new humanized mouse model of Epstein–Barr virus infection that reproduces persistent infection, lymphoproliferative disorder, and cell‐mediated and humoral immune responses. J Infect Dis 2008; 198:673–82. [DOI] [PubMed] [Google Scholar]
- 69. Strowig T, Gurer C, Ploss A, Liu YF, Arrey F, Sashihara J et al Priming of protective T cell responses against virus‐induced tumors in mice with human immune system components. J Exp Med 2009; 206:1423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chijioke O, Marcenaro E, Moretta A, Capaul R, Münz C. Role of the 2B4 receptor in CD8+ T‐cell‐dependent immune control of Epstein–Barr virus infection in mice with reconstituted human immune system components. J Infect Dis 2015; 212:803–7. [DOI] [PubMed] [Google Scholar]
- 71. Dunmire SK, Grimm JM, Schmeling DO, Balfour HH, Hogquist KA. The incubation period of primary Epstein–Barr virus infection: viral dynamics and immunologic events. PLoS Pathog 2015; 11:e1005286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chijioke O, Müller A, Feederle R, Barros MHM, Krieg C, Emmel V et al Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein–Barr virus infection. Cell Rep 2013; 5:1489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. White RE, Rämer PC, Naresh KN, Meixlsperger S, Pinaud L, Rooney C et al EBNA3B‐deficient EBV promotes B cell lymphomagenesis in humanized mice and is found in human tumors. J Clin Invest 2012; 122:1487–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang LX, Kang G, Kumar P, Lu W, Li Y, Zhou Y et al Humanized‐BLT mouse model of Kaposi's sarcoma‐associated herpesvirus infection. Proc Natl Acad Sci U S A 2014; 111:3146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McHugh D, Caduff N, Barros MHM, Rämer PC, Raykova A, Murer A et al Persistent KSHV infection increases EBV‐associated tumor formation in vivo via enhanced EBV lytic gene expression. Cell Host Microbe 2017; 22:61–73.e67. [DOI] [PubMed] [Google Scholar]
- 76. Smith MS, Goldman DC, Bailey AS, Pfaffle DL, Kreklywich CN, Spencer DB et al Granulocyte‐colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model. Cell Host Microbe 2010; 8:284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hakki M, Goldman DC, Streblow DN, Hamlin KL, Krekylwich CN, Fleming WH et al HCMV infection of humanized mice after transplantation of G‐CSF‐mobilized peripheral blood stem cells from HCMV‐seropositive donors. Biol Blood Marrow Transplant 2014; 20:132–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mota J, Rico‐Hesse R. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J Virol 2009; 83:8638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kuruvilla JG, Troyer RM, Devi S, Akkina R. Dengue virus infection and immune response in humanized RAG2–/– γ(c)–/– (RAG‐hu) mice. Virology 2007; 369:143–52. [DOI] [PubMed] [Google Scholar]
- 80. Costa VV, Ye W, Chen Q, Teixeira MM, Preiser P, Ooi EE et al Dengue virus‐infected dendritic cells, but not monocytes, activate natural killer cells through a contact‐dependent mechanism involving adhesion molecules. MBio 2017; 8:e00741–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Marsden MD, Zack JA. Studies of retroviral infection in humanized mice. Virology 2015; 479–480:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ginwala R, Caruso B, Khan ZK, Pattekar A, Chew GM, Corley MJ et al HTLV‐1 infection and neuropathogenesis in the context of Rag1–/– γc–/– (RAG1‐Hu) and BLT mice. J Neuroimmune Pharmacol 2017; 12:504–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Feuer G, Zack JA, Harrington WJ, Valderama R, Rosenblatt JD, Wachsman W et al Establishment of human T‐cell leukemia virus type I T‐cell lymphomas in severe combined immunodeficient mice. Blood 1993; 82:722–31. [PubMed] [Google Scholar]
- 84. Tezuka K, Xun R, Tei M, Ueno T, Tanaka M, Takenouchi N et al An animal model of adult T‐cell leukemia: humanized mice with HTLV‐1‐specific immunity. Blood 2014; 123:346–55. [DOI] [PubMed] [Google Scholar]
- 85. Miyazato P, Yasunaga JI, Taniguchi Y, Koyanagi Y, Mitsuya H, Matsuoka M. De novo human T‐cell leukemia virus type 1 infection of human lymphocytes in NOD‐SCID, common γ‐chain knockout mice. J Virol 2006; 80:10683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rodríguez E, Ip WH, Kolbe V, Hartmann K, Pilnitz‐Stolze G, Tekin N et al Humanized mice reproduce acute and persistent human adenovirus infection. J Infect Dis 2017; 215:70–9. [DOI] [PubMed] [Google Scholar]
- 87. Spengler JR, Lavender KJ, Martellaro C, Carmody A, Kurth A, Keck JG et al Ebola virus replication and disease without immunopathology in mice expressing transgenes to support human myeloid and lymphoid cell engraftment. J Infect Dis 2016; 214:S308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Spengler JR, Saturday G, Lavender KJ, Martellaro C, Keck JG, Nichol ST et al Severity of disease in humanized mice infected with Ebola Virus or Reston Virus is associated with magnitude of early viral replication in liver. J Infect Dis 2017; 217:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kobak L, Raftery MJ, Voigt S, Kühl AA, Kilic E, Kurth A et al Hantavirus‐induced pathogenesis in mice with a humanized immune system. J Gen Virol 2015; 96:1258–63. [DOI] [PubMed] [Google Scholar]
- 90. Spengler JR, Kelly Keating M, McElroy AK, Zivcec M, Coleman‐McCray JD, Harmon JR et al Crimean–Congo hemorrhagic fever in humanized mice reveals glial cells as primary targets of neurological infection. J Infect Dis 2017; 216:1386–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yi G, Xu X, Abraham S, Petersen S, Guo H, Ortega N et al A DNA vaccine protects human immune cells against Zika Virus infection in humanized mice. EBioMedicine 2017; 25:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Richards KA, Chaves FA, Sant AJ. Infection of HLA‐DR1 transgenic mice with a human isolate of influenza a virus (H1N1) primes a diverse CD4 T‐cell repertoire that includes CD4 T cells with heterosubtypic cross‐reactivity to avian (H5N1) influenza virus. J Virol 2009; 83:6566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tu W, Zheng J, Liu Y, Sia SF, Liu M, Qin G et al The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a γδ T cell population in humanized mice. J Exp Med 2011; 208:1511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kamal RP, Katz JM, York IA. Molecular determinants of influenza virus pathogenesis in mice. Curr Top Microbiol Immunol 2014; 385:243–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bem RA, Domachowske JB, Rosenberg HF. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol 2011; 301:L148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hofer U, Schlaepfer E, Baenziger S, Nischang M, Regenass S, Schwendener R et al Inadequate clearance of translocated bacterial products in HIV‐infected humanized mice. PLoS Pathog 2010; 6:e1000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Monaco CL, Gootenberg DB, Zhao G, Handley SA, Ghebremichael MS, Lim ES et al Altered virome and bacterial microbiome in human immunodeficiency virus‐associated acquired immunodeficiency syndrome. Cell Host Microbe 2016; 19:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


