Summary
Psoriasis is characterized by widespread scaly erythematous plaques that cause significant physical and psychological burdens for the affected individuals. Accelerated inflammation driven by the tumour necrosis factor‐α/interleukin‐23/interleukin‐17 axis is now known to be the major mechanism in the development of psoriasis. In addition, psoriasis has an autoimmune nature that manifests as autoreactive T cells and is co‐morbid with other autoimmune diseases, such as autoimmune bullous diseases, vitiligo, alopecia and thyroiditis. In this article, we review the recent topics on autoimmunity and autoimmune co‐morbidities in psoriasis.
Keywords: alopecia, autoimmunity, bullous pemphigoid, psoriasis, thyroiditis, vitiligo
Introduction
Psoriasis is a chronic, immune‐mediated, inflammatory skin disorder that affects approximately 0·1–3% of the general population worldwide.1, 2, 3 Given the visible nature of skin lesions, including those on the face, scalp, hands and nails,4, 5, 6, 7 psoriasis is a significant physical and psychological burden to afflicted patients, decreases patient quality of life, and induces feelings of internalized stigma.8, 9, 10, 11, 12
The disease burden of psoriasis is further increased by its association with psoriatic arthritis, which is characterized by seronegative spondyloarthropathies, enthesitis and elevated C‐reactive protein levels.1, 13, 14, 15, 16 Approximately 15–30% of Caucasians with psoriasis eventually develop psoriatic arthritis.17 Although the prevalence is lower in Asian populations (Chinese 5·3–7·3%, Japanese 10·5% and Korean 11·2%), psoriatic arthritis significantly hampers patients’ daily lives.16, 17 Additionally, psoriasis has significant co‐morbidity with systemic inflammatory diseases, such as cardiovascular diseases and metabolic syndrome, and these conditions are currently receiving increasing attention.18, 19, 20, 21, 22, 23, 24, 25, 26, 27 In parallel, a plethora of inflammatory biomarkers are elevated in psoriasis.28, 29, 30, 31, 32
In addition to these co‐morbid diseases that have a systemic inflammatory nature,33 psoriasis presents with autoimmune aspects and co‐exists with a variety of autoimmune disorders.34, 35, 36, 37, 38, 39 In this article, we summarize recent topics on autoimmunity and autoimmune co‐morbidities in psoriasis.
Current pathogenesis of psoriasis and its treatments
Pathogenesis of psoriasis is multifactorial, including environmental,40, 41, 42 genetic43, 44 and immune‐related factors.45, 46, 47, 48, 49 Recent genome‐wide association studies have identified numerous risk‐associated variants within 44 susceptibility loci for psoriasis, including HLAC*06:02, LCE3D, IL23R and CARD14.1, 43, 44, 50, 51, 52 These susceptibility genes are predominantly related to the innate and adaptive immune system as well as to skin barrier function.1, 43, 44
Cutaneous trauma, such as incisional injury, induces the expression of functional cathelicidin at the injured site.53 Cathelicidin (LL37) is one of the anti‐microbial peptides that are produced by keratinocytes and neutrophils.53 An initial trigger of psoriasis is believed to involve activation of plasmacytoid dendritic cells (DCs) upon stimulation by complexes of host DNA and LL37.45, 46, 47, 48 Activated plasmacytoid DCs and damaged keratinocytes produce interferon‐α (IFN‐α), IFN‐β and tumour necrosis factor‐α (TNF‐α), which result in further production of TNF‐α and interleukin‐23 (IL‐23) by plasmacytoid and recruited inflammatory DCs [TNF/inducible nitric oxide synthase‐producing DCs (TIP‐DCs)].45, 46, 47, 48 Interleukin‐23 is critically involved in the generation and activation of IL‐17‐ and IL‐22‐producing effector T helper type 17 (Th17) and Th22 cells.48, 49
Interleukin‐17 up‐regulates the proliferation of keratinocytes and down‐regulates their differentiation.54 Interleukin‐22 also drives epidermal hyperplasia, primarily by down‐regulation of genes involved in terminal differentiation.55, 56 Interleukin‐17 acts on keratinocytes to induce chemokines that lead to neutrophil, TIP‐DC and Th17 cell influx into the skin;57 and up‐regulates production of the neutrophil‐attractive chemokines CXCL1, CXCL2 and CXCL8 by keratinocytes.58, 59, 60
The accelerated TNF‐α/IL‐23/IL‐17 axis coincides with the characteristic histopathology of psoriasis, such as epidermal hyperproliferation, aberrant differentiation and neutrophilic microabscess. Regarding treatments, topical application of steroids and vitamin D3 analogues inhibits psoriatic inflammation and normalizes epidermal differentiation.61, 62, 63, 64, 65 Systemic treatments, such as cyclosporine, methotrexate and the phosphodiesterase 4 inhibitor apremilast, are useful for patients with extensive lesions.66, 67, 68 Clinical trials have demonstrated the efficacy of the oral janus kinase inhibitor tofacitinib for psoriasis.69, 70 Biological agents targeting TNF‐α, IL‐23 and IL‐17 signalling exhibit high efficacy for patients with severe lesions, arthritis or disturbed quality of life.71, 72, 73, 74, 75, 76, 77, 78, 79 However, development of the anti‐IL‐22 antibody fezakinumab (ILV‐094) has been discontinued due to treatment failure, probably because of the redundancy of IL‐22 in humans.48 The biologicals are applicable in both paediatric and elderly patients, in patients undergoing haemodialysis and patients with perioperational circumstances.80, 81, 82, 83 They are also used in pregnant women, although their safety warrants further evaluation.84, 85 Switching of biologicals is recommended for patients with primary failure, secondary failure and infusion reactions.86
Genetic backgrounds are also related to the treatment response to biologicals.87, 88 The high cost of biologicals limits access to these medications; however, cost‐saving biosimilars are rapidly advancing to the market worldwide.89, 90, 91 Biologicals are effective not only for psoriasis but also for co‐morbid cardiovascular and metabolic diseases.92, 93 In addition, monitoring of infection is mandatory to avoid serious consequences.94, 95, 96 The emergence of antidrug antibodies is another important issue that reduces the treatment response and promotes treatment failure during biological therapy.97, 98
Autoimmunity in psoriasis
In addition to the TNF‐α/IL‐23/IL‐17‐shifted immune deviation, psoriasis is likely to be associated with an autoimmune background.34, 35 Several studies have revealed the presence of autoreactive T cells in psoriasis (Fig. 1).99, 100, 101
Figure 1.
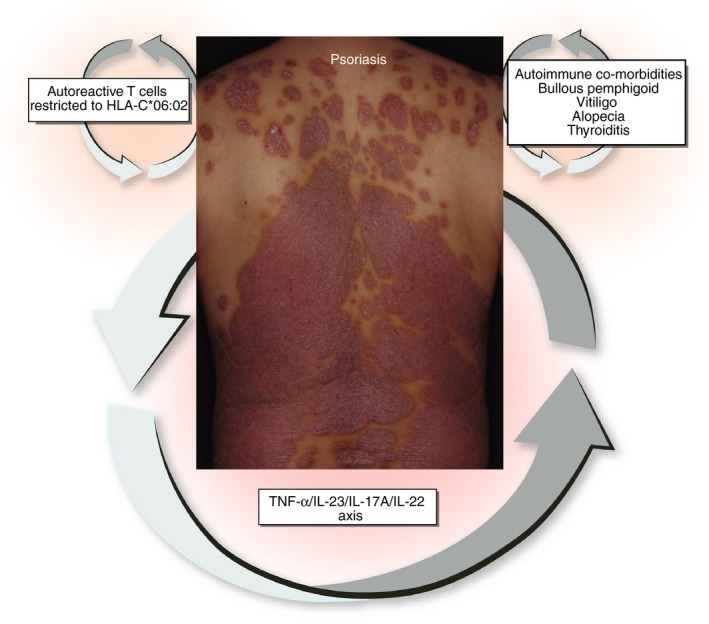
Based on clear‐cut effectiveness of biologicals, the tumour necrosis factor‐α (TNF‐α)/ interleukin‐23 (IL‐23)/ IL‐17A/ IL‐22 axis plays a major role in the pathogenesis of psoriasis. In addition, psoriasis possesses an autoimmune nature manifesting autoreactive T cells that are frequently restricted to HLA‐C*06:02. Psoriasis is also co‐morbid with other autoimmune diseases, such as bullous pemphigoid, vitiligo, alopecia and thyroiditis.
Exacerbation of chronic psoriasis can be associated with streptococcal throat infections.99, 100 Lesional skin and tonsils of patients with psoriasis harbour T cells co‐reactive to streptococcal M protein and type I keratin.99, 100 Streptococcal M protein exhibits structural homology with type I keratins in epidermal keratinocytes.100 These co‐reactive T cells elicited by molecular mimicry are cutaneous lymphocyte antigen (CLA)+, CD8+ and IL‐17 producers.100 The co‐reactive T cells may be pathogenic because tonsillectomy improves clinical symptoms in concert with a significant reduction of this T‐cell population.100 Type I keratin‐specific T cells are also reported in psoriasis by another research group.101
The antimicrobial peptide LL37 is over‐expressed in psoriatic epidermis.46, 101, 102 LL37 not only triggers the TNF‐α/IL‐23/IL‐17 axis by activating DCs but also works as an autoantigen to activate the T‐cell adaptive immune system.46, 101, 102 LL37 is recognized as an autoantigen by circulating T cells in 46% of psoriasis patients and more frequently (75%) in moderate‐to‐severe psoriasis.101 In 54% of LL37‐responder patients, LL37‐specific T cells are exclusively CD4+, 29% are both CD4+ and CD8+, and 17% are exclusively CD8+. These LL37‐specific T cells express CLA and produce variable amounts of IFN‐γ, IL‐17 and IL‐22 but not IL‐4. The frequency of LL37‐specific T cells is significantly correlated with disease severity and is reduced by anti‐TNF‐α therapy.101
The pivotal role of intra‐epidermal CD8+ T cells has been documented in psoriasis.103, 104, 105, 106 These intra‐epidermal CD8+ T cells produce pathogenic IL‐17A, and neutralization of CD8+ T cells effectively prevents psoriasis development in xenotransplanted mice models in vivo.105 Moreover, a recent study by Arakawa et al.106 demonstrated that intraepidermal CD8+ T cells recognize ADAMTS‐like protein 5 (ADAMTSL5) on melanocytes in concert with HLA‐C*06:02, which is the main psoriasis risk allele. Of note, LL37‐specific CD8+ T cells are also restricted to HLAC*06:02.101
In addition to the autoreactive T‐cell response, several autoantibodies have been demonstrated in patients with psoriasis, including anti‐stratum corneum antibody,107 anti‐squamous cell carcinoma antigen108 and anti‐heatshock protein 65.109 However, the clinical significance of the autoantibody production remains elusive.
HLA class I and class II polymorphisms are associated with immune‐mediated diseases typically in a disease‐specific manner.110, 111 Among susceptibility genes in psoriasis, HLAC*06:02 has a fundamental importance and defines familial clustering, early onset and a more severe course of psoriasis.112, 113 The fact that patients with psoriasis harbour autoreactive T cells that are restricted to HLAC*06:02 stress a potential role for these T cells in the development and perpetuation of psoriasis.
Intriguingly, the occurrence of psoriasis is commonly noted in human immunodeficiency virus (HIV) infection.114, 115, 116, 117, 118 Psoriasis with HIV infection may have a more severe course with sudden exacerbations and may be refractory to treatment.114, 115, 116, 117, 118 Paradoxical onset of psoriasis and arthritis is also demonstrated in patients treated with biologicals.119, 120 Anti‐TNF‐α antibodies frequently induce increased levels of KL‐6, which is a serum marker for interstitial pneumonia.121 In addition, the onset and exacerbation of psoriasis have been reported in melanoma patients treated with the anti‐programmed cell death protein 1 antibody nivolumab.122, 123 These phenomena may be related to a disruption in cytokine balance following treatment with biologicals, resulting in the up‐regulation of plasmacytoid DCs and the subsequent production of unopposed IFNs, leading to the bypassed IL‐17 over‐production.124
Autoimmune diseases co‐morbid with psoriasis
Consistent with the autoimmune nature of psoriasis, numerous other autoimmune diseases, including autoimmune bullous diseases,125 vitiligo,126 alopecia127 and thyroiditis,128 are co‐morbid with psoriasis. Among them, autoimmune bullous diseases, such as bullous pemphigoid and anti‐laminin γ1 (p200) pemphigoid, are consistently documented.37, 125, 129, 130 In a nationwide population‐based study of 3485 patients with bullous pemphigoid and 17425 matched controls, psoriasis is significantly associated with bullous pemphigoid [odds ratio (OR) 2·02; 95% CI 1·54–2·66].130 In a case–control study of 287 patients with bullous pemphigoid and 1373 matched controls, the prevalence rate of psoriasis is greater in patients with bullous pemphigoid compared with control subjects (OR 4·4; 95% CI 2·2–8·9).125 This association is also significant among both sexes.125 In a case series of coexisting psoriasis and autoimmune bullous diseases, bullous pemphigoid is the most prevalent (63·4%) followed by anti‐laminin γ1 pemphigoid (37·2%).129
Vitiligo is an autoimmune hypopigmentation disorder that is associated with psoriasis. Arunachalam et al.126 reported that 27 of 463 (5·8%) patients with vitiligo have co‐morbid psoriasis. A cross‐sectional study of 1925 children and adolescents with psoriasis and 1 194 712 controls without psoriasis reveals a strong association of vitiligo with psoriasis (adjusted OR 4·76; 95% CI 1·71–13·20).131 A significant association of vitiligo is evident in a nationwide study including 51 800 psoriasis patients with an OR of 5·94 (95% CI 3·79–9·31).132 In parallel, it is interesting that vitiligo and psoriasis share a common genetic susceptible locus (rs9468925 in HLA‐C/HLA‐B).133
Psoriasis is also associated with autoimmune hair loss, namely alopecia areata (OR 4·71; 95% CI 2·98–7·45), in a national database in Taiwan.132 In a cohort study including 25 341 patients with psoriasis, a significant association of alopecia areata and psoriasis was also noted (OR 2·5; 95% CI 2·0–3·0).127
Hashimoto's thyroiditis and thyroid autoimmunity are frequently observed in the general population.134 Free thyroxin levels are significantly increased in 105 psoriasis patients without arthritis compared with control 96 patients with tinea pedis.135 In a hospital‐based cross‐sectional study of 856 615 patients, 9615 patients were diagnosed with psoriasis, and 1745 patients had Hashimoto's thyroiditis. A significant association exists for psoriasis and Hashimoto's thyroiditis even after adjusting for confounding variables, including gender, age, psoriatic arthropathy and the use of systemic anti‐psoriatic agents (OR 2·49; 95% CI 1·79–3·48).128 However, Vassilatou et al.136 did not identify any tendency of association between psoriasis and autoimmune thyroiditis in their 114 patients with psoriasis.
Although the precise mechanisms underlying the autoimmune co‐morbidities are not completely understood, damage to the basement membrane, melanocytes and hairs by chronic psoriatic inflammation may initiate and accelerate skin‐related autoimmune diseases. An association of Crohn's disease and collagen diseases, such as systemic lupus erythematosus, rheumatoid arthritis, Sjögren's syndrome and systemic sclerosis, has been demonstrated in psoriasis patients.127, 137, 138 Some patients with psoriasis develop collagen diseases upon treatment with biologicals.139, 140, 141
Similar to psoriasis, IFNs, DNA–LL‐37 complex and plasmacytoid DCs play a pivotal role in triggering and perpetuating systemic lupus erythematosus.142, 143, 144 Interferons accelerate the formation of DNA–LL37 complex that activates plasmacytoid DCs to produce high levels of IFNs and IL‐23.142, 144 Interleukin‐23 induces IL‐17 production, which promotes autoimmunity.144 Crohn's disease is also deeply linked to TNF‐α/IL‐23 signalling because both anti‐TNF‐α and anti‐IL‐23 biologicals are effective in this disease.145, 146 In vitiligo, IFN signature is also involved in the initiation of its immune response with the activation of plasmacytoid DCs.147 Serum levels of IL‐23 are also elevated in vitiligo.148 These results supports a notion that common pathogenic pathways or shared autoantigens exist in psoriasis and other autoimmune diseases.
Conclusion
Psoriasis is a common chronic inflammatory skin disease that significantly decreases the physical and psychological quality of life of afflicted patients. The underlying inflammatory process results in increased co‐morbidity with systemic inflammatory diseases, such as cardiovascular diseases and metabolic syndrome. In addition, numerous pathogenic traits are shared by psoriasis and other autoimmune diseases, such as autoimmune bullous diseases, vitiligo and alopecia areata. Future studies are warranted to explore the distinct pathways that underpin the inflammatory and autoimmune co‐morbidity.
Disclosures
The authors declare that they have no conflict of interest.
References
- 1. Boehncke WH, Schön MP. Psoriasis. Lancet 2015; 386:983–94. [DOI] [PubMed] [Google Scholar]
- 2. Furue M, Kadono T. Psoriasis: behind the scenes. J Dermatol 2016; 43:4–8. [DOI] [PubMed] [Google Scholar]
- 3. Furue M, Kadono T. “Inflammatory skin march” in atopic dermatitis and psoriasis. Inflamm Res 2017; 66:833–42. [DOI] [PubMed] [Google Scholar]
- 4. Kwon HH, Kim MW, Park GH, Bae YI, Kuk SK, Suh DH et al Facial Psoriasis Log‐based Area and Severity Index: a valid and reliable severity measurement method detecting improvement of facial psoriasis in clinical practice settings. J Dermatol 2016; 43:894–9. [DOI] [PubMed] [Google Scholar]
- 5. Choi JW, Kim BR, Seo E, Youn SW. Identification of nail features associated with psoriasis severity. J Dermatol 2017; 44:147–53. [DOI] [PubMed] [Google Scholar]
- 6. Errichetti E, Zabotti A, Stinco G, Quartuccio L, Sacco S, De Marchi G et al Dermoscopy of nail fold and elbow in the differential diagnosis of early psoriatic arthritis sine psoriasis and early rheumatoid arthritis. J Dermatol 2016; 43:1217–20. [DOI] [PubMed] [Google Scholar]
- 7. Errichetti E, Stinco G. Dermoscopy in differential diagnosis of palmar psoriasis and chronic hand eczema. J Dermatol 2016; 43:423–5. [DOI] [PubMed] [Google Scholar]
- 8. Atakan N, Yazici AC, Özarmağan G, İnalÖz HS, Gürer MA, Sabuncu İ et al TUR‐PSO: a cross‐sectional, study investigating quality of life and treatment status of psoriasis patients in Turkey. J Dermatol 2016; 43:298–304. [DOI] [PubMed] [Google Scholar]
- 9. López‐Estebaranz JL, Sánchez‐Carazo JL, Sulleiro S. Effect of a family history of psoriasis and age on comorbidities and quality of life in patients with moderate to severe psoriasis: results from the ARIZONA study. J Dermatol 2016; 43:395–401. [DOI] [PubMed] [Google Scholar]
- 10. Alpsoy E, Polat M, FettahlıoGlu‐Karaman B, Karadag AS, Kartal‐Durmazlar P, YalCın B et al Internalized stigma in psoriasis: a multicenter study. J Dermatol 2017; 44:885–91. [DOI] [PubMed] [Google Scholar]
- 11. Hawro M, Maurer M, Weller K, Maleszka R, Zalewska‐Janowska A, Kaszuba A et al Lesions on the back of hands and female gender predispose to stigmatization in patients with psoriasis. J Am Acad Dermatol 2017; 76:648–54. [DOI] [PubMed] [Google Scholar]
- 12. Masaki S, Tatsukawa R, Uryu M, Takahara M, Furue M, Ohata C et al Treatment satisfaction, willingness to pay and quality of life in Japanese patients with psoriasis. J Dermatol 2017; 44:143–6. [DOI] [PubMed] [Google Scholar]
- 13. Asahina A, Umezawa Y, Yanaba K, Nakagawa H. Serum C‐reactive protein levels in Japanese patients with psoriasis and psoriatic arthritis: long‐term differential effects of biologics. J Dermatol 2016; 43:779–84. [DOI] [PubMed] [Google Scholar]
- 14. Takata T, Takahashi A, Taniguchi Y, Terada Y, Sano S. Detection of asymptomatic enthesitis in psoriasis patients: an onset of psoriatic arthritis? J Dermatol 2016; 43:650–4. [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto T, Ohtsuki M, Sano S, Igarashi A, Morita A, Okuyama R et al Epidemiological analysis of psoriatic arthritis patients in Japan. J Dermatol 2016; 43:1193–6. [DOI] [PubMed] [Google Scholar]
- 16. Yamamoto T, Ohtsuki M, Sano S, Igarashi A, Morita A, Okuyama R et al Prevalence and current therapies of psoriatic arthritis in Japan: a survey by the Japanese Society of Psoriasis Research in 2016. J Dermatol 2017; 44:e121. [DOI] [PubMed] [Google Scholar]
- 17. Stuart PE, Nair RP, Tsoi LC, Tejasvi T, Das S, Kang HM et al Genome‐wide association analysis of psoriatic arthritis and cutaneous psoriasis reveals differences in their genetic architecture. Am J Hum Genet 2015; 97:816–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koch M, Baurecht H, Ried JS, Rodriguez E, Schlesinger S, Volks N et al Psoriasis and cardiometabolic traits: modest association but distinct genetic architectures. J Invest Dermatol 2015; 135:1283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parisi R, Rutter MK, Lunt M, Young HS, Symmons DPM, Griffiths CEM et al Psoriasis and the risk of major cardiovascular events: cohort study using the Clinical Practice Research Datalink. J Invest Dermatol 2015; 135:2189–97. [DOI] [PubMed] [Google Scholar]
- 20. Lonnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF. Association of psoriasis with the risk for type 2 diabetes mellitus and obesity. JAMA Dermatol 2016; 152:761–7. [DOI] [PubMed] [Google Scholar]
- 21. Cohen AD, Dreiher J, Shapiro Y, Vidavsky L, Vardy DA, Davidovici B et al Psoriasis and diabetes: a population‐based cross‐sectional study. J Eur Acad Dermatol Venereol 2008; 22:585–9. [DOI] [PubMed] [Google Scholar]
- 22. Naito R, Imafuku S. Distinguishing features of body mass index and psoriasis in men and women in Japan: a hospital‐based case–control study. J Dermatol 2016; 43:1406–11. [DOI] [PubMed] [Google Scholar]
- 23. Liu JH, Chen Y, Zhen Z, Yeung CK, Chan J, Chan HH et al Relation between endothelial progenitor cells and arterial stiffness in patients with psoriasis. J Dermatol 2016; 43:888–93. [DOI] [PubMed] [Google Scholar]
- 24. Honma M, Shibuya T, Iinuma S, Kishibe M, Takahashi H, Ishida‐Yamamoto A. Close correlation of bone mineral density and body mass index in Japanese psoriasis patients. J Dermatol 2017; 44:e1–2. [DOI] [PubMed] [Google Scholar]
- 25. Chularojanamontri L, Wongpraparut C, Silpa‐Archa N, Chaweekulrat P. Metabolic syndrome and psoriasis severity in South‐East Asian patients: an investigation of potential association using current and chronological assessments. J Dermatol 2016; 43:1424–8. [DOI] [PubMed] [Google Scholar]
- 26. Furue M, Tsuji G, Chiba T, Kadono T. Cardiovascular and metabolic diseases comorbid with psoriasis: beyond the skin. Intern Med 2017; 56:1613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oh EH, Ro YS, Kim JE. Epidemiology and cardiovascular comorbidities in patients with psoriasis: a Korean nationwide population‐based cohort study. J Dermatol 2017; 44:621–9. [DOI] [PubMed] [Google Scholar]
- 28. Røpke M, Bulai Livideanu C, Kaldate R, Snel A, Paul C. Changes in IL‐17A, macrophage‐derived chemokine and adiponectin following treatment of psoriasis with calcipotriol plus betamethasone dipropionate aerosol foam: results from the PSO‐ABLE study. Br J Dermatol 2017; https://doi.org/10.1111/bjd.15814. [DOI] [PubMed] [Google Scholar]
- 29. Kim DS, Shin D, Lee MS, Kim HJ, Kim DY, Kim SM et al Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol 2016; 43:305–10. [DOI] [PubMed] [Google Scholar]
- 30. Pina T, Genre F, Lopez‐Mejias R, Armesto S, Ubilla B, Mijares V et al Asymmetric dimethylarginine but not osteoprotegerin correlates with disease severity in patients with moderate‐to‐severe psoriasis undergoing anti‐tumor necrosis factor‐α therapy. J Dermatol 2016; 43:389–94. [DOI] [PubMed] [Google Scholar]
- 31. Sato Y, Kajihara I, Yamada‐Kanazawa S, Jinnin M, Ihn H. S100A7 expression levels in coordination with interleukin‐8 indicate the clinical response to infliximab for psoriasis patients. J Dermatol 2017; 44:838–9. [DOI] [PubMed] [Google Scholar]
- 32. Takahashi T, Asano Y, Shibata S, Tada Y, Sato S. Serum angiopoietin‐2 level as a potential biomarker in psoriasis vulgaris. J Dermatol 2017; 44:205–6. [DOI] [PubMed] [Google Scholar]
- 33. Davidovici BB, Sattar N, Prinz J, Puig L, Emery P, Barker JN et al Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co‐morbid conditions. J Invest Dermatol 2010; 130:1785–96. [DOI] [PubMed] [Google Scholar]
- 34. Sticherling M. Psoriasis and autoimmunity. Autoimmun Rev 2016; 15:1167–70. [DOI] [PubMed] [Google Scholar]
- 35. Prinz JC. Autoimmune aspects of psoriasis: heritability and autoantigens. Autoimmun Rev 2017; 16:970–9. [DOI] [PubMed] [Google Scholar]
- 36. Akiyama M, Ueno T, Kanzaki A, Kuwana M, Nagao M, Saeki H. Association of psoriasis with Hashimoto's thyroiditis, Sjögren's syndrome and dermatomyositis. J Dermatol 2016; 43:711–2. [DOI] [PubMed] [Google Scholar]
- 37. Maki N, Demitsu T, Umemoto N, Nagashima K, Nakamura T, Kakurai M et al Possible paraneoplastic syndrome case of bullous pemphigoid with immunoglobulin G anti‐BP180 C‐terminal domain antibodies associated with psoriasis and primary macroglobulinemia. J Dermatol 2016; 43:571–4. [DOI] [PubMed] [Google Scholar]
- 38. Matsuo H, Asahina A, Fukuda T, Umezawa Y, Nakagawa H. Relapsing polychondritis associated with psoriasis vulgaris successfully treated with adalimumab: a case report with published work review. J Dermatol 2017; 44:826–9. [DOI] [PubMed] [Google Scholar]
- 39. Gál B, Dulic S, Kiss M, Groma G, Kovács L, Kemény L et al Increased circulating anti‐α6‐integrin autoantibodies in psoriasis and psoriatic arthritis but not in rheumatoid arthritis. J Dermatol 2017; 44:370–4. [DOI] [PubMed] [Google Scholar]
- 40. Wolters M. Diet and psoriasis: experimental data and clinical evidence. Br J Dermatol 2005; 153:706–14. [DOI] [PubMed] [Google Scholar]
- 41. Mattozzi C, Paolino G, Richetta AG, Calvieri S. Psoriasis, vitamin D and the importance of the cutaneous barrier's integrity: an update. J Dermatol 2016; 43:507–14. [DOI] [PubMed] [Google Scholar]
- 42. Zeng J, Luo S, Huang Y, Lu Q. Critical role of environmental factors in the pathogenesis of psoriasis. J Dermatol 2017; 44:863–72. [DOI] [PubMed] [Google Scholar]
- 43. Tang H, Jin X, Li Y, Jiang H, Tang X, Yang X et al A large‐scale screen for coding variants predisposing to psoriasis. Nat Genet 2014; 46:45–50. [DOI] [PubMed] [Google Scholar]
- 44. Baurecht H, Hotze M, Brand S, Büning C, Cormican P, Corvin A et al Genome‐wide comparative analysis of atopic dermatitis and psoriasis gives insight into opposing genetic mechanisms. Am J Hum Genet 2015; 96:104–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nestle FO, Conrad C, Tun‐Kyi A, Homey B, Gombert M, Boyman O et al Plasmacytoid predendritic cells initiate psoriasis through interferon‐α production. J Exp Med 2005. Jul 4; 202:135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B et al Plasmacytoid dendritic cells sense self‐DNA coupled with antimicrobial peptide. Nature 2007; 449:564–9. [DOI] [PubMed] [Google Scholar]
- 47. Lowes MA, Suárez‐Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol 2014; 32:227–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM et al IL‐12 and IL‐23 cytokines: from discovery to targeted therapies for immune‐mediated inflammatory diseases. Nat Med 2015; 21:719–29. [DOI] [PubMed] [Google Scholar]
- 49. Guttman‐Yassky E, Krueger JG, Lebwohl MG. Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment. Exp Dermatol 2017; https://doi.org/10.1111/exd.13336. [DOI] [PubMed] [Google Scholar]
- 50. Inoue N, Dainichi T, Fujisawa A, Nakano H, Sawamura D, Kabashima K. CARD14 Glu138 mutation in familial pityriasis rubra pilaris does not warrant differentiation from familial psoriasis. J Dermatol 2016; 43:187–9. [DOI] [PubMed] [Google Scholar]
- 51. Feng C, Wang T, Li SJ, Fan YM, Shi G, Zhu KJ. CARD14 gene polymorphism c.C2458T (p.Arg820Trp) is associated with clinical features of psoriasis vulgaris in a Chinese cohort. J Dermatol 2016; 43:294–7. [DOI] [PubMed] [Google Scholar]
- 52. Takeo N, Fujiwara S, Sakai T, Saito‐Shono T, Ishikawa K, Hatano Y. Hereditary lactate dehydrogenase M‐subunit deficiency with late‐developing pustular psoriasis‐like lesions. J Dermatol 2016; 43:1429–32. [DOI] [PubMed] [Google Scholar]
- 53. Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V et al Cutaneous injury induces the release of cathelicidin anti‐microbial peptides active against group A Streptococcus. J Invest Dermatol 2001; 117:91–7. [DOI] [PubMed] [Google Scholar]
- 54. Ha HL, Wang H, Pisitkun P, Kim JC, Tassi I, Tang W et al IL‐17 drives psoriatic inflammation via distinct, target cell‐specific mechanisms. Proc Natl Acad Sci USA 2014; 111:E3422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL‐22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 2005; 174:3695–702. [DOI] [PubMed] [Google Scholar]
- 56. Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L et al The effects of IL‐20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol 2007; 178:2229–40. [DOI] [PubMed] [Google Scholar]
- 57. Nograles KE, Zaba LC, Guttman‐Yassky E, Fuentes‐Duculan J, Suárez‐Fariñas M, Cardinale I et al Th17 cytokines interleukin (IL)‐17 and IL‐22 modulate distinct inflammatory and keratinocyte‐response pathways. Br J Dermatol 2008; 159:1092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Johnston A, Xing X, Wolterink L, Barnes DH, Yin Z, Reingold L et al IL‐1 and IL‐36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol 2017; 140:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reich K, Papp KA, Matheson RT, Tu JH, Bissonnette R, Bourcier M et al Evidence that a neutrophil‐keratinocyte crosstalk is an early target of IL‐17A inhibition in psoriasis. Exp Dermatol 2015; 24:529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Furue K, Yamamura K, Tsuji G, Mitoma C, Uchi H, Nakahara T et al Highlighting interleukin‐36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol 2018; 98:5–13. [DOI] [PubMed] [Google Scholar]
- 61. Ito K, Koga M, Shibayama Y, Tatematsu S, Nakayama J, Imafuku S. Proactive treatment with calcipotriol reduces recurrence of plaque psoriasis. J Dermatol 2016; 43:402–5. [DOI] [PubMed] [Google Scholar]
- 62. Karakawa M, Komine M, Kishimoto M, Maki N, Matsumoto A, Sugai J et al Effects of maxacalcitol ointment on skin lesions in patients with psoriasis receiving treatment with adalimumab. J Dermatol 2016; 43:1354–7. [DOI] [PubMed] [Google Scholar]
- 63. Usui K, Okubo Y, Hirano T, Tsuboi R. Vitamin D3 derivatives, alone or in combination with glucocorticoids, suppress streptococcal pyrogenic enterotoxin A‐stimulated proliferation of peripheral blood mononuclear cells in patients with psoriasis. J Dermatol 2017; 44:567–72. [DOI] [PubMed] [Google Scholar]
- 64. Takahashi H, Ishida‐Yamamoto A, Iizuka H. Linear psoriasis treated with topical glucocorticoid and active vitamin D3 ointments. J Dermatol 2016; 43:1438–9. [DOI] [PubMed] [Google Scholar]
- 65. Ergun T, Seckin Gencosmanoglu D, Alpsoy E, Bulbul‐Baskan E, Saricam MH, Salman A et al Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol 2017; 44:630–4. [DOI] [PubMed] [Google Scholar]
- 66. Nast A, Gisondi P, Ormerod AD, Saiag P, Smith C, Spuls PI et al European S3‐Guidelines on the systemic treatment of psoriasis vulgaris–Update 2015–Short version–EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol 2015; 29:2277–94. [DOI] [PubMed] [Google Scholar]
- 67. Bae SH, Yun SJ, Lee JB, Kim SJ, Won YH, Lee SC. Algorithm to select optimal systemic anti‐psoriatic drugs in relation with patients’ Psoriasis Area and Severity Index score for plaque psoriasis. J Dermatol 2016; 43:643–9. [DOI] [PubMed] [Google Scholar]
- 68. Ohtsuki M, Okubo Y, Komine M, Imafuku S, Day RM, Chen P et al Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of Japanese patients with moderate to severe plaque psoriasis: efficacy, safety and tolerability results from a phase 2b randomized controlled trial. J Dermatol 2017; 44:873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Abe M, Nishigori C, Torii H, Ihn H, Ito K, Nagaoka M et al Tofacitinib for the treatment of moderate to severe chronic plaque psoriasis in Japanese patients: subgroup analyses from a randomized, placebo‐controlled phase 3 trial. J Dermatol 2017; 44:1228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Asahina A, Etoh T, Igarashi A, Imafuku S, Saeki H, Shibasaki Y, et al Oral tofacitinib efficacy, safety and tolerability in Japanese patients with moderate to severe plaque psoriasis and psoriatic arthritis: a randomized, double‐blind, phase 3 study. J Dermatol 2016; 43:869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB et al Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case‐based presentations and evidence‐based conclusions. J Am Acad Dermatol 2011; 65:137–74. [DOI] [PubMed] [Google Scholar]
- 72. Jabbar‐Lopez ZK, Yiu ZZN, Ward V, Exton LS, Mohd Mustapa MF, Samarasekera E et al Quantitative evaluation of biologic therapy options for psoriasis: a systematic review and network meta‐analysis. J Invest Dermatol 2017; 137:1646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Torii H, Nakano M, Yano T, Kondo K, Nakagawa H. Efficacy and safety of dose escalation of infliximab therapy in Japanese patients with psoriasis: results of the SPREAD study. J Dermatol 2017; 44:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Torii H, Terui T, Matsukawa M, Takesaki K, Ohtsuki M, Nakagawa H. Safety profiles and efficacy of infliximab therapy in Japanese patients with plaque psoriasis with or without psoriatic arthritis, pustular psoriasis or psoriatic erythroderma: results from the prospective post‐marketing surveillance. J Dermatol 2016; 43:767–78. [DOI] [PubMed] [Google Scholar]
- 75. Imafuku S, Honma M, Okubo Y, Komine M, Ohtsuki M, Morita A et al Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52‐week analysis from phase III open‐label multicenter Japanese study. J Dermatol 2016; 43:1011–7. [DOI] [PubMed] [Google Scholar]
- 76. Asahina A, Torii H, Ohtsuki M, Tokimoto T, Hase H, Tsuchiya T et al Safety and efficacy of adalimumab treatment in Japanese patients with psoriasis: results of SALSA study. J Dermatol 2016; 43:1257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Saeki H, Nakagawa H, Nakajo K, Ishii T, Morisaki Y, Aoki T et al Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52‐week, open‐label, phase 3 study (UNCOVER‐J). J Dermatol 2017; 44:355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Matsumoto A, Komine M, Karakawa M, Kishimoto M, Ohtsuki M. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol 2017; 44:202–4. [DOI] [PubMed] [Google Scholar]
- 79. Hwang YJ, Youn SW, Kim BR, Yu DY, Kim Y, Pires A et al Clinical factors predicting the therapeutic response to ustekinumab in patients with moderate to severe chronic plaque psoriasis. J Dermatol 2017; 44:560–6. [DOI] [PubMed] [Google Scholar]
- 80. Kusakari Y, Yamasaki K, Takahashi T, Tsuchiyama K, Shimada‐Omori R, Nasu‐Tamabuchi M et al Successful adalimumab treatment of a psoriasis vulgaris patient with hemodialysis for renal failure: a case report and a review of the previous reports on biologic treatments for psoriasis patients with hemodialysis for renal failure. J Dermatol 2015; 42:727–30. [DOI] [PubMed] [Google Scholar]
- 81. Nimmannitya K, Tateishi C, Mizukami Y, Hamamoto K, Yamada S, Goto H et al Successful treatment with ustekinumab of psoriasis vulgaris in a patient undergoing hemodialysis. J Dermatol 2016; 43:92–4. [DOI] [PubMed] [Google Scholar]
- 82. Kawakami H, Matsumoto Y, Abe N, Katori Y, Takahashi K, Tsuboi R et al Perioperative management of tumor necrosis factor‐α blocker‐treated psoriatic patients: case reports and review. J Dermatol 2016; 43:190–3. [DOI] [PubMed] [Google Scholar]
- 83. Momose M, Asahina A, Hayashi M, Yanaba K, Umezawa Y, Nakagawa H. Biologic treatments for elderly patients with psoriasis. J Dermatol 2017; 44:1020–3. [DOI] [PubMed] [Google Scholar]
- 84. Rademaker M, Agnew K, Andrews M, Armour K, Baker C, Foley P et al Psoriasis in those planning a family, pregnant or breast‐feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol 2017; https://doi.org/10.1111/ajd.12641. [DOI] [PubMed] [Google Scholar]
- 85. Adachi A, Komine M, Hirano T, Tsuda H, Karakawa M, Murata S et al Case of generalized pustular psoriasis exacerbated during pregnancy, successfully treated with infliximab. J Dermatol 2016; 43:1439–40. [DOI] [PubMed] [Google Scholar]
- 86. Honda H, Umezawa Y, Kikuchi S, Yanaba K, Fukuchi O, Ito T et al Switching of biologics in psoriasis: reasons and results. J Dermatol 2017; 44:1015–9. [DOI] [PubMed] [Google Scholar]
- 87. Tejasvi T, Stuart PE, Chandran V, Voorhees JJ, Gladman DD, Rahman P et al TNFAIP3 gene polymorphisms are associated with response to TNF blockade in psoriasis. J Invest Dermatol 2012; 132:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nishikawa R, Nagai H, Bito T, Ikeda T, Horikawa T, Adachi A et al Genetic prediction of the effectiveness of biologics for psoriasis treatment. J Dermatol 2016; 43:1273–7. [DOI] [PubMed] [Google Scholar]
- 89. de la Cruz C, de Carvalho AV, Dorantes GL, Londoño Garcia AM, Gonzalez C, Maskin M et al Biosimilars in psoriasis: clinical practice and regulatory perspectives in Latin America. J Dermatol 2017; 44:3–12. [DOI] [PubMed] [Google Scholar]
- 90. Cohen AD, Wu JJ, Puig L, Chimenti S, Vender R, Rajagopalan M et al Biosimilars for psoriasis: worldwide overview of regulatory guidelines, uptake and implications for dermatology clinical practice. Br J Dermatol 2017; 117:1495–502. [DOI] [PubMed] [Google Scholar]
- 91. Takahashi H, Satoh K, Takagi A, Iizuka H. Economic burden of psoriatic patients in Japan: analysis from a single outpatient clinic. J Dermatol 2017; 44:1024–6. [DOI] [PubMed] [Google Scholar]
- 92. Gkalpakiotis S, Arenbergerova M, Gkalpakioti P, Potockova J, Arenberger P, Kraml P. Impact of adalimumab treatment on cardiovascular risk biomarkers in psoriasis: results of a pilot study. J Dermatol 2017; 44:363–9. [DOI] [PubMed] [Google Scholar]
- 93. Pina T, Corrales A, Lopez‐Mejias R, Armesto S, Gonzalez‐Lopez MA, Gómez‐Acebo I et al Anti‐tumor necrosis factor‐α therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6‐month prospective study. J Dermatol 2016; 43:1267–72. [DOI] [PubMed] [Google Scholar]
- 94. Dávila‐Seijo P, Dauden E, Descalzo MA, Carretero G, Carrascosa JM, Vanaclocha F et al Infections in moderate to severe psoriasis patients treated with biological drugs compared to classic systemic drugs: findings from the BIOBADADERM Registry. J Invest Dermatol 2017; 137:313–21. [DOI] [PubMed] [Google Scholar]
- 95. Miyachi H, Nakamura Y, Wakabayashi S, Iwasawa MT, Oikawa A, Watanabe A et al Case of recurrent severe cellulitis and cutaneous candidiasis during psoriasis treatment with ustekinumab. J Dermatol 2017; 44:e206–7. [DOI] [PubMed] [Google Scholar]
- 96. Stöllberger C, Finsterer J. Varicella zoster virus meningitis under ustekinumab because of plaque psoriasis. J Dermatol 2017; 44:703–5. [DOI] [PubMed] [Google Scholar]
- 97. Kui R, Gál B, Gaál M, Kiss M, Kemény L, Gyulai R. Presence of antidrug antibodies correlates inversely with the plasma tumor necrosis factor (TNF)‐α level and the efficacy of TNF‐inhibitor therapy in psoriasis. J Dermatol 2016; 43:1018–23. [DOI] [PubMed] [Google Scholar]
- 98. Gyldenløve M, Zachariae C, Jensen P, Griehsel H, Ståhle M, Skov L. Drug concentration and antidrug antibodies in patients with psoriasis treated with adalimumab or etanercept. J Eur Acad Dermatol Venereol 2017; 31:e518–9. [DOI] [PubMed] [Google Scholar]
- 99. Diluvio L, Vollmer S, Besgen P, Ellwart JW, Chimenti S, Prinz JC. Identical TCR β‐chain rearrangements in streptococcal angina and skin lesions of patients with psoriasis vulgaris. J Immunol 2006; 176:7104–11. [DOI] [PubMed] [Google Scholar]
- 100. Thorleifsdottir RH, Sigurdardottir SL, Sigurgeirsson B, Olafsson JH, Sigurdsson MI, Petersen H et al Improvement of psoriasis after tonsillectomy is associated with a decrease in the frequency of circulating T cells that recognize streptococcal determinants and homologous skin determinants. J Immunol 2012; 188:5160–5. [DOI] [PubMed] [Google Scholar]
- 101. Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C et al The antimicrobial peptide LL37 is a T‐cell autoantigen in psoriasis. Nat Commun 2014; 5:5621. [DOI] [PubMed] [Google Scholar]
- 102. Lande R, Chamilos G, Ganguly D, Demaria O, Frasca L, Durr S et al Cationic antimicrobial peptides in psoriatic skin cooperate to break innate tolerance to self‐DNA. Eur J Immunol 2015; 45:203–13. [DOI] [PubMed] [Google Scholar]
- 103. Chang JC, Smith LR, Froning KJ, Schwabe BJ, Laxer JA, Caralli LL et al CD8+ T cells in psoriatic lesions preferentially use T‐cell receptor Vβ3 and/or Vβ13.1 genes. Proc Natl Acad Sci USA 1994; 91:9282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Conrad C, Boyman O, Tonel G, Tun‐Kyi A, Laggner U, de Fougerolles A et al α1β1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med 2007; 13:836–42. [DOI] [PubMed] [Google Scholar]
- 105. Di Meglio P, Villanova F, Navarini AA, Mylonas A, Tosi I, Nestle FO et al Targeting CD8+ T cells prevents psoriasis development. J Allergy Clin Immunol 2016; 138:274–6. [DOI] [PubMed] [Google Scholar]
- 106. Arakawa A, Siewert K, Stöhr J, Besgen P, Kim SM, Rühl G et al Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med 2015; 212:2203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jablonska S, Chorzelski TP, Beutner EH, Maciejowska E Jarzabek‐Chorzelska M, Rzesa G. Autoimmunity in psoriasis. Relation of disease activity and forms of psoriasis to immunofluorescence findings. Arch Dermatol Res 1978; 261:135–46. [DOI] [PubMed] [Google Scholar]
- 108. El‐Rachkidy RG, Young HS, Griffiths CE, Camp RD. Humoral autoimmune responses to the squamous cell carcinoma antigen protein family in psoriasis. J Invest Dermatol 2008; 128:2219–24. [DOI] [PubMed] [Google Scholar]
- 109. Rambukkana A, Das PK, Witkamp L, Yong S, Meinardi MM, Bos JD. Antibodies to mycobacterial 65‐kDa heat shock protein and other immunodominant antigens in patients with psoriasis. J Invest Dermatol 1993; 100:87–92. [DOI] [PubMed] [Google Scholar]
- 110. Stokkers PC, Reitsma PH, Tytgat GN, van Deventer SJ. HLA‐DR and ‐DQ phenotypes in inflammatory bowel disease: a meta‐analysis. Gut 1999; 45:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fernando MMA, Stevens CR, Walsh EC, De Jager PL, Goyette P, Plenge RM et al Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet 2008; 4:e1000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gudjónsson JE, Kárason A, Antonsdóttir AA, Rúnarsdóttir EH, Gulcher JR, Stefánsson K et al HLA‐Cw6‐positive and HLA‐Cw6‐negative patients with Psoriasis vulgaris have distinct clinical features. J Invest Dermatol 2002; 118:362–5. [DOI] [PubMed] [Google Scholar]
- 113. Gudjonsson JE, Karason A, Antonsdottir A, Runarsdottir EH, Hauksson VB, Upmanyu R et al Psoriasis patients who are homozygous for the HLA‐Cw*0602 allele have a 2.5‐fold increased risk of developing psoriasis compared with Cw6 heterozygotes. Br J Dermatol 2003; 148:233–5. [DOI] [PubMed] [Google Scholar]
- 114. Duvic M, Johnson TM, Rapini RP, Freese T, Brewton G, Rios A. Acquired immunodeficiency syndrome‐associated psoriasis and Reiter's syndrome. Arch Dermatol 1987; 123:1622–32. [PubMed] [Google Scholar]
- 115. Obuch ML, Maurer TA, Becker B, Berger TG. Psoriasis and human immunodeficiency virus infection. J Am Acad Dermatol 1992; 27:667–73. [DOI] [PubMed] [Google Scholar]
- 116. Yen YF, Jen IA, Chen M, Lan YC, Lee CY, Chuang PH et al HIV infection increases the risk of incident psoriasis: a nationwide population‐based cohort study in Taiwan. J Acquir Immune Defic Syndr 2017; 75:493–9. [DOI] [PubMed] [Google Scholar]
- 117. Johnson TM, Duvic M, Rapini RP, Rios A. AIDS exacerbates psoriasis. N Engl J Med 1985; 313:1415. [PubMed] [Google Scholar]
- 118. Itoi‐Ochi S, Hayashi M, Yamaoka T, Kobayashi Y, Isei T, Shirasaka T et al Occult HIV infection in Japanese rupioid psoriasis. J Dermatol 2017; 44:e172–3. [DOI] [PubMed] [Google Scholar]
- 119. Ruiz‐Genao D, Perez‐Zafrilla B, Lopez‐Estebaranz JL, Belinchón‐Romero I, Carrascosa JM, Ferrán M et al Possible paradoxical occurrence of inflammatory arthritis in patients with psoriasis treated with biologics: findings in the Biobadaderm cohort. Br J Dermatol 2017; 176:797–9. [DOI] [PubMed] [Google Scholar]
- 120. Koizumi H, Tokuriki A, Oyama N, Ido H, Sugiura K, Akiyama M et al Certolizumab pegol, a pegylated anti‐TNF‐α antagonist, caused de novo‐onset palmoplantar pustulosis followed by generalized pustular psoriasis in a patient with rheumatoid arthritis. J Dermatol 2017; 44:723–4. [DOI] [PubMed] [Google Scholar]
- 121. Hayashi M, Yanaba K, Umezawa Y, Asahina A, Nakagawa H. Impact of anti‐tumor necrosis factor‐α agents on serum levels of KL‐6 and surfactant protein‐D in patients with psoriasis. J Dermatol 2017; 44:1063–6. [DOI] [PubMed] [Google Scholar]
- 122. Kato Y, Otsuka A, Miyachi Y, Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J Eur Acad Dermatol Venereol 2016; 30:e89–91. [DOI] [PubMed] [Google Scholar]
- 123. Murata S, Kaneko S, Harada Y, Aoi N, Morita E. Case of de novo psoriasis possibly triggered by nivolumab. J Dermatol 2017; 44:99–100. [DOI] [PubMed] [Google Scholar]
- 124. Collamer AN, Guerrero KT, Henning JS, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum 2008; 59:996–1001. [DOI] [PubMed] [Google Scholar]
- 125. Kridin K, Bergman R. Association between bullous pemphigoid and psoriasis: a case–control study. J Am Acad Dermatol 2017; 77:370–2. [DOI] [PubMed] [Google Scholar]
- 126. Arunachalam M, Dragoni F, Colucci R, Berti S, Crocetti E, Galeone M et al Non‐segmental vitiligo and psoriasis comorbidity – a case–control study in Italian patients. J Eur Acad Dermatol Venereol 2014; 28:433–7. [DOI] [PubMed] [Google Scholar]
- 127. Wu JJ, Nguyen TU, Poon KY, Herrinton LJ. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol 2012; 67:924–30. [DOI] [PubMed] [Google Scholar]
- 128. Kiguradze T, Bruins FM, Guido N, Bhattacharya T, Rademaker A, Florek AG et al Evidence for the association of Hashimoto's thyroiditis with psoriasis: a cross‐sectional retrospective study. Int J Dermatol 2017; 56:553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ohata C, Ishii N, Koga H, Fukuda S, Tateishi C, Tsuruta D et al Coexistence of autoimmune bullous diseases (AIBDs) and psoriasis: a series of 145 cases. J Am Acad Dermatol 2015; 73:50–5. [DOI] [PubMed] [Google Scholar]
- 130. Chen YJ, Wu CY, Lin MW, Chen TJ, Liao KK, Chen YC et al Comorbidity profiles among patients with bullous pemphigoid: a nationwide population‐based study. Br J Dermatol 2011; 165:593–9. [DOI] [PubMed] [Google Scholar]
- 131. Blegvad C, Egeberg A, Nielsen TE, Gislason GH, Zachariae C, Nybo Andersen AM et al Autoimmune disease in children and adolescents with psoriasis: a cross‐sectional study in Denmark. Acta Derm Venereol 2017; 97:1225–29. [DOI] [PubMed] [Google Scholar]
- 132. Tsai TF, Wang TS, Hung ST, Tsai PI, Schenkel B, Zhang M et al Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci 2011; 63:40–6. [DOI] [PubMed] [Google Scholar]
- 133. Zhu KJ, Lv YM, Yin XY, Wang ZX, Sun LD, He SM et al Psoriasis regression analysis of MHC loci identifies shared genetic variants with vitiligo. PLoS One 2011; 6:e23089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chistiakov DA. Immunogenetics of Hashimoto's thyroiditis. J Autoimmune Dis 2005; 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gul U, Gonul M, Kaya I, Aslan E. Autoimmune thyroid disorders in patients with psoriasis. Eur J Dermatol 2009; 19:221–3. [DOI] [PubMed] [Google Scholar]
- 136. Vassilatou E, Papadavid E, Papastamatakis P, Alexakos D, Koumaki D, Katsimbri P et al No association of psoriasis with autoimmune thyroiditis. J Eur Acad Dermatol Venereol 2017; 31:102–6. [DOI] [PubMed] [Google Scholar]
- 137. Eppinga H, Poortinga S, Thio HB, Nijsten TEC, Nuij VJAA, van der Woude CJ et al Prevalence and phenotype of concurrent psoriasis and inflammatory bowel disease. Inflamm Bowel Dis 2017; 23:1783–9. [DOI] [PubMed] [Google Scholar]
- 138. Tselios K, Yap KS, Pakchotanon R, Polachek A, Su J, Urowitz MB et al Psoriasis in systemic lupus erythematosus: a single‐center experience. Clin Rheumatol 2017; 36:879–84. [DOI] [PubMed] [Google Scholar]
- 139. Cemil BC, Atas H, Canpolat F, Akca Y, Sasmaz R. Infliximab‐induced discoid lupus erythematosus. Lupus 2013; 22:515–8. [DOI] [PubMed] [Google Scholar]
- 140. Poulin Y, Thérien G. Drug‐induced hepatitis and lupus during infliximab treatment for psoriasis: case report and literature review. J Cutan Med Surg 2010; 14:100–4. [DOI] [PubMed] [Google Scholar]
- 141. Kikuchi S, Umezawa Y, Hayashi M, Yanaba K, Fukuchi O, Ito T et al Interstitial pneumonia in two patients with psoriasis during ustekinumab treatment. J Dermatol 2016; 43:712–3. [DOI] [PubMed] [Google Scholar]
- 142. Garcia‐Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z et al Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011; 3:73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chasset F, Arnaud L. Targeting interferons and their pathways in systemic lupus erythematosus. Autoimmun Rev 2018; 17:44–52. [DOI] [PubMed] [Google Scholar]
- 144. Dai H, He F, Tsokos GC, Kyttaris VC. IL‐23 limits the production of IL‐2 and promotes autoimmunity in lupus. J Immunol 2017; 199:903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF et al Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002; 359:1541–9. [DOI] [PubMed] [Google Scholar]
- 146. Wils P, Bouhnik Y, Michetti P, Flourie B, Brixi H, Bourrier A et al Subcutaneous ustekinumab provides clinical benefit for two‐thirds of patients with Crohn's disease refractory to anti‐tumor necrosis factor agents. Clin Gastroenterol Hepatol 2016; 14:242–50. [DOI] [PubMed] [Google Scholar]
- 147. Bertolotti A, Boniface K, Vergier B, Mossalayi D, Taieb A, Ezzedine K et al Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res 2014; 27:398–407. [DOI] [PubMed] [Google Scholar]
- 148. Vaccaro M, Cannavò SP, Imbesi S, Cristani M, Barbuzza O, Tigano V et al Increased serum levels of interleukin‐23 circulating in patients with non‐segmental generalized vitiligo. Int J Dermatol 2015; 54:672–4. [DOI] [PubMed] [Google Scholar]


