Summary
Despite major advances in recent years, immunosuppressive regimens for multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosus and graft‐versus‐host disease still have major adverse effects and immunomodulation rather than immune paralysis would be desirable. Statins inhibit the rate‐limiting enzyme of the l‐mevalonate pathway, the 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A reductase. It was shown that blocking the l‐mevalonate pathway reduces inflammation through effects on downstream metabolites of the pathway including farnesylpyrophosphates and geranylgeranylpyrophosphates, which are essential for the attachment of GTPases like RhoA, Rac and Ras to the cell membrane. Therefore, l‐mevalonate pathway downstream products play critical roles in the different steps of an immune response including immune cell activation, migration, cytokine production, immune metabolism and survival. This review discusses the relevance of the different metabolites for the immunomodulatory effect of statins and connects preclinical results with data from clinical studies that tested statins for the treatment of different inflammatory diseases.
Keywords: inflammation, T helper type 2, tolerance, transplantation
Key Points.
Statins regulate the immune response on several levels.
Recent advances in the clinical application of statins in different inflammatory diseases are being highlighted.
Introduction
Inhibition of the of 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A reductase with statins leads to depletion of l‐mevalonate pathway downstream metabolites, which reduces disease severity in several preclinical models of auto‐immunity and allo‐immunity.1, 2, 3, 4, 5, 6 The mechanism by which statins inhibit aberrant immune responses can vary depending on the disease model and the type of statin that is investigated.6
Here we summarize preclinical studies and clinical trials that have evaluated the efficacy and safety of statins in inflammatory diseases including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), multiple sclerosis (MS) and graft‐versus‐host disease (GVHD) (Table 1).
Table 1.
Statins in selected clinical trials for inflammatory diseases
| Disease | Type of the clinical trial | Main conclusion | Publication time (year) | References |
|---|---|---|---|---|
| RA | Post hoc analysis on the effect of statins in patients treated for RA with tocilizumab | Concomitant statin use reduced tocilizumab‐mediated lipid increases | 2017 | 22 |
| RA | Cohort study that included RA patients with a diagnosis of hyperlipidaemia who started statin intake | The connection between lowering LDL‐C and cardiovascular events in RA was comparable to the association found in matched general controls | 2016 | 24 |
| RA | Randomized, double‐blind placebo‐controlled trial on patients with RA treated with atorvastatin versus placebo given in addition to current disease‐modifying anti‐rheumatic drugs treatment | Disease activity score 28, CRP and ESR decreased in the statin group | 2016 | 26 |
| RA | Randomized, placebo controlled, multi‐centre phase II study, on a combination of tofacitinib and atorvastatin | Tofacitinib‐associated elevated LDL‐C in RA patients were reduced by atorvastatin | 2014 | 25 |
| RA | Randomized, double‐blind placebo‐controlled trial on patients with RA treated with atorvastatin versus placebo | Clinical improvement, reduced CRP levels and lower ESRs were seen in the atorvastatin group compared with the placebo group | 2004 | 27 |
| SLE | Controlled phase II trial | Atorvastatin therapy improved endothelium‐dependent vasodilatation in SLE patients | 2007 | 15 |
| SLE | Case studies on three systemic SLE patients | Reduction in proteinuria levels upon statin treatment | 2003 | 18 |
| MS | Double‐blind, controlled clinical trial of patients with secondary progressive multiple sclerosis | At 2 years, the FAB score and the physical component score were higher in the group that was simvastatin‐treated compared with the placebo group39 | 2017 | 39 |
| MS | Phase II open‐label baseline‐to‐treatment trial | Atorvastatin given to MS patient was safe and reduced the number and volume of CEL | 2008 | 41 |
| MS | Phase I/II trial on the safety and efficacy of simvastatin for MS | Significant reduction of CEL in magnetic resonance imaging of the brain in remitting–relapsing MS patients | 2004 | 40 |
| GVHD | Retrospective analysis | Statin intake of the recipient correlates with lower GVHD incidence | 2008 | 60 |
| GVHD | Retrospective analysis | Donor statin treatment is connected to reduced GVHD incidence in patients | 2010 | 55 |
| GVHD | Retrospective analysis. | Recipient statin treatment is connected to reduced GVHD incidence in patients | 2010 | 56 |
| GVHD | Prospective phase II trial of atorvastatin for aGVHD prophylaxis | Donor and recipient treatment with atorvastatin is effective as prophylaxis for GVHD | 2013 | 57 |
| GVHD | Phase II study of atorvastatin for aGVHD prophylaxis | Atorvastatin did not provide any benefit to standard GVHD prophylaxis alone | 2016 | 59 |
| GVHD | Phase II study to evaluate the safety and efficacy of atorvastatin‐based aGVHD prophylaxis | Preliminary efficacy of atorvastatin for aGVHD prevention was seen | 2017 | 58 |
Abbreviations: aGVHD, acute graft‐versus‐host disease; CEL, contrast‐enhancing lesions; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; GVHD, graft‐versus‐host disease; LDL‐C, low‐density lipoprotein cholesterol; MS, multiple sclerosis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Mechanisms of actions of statins on different immune cells
Statins inhibit the rate‐limiting enzyme of the l‐mevalonate pathway and thereby reduce farnesyl and geranylgeranyl residues that are required for the correct attachment of different small GTPases to the cell membrane. This modification modulates the immune response at different levels including T‐cell signalling, antigen presentation, immune cell migration and cytokine production.7 In vitro studies showed that lovastatin inhibited T‐cell proliferation, Ca2+ influx and interleukin‐2 (IL‐2) production in T cells. Additionally recent data indicate that lovastatin blocks the Kv1.3 channel in human T cells, which presents a novel mechanism for the immunomodulatory properties of statins.8 Besides these direct cellular effects on signalling via blockade of GTPase isoprenylation, statins impact gene expression of pro‐inflammatory genes in the innate and adaptive immune systems and also in non‐haematopoietic cells, including endothelial cells and fibroblasts. An important novel observation is that statins also impact the immune system through effects on immune cell metabolism. Recently a study showed that activation of the cholesterol synthesis pathway was essential for immunological training of myeloid immune cells.9 The study also reported that the metabolite mevalonate is the mediator of training through activation of insulin‐like growth factor 1 receptor and mammalian target of rapamycin and subsequent histone modifications in inflammatory pathways.9 Consistent with a role of statins in immune metabolism, another study reported that lovastatin caused potent protection against inflammation‐induced loss and dysfunction of mitochondria and peroxisomes in a mouse model of MS.10
These different approaches show that statins affect the immune response on multiple layers including signalling, gene transcription, epigenetic modifications and immune metabolism.
Statins in SLE
Statins haven been tested for their efficacy in preclinical models of SLE3, 11, 12, 13, 14 and in clinical trials.15, 16 Oral atorvastatin treatment of NZB/NZW mice reduced T‐cell proliferation and cytokine production when the T cells were isolated from the treated mice and tested in vitro. Conversely, the disease severity of SLE in this mouse model was not reduced by atorvastatin.11
A preclinical study analysed female LDLr−/− mice that underwent lethal irradiation and bone marrow transplantation from C57BL/6 mice (LDLr.B6) or SLE‐susceptible B6.Sle1.2.3 mice (LDLr.Sle).12 Sixteen weeks after bone marrow transplantation, mice were treated with atorvastatin versus mycophenolate mofetil with one end‐point being the presence of SLE signs.12 The authors reported that atorvastatin caused a decrease in cholesterol levels and atherogenesis in LDLr.B6 mice but did not diminish atherosclerotic lesion size or SLE signs in LDLr.Sle mice.12 A study performed in the mouse model analysed the effects of the apolipoprotein A‐1 mimetic peptide L‐4F, alone or in combination with pravastatin in apoE−/− Fas−/− C57BL/6 mice that spontaneously develop glomerulonephritis, IgG autoantibodies, atherosclerotic lesions and osteopenia.13 Mice treated with L‐4F alone or combined with pravastatin had significantly reduced IgG anti‐dsDNA levels, proteinuria, glomerulonephritis and osteopenia.13 In mice treated with L‐4F plus pravastatin the authors found less macrophage infiltration and lower levels of pro‐atherogenic chemokines.13 A preclinical study performed in an SLE model reported reduced HLA class II expression upon statin treatment and reduced T helper type 1 (Th1) ‐driven autoimmunity.3
In clinical studies including SLE patients, atorvastatin therapy improved endothelial function,15 which was also reported for simvastatin.16 The authors reported reduced TNF serum levels in SLE patients treated with simvastatin.16 A study on the dose effectiveness and tolerability of pravastatin in SLE patients reported no major impact on disease severity.17 Case studies on three patients with SLE reported a reduction in proteinuria levels upon statin treatment.18 A major clinical problem in patients with SLE is that the atherosclerotic process is accelerated. Therefore, in addition to the immunomodulatory functions of statins, their lipid‐lowering effects may be beneficial in SLE.19 In agreement with this concept, simvastatin improved disease signs of SLE associated with accelerated atherosclerosis in a murine model.14 The controversial results between different trials on statins in SLE could be connected to the type of statin applied. A recent meta‐analysis of five controlled trials that had studied the effect of statin intake on SLE disease activity showed no significant effect of statin treatment on the Systemic Lupus Erythematosus Disease Activity Index.20 However, analysis of seven controlled trials showed a reduction of plasma C‐reactive protein concentrations in patients with SLE by statin treatment.20 This observation was dependent on the type of statin applied, as the plasma C‐reactive protein concentration declined significantly with lipophilic (atorvastatin) but not hydrophilic (pravastatin and rosuvastatin) statins.20
Statins in RA
Multiple preclinical and clinical studies have analysed the impact of different statins on RA.21, 22, 23 Statins are given to patients with RA because of their increased risk for cardiovascular complications.24 A cohort study that included patients with a diagnosis of hyperlipidaemia who started statin intake showed that the connection between lowering low‐density lipoprotein cholesterol and cardiovascular events in RA was comparable to the association found in matched general controls.24 Another study analysed the effect of statins in patients treated for RA with tocilizumab.22 The rationale was that treatment with tocilizumab increases lipid levels. The authors reported that concomitant statin use reduced tocilizumab‐mediated lipid increases.22 A randomized, placebo‐controlled, multi‐centre phase II study, on a combination of tofacitinib and atorvastatin showed that tofacitinib‐associated elevated low‐density lipoprotein cholesterol was reduced by atorvastatin.25 The RA responses were numerically greater in the group that received tofacitinib and atorvastatin compared with tofacitinib and placebo; however, this did not reach statistical significance.25
A randomized, double‐blind placebo‐controlled trial that included 80 patients with RA aged between 19 and 75 years analysed the effects of atorvastatin versus placebo given in addition to current disease‐modifying anti‐rheumatic drugs.26 Disease Activity Score‐28, C‐reactive protein and erythrocyte sedimentation rate decreased in the statin group.26 These response rates are consistent with those reported in a previous randomized, double‐blind placebo‐controlled trial,27 which also reported clinical improvement, reduced C‐reactive protein levels and lower erythrocyte sedimentation rates in the atorvastatin group compared with the placebo group.27
Besides these trials on atorvastatin, smaller trials compared simvastatin with chloroquine and found superiority of the statin group.18 Clinical studies on the anti‐inflammatory and immunomodulatory effects of low‐dosage simvastatin on RA demonstrated that the Th1/Th2 and CD4/CD8 ratios in peripheral blood were significantly reduced by simvastatin.28 Simvastatin was shown to reduce cytokine production and nuclear factor‐κB activation in IL‐1β‐stimulated synoviocytes from patients with RA.29
The type of statin applied in RA may influence the clinical response. A preclinical study showed that atorvastatin and rosuvastatin had no in vivo efficacy against RA with respect to synovial hyperplasia, exudate and cartilage damage.30 However, the authors could reproduce the previously described beneficial effects of simvastatin on RA.30
Overall novel targeted therapies of RA such as IL‐6R blockade with tocilizumab or Janus‐activated kinase (JAK) inhibition with tofacitinib are most likely more potent than statins but they induce hyperlipidaemia and hypercholesteraemia. The treatment of this side effect with a drug that itself has anti‐inflammatory activity is promising and needs to be evaluated in future prospective trials in the RA field.
Statins in MS
Multiple sclerosis was one of the first autoimmune models in which the anti‐inflammatory effects of statins were reported. Meanwhile multiple preclinical studies and clinical trials have investigated the impact of statins on MS. The results vary depending on the type of statin used, the disease model and the clinical setting, respectively.
Statin treatment in mice developing experimental autoimmune encephalomyelitis (EAE), which is a standard animal model of MS, showed potent clinical response rates.1, 31, 32 After these initial pivotal reports, others have shown that statin treatment in EAE reduced central nervous system lesion formation and delayed disease onset.1, 31, 33, 34, 35 Statin effects in EAE were mediated via tolerogenic modification of antigen‐presenting cells, the Th‐1/Th1 cytokine profile, IL‐6 and IL‐23 transcription.36 A more recent study in which mice were immunized with myelin oligodendrocyte glycoprotein,35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 showed that the treatment with simvastatin improved clinical disease scores.37 Additionally, anti‐inflammatory transforming growth factor‐β mRNA expression was increased while IL‐6, IL‐12p40, IL‐12p70, RANTES and macrophage inflammatory protein‐1β were decreased.37 In agreement with these findings the authors also reported lower central nervous system inflammatory mononuclear cell levels and less Th1 and Th17 cell infiltration in the central nervous system.37 Furthermore, simvastatin diminished the proliferation of T cells co‐cultured with primary microglial cells. Although the initial studies had suggested that statin treatment causes a Th2 induction, follow‐up reports showed that atorvastatin treatment did not induce IL‐4‐producing Th2 cells in vivo. Conversely T cells from atorvastatin‐treated IL‐4 reporter mice preferentially differentiated into Th2 cells when reactivated in vitro.38 The studies confirmed that atorvastatin reduced antigen‐specific T‐cell proliferation and secretion of interferon‐γ, tumour necrosis factor, IL‐17 and IL‐12. Signal transducer and activator of transcription 6 (STAT6) is required for Th2 induction but the studies showed that also in STAT6‐deficient mice atorvastatin treatment prevented EAE, although these animals cannot generate IL‐4‐producing Th2 cells.38 The studies also showed that Foxp3+ regulatory T cells were not required for the statin effects.38
In a recent double‐blind, controlled clinical trial of patients with secondary progressive MS, patients were randomly assigned to either simvastatin (n = 70) or placebo (n = 70).39 At 2 years, the frontal assessment battery score was 1·2 points higher in the group that was simvastatin‐treated compared with the placebo group.39 Furthermore, the authors reported that the simvastatin group also had a 2·5‐point improved mean physical component score of the SF‐36.39 This study provides solid evidence of a positive effect of simvastatin on frontal lobe function and a physical quality‐of‐life measure in patients with secondary progressive MS.
Earlier clinical trials on the safety and efficacy of simvastatin for MS had shown a significant reduction of contrast‐enhancing lesions in magnetic resonance imaging of the brain in patients with remitting–relapsing MS.40 A phase II open‐label baseline‐to‐treatment trial reported that atorvastatin given to patients with MS was safe and reduced the number and volume of contrast‐enhancing lesions.41 Mechanistically increased levels of anti‐inflammatory IL‐10 were reported in patients treated with atorvastatin.41 A meta‐analysis on data available in EMBASE, PubMed and CINAHL databases, clinical trials registries, and unpublished conference meeting abstracts showed that in secondary progressive MS, statin monotherapy showed significant reduction in brain atrophy and disability progression but no effect on relapse rate.42 There was no clear evidence that certain statins were more effective than others for MS treatment.42 The reported effects of statins in neuroinflammation warrant confirmation but underline the potential of this class of drugs in MS.
Statins in GVHD
Acute and chronic GVHD occur in patients undergoing allogeneic haematopoietic cell transplantation and cause a high morbidity and mortality.43, 44 Different groups have shown in preclinical models that statins reduce the severity of acute GVHD (aGVHD).4, 45 In murine GVHD, model statin treatment caused increased levels of intracellular IL‐4 in CD4+ T cells, indicating that a shift towards Th2 was protective. Another group showed that lovastatin reduced GVHD through its inhibitory effect on lymphocyte function‐associated antigen 1.45 Statins inhibited allogeneic immune responses in human cell systems in vitro.46 Mechanistically, statins inhibit the rate‐limiting enzyme of the l‐mevalonate pathway and were shown to reduce farnesyl and geranylgeranyl residues that are required for the attachment of different small GTPases to the cell membrane.7 Modifications of the anchoring of these GTPases can modulate the allogeneic immune response.7 In vitro studies showed that interfering with protein geranylgeranylation or farnesylation reduced both pro‐inflammatory cytokines, whereas IL‐10 was increased when a farnesyltransferase inhibitor was used.47 Consistent with this finding, in vitro studies on human and murine T cells have demonstrated that different types of farnesyltransferase inhibitor block cytokine production of T cells in response to activating stimuli at the post‐transcriptional level.47 The reduction in the migratory capacity of antigen‐presenting cells in vitro and in vivo when protein geranylgeranylation or farnesylation was blocked7 was in agreement with reports showing that depletion of geranylgeranylpyrophosphates and farnesylpyrophosphates reduced monocytes and T‐cell migration (Fig. 1).48, 49 More recently, a study analysed the effects of simvastatin on angiopoietin‐1 (Ang‐1) and Ang‐2 expression in a mouse model of aGVHD.50 The authors found that simvastatin increased Ang‐1 production and simultaneously inhibited Ang‐2 release from EA.hy926 endothelial cells.50 Furthermore, treatment with simvastatin reduced aGVHD‐related death and histopathological aGVHD grades. Simvastatin also increased the plasma levels and aortic endothelial levels of Ang‐1.50 Given the role of neovascularization in GVHD51, 52 these studies connect endothelial protective function of statins with aGVHD severity. Statins are also interesting for combination therapies with recently evolving GVHD treatments such as JAK inhibitors,53, 54 which may affect the cholesterol levels.
Figure 1.
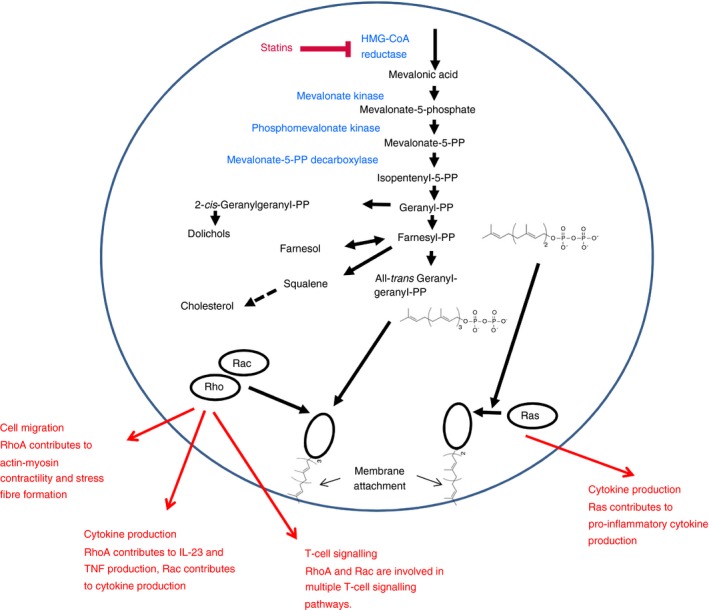
Simplified sketch showing the mode of action of statins as immunosuppressive strategy. Statins inhibit the rate‐limiting enzyme of the mevalonate pathway leading to reduced levels of its downstream products. The pyrophosphate downstream products are critical for geranylgeranylation or farnesylation of GTPases that mediate multiple steps in the immune response such as cell migration, activation, signalling and cytokine production.
In patients undergoing allogeneic haematopoietic cell transplantation, some studies showed that statin intake by the donor55 or host56 was connected to a reduced GVHD incidence.55, 56, 57, 58 In contrast, one trial showed that the addition of atorvastatin to standard aGVHD prophylaxis did not provide a benefit with respect to GVHD rates.59
Summary
The immunomodulatory effects of statins in different disease models have meanwhile been supported by robust data from multiple independent groups. The potency of statins with respect to immunosuppression is lower than that of many of the established immunosuppressive drugs, which may be an advantage when immune‐modulation rather than strong immunosuppression is required. Also, based on their mode of action, which is mainly via inhibition of protein geranylgeranylation and protein farnesylation, a combination strategy with inhibitors of different pathways may result in a synergism. In agreement with this, early studies on such combination therapies have been completed, combining statins with cytokine receptor inhibitors such as tocilizumab against IL‐6R or JAK inhibitors. Interesting novel aspects are the increased Production of Ang‐1 under certain statins, which thereby impact endothelial function, and the direct effects on T cells via calcium ion influx and IL‐2 production. Overall, statins hold promise for combination therapies as they reduce cholesterol levels induced by certain immunosuppressive drugs and modulate the immune response.
Disclosures
The author has received an honorarium from Novartis and research funding from Jazz Pharma.
Author contribution
RZ collected literature, discussed the studies and wrote the manuscript.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft, Germany, Heisenberg Professorship to R.Z. (DFG ZE 872/3‐1) and ERC Consolidator grant (681012 GvHDCure to R.Z.). We apologize to those investigators whose work could not be cited due to space restrictions.
References
- 1. Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM et al The HMG‐CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002; 420:78–84. [DOI] [PubMed] [Google Scholar]
- 2. Greenwood J, Walters CE, Pryce G, Kanuga N, Beraud E, Baker D et al Lovastatin inhibits brain endothelial cell Rho‐mediated lymphocyte migration and attenuates experimental autoimmune encephalomyelitis. FASEB J 2003; 17:905–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leung BP, Sattar N, Crilly A, Prach M, McCarey DW, Payne H et al A novel anti‐inflammatory role for simvastatin in inflammatory arthritis. J Immunol 2003; 170:1524–30. [DOI] [PubMed] [Google Scholar]
- 4. Zeiser R, Youssef S, Baker J, Kambhan N, Steinman L, Negrin RS. HMG‐CoA reductase inhibitors (statins) provide acute‐graft‐versus‐host disease protection by Th‐2 cytokine induction while sparing graft‐versus‐leukemia activity. Blood 2007; 110:4588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Broady R, Levings MK. Graft‐versus‐host disease: suppression by statins. Nat Med 2008; 14:1155–6. [DOI] [PubMed] [Google Scholar]
- 6. Zeiser R, Maas K, Youssef S, Dürr C, Steinman L, Negrin RS. Regulation of different inflammatory diseases by impacting the mevalonate pathway. Immunology 2009; 127:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hechinger AK, Maas K, Dürr C, Leonhardt F, Prinz G, Marks R et al Inhibition of protein geranylgeranylation and farnesylation protects against GvHD via effects on CD4 effector T cells. Haematologica 2013; 98:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao N, Dong Q, Qian C, Li S, Wu QF, Ding D et al Lovastatin blocks Kv1.3 channel in human T cells: a new mechanism to explain its immunomodulatory properties. Sci Rep 2015; 5:17381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bekkering S, Arts RJW, Novakovic B, Kourtzelis I, van der Heijden CDCC, Li Y et al Metabolic induction of trained immunity through the mevalonate pathway. Cell 2018; 172:135–46. [DOI] [PubMed] [Google Scholar]
- 10. Singh I, Samuvel DJ, Choi S, Saxena N, Singh AK, Won J. Combination therapy of lovastatin and AMPK activator improves mitochondrial and peroxisomal functions and clinical disease in EAE model. Immunology 2018; https://doi.org/10.1111/imm.12893. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graham KL, Lee LY, Higgins JP, Steinman L, Utz PJ, Ho PP. Failure of oral atorvastatin to modulate a murine model of systemic lupus erythematosus. Arthritis Rheum 2008; 58:2098–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Leuven SI, Mendez‐Fernandez YV, Wilhelm AJ, Wade NS, Gabriel CL, Kastelein JJ et al Mycophenolate mofetil but not atorvastatin attenuates atherosclerosis in lupus‐prone LDLr−/− mice. Ann Rheum Dis 2012; 71:408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woo JM, Lin Z, Navab M, Van Dyck C, Trejo‐Lopez Y, Woo KM et al Treatment with apolipoprotein A‐1 mimetic peptide reduces lupus‐like manifestations in a murine lupus model of accelerated atherosclerosis. Arthritis Res Ther 2010; 12:R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aprahamian T, Bonegio R, Rizzo J, Perlman H, Lefer DJ, Rifkin IR et al Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model. J Immunol 2006; 177:3028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferreira GA, Navarro TP, Telles RW, Andrade LEC, Sato I. Atorvastatin therapy improves endothelial‐dependent vasodilatation in patients with systemic lupus erythematosus: an 8 weeks controlled trial. Rheumatology 2007; 46:1560–5. [DOI] [PubMed] [Google Scholar]
- 16. Kotyla PJ. Comment on: atorvastatin therapy improves endothelial‐dependent vasodilation in patients with systemic lupus erythematosus: an 8 week controlled trial. Rheumatology 2008; 47:381–2. [DOI] [PubMed] [Google Scholar]
- 17. Costenbader KH, Liang MH, Chibnik LB, Aizer J, Kwon H, Gall V et al A pravastatin dose‐escalation study in systemic lupus erythematosus. Rheumatol Int 2007; 27:1071–7. [DOI] [PubMed] [Google Scholar]
- 18. Abud‐Mendoza C, de la Fuente H, Cuevas‐Orta E, Baranda L, Cruz‐Rizo J, González‐Amaro R. Therapy with statins in patients with refractory rheumatic diseases: a preliminary study. Lupus 2003; 12:607–11. [DOI] [PubMed] [Google Scholar]
- 19. van Leuven SI, Mendez‐Fernandez YV, Stroes ES, Tak PP, Major AS. Statin therapy in lupus‐mediated atherogenesis: two birds with one stone? Ann Rheum Dis 2011; 70:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sahebkar A, Rathouska J, Derosa G, Maffioli P, Nachtigal P. Statin impact on disease activity and C‐reactive protein concentrations in systemic lupus erythematosus patients: a systematic review and meta‐analysis of controlled trials. Autoimmun Rev 2016; 15:344–53. [DOI] [PubMed] [Google Scholar]
- 21. Oza A, Lu N, Schoenfeld SR, Fisher MC, Dubreuil M, Rai SK et al Survival benefit of statin use in ankylosing spondylitis: a general population‐based cohort study. Ann Rheum Dis 2017; 76:1737–42. [DOI] [PubMed] [Google Scholar]
- 22. Soubrier M, Pei J, Durand F, Gullestad L, John A. Concomitant use of statins in tocilizumab‐treated patients with rheumatoid arthritis: a post hoc analysis. Rheumatol Ther 2017; 4:133–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin CH, Hsu KW, Chen CH, Uang YS, Lin CJ. Differential changes in the pharmacokinetics of statins in collagen‐induced arthritis rats. Biochem Pharmacol 2017; 142:216–28. [DOI] [PubMed] [Google Scholar]
- 24. An J, Alemao E, Reynolds K, Kawabata H, Solomon DH, Liao KP et al Cardiovascular outcomes associated with lowering low‐density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol 2016; 43:1989–96. [DOI] [PubMed] [Google Scholar]
- 25. McInnes IB, Kim HY, Lee SH, Mandel D, Song YW, Connell CA et al Open‐label tofacitinib and double‐blind atorvastatin in rheumatoid arthritis patients: a randomised study. Ann Rheum Dis 2014; 73:124–31. [DOI] [PubMed] [Google Scholar]
- 26. Mowla K, Rajai E, Ghorbani A, Dargahi‐Malamir M, Bahadoram M, Mohammadi S. Effect of atorvastatin on the disease activity and severity of rheumatoid arthritis: double‐blind randomized controlled trial. J Clin Diagn Res 2016; 10:OC32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I et al Trial of atorvastatin in Rheumatoid Arthritis (TARA): double‐blind, randomised placebo‐controlled trial. Lancet 2004; 363:2015–21. [DOI] [PubMed] [Google Scholar]
- 28. Kanda H, Yokota K, Kohno C, Sawada T, Sato K, Yamaguchi M et al Effects of low‐dosage simvastatin on rheumatoid arthritis through reduction of Th1/Th2 and CD4/CD8 ratios. Mod Rheumatol 2007; 17:364–8. [DOI] [PubMed] [Google Scholar]
- 29. Lazzerini PE, Lorenzini S, Selvi E, Capecchi PL, Chindamo D, Bisogno S et al Simvastatin inhibits cytokine production and nuclear factor‐κB activation in interleukin 1β‐stimulated synoviocytes from rheumatoid arthritis patients. Clin Exp Rheumatol 2007; 25:696–700. [PubMed] [Google Scholar]
- 30. Palmer G, Chobaz V, Talabot‐Ayer D, Taylor S, So A, Gabay C et al Assessment of the efficacy of different statins in murine collagen‐induced arthritis. Arthritis Rheum 2004; 50:4051–9. [DOI] [PubMed] [Google Scholar]
- 31. Nath N, Giri S, Prasad R, Singh AK, Singh I. Potential targets of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitor for multiple sclerosis therapy. J Immunol 2004; 172:1273–86. [DOI] [PubMed] [Google Scholar]
- 32. Stüve O, Youssef S, Weber MS, Nessler S, von Büdingen HC, Hemmer B et al Immunomodulatory synergy by combination of atorvastatin and glatiramer acetate in treatment of CNS autoimmunity. J Clin Invest 2006; 116:1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aktas O, Waiczies S, Smorodchenko A, Dorr J, Seeger B, Prozorovski T et al Treatment of relapsing paralysis in experimental encephalomyelitis by targeting TH1 cells through atorvastatin. J Exp Med 2003; 197:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dunn SE, Youssef S, Goldstein MJ, Prod'homme T, Weber MS, Zamvil SS et al Isoprenoids determine Th1/Th2 fate in pathogenic T cells, providing a mechanism of modulation of autoimmunity by atorvastatin. J Exp Med 2006; 203:401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zipp F, Waiczies S, Aktas O, Neuhaus O, Hemmer B et al Impact of HMG‐CoA reductase inhibition on brain pathology. Trends Pharmacol Sci 2007; 28:342–9. [DOI] [PubMed] [Google Scholar]
- 36. Zhang X, Jin J, Peng X, Ramgolam VS, Markovic‐Plese S. Simvastatin inhibits IL‐17 secretion by targeting multiple IL‐17‐regulatory cytokines and by inhibiting the expression of IL‐17 transcription factor RORC in CD4+ lymphocytes. J Immunol 2008; 180:6988–96. [DOI] [PubMed] [Google Scholar]
- 37. de Oliveira DM, de Oliveira EM, Ferrari Mde F, Semedo P, Hiyane MI, Cenedeze MA et al Simvastatin ameliorates experimental autoimmune encephalomyelitis by inhibiting Th1/Th17 response and cellular infiltration. Inflammopharmacology 2015; 23:343–54. [DOI] [PubMed] [Google Scholar]
- 38. Weber MS, Prod'homme T, Youssef S, Dunn SE, Steinman L, Zamvil SS. Neither T‐helper type 2 nor Foxp3+ regulatory T cells are necessary for therapeutic benefit of atorvastatin in treatment of central nervous system autoimmunity. J Neuroinflammation 2014; 11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chan D, Binks S, Nicholas JM, Frost C, Cardoso MJ, Ourselin S et al Effect of high‐dose simvastatin on cognitive, neuropsychiatric, and health‐related quality‐of‐life measures in secondary progressive multiple sclerosis: secondary analyses from the MS‐STAT randomised, placebo‐controlled trial. Lancet Neurol 2017; 16:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vollmer T, Key L, Durkalski V, Tyor W, Corboy J. Oral simvastatin treatment in relapsing‐remitting multiple sclerosis. Lancet 2004; 363:1607–8. [DOI] [PubMed] [Google Scholar]
- 41. Paul F, Waiczies S, Wuerfel J, Bellmann‐Strobl J, Dörr J, Waiczies H et al Oral high‐dose atorvastatin treatment in relapsing–remitting multiple sclerosis. PLoS ONE 2008; 3:1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pihl‐Jensen G, Tsakiri A, Frederiksen JL. Statin treatment in multiple sclerosis: a systematic review and meta‐analysis. CNS Drugs 2015; 29:277–91. [DOI] [PubMed] [Google Scholar]
- 43. Zeiser R, Blazar BR. Pathophysiology of chronic graft‐versus‐host disease and therapeutic targets. N Engl J Med 2017; 377:2565–79. [DOI] [PubMed] [Google Scholar]
- 44. Zeiser R, Blazar BR. Acute graft‐versus‐host disease – biologic process, prevention, and therapy. N Engl J Med 2017; 377:2167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang Y, Li D, Jones D, Bassett R, Sale GE, Khalili J et al Blocking LFA‐1 activation with lovastatin prevents graft‐versus‐host disease in mouse bone marrow transplantation. Biol Blood Marrow Transplant 2009; 15:1513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shimabukuro‐Vornhagen A, Liebig T, von Bergwelt‐Baildon M. Statins inhibit human APC function: implications for the treatment of GVHD. Blood 2008; 112:1544–5. [DOI] [PubMed] [Google Scholar]
- 47. Marks R, Ho AW, Robbel C, Kuna T, Berk S, Gajewski TF. Farnesyltransferase inhibitors inhibit T cell cytokine production at the post‐transcriptional level. Blood 2007; 110:1982–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Montecucco F, Burger F, Pelli G, Poku NK, Berlier C, Steffens S et al Statins inhibit C‐reactive protein‐induced chemokine secretion, ICAM‐1 upregulation and chemotaxis in adherent human monocytes. Rheumatology 2009; 48:233–42. [DOI] [PubMed] [Google Scholar]
- 49. Waiczies S, Bendix I, Prozorovski T, Ratner M, Nazarenko I, Pfueller CF et al Geranylgeranylation but not GTP loading determines rho migratory function in T cells. J Immunol 2007; 179:6024–32. [DOI] [PubMed] [Google Scholar]
- 50. Zheng P, Wu QL, Li BB, Chen P, Nie DM, Zhang R et al Simvastatin ameliorates graft‐vs‐host disease by regulating angiopoietin‐1 and angiopoietin‐2 in a murine model. Leuk Res 2017; 55:49–54. [DOI] [PubMed] [Google Scholar]
- 51. Leonhardt F, Grundmann S, Behe M, Bluhm F, Dumont RA, Braun F et al Inflammatory neovascularization during graft‐versus‐host disease is regulated by αv integrin and miR‐100. Blood 2013; 121:3307–18. [DOI] [PubMed] [Google Scholar]
- 52. Riesner K, Shi Y, Jacobi A, Kräter M, Kalupa M, McGearey A et al Initiation of acute graft‐versus‐host disease by angiogenesis. Blood 2017; 129:2021–32. [DOI] [PubMed] [Google Scholar]
- 53. Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas‐Bauer K, Metzelder SK et al Ruxolitinib in corticosteroid‐refractory graft‐versus‐host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia 2015; 29:2062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stickel N, Hanke K, Marschner D, Prinz G, Köhler M, Melchinger W et al MicroRNA‐146a reduces MHC‐II expression via targeting JAK/STAT‐signaling in dendritic cells after stem cell transplantation. Leukemia 2017; 31:2732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rotta M, Storer BE, Storb RF, Martin PJ, Heimfeld S, Peffer A et al Donor statin treatment protects against severe acute graft‐versus‐host disease after related allogeneic hematopoietic cell transplantation. Blood 2010; 115:1288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rotta M, Storer BE, Storb R, Martin PJ, Flowers ME, Vernon MS et al Impact of recipient statin treatment on graft‐versus‐host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2010; 16:1463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hamadani M, Gibson LF, Remick SC, Wen S, Petros W, Tse W et al Sibling donor and recipient immune modulation with atorvastatin for the prophylaxis of acute graft‐versus‐host disease. J Clin Oncol 2013; 31:4416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kanate AS, Hari PN, Pasquini MC, Visotcky A, Ahn KW, Boyd J et al Recipient immune modulation with atorvastatin for acute graft‐versus‐host disease prophylaxis after allogeneic transplantation. Biol Blood Marrow Transplant 2017; 23:1295–302. [DOI] [PubMed] [Google Scholar]
- 59. Efebera YA, Geyer S, Andritsos L, Vasu S, Jaglowski S, Bingman A et al Atorvastatin for the prophylaxis of acute graft‐versus‐host disease in patients undergoing HLA‐matched related donor allogeneic hematopoietic stem cell transplantation (allo‐HCT). Biol Blood Marrow Transplant 2016; 22:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hamadani M, Awan FT, Devine SM. The impact of HMG‐CoA reductase inhibition on the incidence and severity of graft‐versus‐host disease in patients with acute leukemia undergoing allogeneic transplantation. Blood 2008; 111:3901–2. [DOI] [PubMed] [Google Scholar]


