Summary
Cigarette smoke contains toxic and carcinogenic substances that contribute to the development of cancer and various diseases. Genetic variation might be important, because not all smokers develop smoking‐related disease. The current study addressed the possible interactions among selected single nucleotide polymorphisms (SNPs) in genes related to systemic inflammation, smoking status, the levels of circulating immune response cells and plasma biomarkers of systemic inflammation. Sixty‐four healthy blood donors were recruited, 31 of whom were current smokers and 33 were never‐users of tobacco products, references. Compared to references, the smokers showed significantly increased levels of circulating total white blood cells, lymphocytes, monocytes, neutrophils, basophils and C‐reactive protein (CRP). Smokers also more frequently exhibited circulating cell phenotypes that are associated with an immunocompromised state: CD8dim cells in the lymphocyte group, CD13+ CD11+, CD13+ CD14+, CD13+ CD56+ cells in the monocyte group and CD13+ CD11+, CD13+ CD56+ cells in the neutrophil group. We observed an interaction among SNPs, smoking status and some of the studied biomarkers. The average plasma CRP level was significantly higher among the smokers, with the highest level found among those with the CRP rs1800947 CC genotype. Additionally, an increased CD8+ GZB + cells in the CD8dim group were found among smokers with the GZB rs8192917 AA genotype. Thus, smoking appears to be associated with systemic inflammation and increased levels of circulating immunosuppressive cells. The extent of these effects was associated with SNPs among the smokers. This observation may contribute to a better understanding of the genetic susceptibility of smoking‐related disease and the variations observed in clinical outcomes.
Keywords: cigarette smoking, immune response, single nucleotide polymorphisms, systemic inflammation
Introduction
Cigarette smoking is a significant risk factor for diseases in the cardiovascular system, respiratory system and several types of cancer.1, 2, 3 However, not all smokers develop smoking‐related disease. Genetic susceptibility might play a role because an increased risk – at least for some types of cancer – has also been reported among non‐smoking, first‐degree relatives.4, 5 In vitro, smoke extract alters normal cell phenotypes and gene expression profiles and can induce massive cell death. Such effects were found to be associated with certain single nucleotide polymorphism (SNP) genotypes.6, 7
Cell death induced by smoking may trigger a local or systemic inflammatory response that results in endothelial cell activation, the promotion of a prothrombotic stage and atherosclerotic plaque formation.8, 9 The extent of systemic inflammation can be assessed by the plasma C‐reactive protein (CRP) levels.10 Increased plasma CRP levels are associated with a poor clinical outcome among head and neck cancer patients, independently of tumour–node–metastasis (TNM) staging.11
Elevated levels of circulating blood lymphocytes, monocytes and neutrophils are associated with inflammation and poor survival in cancer patients.12 Monocytes and neutrophils play a major role in the innate immune response and interact with adaptive immune response cells in the lymphocyte group.13 Such interactions could result in either the activation or suppression of the host immune response.14
CD8+ cells in the T‐lymphocyte group could be divided into a highly expressed CD8 level (CD8bright) based on the α/β heterodimers or a lower expressed CD8 level (CD8dim) based on the α/α homodimers receptor. The function of these CD8 subpopulations might differ.15, 16 Increased levels of CD8dim suggest an immunosuppressed state17 and cytotoxic T‐lymphocyte (CTL) impairment,18 and have been associated with disease progression in human immunodeficiency virus (HIV)‐positive patients.19
Perforin (PRF) and granzyme B (GZB) are cytotoxic granules that mediate cell death.20 Over‐expression of granzyme B (GZB) in CD8+ T‐lymphocytes is a putative biomarker of impaired immunity detected in patients with systemic lupus erythematosus.21
The current study addressed the possible interactions among selected SNPs in genes related to systemic inflammation, smoking status and the levels of circulating immune response cells and plasma biomarkers of systemic inflammation.
Materials and methods
Subjects
We recruited 31 healthy blood donors who, according to self‐reports, were current smokers (eight males and 23 females with a median age of 57 years). The preponderance of females was probably a reflection of the fact that in Sweden, contrary to the situation in most other countries, the prevalence of smoking is higher among females than it is among males.3 A sample of donors who reported having never used any type of tobacco product was matched to the group of smokers on the basis of age and gender. This resulted in a reference group with a total of 33 individuals (six males and 27 females, with a median age of 54 years).
All study subjects provided informed consent to participate in this study, according to the Declaration of Helsinki. Ethics approval was obtained from the regional ethical review board of Linköping, Sweden.
Blood samples and flow cytometry
Thirty millilitres of venous blood was collected from all participants. The levels of circulating total white blood cells (WBCs) and subpopulations were analysed with a Sysmex XE5000 instrument (Sysmex Corporation, Kobe, Japan).
The phenotypes of ex‐vivo fresh peripheral WBC subpopulations were analysed using a Becton Dickinson FACSCanto II flow cytometer (BD Biosciences, San Jose, CA). All monoclonal antibodies were purchased from BD Biosciences. The cells were directly stained with surface markers for CD3, CD4, CD8, CD11, CD13, CD14 and CD56, and were intracellularly stained for GZB and PRF according to the company protocol. Peripheral blood lymphocyte, monocyte and neutrophil subpopulations were analysed after gating according to their SSC/FSC location (Fig. 1). Data analysis was performed with the bd facsdiva software program (BD Biosciences).
Figure 1.
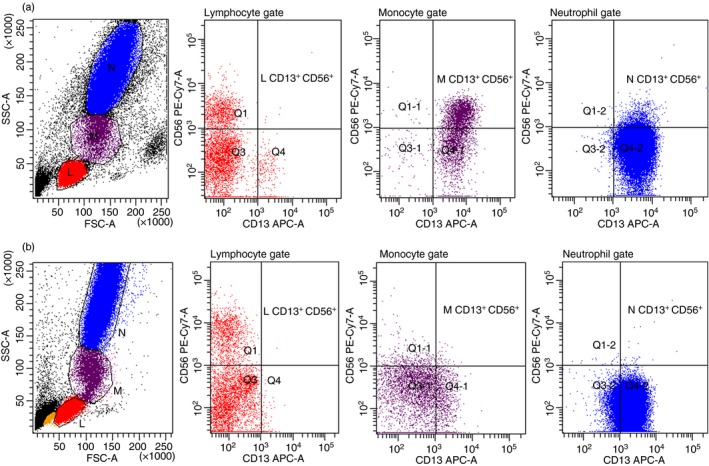
Smoking status was significantly related to up‐regulated circulating CD13+ CD56+ cells in monocyte (M) and neutrophil (N) but not lymphocyte (L) populations. Flow cytometry dot‐plot results presented ex‐vivo fresh blood cells of one healthy smoker (a) and one reference (b). [Colour figure can be viewed at http://wileyonlinelibrary.com]
Plasma CRP analysis
Plasma CRP levels were analysed using Siemens Advia 1800 (Siemens Healthcare, Erlangen, Germany).
DNA samples and SNP analysis
High molecular‐weight DNA was extracted from the blood samples using a Qiagen Bio Robot M48 with MagAttract DNA Blood M48 kit (http://www.qiagen.com). The quantity and quality of DNA were determined by spectrophotometric analysis (Nanodrop ND‐1000; Nanodrop Technologies, Wilmington, DE, USA). These DNA samples were used as templates in the SNP detection.
Four SNPs located in CRP rs1800947, GZB rs8192917, PRF rs10999426 and PRF rs3758562 were analysed. These SNPs were tested and passed the two‐hits in the dbSNP database or HapMap‐validated SNPs with an Illumina design ability score. Genotyping of the candidate SNPs was performed by the Golden Gate assay (http://www.illumina.com), according to the manufacturer's protocol22 at the SNP and SEQ Technology Platform, Uppsala University, Sweden (http://www.genotyping.se).
Statistics
The Mann–Whitney U‐test was used for distributional comparisons using SPSS 18 : SPSS Inc., Chicago, Illinois, USA. All comparisons were two‐sided, with a P‐value ≤ 0·05 considered statistically significant. Correction for multiple testing was performed according to Benjamini and Hochberg23, 24 with a false discovery rate of 0·05.
Results
WBCs and WBC subpopulations
A significantly increased level of circulating total WBCs, lymphocytes, monocytes, neutrophils and basophils was detected among the smokers compared to the reference subjects (Table 1a).
Table 1.
Number of circulating white blood cells (WBCs) in smokers compared to references. (a) The number of WBCs and its subpopulations. (b) Phenotypes of lymphocytes. (c) Phenotypes of myelocyte subpopulations
| Smokers (n = 31) | References (n = 33) | P‐valuea | |||
|---|---|---|---|---|---|
| Median | Min–max | Median | Min–max | ||
| (a) WBCs (106 cells/l) | |||||
| Total WBCs | 7850 | 3070–12 200 | 5220 | 3400–9450 | < 0·001 |
| Lymphocytes | 2230 | 1120–2980 | 1620 | 970–4300 | 0·006 |
| Monocytes | 600 | 280–1060 | 460 | 300–800 | 0·001 |
| Neutrophils | 4500 | 1500–9320 | 3070 | 1560–5960 | 0·001 |
| Eosinophils | 140 | 10–520 | 150 | 40–360 | 0·47 |
| Basophils | 40 | 10–130 | 20 | 0–100 | 0·03 |
| (b) Myelocytes (106 cells/l) | |||||
| Monocytes | |||||
| CD13+ | 520 | 156–1007 | 330 | 115–708 | 0·002 |
| CD13+ CD11+ | 450 | 188–876 | 290 | 22–616 | 0·03 |
| CD13+ CD14+ | 450 | 59–906 | 190 | 1–567 | 0·001 |
| CD13+ CD56+ | 90 | 8–327 | 30 | 1–120 | < 0·001 |
| Neutrophils | |||||
| CD13+ | 4880 | 1920–8730 | 2850 | 1520–5460 | < 0·001 |
| CD13+ CD11+ | 4900 | 2940–5762 | 2650 | 714–4969 | 0·001 |
| CD13+ CD56+ | 112 | 0–611 | 0 | 0–78 | < 0·001 |
| (c) Lymphocytes (106 cells/l) | |||||
| CD3+ | 1590 | 728–2444 | 1200 | 280–2178 | 0·03 |
| CD56+ | 300 | 69–680 | 270 | 156–1720 | 0·85 |
| CD3+ CD56+ | 50 | 7–219 | 90 | 22–1238 | 0·09 |
| CD4+ | 990 | 414–1711 | 790 | 352–2150 | 0·1 |
| CD4+ PRF+ | 30 | 0–319 | 30 | 0–189 | 0·85 |
| CD4+ GZB+ | 100 | 0–402 | 90 | 10–1140 | 0·85 |
| CD4 PRF+ GZB+ | 20 | 0–319 | 30 | 0–189 | 0·85 |
| CD8+ | 640 | 258–1010 | 420 | 238–1161 | 0·02 |
| CD8bright | 360 | 159–950 | 310 | 144–903 | 0·39 |
| CD8bright PRF+ | 20 | 0–365 | 30 | 0–168 | 0·43 |
| CD8bright GZB+ | 160 | 12–559 | 120 | 17–757 | 0·86 |
| CD8bright PRF+ GZB+ | 20 | 0–365 | 30 | 0–168 | 0·43 |
| CD8dim | 160 | 52–420 | 110 | 39–264 | 0·05 |
| CD8dim PRF+ | 73 | 21–420 | 66 | 0–232 | 0·43 |
| CD8dim GZB+ | 113 | 32–445 | 93 | 16–378 | 0·39 |
| CD8dim PRF+ GZB+ | 70 | 21–420 | 70 | 0–232 | 0·43 |
| CD4/CD8 ratio | 1·7 | 0·6–3·6 | 1·85 | 0·9–3·8 | 0·6 |
PRF, perforin; GZB, granzyme B.
P‐value after correction for multiple testing according to Benjamini and Hochberg's method.
In the monocyte and neutrophil gate, the levels of cells with the CD13+ CD13+ CD11+ and CD13+ CD56+ phenotypes were also significantly increased among the smokers, as was the level of monocytes with CD13+ CD14+ expression (Fig. 1 and Table 1b).
In the lymphocyte gate, the level of CD3+, CD8+ and CD8dim cells was significantly increased among the smokers (Fig. 2 and Table 1c).
Figure 2.
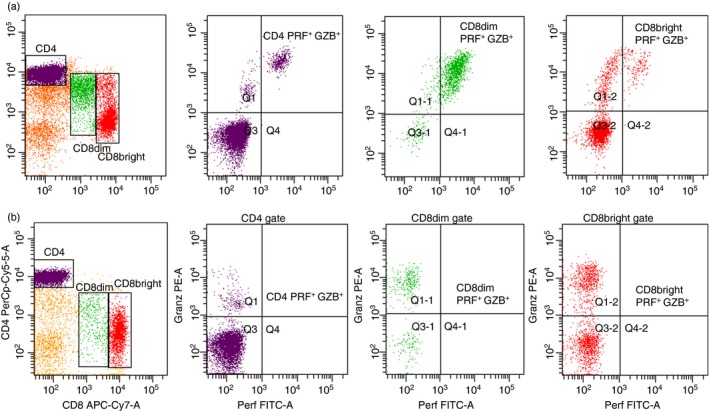
Expression of perforin (PRF+) and granzyme B (GZB+) positive cells in CD4+, CD8dim and CD8bright populations. Flow cytometry dot‐plot results presented ex‐vivo fresh blood cells of one healthy smoker (a) and one reference (b). [Colour figure can be viewed at http://wileyonlinelibrary.com]
No statistically significant differences were detected in terms of the level or phenotype of WBCs or WBC subpopulations between young and old subjects (≤ 55 versus > 55 years). This observation applied to both smokers and never‐smoking subjects, references (data not shown).
Plasma CRP
There was a highly significant difference (P = 0·009) between the level of plasma CRP among the smokers (median = 2·57 mg/l, min–max: 0·27–16·16) compared to the reference subjects (median = 1·34 mg/l, min–max: 0·23–7·38).
SNPs and smoking status
No difference in the distribution SNP frequencies of gene CRP rs1800947, GZB rs8192917, PRF rs10999426 and PFR rs3758562 was observed among the smokers compared to the reference subjects (data not shown). However, the highest level of CRP was noted among smokers who had the CRP rs1800947 CC genotype (P = 0·03, Table 2).
Table 2.
Influence of single nucleotide polymorphism (SNP) genotype on levels of plasma CRP and numbers of CD8+ GZB+ cells in healthy smokers compared with references
| Gene | rs | Sequence | Smokers (n) | References (n) | P‐valuea | |||
|---|---|---|---|---|---|---|---|---|
| Median | Min–max | Median | Min–max | |||||
| Plasma CRP (mg/l) | CRP | 1800947 | CC | 2·65 (25) | 0·49–7·9 | 1·49 (29) | 0·32–7·38 | 0·03 |
| CG/GG | 1·67 (6) | 0·27–16·2 | 1·1 (4) | 0·26–3·69 | 0·67 | |||
| CD8+ (106cells/l) | GZB | 8192917 | AA | 341 (23) | 49–690 | 212 (26) | 92–797 | 0·05 |
| AG/GG | 184 (8) | 95–357 | 406 (7) | 134–1135 | 0·10 | |||
| CD8bright (106 cells/l) | GZB | 8192917 | AA | 167 (23) | 12–558 | 118 (26) | 17–693 | 0·24 |
| AG/GG | 110 (8) | 21–182 | 191 (7) | 61–756 | 0·10 | |||
| CD8dim (106 cells/l) | GZB | 8192917 | AA | 131 (23) | 36–445 | 80 (26) | 16–234 | 0·03 |
| AG/GG | 78 (8) | 32–212 | 142 (7) | 36–378 | 0·24 | |||
GZB, Granzyme B; CRP, C‐reactive protein.
P value after correction for multiple testing according to Benjamini and Hochberg's method.
A significantly increased level of CD8+ GZB+ cells was observed among the smokers exhibiting the GZB rs8192917 AA genotype. This increase mainly concerned CD8dim GZB+ cells with the GZB rs8192917 AA genotype (Table 2).
No difference was observed between smokers and references in terms of PRF expression in CD4+, CD8+, CD8bright or CD8dim cells related to the PRF rs10999426 or PRF rs3758562 genotype polymorphism (data not shown).
Discussion
We have previously shown that cigarette smoke condensate induced massive cell death in vitro.6 Based on this observation, we hypothesized that the autoantigens released from dead cells in vivo may provoke a local host immune response. In this investigation, we observed significantly increased levels of circulating total WBCs, lymphocytes, monocytes, neutrophils, basophils and plasma CRP among healthy smokers. This observation confirms that cigarette smoke can, in fact, induce a systemic inflammatory host immune response in vivo. Chronic systemic inflammation has been suggested to contribute not only to the increased risk of cancer among smokers, but also to smoking‐related cardiovascular disease.2, 8
It has been reported that young smokers exhibit increased levels of circulating CD4+ (but not CD8+) cells compared to their non‐smoking monozygotic twins.25 We observed elevated levels of circulating CD8dim cells among the smokers compared to the reference subjects. The discrepancy between our findings and the mentioned results might be related to the different composition of the reference groups (identical monozygotic twins versus unrelated healthy blood donors). However, it is also possible that monozygotic twins of smokers might be exposed to second‐hand smoke to a greater extent than non‐smoking, unrelated individuals.25
The intensity of CD8 expression has been suggested to constitute an indicator of the host immunological profile.15, 17 Increased circulating CD8dim cells have been documented among HIV‐infected individuals and are associated with peripheral T‐cell exhaustion or impairment of effective CTL.19 Additionally, among laboratory animals with an experimental simian immunodeficiency virus infection, an increased level of CD8dim cells was correlated with impaired immune function.18
It has been reported that increased levels of CD8+ GZB+ cells constitute an abnormal adaptive immune response mediator.26 The increase in circulating CD8dim GZB+ cells observed in our study provides further support for the hypothesis that smoking may also induce a state of immunosuppression among apparently healthy individuals.
Our results extend previous findings showing that smoke extracts in vitro or smoking status in vivo affect various immunological functions. For instance, in‐vitro exposure to cigarette smoke was found to impair myeloid cell differentiation.27 An increased level of circulating CD13+ CD11+ or CD13+ CD56+ cells in monocytes and neutrophils among smokers, which was detected in our investigation, suggested a smoking related‐impairment of the host systemic innate immune response.27, 28
We observed an increased level of CD8dim GZB+ cells among smokers that was associated with the GZB rs8192917 AA genotype. We also observed an increased plasma CRP level among the smoking subjects that was associated with the CRP rs1800947 CC genotype. The effect of smoking was more pronounced among individuals exhibiting the studied SNPs, and was not observed for all smokers. Thus, the risk among smokers to develop smoking‐ related disease might be associated with specific SNPs that are related to immune function.29, 30, 31
In conclusion, our investigation suggests that genetic variation at SNPs sequences might predict the risk of smoking‐related disease. Thus, using SNPs as a predictive biomarker of individual risk or as additional motivation for smoking cessation merit further investigation.
Author contributions
NL, LER, OT, KU and SL designed the study and analysed the data. TL and B‐ÅA carried out the experiments and was responsible for all analyses. All authors wrote, read and approved the final manuscript.
Ethics approval and informed consent
All study subjects provided informed consent to participate in this study according to the Declaration of Helsinki. Ethics approval for this investigation was obtained from the regional ethical review board of Linköping, Sweden.
Disclosure
None.
Acknowledgements
We would like to thank the study participants and the staff at Ryhov Hospital, Jönköping for providing blood samples. We are indebted to the hospital for use of the FACS facility. We also thank Tomas Axelsson and Mats Nilsson for practical support throughout this project. This study was supported by the Jönköping Clinical Cancer Research Foundation (Grant 110426‐1), Futurum (Grant 144631), FORSS (Grant 567001), the Swedish Laryngeal Foundation, the Thai Office of Science and Technology in Brussels, Ministry of Science and Technology, Research and Researchers for Industries (Grant PHD60I0017). The funders had no role in the study design, data collection, analysis, decision to publish or in the preparation of this manuscript.
References
- 1. Rom O, Avezov K, Aizenbud D, Reznick AZ. Cigarette smoking and inflammation revisited. Respir Physiol Neurobiol 2013; 187:5–10. [DOI] [PubMed] [Google Scholar]
- 2. Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking‐associated cancers. Oncogene 2002; 21:7435–51. [DOI] [PubMed] [Google Scholar]
- 3. Global Burden of Disease (GBD) Tobacco Collaborators . Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017; 389:1885–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foulkes WD, Brunet JS, Sieh W, Black MJ, Shenouda G, Narod SA. Familial risks of squamous cell carcinoma of the head and neck: retrospective case‐control study. BMJ 1996; 313:716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu Z, Fang F, Chang ET, Ye W. Cancer risk in the relatives of patients with nasopharyngeal carcinoma – a register‐based cohort study in Sweden. Br J Cancer 2015; 112:1827–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laytragoon‐Lewin N, Bahram F, Rutqvist LE, Turesson I, Lewin F. Direct effects of pure nicotine, cigarette smoke extract, Swedish‐type smokeless tobacco (Snus) extract and ethanol on human normal endothelial cells and fibroblasts. Anticancer Res 2011; 31:1527–34. [PubMed] [Google Scholar]
- 7. Cederblad L, Thunberg U, Engstrom M, Castro J, Rutqvist LE, Laytragoon‐Lewin N. The combined effects of single‐nucleotide polymorphisms, tobacco products, and ethanol on normal resting blood mononuclear cells. Nicotine Tob Res 2013; 15:890–5. [DOI] [PubMed] [Google Scholar]
- 8. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–95. [DOI] [PubMed] [Google Scholar]
- 9. Murray LA, Dunmore R, Camelo A, Da Silva CA, Gustavsson MJ, Habiel DM et al Acute cigarette smoke exposure activates apoptotic and inflammatory programs but a second stimulus is required to induce epithelial to mesenchymal transition in COPD epithelium. Respir Res 2017; 18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010; 6:149–63. [DOI] [PubMed] [Google Scholar]
- 11. Andersson BA, Lewin F, Lundgren J, Nilsson M, Rutqvist LE, Lofgren S et al Plasma tumor necrosis factor‐alpha and C‐reactive protein as biomarker for survival in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol 2014; 140:515–9. [DOI] [PubMed] [Google Scholar]
- 12. Fox P, Hudson M, Brown C, Lord S, Gebski V, De Souza P et al Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br J Cancer 2013; 109:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11:723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011; 11:519–31. [DOI] [PubMed] [Google Scholar]
- 15. Falanga YT, Frascoli M, Kaymaz Y, Forconi C, Ong'echa JM, Bailey JA et al High pathogen burden in childhood promotes the development of unconventional innate‐like CD8+ T cells. JCI Insight 2017; 2:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jorgensen PB, Livbjerg AH, Hansen HJ, Petersen T, Hollsberg P. Epstein–Barr virus peptide presented by HLA‐E is predominantly recognized by CD8(bright) cells in multiple sclerosis patients. PLOS ONE 2012; 7:e46120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naji A, Le Rond S, Durrbach A, Krawice‐Radanne I, Creput C, Daouya M et al CD3+CD4low and CD3+CD8low are induced by HLA‐G: novel human peripheral blood suppressor T‐cell subsets involved in transplant acceptance. Blood 2007; 110:3936–48. [DOI] [PubMed] [Google Scholar]
- 18. Xu H, Wang X, Lackner AA, Veazey RS. CD8 down‐regulation and functional impairment of SIV‐specific cytotoxic T lymphocytes in lymphoid and mucosal tissues during SIV infection. J Leukoc Biol 2013; 93:943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmitz JE, Forman MA, Lifton MA, Concepcion O, Reimann KA Jr, Crumpacker CS et al Expression of the CD8alpha beta‐heterodimer on CD8(+) T lymphocytes in peripheral blood lymphocytes of human immunodeficiency virus– and human immunodeficiency virus+ individuals. Blood 1998; 92:198–206. [PubMed] [Google Scholar]
- 20. Trapani JA, Davis J, Sutton VR, Smyth MJ. Proapoptotic functions of cytotoxic lymphocyte granule constituents in vitro and in vivo . Curr Opin Immunol 2000; 12:323–9. [DOI] [PubMed] [Google Scholar]
- 21. Blanco P, Pitard V, Viallard JF, Taupin JL, Pellegrin JL, Moreau JF. Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum 2005; 52:201–11. [DOI] [PubMed] [Google Scholar]
- 22. Steemers FJ, Chang W, Lee G, Barker DL, Shen R, Gunderson KL. Whole‐genome genotyping with the single‐base extension assay. Nat Methods 2006; 3:31–3. [DOI] [PubMed] [Google Scholar]
- 23. Bacchetti P, Deeks SG, McCune JM. Breaking free of sample size dogma to perform innovative translational research. Sci Transl Med 2011; 3:87ps24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 1995; 57:289–300. [Google Scholar]
- 25. Andreoli C, Bassi A, Gregg EO, Nunziata A, Puntoni R, Corsini E. Effects of cigarette smoking on circulating leukocytes and plasma cytokines in monozygotic twins. Clin Chem Lab Med 2015; 53:57–64. [DOI] [PubMed] [Google Scholar]
- 26. Mellor‐Heineke S, Villanueva J, Jordan MB, Marsh R, Zhang K, Bleesing JJ et al Elevated granzyme B in cytotoxic lymphocytes is a signature of immune activation in hemophagocytic lymphohistiocytosis. Front Immunol 2013; 4:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lerner L, Weiner D, Katz R, Reznick AZ, Pollack S. Increased pro‐inflammatory activity and impairment of human monocyte differentiation induced by in vitro exposure to cigarette smoke. J Physiol Pharmacol 2009; 60(Suppl 5):81–6. [PubMed] [Google Scholar]
- 28. Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol 2006; 16:53–65. [DOI] [PubMed] [Google Scholar]
- 29. Engels EA, Wu X, Gu J, Dong Q, Liu J, Spitz MR. Systematic evaluation of genetic variants in the inflammation pathway and risk of lung cancer. Cancer Res 2007; 67:6520–7. [DOI] [PubMed] [Google Scholar]
- 30. Wang L, Habuchi T, Mitsumori K, Li Z, Kamoto T, Kinoshita H et al Increased risk of prostate cancer associated with AA genotype of cyclin D1 gene A870G polymorphism. Int J Cancer 2003; 103:116–20. [DOI] [PubMed] [Google Scholar]
- 31. Pouwels SD, Heijink IH, Brouwer U, Gras R, den Boef LE, Boezen HM et al Genetic variation associates with susceptibility for cigarette smoke‐induced neutrophilia in mice. Am J Physiol Lung Cell Mol Physiol 2015; 308:L693–709. [DOI] [PubMed] [Google Scholar]


