Summary
The tumour‐like growth of larval Echinococcus multilocularis tissue (causing alveolar echinococcosis, AE) is directly linked to the nature/orientation of the periparasitic host immune‐mediated processes. Parasite‐mediated immune suppression is a hallmark triggering infection outcome in both chronic human and murine AE. So far, little is known about secondary systemic immune effects of this pathogen on other concomitant diseases, e.g. endogenous gut inflammation. We examined the influence of E. multilocularis infection on murine dextran sodium sulphate (DSS) ‐induced colitis. At 3 months after E. multilocularis infection (chronic stage), the mice were challenged with 3% DSS in the drinking water for 5 days plus subsequently with tap water (alone) for another 4 days. After necropsy, fixed tissues/organs were sectioned and stained with haematoxylin & eosin for assessing inflammatory reactions. Cytokine levels were measured by flow cytometry and quantitative RT‐PCR. Colitis severity was assessed (by board‐certified veterinary pathologists) regarding (i) colon length, (ii) weight loss and (iii) a semi‐quantitative score of morphological changes. The histopathological analysis of the colon showed a significant reduction of DSS‐induced gut inflammation by concomitant E. multilocularis infection, which correlated with down‐regulation of T helper type 1 (Th1)/Th17 T‐cell responses in the colon tissue. Echinococcus multilocularis infection markedly reduced the severity of DSS‐induced gut inflammation upon down‐regulation of Th1/Th17 cytokine expression and attenuation of CD11b+ cell activation. In conclusion, E. multilocularis infection remarkably reduces DSS‐induced colitis in mice by attenuating Th1/Th17‐mediated immune reactions.
Keywords: inflammation, parasitic‐helminth, T helper type 1/type 17 cells
Introduction
Alveolar echinococcosis (AE) is a very severe zoonotic helminthic disease in humans, which is fatal if not appropriately treated.1 AE is characterized by chronic and progressively developing hepatic damage caused by the continuously proliferating parasite tissue (metacestode) of Echinococcus multilocularis,2 clinically mimicking a slowly growing and metastasizing liver cancer.3 The type of immune response elicited during E. multilocularis infection predetermines the outcome of infection (resistance versus susceptibility)4 and, if disease occurs, the kinetics of progression of disease.5 In humans, T helper type 2 (Th2) ‐oriented immunity is associated with increased susceptibility to disease, leading to chronic AE, whereas Th1 cell activation has been linked to reduced or abrogated metacestode proliferation, or even protection, which occurs when the parasite becomes aborted, resulting in ‘died‐out’ lesions.2, 3 Experimental murine AE is immunologically characterized by an initial Th1‐oriented response during the early stage of infection (till approximately 1 month after infection), which gradually acquires a greater Th2 emphasis alongside Th1 activation, so becoming a mixed Th1/Th2 response during the chronic phase of AE (2–4 months after infection). This mixed Th1/Th2 profile in immunocompetent mice is associated with the expression of pro‐inflammatory cytokines in the periparasitic granuloma and partial/relative protective immunity (restriction of parasite growth) through fibrosis and necrosis,6 whereas in immunodeficient hosts uncontrolled susceptibility results in an extremely rapid progressing parasite proliferation.7, 8, 9 It has been previously reported that CD4+ CD25+ T regulatory (Treg) cells appeared quantitatively up‐regulated in human AE. It was also shown that this up‐regulation is associated with the blunting of the immune response to specific antigens, and/or to the suppression of the secretion of pro‐inflammatory cytokines, especially through high interleukin‐10 (IL‐10) and transforming growth factor‐β 1 (TGF‐β 1) production in cystic echinococcosis.10 In the experimental mouse model, increased CD4+ CD25+ Treg cells were also observed within the peritoneal cell population. Such results concurred with other findings to demonstrate that E. multilocularis antigens promote T‐cell differentiation into Treg cells11 and so finally induce immune tolerance and anergy towards the parasitic infection.
The ‘hygiene hypothesis’ proposes that the stimulation of the immune system by microbes or microbial products protects from the development of inflammatory diseases. Reflecting the anti‐inflammatory actions of parasite‐derived immunomodulators, an inverse correlation exists between helminthic infections and the occurrence of autoimmune diseases, best documented in the industrially developed world.12 Furthermore, it has become evident that many helminthic infections are associated with reduced allergic reactivities, and most respective studies demonstrated an increased Treg cell activity in infected murine or human hosts.13
Crohn's disease and ulcerative colitis are the main and distinct clinical and pathophysiological entities of inflammatory bowel diseases (IBD).14 Current evidence suggests that aberrant immune reactions against intestinal lumen‐derived microbial antigens can lead to their onset in genetically susceptible individuals.15 During the last 15 years, the therapy of patients with IBD was revolutionized with the development of specific biologicals, notably of tumour necrosis factor (TNF) inhibitors of various types.16, 17 However, approximately 20% of patients do not respond to anti‐TNFs, and over 30% eventually lose response.16, 18, 19 In addition, these biological compounds and the often‐associated chemical immunosuppressants have been shown to be responsible for adverse effects and especially for increasing the risk of infections and malignancies.20, 21
Much experimental data support the hypothesis that helminths are able to modulate colitis. The possible beneficial effect of helminth infections on the development and course of colitis has been investigated in different animal models.22 It was shown that an infection with Schistosoma mansoni cercariae exerted preventive effects on the course of trinitrobenzene sulphonic acid (TNBS) ‐induced colitis in rats and dextran sodium sulphate (DSS) ‐induced colitis in mice.14, 23, 24 Furthermore, S. mansoni eggs prevented mice from developing TNBS‐induced colitis, but did not prevent mice from developing DSS colitis.15, 24 Schistosoma japonicum eggs also exerted preventive effects on TNBS‐induced colitis in mice.16, 17 A previous infection of mice with Trichinella spiralis larvae and Trichinella papuae larvae reduced the severity of DNBS‐induced and DSS‐induced colitis, respectively.18, 19 Infection with Hymenolepis diminuta larvae in DNBS mice had a profound anti‐colitis effect (both prophylactically and as a treatment), which was not seen in semi‐permissive rats.20, 21 In contrast, Heligmosomoides polygyrus bakeri larvae enhanced Citrobacter rodentium‐induced infectious colitis in mice,25, 26 and Hymenolepis diminuta infection caused an exacerbation of oxazolone‐induced colitis in mice.27, 28 Soon after the first promising findings of helminth infections on experimental colitis were published, clinical trials were started to explore whether helminths could alter the course of disease in patients, with the use of the pig whipworm Trichuris suis 29, 30, 31 and larvae of the human hookworm Necator.32 After these promising results, the US Food and Drug Administration requested the development of T. suis ova under good manufacturing practice and appropriate safety testing in order to continue clinical tests.33 Helminthic immunomodulators therefore may be highly attractive molecules with great potential to be used as therapeutic compounds for the treatment of colitis, as exemplified by the recent development of a transgenic probiotic secreting cystatin, an immunomodulator from nematodes, for site‐directed treatment of gut inflammation.34
However, in clinical trials, the use of intestinal helminths/helminth eggs was not as convincing in patients with IBD as in the experimental models;35 this could be due to the schedule of administration and/or the voluntary use of low doses of worms to limit possible adverse effects. Other types of helminths and/or their products could be an alternative. Regarding the influence of cestode infections on immunologically mediated diseases, it has been shown that, in experimental models, Echinococcus granulosus infection reduced airway inflammation36 and also alleviated the severity of experimental DSS‐induced colitis.37 However, the influence of E. multilocularis, which generates a more complex and sustained immunoregulatory profile than E. granulosus, has never been studied, and the role of systemic T‐cell regulation, its related cytokine production, and antigen‐presenting process have not been clarified.
The major aims of the present study were: (i) to address whether infection by the cestode E. multilocularis could modulate the phenotype of the well‐known DSS‐induced colitis; and (ii) to further explore possible mechanisms that would explain the immunotherapeutic properties of E. multilocularis infection acting on DSS‐induced colitis. To achieve these goals, we investigated the differential levels of pathological changes in the colon and its related pro‐inflammatory/inflammatory cytokines, Th1/Th2 and Treg/Th17‐related cytokine expression levels in both spleen and colon, together with CD11b+ CD11c+ cell activation in the spleen, in E. multilocularis‐infected mice subsequently challenged with a DSS‐induced colitis.
Materials and methods
Ethical statements
The animal study was performed in strict accordance with the requirements of the Swiss Guidelines for the Care and Use of Laboratory Animals. The protocol was approved by the governmental Commission for Animal Experimentation of the Canton of Bern (approval nos BE103/11 and BE112/14).
Experimental design, parasite sampling and histological examination
Mice
Female C57BL/6 mice, all aged between 8 and 10 weeks, were bred and housed under specific pathogen‐free conditions according to recommendations of the Federation of European Laboratory Animal Science Association, and additionally monitored by daily inspection, including the assessment of the appearance of health status, putative weight loss or gain during the whole course of the experiment. Facility supervision for infectious diseases specifically included also the demonstration of absence of Helicobacter, Citrobacter, norovirus, rotavirus and Oxyuris. All experiments with animals were performed within a laminar flow safety enclosure. The mice were randomly divided into four groups with six mice in each group comprising: (i) negative control group (NC); (ii) E. multilocularis infection group (Em); (iii) DSS‐induced colitis group (DSS); (iv) E. multilocularis infection plus DSS‐induced colitis group (Em+DSS).
Parasite and experimental infection
Echinococcus multilocularis (H95) was isolated and maintained by serial passages (vegetative transfer) in C57BL/6 mice as previously described.38 In order to prepare the infection material for mice, metacestode tissue was obtained from previously infected mice by aseptic removal from the peritoneal cavity. After grinding the tissue through a sterile 50‐μm sieve, approximately 100 freshly prepared vesicular cysts were suspended in 100 μl RPMI‐1640 (Gibco, Basel, Switzerland) and injected intraperitoneally.
DSS‐induced colitis
At 3 months after E. multilocularis infection, or in non‐infected mice used as controls, experimental acute colitis was induced by administration of 3% 36 000–50 000 MW DSS (MP Biomedical, Solon, OH) in the drinking water for 5 days, followed by 4 days of regular tap water, animals were finally killed at day 9.
Collection of host tissue
Mice were killed by Isofluran anaesthesia followed by CO2 euthanasia. Blood was collected by cardiac puncture, and serum samples were stored at −80°.
Colonic cells
Approximately 1·5‐cm lengths of the anterior colons were opened longitudinally and cut into small pieces. The epithelium was removed by incubation in Hanks' balanced salt solution/HEPES containing 5% horse serum, 5 mm EDTA and 2 mm dithiothreitol at 37° for 30 min under magnetic stirring. Colonic cells were obtained by subsequent digestion with 200 U/ml collagenase (Type IV; Sigma‐Aldrich, St Louis, MO, USA) and 50 U/ml DNase (Type I, grade II; Roche, Basel, Switzerland) for 45 min, then filtered through a 40‐μm cell strainer, counted using Trypan Blue staining and further characterized by quantitative RT‐PCR.
Histopathology and histopathological grading
Sternum, thymus, part of the spleen, mesenteric lymph nodes, and small and large intestine from each mouse were fixed in 10% neutral‐buffered formalin for 24 hr and embedded in paraffin. Blocks were sectioned and slides were stained with haematoxylin & eosin. On haematoxylin & eosin‐stained sections, morphological changes of all tissues were recorded and a semi‐quantitative grading was used to score bone marrow, spleen and colon as listed in Table 1. The disease activity index (DAI) for the DSS‐induced colitis scoring system is summarized in Table 2. The macroscopic and microscopic evaluations were performed in a blinded fashion by European‐board‐certified veterinary pathologists.
Table 1.
Histopathological scores to quantify changes in different organ systems
| Organ | Criterion | Score | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Bone marrow | Ratio myelopoiesis/erythropoiesis | 3–4 : 1 | 5 : 1 | 6–10 : 1 | 11–25 : 1 | > 25 : 1 |
| Spleen | % increase in extramedullary haematopoiesis | No increase | < 25 | 26–50 | 51–75 | 76–100 |
| Colon | Inflammation | No pathological change | Mild infiltrates of inflammatory cells in the lamina propria without erosion/ulceration | Marked inflammation and deep ulceration with crypt abscesses | Additional epithelial dysplasia and/or neoplasia | – |
Table 2.
Disease activity index (DAI) for dextran sodium sulphate (DSS) ‐induced colitis
| Weight loss | Score | Stool blood | Score | Stool consistency | Score |
|---|---|---|---|---|---|
| < 1% | 0 | Absence | 0 | Formed and hard | 0 |
| 1–5% | 1 | Formed but soft | 1 | ||
| 5–10% | 2 | Presence | 2 | Loose stools | 2 |
| 10–15% | 3 | Mild diarrhoea (watery) | 3 |
Cell preparations
Spleen cells were collected by grinding individual organs separately with 5 ml RPMI‐1640. Cells were subsequently washed twice and resuspended in RPMI‐1640 (Gibco). Macrophages were removed from each group of mice by plastic adhesion after incubation of spleen cell suspension in 15 ml RPMI‐1640 + 20% fetal calf serum in a Petri dish for 2 hr at 37°, in an atmosphere containing 5% CO2. Subsequent to incubation, non‐adherent cells were separated from macrophage‐enriched adherent cells, and this new cell suspension was used for FACS analyses.
Flow cytometry
Aliquots of 105 cells/100 μl of staining buffer per well were incubated each with 1 μg of purified anti‐CD16/CD32 for 20 min in the dark to block non‐specific binding of antibodies to the FcγIII and FcγII receptors. Subsequently, these cells were separately stained with the following surface markers for 15 min with 1 μg of primary antibodies: allophycocyanin‐labelled anti‐CD4, anti‐CD80, anti‐CD86; phycoerythrin‐labelled anti‐CD11b, anti‐CD11c. All antibodies were from eBioscience (San Diego, CA). For intracellular staining, peritoneal exudate cells (PECs) or spleen cells were first incubated with Inside Fix (Miltenyi Biotec, Bergisch Gladbach, Germany) for 20 min at room temperature and subsequently stained with phycoerythrin‐labelled anti‐interferon‐γ (IFN‐γ), anti‐IL‐4, anti‐IL‐17A, anti‐IL‐10 and anti‐Foxp3 (eBioscience) in Inside Perm (Miltenyi Biotec) for 15 min in the dark. Corresponding fluorochrome‐labelled isotype control antibodies were used for staining controls. Cells, resuspended in 300 μl of buffer (0·15 m NaCl, 1 mm NaH2PO4 H2O, 10 mm Na2HPO4 2H2O and 3 mm NaN3) were analysed in a flow cytometer (Becton Dickinson, Heidelberg, Germany) using the corresponding cell quest software.
Cytokine gene‐expression analyses by quantitative RT‐PCR
Total RNA was extracted from spleen or colon tissue previously put into TRIzol (Invitrogen) according to the manufacturer's instructions. The cDNA was synthesized using the Omniscript Reverse Transcription kit (Qiagen, Hilden, Germany). SYBR‐Green Mix‐based quantitative RT‐PCR was carried out on a Rotor‐Gene 6000 quantitative PCR detection system (Corbett, Sydney, Australia) with the FastStart Essential DNA Green Master (Roche) following the manufacturer's instructions. PCR cycling was performed in triplicates in final volumes of 20 μl containing 2 μl cDNA and 10 pm of each primer (Cycle scheme: initial denaturation at 95° for 15 min, 45 cycles of 95° for 15 seconds, 55° for 30 seconds and 72° for 30 seconds). Fluorescence was measured in every cycle, and a melting curve was analysed after the PCR by increasing the temperature from 55° to 95° in 0·5° increments. The primers used were described earlier,39 and cytokine mRNA levels were quantified relative to the mRNA level of the housekeeping gene β‐actin. Respective mean values from triplicate determinations from six individual mice in each group were taken for the calculation of relative cytokine mRNA levels in relation to β‐actin mRNA levels.
Statistical analyses
All data were analysed by spss 17.0 (IBM Corporation, Armonk, New York, USA). The results are presented as means ± SD. Normality of data was assessed by D'Agostino & Pearson and Shapiro–Wilk test. For normally distributed groups of data, one‐way analysis of variance followed by Bonferroni's post‐test or unpaired two‐tail Student's t‐test were used to compare the differences between groups. Significance was defined as P < 0·05 for all tests, except those subsequently corrected by Bonferroni.
Results
Echinococcus multilocularis infection reduces severity of DSS‐induced colitis in mice
To evaluate the benefits of E. multilocularis infection in abrogating or down‐regulating colitis, we subjected mice to DSS‐induced colitis 3 months after E. multilocularis infection. With administration of 3% DSS, the Em + DSS groups (E. multilocularis infection combined with challenge with 3% DSS) exhibited less severe symptoms including body weight losses, DAI scores and colon length changes compared with the DSS group (challenge with 3% DSS) (Fig. 1). The body weight in the DSS group (acute colitis model) decreased significantly when compared with the negative control (NC) groups, whereas body weight loss was much lower in the Em+DSS group (Fig. 1a). The DAI scores were significantly lower in the Em+DSS group when compared with those of the DSS group (Fig. 1b). Similarly, the average colon length of the DSS group was shortened by approximately 24% when compared with that of the NC groups, whereas it was only reduced by about 4% in mice of the Em+DSS group (Fig. 1c). These results suggest that E. multilocularis infection reduces the severity of colitis.
Figure 1.
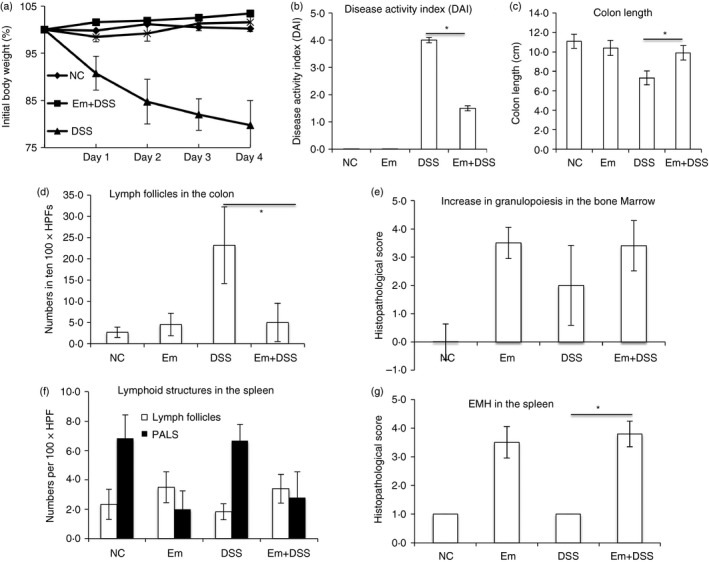
Mice chronically infected with Echinococcus multilocularis are protected from dextran sodium sulphate (DSS) ‐induced colitis. (a) Weight loss relative to the initial body weight. Mean values of n = 15 to n = 18 mice analysed per group are shown with error bars indicating the SD. At 3 months after E. multilocularis infection, experimental colitis was induced by administration of 3% DSS in the drinking water for 5 days followed by 4 days of regular tap water. Day 1, 2, 3, 4 indicates the days after tap water. (b) Disease activity index (DAI) changes among groups after 9 days 3% DSS treatment at the end time‐point. Data represents the mean ± SD (n = 15 to n = 18). (c) Colon lengths were determined in individual mice. Data show mean values for each group of mice. (d–g) Individual parameters of histopathological scoring. (d) Lymph follicles in the colon. (e) Increase in granulopoiesis in the bone marrow. (f) Lymphoid structures in the spleen. (g) Extramedullary haematopoiesis in the spleen. Histopathological scores were determined for individual mice by a pathologist according to parameters defined in the Materials and methods section. Columns show mean values for n = 5 or n = 6 mice analysed per group and error bars indicate the SD. One representative experiment out of three independent experiments is shown. HPF, high‐power field; EMH, extramedullary haematopoiesis. *P < 0·05.
Histopathological effects of E. multilocularis infection on DSS‐induced colitis in mice
Significant morphological changes were recorded in the bone marrow and lymphoid organs of the Em group and of the Em+DSS group when compared with the NC and DSS groups (Fig. 1d–g). In detail, the ratio of granulocytic to erythrocytic precursor cells in the bone marrow was scored 0 (no pathological change) in the NC group, 2 (mild increase) in the DSS group and grade 3 (moderate increase) in groups with Em or Em+DSS, respectively (mean values) (Fig. 1e). Reduced numbers of periarteriolar lymphoid sheaths were noted in the Em and Em+DSS groups in comparison to the NC and DSS groups (Fig. 1f). Additionally, a significant increase in extramedullary haematopoiesis was noted in the spleen of all animals from the Em and Em+DSS groups compared with the NC and DSS groups (Fig. 1g), corresponding to a marked increase in organ weight (data not shown). The large intestine was significantly altered in all animals from the DSS group, displaying multifocal epithelial necrosis and mucosal ulceration, loss of goblet cells, loss and/or proliferation of crypts, crypt abscesses and marked infiltration of the lamina propria by macrophages, neutrophils and lymphocytes, and finally fibrosis (grade 2, Fig. 2). Besides these findings, five animals also revealed epithelial dysplasia/proliferation (grade 3, Fig. 2). In comparison, the large intestine from animals of groups Em and Em+DSS presented fewer lymph follicles in the mucosa and/or submucosa (grade 1, Fig. 2), and no pathological changes were noted in the NC group (grade 0) (Figs 1d and 2).
Figure 2.
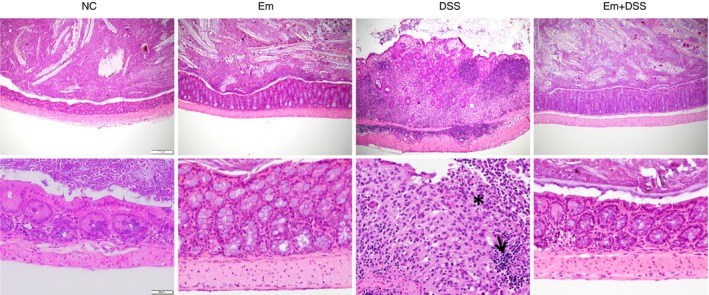
Representative histopathological findings in the colon [haematoxylin & eosin (H&E) staining]. Representative histopathological changes of the colon [H&E stain, magnification 100× (first row) and 400× (second row)]. Distension of mucosa and submucosa by infiltration of inflammatory cells, fibrosis, loss and proliferation of crypts in dextran sodium sulphate (DSS) group compared with a regular colon without pathological changes in NC, Em and Em+DSS groups. Higher magnification reveals ulceration of the mucosal epithelium with infiltration of neutrophils (asterisk) and lymphocytes in the depth of the lamina propria mucosae (arrow). [Colour figure can be viewed at http://wileyonlinelibrary.com]
Echinococcus multilocularis infection reduces DSS‐induced inflammatory/pro‐inflammatory cytokine expression in the colons of mice
Cytokines are principal mediators of the innate and adaptive arms of the immune responses in mucosal inflammation. To analyse the influence of the cytokine patterns in DSS‐induced colitis mice and the effect of E. multilocularis infection on DSS‐induced colitis pathogenesis, the cytokine profile in the colon of the mice was measured by quantitative RT‐PCR. In general, a marked up‐regulation in the pro‐inflammatory/Th1 cytokine (IL‐12, IFN‐γ, IL‐1β, IL‐6, TNF‐α) and Th17 cytokine (IL‐17A) mRNA expression level was observed in the DSS group (Fig. 3). Interestingly, a significant decrease of those cytokine expression levels (IL‐12, IFN‐γ, IL‐6, TNF‐α, IL‐17A,) was noted in the colon of the Em+DSS group, when compared with the DSS group. Interleukin‐1β, responsible for inducing the inflammatory cascade, was not differentially expressed in the colon of the Em+DSS group, when compared with DSS group (Fig. 3). There was no difference in IL‐4 mRNA level between the colons of the Em+DSS and the DSS groups (data not shown).
Figure 3.

Colonic inflammation was attenuated in mice with alveolar echinococcosis (AE) regarding dextran sodium sulphate (DSS‐induced colitis. Inflammatory/inhibitory cytokine gene expression levels in the colon of mice (measured by quantitative RT‐PCR). AU, arbitrary units. β‐Actin was used as housekeeping gene. Data represent mean ± SD of three independent experiments of a total of 15–18 mice in each group (five or six mice per group in each independent experiment). Comparison between groups was performed using a one‐way analysis of variance with Bonferroni's multiple comparison post‐test for statistical analysis. *P < 0·004.
Echinococcus multilocularis infection changed immune direction in the spleen from mice with DSS‐induced colitis
To further explore the effect of E. multilocularis infection on the systemic T‐cell immune response in DSS‐induced colitis in mice, T helper‐related cytokines were comparatively assessed in all the groups (Em, DSS, Em+DSS, and in their respective NC controls). Flow cytometry revealed that Th cells from the Em group were oriented towards the Treg/Th2 pathway at the chronic stage of E. multilocularis infection (Em group), with high expression levels of IL‐10, Foxp3 and IL‐4 (Fig. 4), whereas DSS induced significantly increased expression levels of IFN‐γ when compared with the NC group. CD4+ T cells from Em+DSS mice were oriented towards a Treg pathway, with high expression levels of IL‐10 and Foxp3 in the spleen. Conversely, a lower IFN‐γ expression level was found in the Em+DSS group when compared with the DSS group (Fig. 4).
Figure 4.
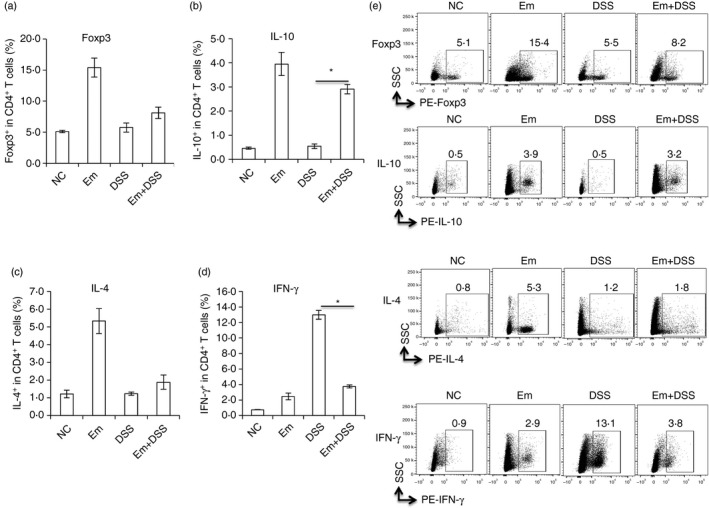
Dextran sodium sulphate (DSS) ‐induced colitis changed the splenic immune response in mice with alveolar echinococcosis (AE). Cells were firstly gated on lymphocytes by size and singularity followed by DAPI exclusion to identify live cells for further analysis. Live lymphocytes were gated on CD4 expression to first identify CD4+ T cells. Then phycoerythrin (PE) ‐labelled Foxp3, interleukin‐10 (IL‐10), IL‐4 and interferon‐γ (IFN‐γ) expression was identified as Foxp3+, IL‐10+, IL‐4+ and IFN‐γ + T cells within CD4+ T cells. (a) Frequency of Foxp3+ T cells within CD4+ T cells in spleen cells from each group. (b) Frequency of CD4+ IL‐10+ T cells in spleen cells from each group. (c) Frequency of IL‐4+ T cells within CD4+ T cells in spleen cells from each group. (d) Frequency of IFN‐γ + T cells within CD4+ T cells in spleen cells from each group. The corresponding isotype control antibodies were identically labelled as staining controls. Data represent mean ± SD of three independent experiments of a total of 15–18 mice in each group (five or six mice per group in each independent experiment). (e) Representative images of Foxp3+, IL‐10+, IL‐4+, IFN‐γ + T cells within CD4+ T cells in spleen cells from each group. Comparison between groups was performed using a one‐way analysis of variance with Bonferroni's multiple comparison post‐test for statistical analysis. *P < 0·006. NS, no significance.
Echinococcus multilocularis infection reduces antigen‐presenting cell activation and co‐stimulation in the spleen from mice with DSS‐induced colitis
To further explore the effect of E. multilocularis infection on antigen‐presenting cell activation and co‐stimulation signals for T cells, we measured CD80, CD86 and CD40 in CD11b+ and CD11c+ cells. Flow cytometry showed that the frequency of maturation markers CD80 and CD86 was higher in CD11c+ cells (Fig. 5a–d), and the frequency of T‐cell co‐stimulation marker CD40 was lower in CD11c+ cells in the Em group than in the NC group (Fig. 6a,b). However, there was no difference either in CD80 and CD86 frequency (Fig. 5a–d), or in CD40 frequency among CD11b+ cells in these groups (Fig. 6a,b). However, DSS challenge led to a significantly increased expression level of CD80, CD86 and CD40 in CD11b+ cells when compared with the NC group (Figs 5 and 6). Conversely, in the Em+DSS group, CD80, CD86 and CD40 levels in CD11b+ cells were significantly lower when compared with the DSS group (Figs 5 and 6). However, there was no difference in the CD80, CD86 and CD40 frequencies among CD11c+ cells between groups DSS and Em+DSS (Figs 5 and 6).
Figure 5.
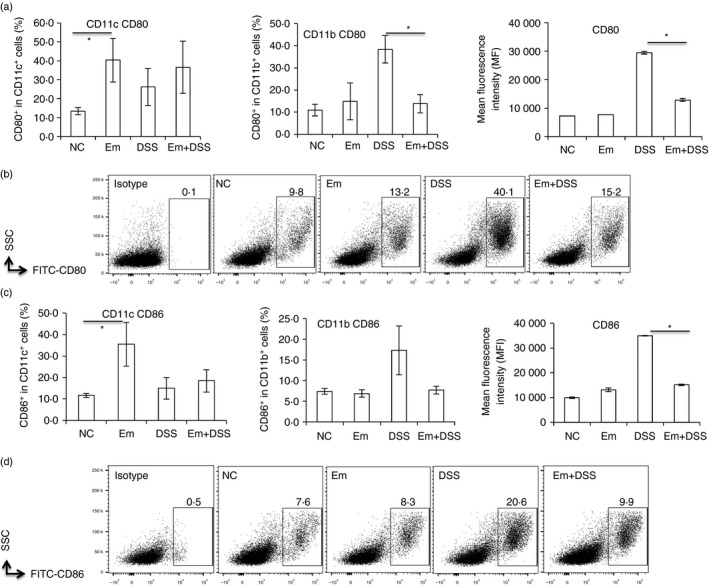
Antigen‐presenting cell (APC) activation was attenuated in the spleen of mice with alveolar echinococcosis (AE) upon dextran sodium sulphate (DSS) ‐induced colitis. Cells were first gated on size and singularity followed by DAPI exclusion to identify live cells for further analysis. Live cells were gated on CD11b or CD11c expression to first identify CD11b+ and CD11c+ cells. Then phycoerythrin‐labelled CD80, CD86 expression was identified as CD80, CD86 frequency within CD11c+ or CD11c+ cells. (a) CD80 frequency within CD11c+ and CD11b+ cells, CD80 mean fluorescence intensity (MFI) within CD11b+ cells in spleen cells from each group. (b) Representative images of CD80+ within CD11b+ cells in spleen cells from each group. (c) CD86 frequency within CD11c+ and CD11b+ cells, CD86 MFI within CD11b+ cells in spleen cells from each group. (d) Representative images of CD86+ within CD11b+ cells in spleen cells from each group. Comparison between groups was performed using a one‐way analysis of variance with Bonferroni's multiple comparison post‐test for statistical analysis. *P < 0·004.
Figure 6.
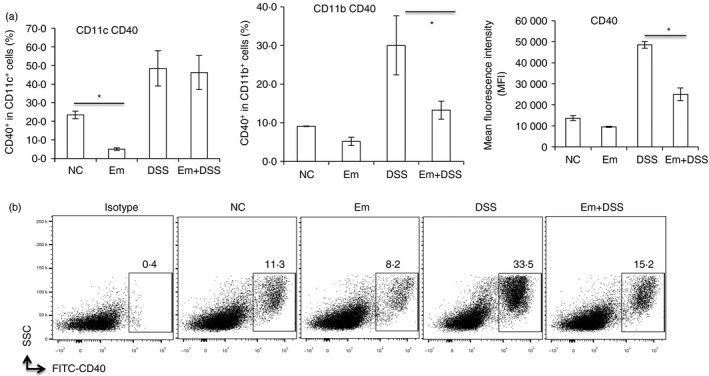
Co‐stimulation was attenuated in the spleen of mice with alveolar echinococcosis (AE) upon dextran sodium sulphate (DSS) ‐induced colitis. Cells were first gated on size and singularity followed by DAPI exclusion to identify live cells for further analysis. Live cells were gated on CD11b or CD11c expression to first identify CD11b+ and CD11c+ cells. Then phycoerythrin‐labelled CD40 expression was identified as CD40 frequency within CD11c+ or CD11c+ cells. (a) CD40 frequency within CD11c+ and CD11b+ cells, CD40 mean fluorescence intensity (MFI) in CD11b+ cells in spleen cells from each group. (b) Representative images of CD40+ within CD11b+ cells in spleen cells from each group. Comparison between groups was performed using a one‐way analysis of variance with Bonferroni's multiple comparison post‐test for statistical analysis. *P < 0·004.
Discussion
Inflammatory bowel diseases are complex diseases caused by a deregulated immune response to intestinal luminal antigens,15 and have increased at an alarming rate in the past two decades.40, 41, 42, 43, 44, 45 Symptoms of IBD are the result of complex interactions among genetic and environmental factors, and the immune response.46, 47 Nowadays, therapy with immunomodulating biological agents is considered the mainstay for moderate‐to‐severe Crohn's disease and severe ulcerative colitis.16 The anti‐TNFs infliximab and adalimumab have been extensively used and shown to induce clinical and endoscopic remission in both diseases.16 Nevertheless, some patients do not respond initially or, more frequently, lose response. Recently, other biological agents targeting other immunological pathways were approved for the treatment of IBD, such as vedolizumab, an α 4 β 7 anti‐integrin that impairs gut homing of lymphocytes in Crohn's disease and ulcerative colitis,37 and ustekinumab, a bivalent anti‐IL‐12–IL‐23 monoclonal antibody for Crohn's disease.38 Other promising therapeutic biologicals targeting specific cellular metabolic pathways are currently in phase III stage of research, such as orally administered janus kinase inhibitors (tofacitinib) in ulcerative colitis,39 or a SMAD7 oral anti‐sense oligonucleotide (Mongersen) that down‐regulates inflammatory cytokine production by restoring TGF‐β1/Smad signalling in Crohn's disease .40 However, altogether these drugs have potential deleterious and sometimes unpredictable paradoxical effects on the immune system and new therapeutic approaches are eagerly awaited. Faecal transplantation has shown promising results in ulcerative colitis and opens a new ‘pathophysiological era’ in IBD treatment management but its long‐term efficacy and modalities of administration are debated.41 Therefore, it is still important to look for other therapeutic pathways against IBD, and systemic immune modulation induced by parasite‐derived compounds is one of these innovative pathways.
Epidemiological observations showed an association between (i) the decrease in infectious diseases, including parasite infections, use of antibiotics, vaccinations and a general improvement in food, water and housing sanitary conditions; and (ii) an increase in the incidence of allergic diseases, and also autoimmune and chronic inflammatory disorders.48 This finding forms the basis of the continually revisited ‘hygiene hypothesis’.43 Within this hypothesis, intestinal worms could play a role by modulating the composition/function of the gut microbiota, which is fundamental to the ‘education’ of the immune system after birth; all types of helminths, including their larval stage in organs other than the gut, might also play a role in the systemic immune balance that prevents abnormal inflammation.43 Several experimental studies using different animal models of colitis have shown the ability of parasitic worms to attenuate intestinal inflammation,28, 29, 30, 31, 32, 33 e.g. T. suis exerts a therapeutic effect in patients with ulcerative colitis and Crohn's disease.29, 30, 31, 49 Although a large body of evidence indicates that regulatory mechanisms that reduce IBD severity may be triggered by intestinal helminths, and especially nematode infections, far less is known about larval cestodes, which cannot exert a direct effect on the gut. Previous experiments using a mouse model of cystic echinococcosis (E. granulosus infection) have suggested that concomitant infection with E. granulosus improved the clinical score, ameliorated the DAI, and prevented the shortening of the colon in experimental DSS‐induced colitis;37 such improvement was associated with a reduced nitric oxide and TNF‐α production in the plasma of experimental E. granulosus‐infected mice with DSS‐induced colitis and decreased inducible nitric oxide synthase and nuclear factor‐κB expression in colonic tissue. It is interesting to note that a crude extract of the laminated layer from an E. granulosus cyst was as able as active E. granulosus infection to attenuate mucosal intestinal damage and inflammatory responses in DSS‐induced colitis in mice;50 in these experiments using the laminated layer, the authors also focused on macrophage‐dependent mechanisms, and confirmed the reduced nitric oxide production and inducible nitric oxide synthase expression in the colon as well as reduced TNF‐α production. However, no complementary studies of the T‐cell‐related immunoregulatory mechanisms were performed.
Chronic infection with E. multilocularis is characterized by a marked parasite‐induced immune tolerance status including a down‐regulation of periparasitic and systemic immunity against the metacestode both in humans51 and in experimentally infected mice.52 Mechanisms of the immune tolerance involved in Echinococcus spp. infection has received more attention in AE, caused by E. multilocularis, than in cystic echinococcosis, caused by E. granulosus, because it is chronically sustained and because the rodents are the natural intermediate host of the parasite, hence making the mouse experimental model highly relevant.2, 3, 4, 5 We therefore hypothesized that the negative immune regulation observed when E. multilocularis infection is fully established would also down‐regulate DSS‐induced colon inflammation, and so prevent or repair DSS‐induced damage; this model would also allow us to explore the T‐cell‐dependent immunoregulatory mechanisms, which have been well delineated in the chronic infection by E. multilocularis in mice. The results of our first experiments fully confirm our hypothesis; taken together, our observations suggest that a pre–established E. multilocularis infection protects mice from DSS‐induced colitis. DSS‐exposed E. multilocularis‐infected mice exhibited significantly less severe colitis than those animals without E. multilocularis infection: colonic improvement included maintained colorectal lengths, and microscopically normal mucosal structures with a nearly normal number and size of goblet cells secreting mucus, in marked contrast to non‐infected animals with DSS‐induced colitis, suggesting that E. multilocularis, like other helminths, can help in preserving/restoring these cells.50 Goblet cells are involved in regulating both the mucosal barrier and the relative composition of the luminal microbiota by mucin production.50 The production of mucus by these cells could limit bacterial access to epithelial cells and prevent chronic inflammation.50
Polymorphonuclear leucocytes such as neutrophils play a critical role in the maintenance of intestinal homeostasis. They display defence mechanisms to eliminate microbes that have translocated across the single layer of mucosal epithelial cells, which form a critical barrier between the gut lumen and the underlying tissue. During the inflammatory response, neutrophils also contribute to the recruitment of other immune cells and facilitate mucosal healing by releasing mediators necessary for the resolution of inflammation. Neutrophil infiltration is a key event in inflammation of the colon.53 Here we found that E. multilocularis infection during DSS‐induced colitis generated a greater infiltration by monocytes than neutrophils, the latter being the main cell type detected in the absence of helminth infection. Inflammatory monocytes accumulate in response to infection or tissue injury, and in most cases they help to clear pathogens.54, 55 However, in IBD, the recruitment of inflammatory monocytes into damaged tissue frequently worsens the inflammation.56 Macrophages from E. multilocularis‐infected mice have been shown to exhibit a reduced ability to present a conventional antigen, such as ovalbumin to specific responder lymph node T cells, when compared with normal macrophages from non‐infected mice; this triggers an unresponsiveness of T cells, which in turn leads to the suppression of their clonal expansion during the chronic phase of E. multilocularis infection.57 High periparasitic nitric oxide production by peritoneal exudate cells, mainly macrophages, were also shown to contribute to E. multilocularis‐induced immunosuppression.58, 59 Functionally modified macrophages might therefore play a critical role in avoiding colonic inflammation and perhaps inhibiting recruitment of inflammatory cells into the lamina propria of the colon; the relevance of this hypothesis is also supported by the results obtained in the studies with E. granulosus infection.23, 49
CD4+ T cells and their associated cytokines play an important role in the development of DSS‐induced experimental colitis;60 however, their role was not studied in the E. granulosus model.47 In E. multilocularis infection, in resistant hosts, Th1 cytokines induce a protective immunity that involves IFN‐α and IL‐1261 as initiating cytokines, and IFN‐γ and TNF‐α as effector cytokines.7, 59 The key‐role for TNF‐α in the protection of mice against E. multilocularis infection was demonstrated by the extreme susceptibility of TNF‐α‐deficient (knockout) mice to the infection.62 The down‐regulation of TNF‐α observed both in the E. granulosus 23 and our E. multilocularis model, associated with reduced intensity of the DCC‐induced colitis, fits well with such observations and with the pathophysiology of experimental colitis, as well as with the spectacular effect of anti‐TNF‐α biological agents in patients with Crohn's disease. In patients with AE and in experimental susceptible mice, the sustained inhibition of Th1‐type effector cells as well as pro‐inflammatory and Th1‐type cytokines at the chronic stage of E. multilocularis infection is associated with up‐regulation of CD4+ CD25+ Treg cells and of Th2/Treg‐cell‐associated cytokines and regulatory factors.7, 60 Our results in the non‐DSS‐challenged E. multilocularis‐infected mice confirmed these observations; conversely, our observations in DSS‐challenged mice confirmed the up‐regulation of Th1‐type cytokine mRNA in acute DSS‐induced colitis, including IL‐12, IFN‐γ, IL‐1β, IL‐6, TNF‐α and the Th17 class cytokine IL‐17A. In addition to TNF‐α inhibition, we could show that E. multilocularis infection markedly reduced the expression level of Th1/Th17‐related cytokines, including IFN‐γ, IL‐6 and IL‐17A in mice with associated experimental colitis. Our results showing that CD4+ T cells in the spleen from Em+DSS mice are oriented towards a Treg cell pathway, with low IFN‐γ and high IL‐10 and FoxP3 expression, strongly suggest that the clinical and histopathological effects on colitis are actually mediated through changes in the systemic immune profile of the mice, as was suggested in experiments with other helminthic infections.62 The reduced levels of the co‐stimulation molecules CD80, CD86 and CD40 that we observed on CD11b+ dendritic cells in E. multilocularis infected mice with colitis compared with the non‐infected mice similarly challenged with DSS, could be involved, at least partially, in skewing the immune profile. Our knowledge of TGF‐β and TGF‐β/Smad signalling in E. multilocularis infection63 and of the efficacy of new biological agents that interfere with Smad signalling in Crohn's disease16 suggests that further studies on the mechanisms of E. multilocularis‐mediated protection against DSS‐induced colitis should focus on the TGF‐β/Smad pathway.
Even more than nematode worms such as Trichuris spp., which have a potential for pathogenicity,64 Echinococcus spp. metacestodes are associated with potentially severe diseases and concomitant infection cannot represent an alternative to the currently used therapies in IBD. Cestode‐derived compounds, and especially those derived from E. multilocularis, might be more appropriate than real infection with nematodes for subsequent therapeutic application in human patients with IBD. Parasite metabolites and/or secreted or excreted parasite molecules appear as crucial elements in the immune modulation at the host–parasite interface to promote parasite survival.6 For E. multilocularis, immune modulatory effects have been mainly attributed to E. multilocularis vesicular fluid,13 and also to the laminated layer, which represents a highly immunogenic mucopolysaccharide interface between the germinal layer of the parasite and adjacent host tissue.3 Experiments using extracts of the non‐infectious laminated layer of E. granulosus are encouraging.49 At this stage, identification and purification of the bioactive molecule(s) responsible for the effective anti‐inflammatory and therapeutic effects of Echinococcus spp. in colitis as well as exploration of their safety aspects will therefore be the next steps towards promising therapeutic agents.
Disclosures
The authors declare no commercial or financial conflict of interest.
Acknowledgement
This work was funded by Swiss National Science Foundation (grant no. 160108/1).
References
- 1. Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis 2010; 4:e722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vuitton DA. The ambiguous role of immunity in echinococcosis: protection of the host or of the parasite? Acta Trop 2003; 85:119–32. [DOI] [PubMed] [Google Scholar]
- 3. Vuitton DA, Zhang SL, Yang Y, Godot V, Beurton I, Mantion G et al Survival strategy of Echinococcus multilocularis in the human host. Parasitol Int 2006; 55(Suppl):S51–5. [DOI] [PubMed] [Google Scholar]
- 4. Gottstein B, Wang J, Boubaker G, Marinova I, Spiliotis M, Muller N et al Susceptibility versus resistance in alveolar echinococcosis (larval infection with Echinococcus multilocularis). Vet Parasitol 2015; 213:103–9. [DOI] [PubMed] [Google Scholar]
- 5. Wang J, Gottstein B. Immunoregulation in larval Echinococcus multilocularis infection. Parasite Immunol 2016; 38:182–92. [DOI] [PubMed] [Google Scholar]
- 6. Wang J, Lin R, Zhang W, Li L, Gottstein B, Blagosklonov O et al Transcriptional profiles of cytokine/chemokine factors of immune cell‐homing to the parasitic lesions: a comprehensive one‐year course study in the liver of E. multilocularis‐infected mice. PLoS One 2014; 9:e91638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sailer M, Soelder B, Allerberger F, Zaknun D, Feichtinger H, Gottstein B. Alveolar echinococcosis of the liver in a six‐year‐old girl with acquired immunodeficiency syndrome. J Pediatr 1997; 130:320–3. [DOI] [PubMed] [Google Scholar]
- 8. Dai WJ, Waldvogel A, Siles‐Lucas M, Gottstein B. Echinococcus multilocularis proliferation in mice and respective parasite 14‐3‐3 gene expression is mainly controlled by an αβ CD4 T‐cell‐mediated immune response. Immunology 2004; 112:481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dai WJ, Waldvogel A, Jungi T, Stettler M, Gottstein B. Inducible nitric oxide synthase deficiency in mice increases resistance to chronic infection with Echinococcus multilocularis . Immunology 2003; 108:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tuxun T, Wang JH, Lin RY, Shan JY, Tai QW, Li T et al Th17/Treg imbalance in patients with liver cystic echinococcosis. Parasite Immunol 2012; 34:520–7. [DOI] [PubMed] [Google Scholar]
- 11. Nono JK, Lutz MB, Brehm K. EmTIP, a T‐Cell immunomodulatory protein secreted by the tapeworm Echinococcus multilocularis is important for early metacestode development. PLoS Negl Trop Dis 2014; 8:e2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pineda MA, Al‐Riyami L, Harnett W, Harnett MM. Lessons from helminth infections: ES‐62 highlights new interventional approaches in rheumatoid arthritis. Clin Exp Immunol 2014; 177:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maizels RM, McSorley HJ, Smyth DJ. Helminths in the hygiene hypothesis: sooner or later? Clin Exp Immunol 2014; 177:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bodammer P, Waitz G, Loebermann M, Holtfreter MC, Maletzki C, Krueger MR et al Schistosoma mansoni infection but not egg antigen promotes recovery from colitis in outbred NMRI mice. Dig Dis Sci 2011; 56:70–8. [DOI] [PubMed] [Google Scholar]
- 15. Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF Jr et al Exposure to schistosome eggs protects mice from TNBS‐induced colitis. Am J Physiol Gastrointest Liver Physiol 2003; 284:G385–91. [DOI] [PubMed] [Google Scholar]
- 16. Zhao Y, Zhang S, Jiang L, Jiang J, Liu H. Preventive effects of Schistosoma japonicum ova on trinitrobenzenesulfonic acid‐induced colitis and bacterial translocation in mice. J Gastroenterol Hepatol 2009; 24:1775–80. [DOI] [PubMed] [Google Scholar]
- 17. Xia CM, Zhao Y, Jiang L, Jiang J, Zhang SC. Schistosoma japonicum ova maintains epithelial barrier function during experimental colitis. World J Gastroenterol 2011; 17:4810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khan WI, Blennerhasset PA, Varghese AK, Chowdhury SK, Omsted P, Deng Y et al Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun 2002; 70:5931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adisakwattana P, Nuamtanong S, Kusolsuk T, Chairoj M, Yenchitsomanas PT, Chaisri U. Non‐encapsulated Trichinella spp., T. papuae, diminishes severity of DSS‐induced colitis in mice. Asian Pac J Allergy Immunol 2013; 31:106–14. [DOI] [PubMed] [Google Scholar]
- 20. Hunter MM, Wang A, Hirota CL, McKay DM. Neutralizing anti‐IL‐10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. J Immunol 2005; 174:7368–75. [DOI] [PubMed] [Google Scholar]
- 21. Melon A, Wang A, Phan V, McKay DM. Infection with Hymenolepis diminuta is more effective than daily corticosteroids in blocking chemically induced colitis in mice. J Biomed Biotechnol 2010; 2010:384523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weinstock LB, Kaleem Z, Gutwein MB. Cryptosporidiosis‐associated lymphocytic colitis. Am J Gastroenterol 2013; 108:1369–71. [DOI] [PubMed] [Google Scholar]
- 23. Moreels TG, Nieuwendijk RJ, De Man JG, De Winter BY, Herman AG, Van Marck EA et al Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut 2004; 53:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie AN, van Rooijen N et al Infection with a helminth parasite prevents experimental colitis via a macrophage‐mediated mechanism. J Immunol 2007; 178:4557–66. [DOI] [PubMed] [Google Scholar]
- 25. Chen CC, Louie S, McCormick B, Walker WA, Shi HN. Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter‐induced colitis in mice. Infect Immun 2005; 73:5468–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen C, Ran ZH, Xiao SD. Therapeutic effects of pravastatin on colitis induced by acetic acid and relevant mechanism: experiment with rats. Zhonghua Yi Xue Za Zhi 2006; 86:1284–8. [PubMed] [Google Scholar]
- 27. Hunter MM, Wang A, McKay DM. Helminth infection enhances disease in a murine TH2 model of colitis. Gastroenterology 2007; 132:1320–30. [DOI] [PubMed] [Google Scholar]
- 28. Wang A, Fernando M, Leung G, Phan V, Smyth D, McKay DM. Exacerbation of oxazolone colitis by infection with the helminth Hymenolepis diminuta: involvement of IL‐5 and eosinophils. Am J Pathol 2010; 177:2850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Summers RW, Elliott DE, Qadir K, Urban JF Jr, Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol 2003; 98:2034–41. [DOI] [PubMed] [Google Scholar]
- 30. Summers RW, Elliott DE, Urban JF Jr, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn's disease. Gut 2005; 54:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Summers RW, Elliott DE, Urban JF Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 2005; 128:825–32. [DOI] [PubMed] [Google Scholar]
- 32. Croese J, O'Neil J, Masson J, Cooke S, Melrose W, Pritchard D et al A proof of concept study establishing Necator americanus in Crohn's patients and reservoir donors. Gut 2006; 55:136–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sandborn WJ, Elliott DE, Weinstock J, Summers RW, Landry‐Wheeler A, Silver N et al Randomised clinical trial: the safety and tolerability of Trichuris suis ova in patients with Crohn's disease. Aliment Pharmacol Ther 2013; 38:255–63. [DOI] [PubMed] [Google Scholar]
- 34. Whelan RA, Rausch S, Ebner F, Gunzel D, Richter JF, Hering NA et al A transgenic probiotic secreting a parasite immunomodulator for site‐directed treatment of gut inflammation. Mol Ther 2014; 22:1730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heylen M, Ruyssers NE, Gielis EM, Vanhomwegen E, Pelckmans PA, Moreels TG et al Of worms, mice and man: an overview of experimental and clinical helminth‐based therapy for inflammatory bowel disease. Pharmacol Ther 2014; 143:153–67. [DOI] [PubMed] [Google Scholar]
- 36. Wang H, Li J, Pu H, Hasan B, Ma J, Jones MK et al Echinococcus granulosus infection reduces airway inflammation of mice likely through enhancing IL‐10 and down‐regulation of IL‐5 and IL‐17A. Parasit Vectors 2014; 7:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khelifi L, Soufli I, Labsi M, Touil‐Boukoffa C. Immune‐protective effect of echinococcosis on colitis experimental model is dependent of down regulation of TNF‐α and NO production. Acta Trop 2017; 166:7–15. [DOI] [PubMed] [Google Scholar]
- 38. Gottstein B. Echinococcus multilocularis infection: immunology and immunodiagnosis. Adv Parasitol 1992; 31:321–80. [DOI] [PubMed] [Google Scholar]
- 39. Matsumoto J, Muller N, Hemphill A, Oku Y, Kamiya M, Gottstein B. 14‐3‐3‐ and II/3‐10‐gene expression as molecular markers to address viability and growth activity of Echinococcus multilocularis metacestodes. Parasitology 2006; 132:83–94. [DOI] [PubMed] [Google Scholar]
- 40. Bashi T, Blank M, Shoenfeld Y. Treating inflammatory bowel disease: from helminths to ova. Isr Med Assoc J 2014; 16:627–8. [PubMed] [Google Scholar]
- 41. Taghipour N, Aghdaei HA, Haghighi A, Mossafa N, Tabaei SJ, Rostami‐Nejad M. Potential treatment of inflammatory bowel disease: a review of helminths therapy. Gastroenterol Hepatol Bed Bench 2014; 7:9–16. [PMC free article] [PubMed] [Google Scholar]
- 42. Garg SK, Croft AM, Bager P. Helminth therapy (worms) for induction of remission in inflammatory bowel disease. Cochrane Database Syst Rev 2014; 1:CD009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buning J, Homann N, von Smolinski D, Borcherding F, Noack F, Stolte M et al Helminths as governors of inflammatory bowel disease. Gut 2008; 57:1182–3. [DOI] [PubMed] [Google Scholar]
- 44. Ruyssers NE, De Winter BY, De Man JG, Loukas A, Herman AG, Pelckmans PA et al Worms and the treatment of inflammatory bowel disease: are molecules the answer? Clin Dev Immunol 2008; 2008:567314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Summers RW, Elliott DE, Weinstock JV. Is there a role for helminths in the therapy of inflammatory bowel disease? Nat Clin Pract Gastroenterol Hepatol 2005; 2:62–3. [DOI] [PubMed] [Google Scholar]
- 46. de Lange KM, Barrett JC. Understanding inflammatory bowel disease via immunogenetics. J Autoimmun 2015; 64:91–100. [DOI] [PubMed] [Google Scholar]
- 47. Ince MN, Elliott DE. Immunologic and molecular mechanisms in inflammatory bowel disease. Surg Clin North Am 2007; 87:681–96. [DOI] [PubMed] [Google Scholar]
- 48. Rook GA. Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol 2012; 42:5–15. [DOI] [PubMed] [Google Scholar]
- 49. Hsu SJ, Tseng PH, Chen PJ. Trichuris suis therapy for ulcerative colitis: nonresponsive patients may need anti‐helminth therapy. Gastroenterology 2005; 129:768–9; author reply 769. [DOI] [PubMed] [Google Scholar]
- 50. Soufli I, Toumi R, Rafa H, Amri M, Labsi M, Khelifi L et al Crude extract of hydatid laminated layer from Echinococcus granulosus cyst attenuates mucosal intestinal damage and inflammatory responses in Dextran Sulfate Sodium induced colitis in mice. J Inflamm (Lond) 2015; 12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harraga S, Godot V, Bresson‐Hadni S, Mantion G, Vuitton DA. Profile of cytokine production within the periparasitic granuloma in human alveolar echinococcosis. Acta Trop 2003; 85:231–6. [DOI] [PubMed] [Google Scholar]
- 52. Bresson‐Hadni S, Liance M, Meyer JP, Houin R, Bresson JL, Vuitton DA. Cellular immunity in experimental Echinococcus multilocularis infection. II. Sequential and comparative phenotypic study of the periparasitic mononuclear cells in resistant and sensitive mice. Clin Exp Immunol 1990; 82:378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Deng Q, Chen H, Liu Y, Xiao F, Guo L, Liu D et al Psychological stress promotes neutrophil infiltration in colon tissue through adrenergic signaling in DSS‐induced colitis model. Brain Behav Immun 2016; 57:243–54. [DOI] [PubMed] [Google Scholar]
- 54. Xiong H, Keith JW, Samilo DW, Carter RA, Leiner IM, Pamer EG. Innate lymphocyte/Ly6Chi monocyte crosstalk promotes Klebsiella pneumoniae clearance. Cell 2016; 165:679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ivanova EA, Orekhov AN. Monocyte activation in immunopathology: cellular test for development of diagnostics and therapy. J Immunol Res 2016; 2016:4789279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gren ST, Grip O. Role of monocytes and intestinal macrophages in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis 2016; 22:1992–8. [DOI] [PubMed] [Google Scholar]
- 57. Mejri N, Gottstein B. Intraperitoneal Echinococcus multilocularis infection in C57BL/6 mice affects CD40 and B7 costimulator expression on peritoneal macrophages and impairs peritoneal T cell activation. Parasite Immunol 2006; 28:373–85. [DOI] [PubMed] [Google Scholar]
- 58. Dai WJ, Gottstein B. Nitric oxide‐mediated immunosuppression following murine Echinococcus multilocularis infection. Immunology 1999; 97:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Andrade MA, Siles‐Lucas M, Espinoza E, Perez Arellano JL, Gottstein B, Muro A. Echinococcus multilocularis laminated‐layer components and the E14t 14‐3‐3 recombinant protein decrease NO production by activated rat macrophages in vitro . Nitric Oxide 2004; 10:150–5. [DOI] [PubMed] [Google Scholar]
- 60. Shintani N, Nakajima T, Okamoto T, Kondo T, Nakamura N, Mayumi T. Involvement of CD4+ T cells in the development of dextran sulfate sodium‐induced experimental colitis and suppressive effect of IgG on their action. Gen Pharmacol 1998; 31:477–81. [DOI] [PubMed] [Google Scholar]
- 61. Emery I, Leclerc C, Sengphommachanh K, Vuitton DA, Liance M. In vivo treatment with recombinant IL‐12 protects C57BL/6J mice against secondary alveolar echinococcosis. Parasite Immunol 1998; 20:81–91. [DOI] [PubMed] [Google Scholar]
- 62. Amiot F, Fitting C, Tracey KJ, Cavaillon JM, Dautry F. Lipopolysaccharide‐induced cytokine cascade and lethality in LT α/TNF α‐deficient mice. Mol Med 1997; 3:864–75. [PMC free article] [PubMed] [Google Scholar]
- 63. Wang J, Zhang C, Wei X, Blagosklonov O, Lv G, Lu X et al TGF‐β and TGF‐β/Smad signaling in the interactions between Echinococcus multilocularis and its hosts. PLoS One 2013; 8:e55379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hayes KS, Cliffe LJ, Bancroft AJ, Forman SP, Thompson S, Booth C et al Chronic Trichuris muris infection causes neoplastic change in the intestine and exacerbates tumour formation in APC min/+ mice. PLoS Negl Trop Dis 2017; 11:e0005708. [DOI] [PMC free article] [PubMed] [Google Scholar]


