Summary
Glycodelin is an immunomodulator, indispensable for the maintenance of pregnancy in humans. The glycoprotein induces apoptosis in activated CD4+ T cells, monocytes and natural killer (NK) cells, and suppresses the activity of cytotoxic T cells, macrophages and dendritic cells. This study explores the immunosuppressive property of glycodelin for its possible use in preventing graft rejection. Because glycodelin is found only in certain primates, the hypothesis was investigated in an allograft nude mouse model. It is demonstrated that treatment of alloactivated mononuclear cells with glycodelin thwarts graft rejection. Glycodelin decreases the number of activated CD4+ and CD8+ cells and down‐regulates the expression of key proteins known to be involved in graft demise such as granzyme‐B, eomesodermin (EOMES), interleukin (IL)‐2 and proinflammatory cytokines [tumour necrosis factor (TNF)‐α and IL‐6], resulting in a weakened cell‐mediated immune response. Immunosuppressive drugs for treating allograft rejection are associated with severe side effects. Glycodelin, a natural immunomodulator in humans, would be an ideal alternative candidate.
Keywords: granzyme‐B, IL‐2, immunosuppression, T cells
Introduction
The fetus is a semi‐allograft and would be rejected if there were to be no modulation of the maternal immune response during pregnancy 1. Of the various known factors/biomolecules that are involved in the protection of the fetus from the onslaught of the mother's immune system, one is the progesterone‐induced endometrial protein, glycodelin‐A (GdA) 2. GdA is secreted by the decidualized endometrium and accumulates in the amniotic fluid 3. GdA levels peak during the 12th to 16th weeks of pregnancy, decrease subsequently to lower levels but remain higher than the circulatory levels present during the luteal phase of the menstrual cycle 3. Clinical studies that have correlated recurring spontaneous abortions to low levels of circulatory GdA 4 lend support to the importance of glycodelin in prevention of fetal rejection.
GdA induces apoptosis in CD4+ T lymphocytes 5, 6, 7, monocytes 8, 9 and natural killer (NK) cells 10, inhibits the proliferation of B cells 10, 11, inhibits the phagocytic activity in monocytic cells 8, skews the T helper type 1 (Th‐1)/Th‐2 balance 7 and induces tolerogenic phenotype in dendritic cells 12. Importantly, GdA suppresses the activity of cytotoxic T lymphocyte cells (CTLs) 13. The immunosuppressive property is regulated by differential glycosylation and, other than GdA, only the GdF glycoform harbours this activity 14. The immunomodulatory activity of Gd lies in its protein backbone; however, the glycans appear to regulate the activity by masking/unmasking the functional domain 15, 16.
Although not very well understood, immunological pathways leading to fetal rejection appear to be similar to those seen in allograft rejection. Transplantation of incompatible grafts results in the donor tissue being attacked by the recipient's immune system 17. T cells are the key mediators of allograft rejection. The donor alloantigens processed by the antigen‐presenting cells are presented to naive CD4+ or CD8+ T cells. Once activated, on one hand, CD4+ T cells secrete cytokines activating other CD8+ T cells and B cells and on the other hand, CD8+ T cells differentiate into CTLs that kill their target graft cells via secretion of granzyme‐B and perforin 18, 19. Moreover, CD8+ T cells can also be activated by engaging directly with the major histocompatibility complex (MHC) class I molecules presented on the graft cells 18, 20. Secretion of proinflammatory cytokines such as interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α and interleukin (IL)‐6 by the T cells stimulates and recruits more immune cells to the site of transplantation 17, 19, leading eventually to the obliteration of the donor tissue.
Taking together all the above prompted us to explore the possibility of using GdA to prevent graft rejection. Because glycodelin is a human/some primates‐specific protein 3, 21, 22, 23, we developed a xenograft nude mouse model, which will be referred to as ‘allograft’, because both the target cells and adoptively transferred immune cells are of human origin. We observed that glycodelin inhibits graft rejection by down‐regulating IL‐2, eomesodermin (EOMES), granzyme‐B, IL‐6 and tumour necrosis factor (TNF)‐α, resulting in a suppressed cell‐mediated immune response against the graft.
Materials and methods
Cells and cell lines
Human hepatocellular carcinoma (HepG2) cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Sigma‐Aldrich, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), 1 mM Glutamax (Gibco, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) and antibiotics (50 µg/ml gentamycin and 5 U/ml nystatin). Cells (107 cells per mouse) were injected subcutaneously into the flanks of nude mice. Tumours were observed in 50% of mice after 45–60 days. Tumours were excised, treated with 1% collagenase to acquire a single‐cell suspension and cultured as above. These cells, named HepG2e (HepG2 explant), established palpable tumours in 100% of mice in ∼6–8 days.
Jurkat cells (human T lymphocyte cell line) were maintained in Rosewell Park Memorial Medium‐1640 (RPMI‐1640; Sigma‐Aldrich) supplemented with 10% FBS, 1 mM glutamax, 50 μM β‐mercaptoethanol and antibiotics.
Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy donors using Histopaque‐1077 (Sigma‐Aldrich) density gradient centrifugation and were cultured in RPMI‐1640 supplemented with 10% FBS, 1 mM glutamax and 50 μM β‐mercaptoethanol and antibiotics. All cells were maintained at 37°C in 5% CO2. The protocol for the isolation was approved by the Institutional Human Ethics Committee, Indian Institute of Science, India (IHEC no: 2–15032017).
Expression and purification of recombinant Gd
The plasmid pASK75 containing the mutated Gd gene (N28E/G77E/F162G) was received as a gift from Professor Skerra, whose group was the first to express and purify soluble Gd from Escherichia coli. Recombinant Gd (rGd) was expressed in the periplasm of the E. coli C43 strain, as described by Schiefner et al. 21. Briefly, cells were transformed with the plasmid and induced with 0·2 mg/l of anhydrotetracycline for 4 h when the optical density of the culture reached 1·6. Cells were pelleted and resuspended in periplasmic extraction buffer containing lysozyme for 30 min. rGd was purified by Ni‐NTA column chromatography and analysed for its apoptotic activity.
Apoptotic activity of rGd
To determine whether the rGd harbours the apoptotic activity described earlier for the native GdA, Jurkat cells were incubated with rGd for 24 h at 37°C, followed by staining with annexin‐V fluorescein isothiocyanate (FITC) and propidium iodide (PI), according to the manufacturer's protocol (BD Biosciences, San Jose, CA, USA). Cells were analysed by flow cytometry.
Alloactivation
The protocol to generate cytotoxic cells was modified from that reported earlier in our laboratory 13. PBMCs were cultured with HepG2e cells at a ratio of 20 : 1 for 96 h in Iscove's modified Dulbecco's medium (IMDM; Sigma‐Aldrich) supplemented with 15% FBS, 1 mM glutamax; 5 U/ml of interleukin‐2 (IL‐2; Sigma‐Aldrich) was added to the culture at 0 and 48 h.
Staining for activation marker
Alloactivation of PBMCs was carried out for different time intervals. Cells were harvested, washed with phosphate‐buffered saline (PBS) (50 mM phosphate buffer, pH 7·2 containing 150 mM NaCl) and stained with 1·5 µg/ml of anti‐CD69 antibody‐phycoerythrin (PE) conjugate on ice for 1 h in the dark. Cells were washed with PBS and fixed with 2% paraformaldehyde before flow cytometry analysis.
Proliferation of alloactivated PBMCs
Proliferation of PBMCs was assessed using MTT [3‐(4,5‐dimethylthiazol‐2‐YI)‐2,5‐diphenyltetrazolium bromide] assay; 2 × 105 unactivated or activated with anti‐CD3 antibody or alloactivated PBMCs were plated. Twenty μl of MTT (4 mg/ml in PBS) was added to each well and incubated for 4 h. The formazan crystals were dissolved in 200 µl of dimethyl sulphoxide (DMSO) and absorbance was estimated at A540 nm in an enzyme‐linked immunosorbent assay (ELISA) microplate reader (VersaMax, Molecular Devices, Sunnyvale, CA, USA).
Cytotoxicity of alloactivated PBMCs
Carboxyfluorescein succinimidyl ester (CFSE)/PI assay was used to examine the cytotoxicity of the alloactivated PBMCs. Target cells (HepG2e) were labelled with 2·5 μM CFSE for 15 min. The reaction was stopped with 5% FBS in PBS followed by two washes with PBS. The CFSE‐labelled target cells were then incubated with the effector cells (alloactivated PBMCs) at different ratios for 4 h at a cell density of 5 × 107/ml. After incubation, cells were harvested, washed and stained with PI for 15 min. Samples were subjected to flow cytometric analysis. The percentage of specific lysis was calculated as [100X (sample lysis‐basal lysis)/(100‐basal lysis)], where basal lysis is the % lysis seen in the absence of effector cells. Alloactivated PBMCs were incubated with rGd for 24 h at the indicated concentrations prior to their addition to the labelled target cells for CFSE/PI assay to determine the effect of rGd.
Mice
Six to eight‐week‐old nude male mice weighing 20–25 g were procured from the Central Animal Facility, Indian Institute of Science, Bangalore, India. Mice were housed in a clean air facility and exposed to 12 h day/night cycle, with food and water ad libitum. The animal experiments were conducted in accordance with the institute guidelines and the experimental protocols were approved by the Institutional Animal Ethics Committee (Project no. CAF/Ethics/198/2010). At the end of the experiments mice were euthanized by CO2 exposure.
In‐vivo effect of rGd
Mice were divided into three groups, each consisting of six mice and injected subcutaneously in the flanks with 1 × 106 HepG2e cells mixed with 1 × 107 viable unactivated or alloactivated PBMCs or rGd‐treated alloactivated PBMCs in 100 µl IMDM. Mice were monitored for 30 days. The tumour diameter was measured periodically using Vernier callipers. Tumour volume was calculated as ab2/2, where ‘a’ is the length and ‘b’ is the width.
For examining the effect of rGd on tumour (graft) rejection, 2 × 106 HepG2e cells were injected subcutaneously into the flanks of 24 mice. After palpable tumours (10–100 mm3) were observed, mice were injected subcutaneously with 1 × 107 viable unactivated /alloactivated PBMCs/rGd‐treated alloactivated PBMCs near the tumour in 50 µl IMDM. Tumour growth was measured periodically for 15 days. Three mice from the AP and APG groups were euthanized on days 3 and 15. Excised tumours were snap‐frozen in liquid nitrogen and stored at −80°C.
For the rGd‐treated group, the alloactivated PBMCs were first incubated with 1 µM rGd for 24 h and then mixed with 1 µM rGd before transfer to mice. All the animal experiments were replicated at least thrice.
Measurement of mRNA levels of marker proteins
Frozen tumours were thawed, total RNA was isolated using TRI reagent (Sigma‐Aldrich) and reverse‐transcribed to cDNA using oligo‐dT (Thermo Fisher Scientific) and reverse transcriptase (Thermo Fisher Scientific). Real‐time polymerase chain reaction (PCR) using SYBR green (Bio‐Rad, Hercules, CA, USA) was performed in a Bio‐Rad cycler iQ5 (Bio‐Rad). The primers used for the amplification of the mRNA corresponding to the marker genes are listed in Table 1. The amplification conditions were: initial denaturation at 95°C for 5 min followed by 40 cycles of denaturation at 94°C for 30 s, primer annealing at 60°C for 30 s and extension at 72°C for 30 s. The final extension was carried out at 72°C for 5 min. The fold change in the expression of test genes was calculated relative to their levels in day 3 alloactivated PBMCs.
Table 1.
List of primers used for real‐time polymerase chain reaction (PCR)
| Gene | Forward primer | Reverse primer |
|---|---|---|
| 18s rRNA | CGACCATAAACGATGCCGAC | GGTGGTGCCCTTCCGTCAAT |
| CD4 | GTCCCTTTTAGGCACTTGCTTCT | TCTTTCCCTGAGTGGCTGCT |
| CD8a | TCTCCCAAAACAAGCCCAAGG | GTTGCTCAGGGCCGAGCAG |
| CD14 | ACTTATCGACCATGGAGCGC | AGCTCACAAGGTTCTGGCGT |
| CD20 | GGGCTGTCCAGATTATGAATG | GAGTTTTTCTCCGTTGCTGC |
| CD56 | TCTGGATGGGCACATGGTG | TGCTCTTCAGGGTCAGCGA |
| IL‐2 | CCCAAGAAGGCCACAGAACTG | CTTAAGTGAAAGTTTTTGCTTTGAG |
| IL‐6 | GATTCAATGAGGAGACTTGCC | TGTTCTGGAGGTACTCTAGGTA |
| EOMES | GGGGAGGTCGAGGTTCTTACCAGA | CTTGAACACAGTGGGGCTTGTTCT |
| Granzyme‐B | GACAGCTGCTCACTGTTGGGG | AGCTCTGGTCCGCTTGGCCT |
| TNF‐α | AGCCTGTAGCCCATGTTGTAG | CTCTCAGCTCCACGCCATTG |
IL = interleukin; EOMES = eomesodermin; TNF = tumour necrosis factor.
Analysis of expression of marker proteins on cells
Frozen tumours were thawed in PBS containing 10% FBS at 37°C and the tissues were disaggregated mechanically to form a single‐cell suspension 24. Cells were stained with CD8‐fluorescein isothiocyanate (FITC) (BioLegend, San Diego, CA, USA), granzyme‐B (Cusabio, College Park, MD, USA), IL‐2 (Cusabio) and EOMES‐alexa 647 (eBiosciences, Thermo Fisher Scientific) according to the manufacturer's instructions and analysed by flow cytometry.
Flow cytometric analysis
BD FACSVerseTM (Becton Dickinson, Franklin Lakes, NJ, USA) was used for flow cytometry. At least 10 000 events per sample were acquired in all the cases. Data were analysed using the software FACSuite™ or Cyflogic.
Statistical analysis
All data were evaluated using GraphPad Prism version 5.0. Unpaired Student's t‐test and one‐way analysis of variance (anova) was performed and P < 0·05 was considered to be statistically significant.
Results
Apoptotic effect of recombinant Gd on Jurkat cells
GdA is known to induce apoptosis in activated T cells 5, 7. To ascertain whether the E. coli‐expressed Gd (rGd; non‐glycosylated) exhibits the same activity, Jurkat cells cultured with varying concentrations of rGd for 24 h were stained with annexin V‐FITC/PI prior to their analysis by flow cytometry. A concentration‐dependent increase in apoptosis was observed, with 200 nM rGd causing more than 50% apoptosis (P < 0·0005) (Fig. 1a). This is the first report, to our knowledge, on the E. coli‐produced Gd harbouring apoptotic activity. Earlier attempts to obtain active Gd had failed because of inappropriate folding of the protein 15. Skerra et al. introduced mutations in the Gd gene which permitted the expression of soluble and crystallizable Gd 21.
Figure 1.
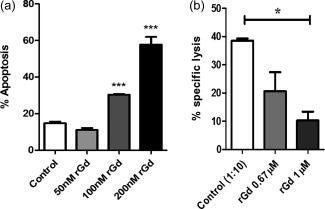
Activity of Escherichia coli produced recombinant glycodelin (rGd). (a) Jurkat cells were treated with the indicated concentration of rGd for 24 h. Cells were then stained with annexin V‐fluorescein isothiocyanate (FITC) and propidium iodide (PI) and the apoptotic population was measured by flow cytometry (Student's t‐test P < 0·0005). (b) The alloactivated PBMCs were pretreated with 0·67 μM and 1 μM rGd for 24 h, then added to (CFSE)‐labelled human hepatocellular carcinoma expansion media (HepG2e) cells at a ratio of 10 : 1 and incubated for 4 h. After incubation, cells were harvested, stained with PI and analysed by flow cytometry (Student's t‐test, P < 0·012). Data are representative of three independent experiments.
Alloactivation of PBMCs
The surface expression of CD69, an early T cell activation marker, was examined on PBMCs that were co‐cultured with HepG2e cells for 96 h using flow cytometry. As seen in Supporting information, Fig. S1a, cells expressing CD69 increased from 22 to 34% by 16 h and declined to basal levels after 36 h. PBMCs isolated from healthy donors were activated either by incubating with anti‐CD3 antibody (as positive control) or by co‐culturing with HepG2e cells. The extent of proliferation of the alloactivated PBMCs (over 96 h) was found to be comparable to that of PBMCs activated (over 48 h) by anti‐CD3 antibody (Supporting information, Fig. S1b,c). Both assays showed that the PBMCs were activated as a result of co‐culturing them with HepG2e cells.
Effect of rGd on the cytotoxicity of alloactivated PBMCs
To determine whether the alloactivation resulted in the generation of cytotoxic PBMCs, the alloactivated cells (effectors) were incubated for 4 h with their target HepG2e cells and their lysis was measured. With increasing numbers of effector cells there was an increase in the percentage of specific lysis of the target cells (Supporting information, Fig. S1d). Lysis of ∼50% of HepG2e cells was observed at an effector : target ratio of 1 : 10 and this ratio was used for all further studies.
Subsequently, the effect of rGd on the in‐vitro‐generated cytotoxic cells was studied. Alloactivated PBMCs were treated with rGd for 24 h prior to the cytotoxicity assay. As shown in Fig. 1b, rGd decreased the cytotoxicity of alloactivated PBMCs four‐fold at 1 μM concentration (P < 0·012), which is in concordance with those reported earlier 13.
Effect of rGd on the cytotoxic activity of PBMCs in vivo
Nude mice were injected with HepG2e cells to establish a palpable tumour and the effect of the cytotoxic cells in the presence and absence of rGd on the establishment of the tumour, and its growth was monitored for a month. As shown in Fig. 2a, when mixed with unactivated PBMCs (UP), HepG2e cells formed tumours in all the injected mice within a week, while no tumour formation was observed for at least 2 weeks when they were injected along with alloactivated PBMCs (AP; P < 0·0001). Moreover, 50% of the mice did not develop any tumours even up to 30 days after injection of the cells. Of interest was the group that received rGd‐treated cytotoxic cells (APG), along with the HepG2e cells, for which AP were treated with 1 µM rGd for 24 h and mixed again with rGd prior to their injection in mice. As can be seen in Fig. 2a, although there was a 2‐day delay in tumour development in this group compared to the UP group, all the mice developed tumours by day 14. By the end of the 30‐day observation period, all the mice receiving HepG2e cells mixed with either UP or APG had developed tumours. A significant increase (P < 0·048) was seen in the tumour growth in the group that received APG compared to the mice that received AP (Fig. 2b). Thus, these observations suggest that rGd inhibits the cytolytic activity of the alloactivated PBMCs, thereby allowing the establishment of HepG2e tumours.
Figure 2.
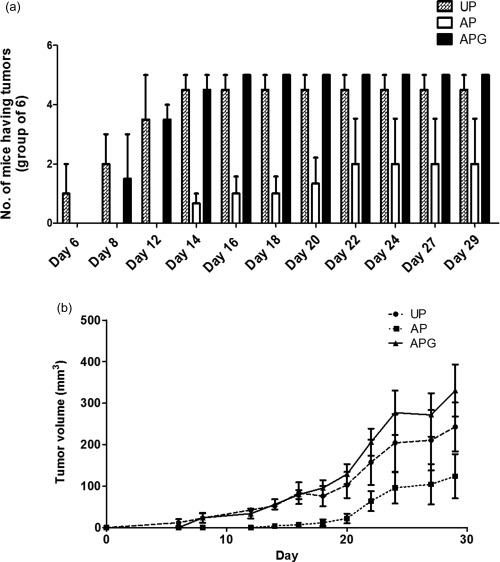
Effect of recombinant glycodelin (rGd) on the in‐vivo cytotoxicity of alloactivated peripheral blood mononuclear cells (PBMCs). Nude mice were injected subcutaneously with HepG2e cells mixed with UP, AP or APG at a ratio of 1 : 10 and the tumour growth was monitored for 4 weeks. (a) Number of mice with tumour [one‐way analysis of variance (anova), P < 0·0001]. (b) The kinetics of tumour growth (one‐way anova, P < 0·048). UP = unactivated PBMCs; AP = alloactivated PBMCs; APG = alloactivated PBMCs treated with rGd. Data presented is cumulative of three independent experiments.
Suppression of tumour rejection by rGd
To determine whether alloactivated PBMCs could bring about tumour rejection, UP or AP were transferred to mice after the HepG2e tumours were established and the tumour volumes were measured for 15 days. In the group injected with UP there was an exponential increase in the tumour volume. However, there was very little increase in the tumour size in the group that received the AP (P < 0·001). When mice were injected with APG, a dose‐dependent increase in the tumour volume was seen (Fig. 3b). Those that received APG treated with a higher concentration of rGd exhibited accelerated tumour growth (P < 0·05).
Figure 3.
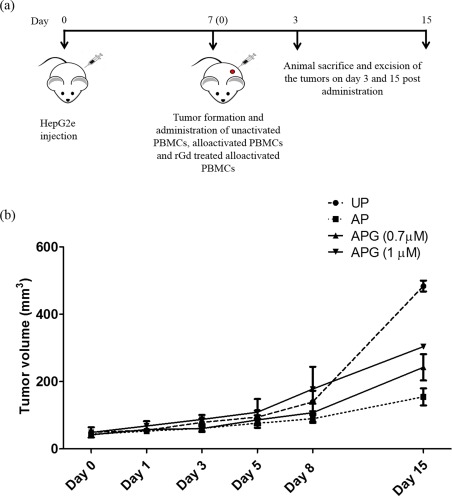
Effect of recombinant glycodelin (rGd) on the inhibition of tumour growth by alloactivated peripheral blood mononuclear cells (PBMCs). (a) Pictorial representation of the injection regimen followed for the experiment. (b) Unactivated PBMCs/alloactivated PBMCs/alloactivated PBMCs treated with two different concentrations of rGd were injected at the site of tumours and the tumour growth was measured for 15 days. UP = unactivated PBMCs; AP = alloactivated PBMCs; APG = alloactivated PBMCs treated with rGd. Data presented is cumulative of three independent experiments. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Mechanistic insights into the suppression of tumour rejection by rGd
To elucidate the mechanism of the effect of rGd on the cytotoxic cells, the mRNA levels of the T cell markers, CD4 and CD8, and key molecules involved in cytotoxicity, namely IL‐2, granzyme‐B and EOMES, were measured. AP or APG were injected at the HepG2e tumour site. Tumours were excised on days 3 and 15, RNA was isolated and real‐time PCR was performed. Amplification of CD4, CD8, CD56, CD20 and CD14 markers confirmed the infiltration of human PBMCs in the tumour by day 3 (Fig. 4). Analysis of the AP population before and after administration in mice revealed that CD4 and CD8 mRNA levels were augmented by 15‐fold and 3‐fold, respectively, while CD14, CD20 and CD56 mRNA levels were decreased (Fig. 4). Apart from day 3, when maximum infiltration of the immune cells could be expected in the tumour, mRNA levels of the interested genes were also assessed on day 15 to determine their correlation with respect to the tumour growth in the AP and APG groups. The mRNA levels of CD4 and CD8, along with IL‐2 and EOMES, were found to increase in the tumours excised on day 15 from the AP‐injected group of mice. Tumours from the APG group had lower levels of all the markers on day 3 compared to the AP group (CD4, 89‐fold; CD8, fourfold; IL‐2, 41‐fold; granzyme‐B, 45‐fold; EOMES, fivefold; Fig. 5a–e). Similarly, day 15 APG tumours also showed reduced mRNA levels of all the genes; however, the difference was less profound except for CD8.
Figure 4.
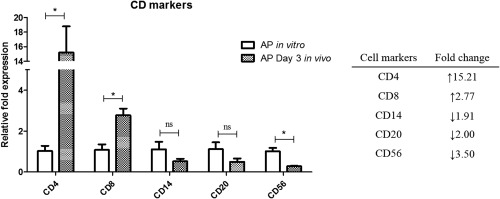
Immune cell population in alloactivated peripheral blood mononuclear cells (PBMCs) before and after administration in mice. RNA was isolated from AP and tumours injected with AP (day 3). Real‐time polymerase chain reaction (PCR) was performed to amplify the cDNA of CD4, CD8, CD14, CD20 and CD56. The inset table tabulates the fold change in mRNA levels of each cell marker in tumour injected with AP on day 3 in comparison to AP. Data are represented as the fold expression relative to AP (before injection in mice) for each protein. AP = alloactivated PBMCs.
Figure 5.
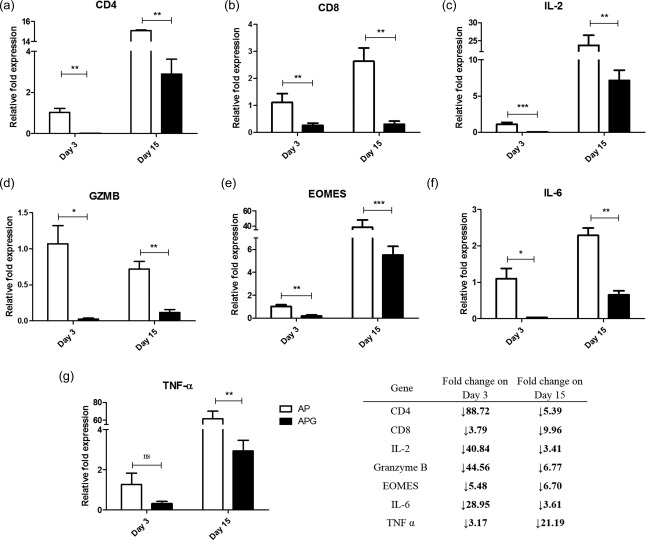
Down‐regulation of genes associated with cytotoxic activity of alloactivated peripheral blood mononuclear cells (PBMCs) by recombinant glycodelin (rGd). RNA was isolated from the tumours after injection of AP or APG. Real‐time polymerase chain reaction (PCR) was performed to detect the mRNA levels of human (a) CD4, (b) CD8, (c) interleukin (IL)‐2, (d) granzyme‐B, (e) eomesodermin (EOMES), (f) tumour necrosis factor (TNF)‐α and (g) IL‐6. The inset table tabulates the fold change in mRNA levels of each protein in APG in comparison to AP on day 3. Data are represented as the fold expression relative to the day 3 AP for each protein (Student's t‐test; *P < 0·05; **P ≤ 0·01; ***P < 0·001). AP = alloactivated PBMCs; APG = alloactivated PBMCs treated with rGd.
Additionally, the relative fold expression of the proinflammatory cytokines, TNF‐α and IL‐6, were down‐regulated significantly by rGd treatment on both days 3 and 15 (Fig. 5f,g).
In order to determine whether the decrease in the RNA levels of the different markers corresponded to the expression of the proteins, cells were isolated from the tumours and stained with antibodies to human CD8, granzyme‐B, IL‐2 and EOMES. For flow cytometric analysis of the protein expression, the HepG2e cells were gated out by using forward‐scatter (FSC)‐H versus FSC‐A scatter‐plots. The small size of the tumours on day 3 posed tissue processing constraints and the immune cells recovered were statistically inadequate for flow cytometric analysis. Therefore, day 3 was excluded from the data to assess changes in protein expression. As seen in Fig. 6, there was a decrease in the expression of CD8 (70%), IL‐2 (55%), granzyme‐B (81%) and EOMES (96%) in the immune cells isolated from the APG tumours compared to the AP tumours.
Figure 6.
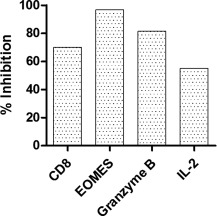
Down‐regulation of proteins associated with cytotoxic activity of alloactivated peripheral blood mononuclear cells (PBMCs) by recombinant glycodelin (rGd). Cells isolated from the tumours excised on day 15 after injection of AP or APG were stained with human antibodies specific to CD8, interleukin (IL)‐2, eomesodermin (EOMES) and granzyme‐B. Protein expression was analysed using flow cytometry. Decrease in the protein expression in APG in comparison to AP is represented as the % inhibition. AP = alloactivated PBMCs; APG = alloactivated PBMCs treated with rGd.
Consolidating all the data, it was concluded that rGd decreases the T cell population as well as abrogating the cytotoxic activity of alloactivated PBMCs in vivo.
Discussion
Results presented in the paper suggest that the immunomodulatory role of glycodelin, which is indispensable for the maintenance of pregnancy, could possibly be explored for the prevention of rejection of donor grafts. Allograft rejection is the major drawback associated with incompatible organ transplantation 17. Use of immunosuppressants is the choice of treatment in most cases to bring respite 25; however, they concomitantly make the recipient susceptible to infections 19, hence the need for a treatment which provides a specific and localized immunosuppression at the site of transplantation, something similar to what is seen during pregnancy. Being a semi‐allograft, the fetus presents the same challenges to the maternal immune system, which needs to be modulated to prevent fetal rejection and yet protect it from pathogenic assault at the same time 1. Therefore, the idea of investigating the potential of natural immunomodulators relevant during pregnancy in preventing graft rejection is reasonable. Indeed, treatment with galectin‐1 in a mouse model following an allogenic bone marrow transplantation was shown to inhibit the graft‐versus‐host response 26. Another such immunomodulator which is essential in abrogating fetal rejection is glycodelin A. In this context, several studies have demonstrated the immunomodulatory activity of GdA on almost all the immune cells at the feto–maternal interface 27, 28, and of relevance to this study is the inhibitory effect of GdA on the activity of CTLs 13, 29. In case of incompatible organ transplantation, CTL‐mediated killing of graft cells is one of the major processes that leads to the destruction of the donor graft 18.
We proposed to utilize the immunomodulatory property of glycodelin for acceptance of grafts. Towards this, human cytotoxic T cells were generated first in vitro to a human cell line, HepG2e (to represent the donor tissue) and demonstrated that the cytotoxic T cells effectively killed their targets. Exposure to glycodelin inhibited the cytotoxic activity of the cells. The studies were then extended to an animal model.
As glycodelin is found to be present only in human and certain primates 21, there is no small animal model to test the activity in vivo. We therefore established a graft rejection model using nude mice. HepG2e tumours were generated in mice which served as the allograft and the human AP generated in vitro against the HepG2e cells served as the rejection machinery consisting primarily of CTLs. It is important to mention here that Gd does not induce apoptosis in either the non‐immune cells such as HepG2e cells or the mouse immune cells (Supporting information, Fig. S2). First, it was determined whether the effect of rGd on the cytotoxic cells could be translated to in‐vivo conditions. HepG2e tumours were established in nude mice in the absence (UP) or presence of human cytotoxic cells, untreated (AP) or treated with rGd (APG). No tumours developed in 70% of the mice receiving AP. All the mice that received APG exhibited tumour development comparable to that of the control group. These results showed that the cytotoxic cells retain their activity when injected in vivo in mice and, most importantly, that the rGd inhibited the activity of these cytotoxic cells.
After having demonstrated that AP can inhibit the establishment of HepG2e tumours and that rGd could abrogate this activity efficiently, we initiated the second set of experiments. Seven days after the tumours of HepG2e were established in mice, they were injected with UP or AP or APG and the tumour growth was measured. The growth of tumours in AP‐injected mice was retarded compared to those in animals injected with UP. These results are in agreement with studies showing limitation of tumour growth by adoptively transferred CTLs in xenograft models 30, 31. In the case of the APG‐injected group, the inhibition of tumour growth mediated by the cytotoxic cells was observed to be rescued. Thus, rGd was able to suppress the cytotoxicity of the alloactivated cells and thereby prevent the regression in the observed tumour growth.
A dynamic immune response exists at the site of transplantation against the graft which needs to be weakened if allograft should survive 25. Infiltration of the activated CTLs to the site of the graft followed by the release of cytotoxic components, perforin and granzyme or FasL‐induced programmed cell death and also release of soluble mediators such as TNF‐α are the key events leading to the rejection or demise of the graft 18, 32. We proposed to investigate the mechanism of the role played by rGd in the prevention of graft rejection at the molecular level. Towards this, we first confirmed the homing of the injected AP to the established tumour (graft) by amplifying the CD markers associated with the human mononuclear cells on day 3. Equal numbers of viable AP or APG were injected in mice, which negated any discrepancies in analysis due to cell death as a result of pretreatment with rGd. As would be expected, injection of AP in mice led to HepG2e‐mediated reactivation and proliferation of both CD4+ and CD8+ T cells, while there was a reduction in the other immune cell populations. This was indicative of an active and adaptive cellular immune response at the tumour site. When compared to the AP group, the APG group tumours showed a decrease in the CD4 and CD8 mRNA levels, the difference being most pronounced on day 3 (CD4, 88·72‐fold; CD8, 3·79‐fold). These findings are consistent with earlier reports demonstrating that activated CD8+ T cells are more resistant to GdA‐mediated apoptosis compared to CD4+ T cells 13. Upon activation, CD4+ and CD8+ T cells secrete IL‐2 which is required for T cell proliferation and survival 33. A 40·84‐fold reduction in IL‐2 mRNA levels was observed on day 3 upon rGd treatment. GdA‐mediated decrease in the IL‐2 secretion by both CD4+ and CD8+ T cells has been shown earlier 29, 34 which supports our observations. Moreover, a decrease in IL‐2 can result in a weakened survival signal for CD8+ T cells leading to their death over a period of time, probably explaining the 10‐fold decline in their numbers by day 15.
IL‐2 up‐regulates the expression of perforin and granzyme‐B in the activated CD8+ T cells via the transcription factor, EOMES 35. When CTLs come into contact with their target cells, release of the serine protease granzyme‐B is triggered which, with the help of the pore‐forming protein, perforin, enters the target cells leading to induction of apoptosis 36. Overall, IL‐2, EOMES and granzyme‐B regulate the cytotoxicity of CTLs. On day 3, the mRNA levels of granzyme‐B were found to decline by 44·56‐fold upon rGd treatment, which is much greater than the levels of CD8, suggesting that the effect is not only dependent upon loss of cell viability but also on the decrease in IL‐2 levels. Treatment with rGd brought about a decrease in EOMES seen in the tumours on both days 3 and 15. The expression of these molecules was also studied at the protein level. CD8, EOMES, IL‐2 and granzyme‐B‐positive infiltrating cells of the tumours were found to be highly reduced in case of the APG group compared to the AP group. These observations are in concurrence with earlier work conducted in our laboratory, where GdA was shown to impede the cytolytic activity of CTLs not only by inhibiting EOMES, granzyme‐B and perforin transcription 13, but also by weakening the IL‐2 signalling 29.
Several studies suggest the importance of the inflammatory cytokines, TNF‐α and IL‐6, as they exhibit a major role in activating T cells leading eventually to the pathogenesis of graft rejection 19, 37. Serum concentrations of IL‐6 were found to be high in patients with acute graft‐versus‐host disease 38. Therefore, in the present study, we measured the TNF‐α and IL‐6 mRNA levels and observed a decrease upon rGd treatment, confirming its anti‐inflammatory role. Intriguingly, maximum reduction in the levels of IL‐6 was seen on day 3 (29‐fold) as seen with IL‐2 and granzyme B, whereas for TNF‐α it was on day 15 (21‐fold). Most probably, TNF‐α is not involved directly in the mechanism by which rGd renders its effect, and thus the differences in its levels between AP and APG group could be attributed to the differential tumour growth seen in the mice from both groups.
We have used CD4 and CD8 mRNA levels as a measure of the corresponding T cell population, but the same does not hold true for the other effector molecules. Different trends in their mRNA levels in the tumours isolated from AP mice on days 3 and 15 demonstrate that their levels are not relative to either CD4+ or CD8+ T cells. Similarly, upon rGd treatment, the down‐regulation of IL‐2, granzyme‐B and EOMES cannot be solely because of decrease in cell viability.
In summary, we generated cytotoxic cells from normal healthy human PBMCs on co‐culturing with the target HepG2e cells, which effectively decreased their growth in mice. Exposure of the cytotoxic cells to rGd suppressed graft rejection mediated by them by decreasing the levels of IL‐2, EOMES and granzyme‐B. These observations, in addition to rGd‐mediated induction of apoptosis in CD4+ T cells, which are the key mediators of both arms of the acquired immune response, lead to the interesting possibility of utilizing glycodelin in the management of graft rejection. A similar suggestion of treatment with Gd in case of lung transplantation to avoid allograft rejection was made recently by Schneider et al 39. They proposed suppressing the immune system of the recipient before lung transplantation by administering Gd in the form of solution/vapours or by ex‐vivo gene delivery and an additional dose of vaporized Gd after transplantation. This is the first report highlighting the immunomodulatory activity of glycodelin in vivo. However, glycodelin is found only in some orders of primates, therefore our observations need to be validated in a primate model.
Disclosure
The authors have no conflicts of interest.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Generation of cytotoxic peripheral blood mononuclear cells (PBMCs). (a) PBMC (responders) were co‐cultured with human hepatocellular carcinoma expansion media (HepG2e) (stimulators) at the ratio of 20 : 1 for 48 h. Cells were harvested at the indicated time‐intervals and stained for surface CD69 marker with anti‐CD69 antibody phycoerythrin (PE) conjugate and analysed by flow cytometry. (b,c) Cells were analysed for their proliferative ability by MTT assay after activation by anti‐CD3 antibody for 48 h (b) or by culturing with HepG2e for 96h (c). (d) CFSE‐labelled HepG2e cells (targets) were incubated with alloactivated PBMCs (effectors) for 4 h in the varying ratios and then stained with PI. The lysis of the target cells was analysed by flow cytometry. Data show mean ± standard error of the mean (s.e.m.) of three experiments.
Fig. S2. Recombinant glycodelin (rGd) does not induce apoptosis in human hepatocellular carcinoma expansion media (HepG2e) and mouse immune cells. HepG2e, Jurkat (human T cell leukaemia), Sp2/O (murine B cell myeloma) and RAW (murine macrophage) cells were treated with 1 µM, 150 nM, 800 nM and 800 nM rGd, respectively, for 24 h. Cells were then stained with annexin V‐fluorescein isothiocyanate (FITC) and propidium iodide (PI) and the apoptotic population was measured by flow cytometry.
Acknowledgements
A. D. and B. B. were involved in the study design, conduction of experiments, data analysis, manuscript drafting and discussions. A. A. K. supervised the study and participated in the design of experiments and critical analysis of the data, manuscript corrections and discussions. We acknowledge Professor Arne Skerra for providing the Gd (N28E/G77E/F162G) construct. We thank the Central Animal Facility and FACS facility, Indian Institute of Science. A. A. K. acknowledges the Department of Biotechnology, Government of India, for the funding. A. D. and B. B. thank the University Grants Commission, New Delhi, for research fellowship.
References
- 1. Arck PC, Hecher K. Fetomaternal immune cross‐talk and its consequences for maternal and offspring's health. Nat Med 2013; 19:548–56. [DOI] [PubMed] [Google Scholar]
- 2. Clark GF, Schust DJ. Manifestations of immune tolerance in the human female reproductive tract. Front Immunol 2013; 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Julkunen M, Rutanen EM, Koskimies A, Ranta T, Bohn H, Seppälä M. Distribution of placental protein 14 in tissues and body fluids during pregnancy. Br J Obstet Gynaecol 1985; 92:1145–51. [DOI] [PubMed] [Google Scholar]
- 4. Dalton CF, Laird SM, Estdale SE, Saravelos HG, Li TC. Endometrial protein PP14 and CA‐125 in recurrent miscarriage patients; correlation with pregnancy outcome. Hum Reprod 1998; 13:3197–202. [DOI] [PubMed] [Google Scholar]
- 5. Mukhopadhyay D, Sundereshan S, Rao C, Karande AA. Placental protein 14 induces apoptosis in T cells but not in monocytes. J Biol Chem 2001; 276:28268–73. [DOI] [PubMed] [Google Scholar]
- 6. SundarRaj S, Mukhopadhyay D, Karande AA. Glycodelin A triggers mitochondrial stress and apoptosis in T cells by a mechanism distinct and independent of TCR signaling. Mol Immunol 2008; 45:2391–400. [DOI] [PubMed] [Google Scholar]
- 7. Lee C‐L, Chiu PCN, Lam KKW et al Differential actions of glycodelin‐A on Th‐1 and Th‐2 cells: a paracrine mechanism that could produce the Th‐2 dominant environment during pregnancy. Hum Reprod 2011; 26:517–26. [DOI] [PubMed] [Google Scholar]
- 8. Alok A, Mukhopadhyay D, Karande AA. Glycodelin A, an immunomodulatory protein in the endometrium, inhibits proliferation and induces apoptosis in monocytic cells. Int J Biochem Cell Biol 2009; 41:1138–47. [DOI] [PubMed] [Google Scholar]
- 9. Tee MK, Vigne JL, Yu J, Taylor RN. Natural and recombinant human glycodelin activate a proapoptotic gene cascade in monocyte cells. J Leukoc Biol 2008; 83:843–52. [DOI] [PubMed] [Google Scholar]
- 10. Alok A, Karande AA. The role of glycodelin as an immune‐modulating agent at the feto‐maternal interface. J Reprod Immunol 2009; 83:124–7. [DOI] [PubMed] [Google Scholar]
- 11. Yaniv E, Borovsky Z, Mishan‐Eisenberg G, Rachmilewitz J. Placental protein 14 regulates selective B cell responses. Cell Immunol 2003; 222:156–63. [DOI] [PubMed] [Google Scholar]
- 12. Scholz C, Toth B, Brunnhuber R et al Glycodelin A induces a tolerogenic phenotype in monocyte‐derived dendritic cells in vitro . Am J Reprod Immunol 2008; 60:501–12. [DOI] [PubMed] [Google Scholar]
- 13. Soni C, Karande AA. Glycodelin A suppresses the cytolytic activity of CD8+ T lymphocytes. Mol Immunol 2010; 47:2458–66. [DOI] [PubMed] [Google Scholar]
- 14. Lee CL, Pang PC, Yeung WS et al Effects of differential glycosylation of glycodelins on lymphocyte survival. J Biol Chem 2009; 284:15084–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jayachandran R, Shaila MS, Karande AA. Analysis of the role of oligosaccharides in the apoptotic activity of glycodelin A. J Biol Chem 2004; 279:8585–91. [DOI] [PubMed] [Google Scholar]
- 16. Jayachandran R, Radcliffe CM, Royle L et al Oligosaccharides modulate the apoptotic activity of glycodelin. Glycobiology 2006; 16:1052–63. [DOI] [PubMed] [Google Scholar]
- 17. Afzali B, Lechler RI, Hernandez‐Fuentes MP. Allorecognition and the alloresponse: clinical implications. Tissue Antigens 2007; 69:545–56. [DOI] [PubMed] [Google Scholar]
- 18. Bueno V, Pestana JO. The role of CD8+ T cells during allograft rejection. Braz J Med Biol Res 2002; 35:1247–58. [DOI] [PubMed] [Google Scholar]
- 19. Sanchez‐Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology 2011; 140:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schenk AD, Nozaki T, Rabant M, Valujskikh A, Fairchild RL. Donor‐reactive CD8 memory T cells infiltrate cardiac allografts within 24‐h posttransplant in naive recipients. Am J Transplant 2008; 8:1652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schiefner A, Rodewald F, Neumaier I, Skerra A. The dimeric crystal structure of the human fertility lipocalin glycodelin reveals a protein scaffold for the presentation of complex glycans. Biochem J 2015; 466:95–104. [DOI] [PubMed] [Google Scholar]
- 22. Inaba N, Sato N, Fukazawa I et al The immunocytochemical localization of new soluble placental tissue proteins (PP14, 16, 17, 19, 20 and PP21) in human and Cynomolgus monkey placentae. Arch Gynecol 1987; 240:13–9. [DOI] [PubMed] [Google Scholar]
- 23. Fazleabas AT, Donnelly KM, Hild‐Petito S, Hausermann HM, Verhage HG. Secretory proteins of the baboon (Papio anubis) endometrium: regulation during the menstrual cycle and early pregnancy. Hum Reprod Update 1997; 3:553–9. [DOI] [PubMed] [Google Scholar]
- 24. van Dam PA, Watson JV, Lowe DG, Chard T, Shepherd JH. Comparative evaluation of fresh, fixed, and cryopreserved solid tumour cells for reliable flow cytometry of DNA and tumour associated antigen. Cytometry 1992; 13:722–9. [DOI] [PubMed] [Google Scholar]
- 25. Grinyó JM, Cruzado JM. Mycophenolate mofetil and calcineurin‐inhibitor reduction: recent progress. Am J Transplant 2009; 9:2447–52. [DOI] [PubMed] [Google Scholar]
- 26. Baum LG, Blackall DP, Arias‐Magallano S et al Amelioration of graft versus host disease by galectin‐1. Clin Immunol 2003; 109:295–307. [DOI] [PubMed] [Google Scholar]
- 27. Dixit A, Karande AA. Glycodelin A and galectin‐1: role in foetal tolerance. J Reprod Health Med 2016; 2:S1–8. [Google Scholar]
- 28. Seppala M, Taylor RN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev 2002; 23:401–30. [DOI] [PubMed] [Google Scholar]
- 29. Soni C, Karande AA. Glycodelin‐A interferes with IL‐2/IL‐2R signalling to induce cell growth arrest, loss of effector functions and apoptosis in T‐lymphocytes. Hum Reprod 2012; 27:1005–15. [DOI] [PubMed] [Google Scholar]
- 30. Oflazoglu E, Elliott M, Takita H, Ferrone S, Henderson RA, Repasky EA. Adoptively transferred human lung tumour specific cytotoxic T cells can control autologous tumour growth and shape tumour phenotype in a SCID mouse xenograft model. J Transl Med 2007; 5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crowley NJ, Vervaert CE, Seigler HF. Human xenograft–nude mouse model of adoptive immunotherapy with human melanoma‐specific cytotoxic T‐cells. Cancer Res 1992; 52:394–9. [PubMed] [Google Scholar]
- 32. Wood KJ, Goto R. Mechanisms of rejection: current perspectives. Transplantation 2012; 93:1–10. [DOI] [PubMed] [Google Scholar]
- 33. Nelson BH. IL‐2, regulatory T cells, and tolerance. J Immunol 2004; 172:3983–8. [DOI] [PubMed] [Google Scholar]
- 34. Pockley AG, Bolton AE. Placental protein 14 (PP14) inhibits the synthesis of interleukin‐2 and the release of soluble interleukin‐2 receptors from phytohaemagglutinin‐stimulated lymphocytes. Clin Exp Immunol 1989; 77:252–6. [PMC free article] [PubMed] [Google Scholar]
- 35. Janas ML, Groves P, Kienzle N, Kelso A. IL‐2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. J Immunol 2005; 175:8003–10. [DOI] [PubMed] [Google Scholar]
- 36. Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol 2015; 15:388–400. [DOI] [PubMed] [Google Scholar]
- 37. Ferrara JL, Cooke KR, Teshima T. The pathophysiology of acute graft‐versus‐host disease. Int J Hematol 2003; 78:181–7. [DOI] [PubMed] [Google Scholar]
- 38. Fujii N, Hiraki A, Aoe K et al Serum cytokine concentrations and acute graft‐versus‐host disease after allogeneic peripheral blood stem cell transplantation: concurrent measurement of ten cytokines and their respective ratios using cytometric bead array. Int J Mol Med 2006; 17:881–5. [PubMed] [Google Scholar]
- 39. Schneider M, Meister M, Muley T. Glycodelin as diagnostic and prognostic marker and for monitoring treatment of lung diseases. Google Patents 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Generation of cytotoxic peripheral blood mononuclear cells (PBMCs). (a) PBMC (responders) were co‐cultured with human hepatocellular carcinoma expansion media (HepG2e) (stimulators) at the ratio of 20 : 1 for 48 h. Cells were harvested at the indicated time‐intervals and stained for surface CD69 marker with anti‐CD69 antibody phycoerythrin (PE) conjugate and analysed by flow cytometry. (b,c) Cells were analysed for their proliferative ability by MTT assay after activation by anti‐CD3 antibody for 48 h (b) or by culturing with HepG2e for 96h (c). (d) CFSE‐labelled HepG2e cells (targets) were incubated with alloactivated PBMCs (effectors) for 4 h in the varying ratios and then stained with PI. The lysis of the target cells was analysed by flow cytometry. Data show mean ± standard error of the mean (s.e.m.) of three experiments.
Fig. S2. Recombinant glycodelin (rGd) does not induce apoptosis in human hepatocellular carcinoma expansion media (HepG2e) and mouse immune cells. HepG2e, Jurkat (human T cell leukaemia), Sp2/O (murine B cell myeloma) and RAW (murine macrophage) cells were treated with 1 µM, 150 nM, 800 nM and 800 nM rGd, respectively, for 24 h. Cells were then stained with annexin V‐fluorescein isothiocyanate (FITC) and propidium iodide (PI) and the apoptotic population was measured by flow cytometry.


