Summary
CD3+CD20+ T cells are a population of CD3+ T cells that express CD20 and identified in healthy donors and autoimmune diseases. However, the nature and role of these cells in patients with psoriasis remain unclear. In this study, we aimed to investigate the level, phenotype, functional and clinical relevance of CD3+CD20+ T cells in the peripheral blood of patients with psoriasis. We found that a small subset of CD3+ T cells expressed CD20 molecule in the peripheral blood of patients with psoriasis, and their levels were similar to those in healthy donors. Circulating CD3+CD20+ T cells in patients with psoriasis were enriched in CD4+ cells and displayed an activated effector phenotype, as these cells contained fewer CD45RA+‐naive and CCR7+ cells with increased activity than those of CD3+ T cells lacking CD20. In addition, compared with healthy donors, circulating CD3+CD20+ T cells in patients with psoriasis produced more cytokines, interleukin (IL)‐17A, tumour necrosis factor (TNF)‐α and IL‐21, but not IL‐4 and IFN‐γ. Furthermore, a significantly positive correlation was found between the levels of IL‐17A, TNF‐α and IL‐21‐production CD3+CD20+ T cells with Psoriasis Area and Severity Index scores. Our findings suggest that CD3+CD20+ T cells may play a role in the pathogenesis of psoriasis.
Keywords: CD3+CD20+ T cells, disease severity, psoriasis
Introduction
Psoriasis is one of the most common chronic inflammatory diseases, affecting approximately 0·5–1% of children and 2–3% of adults 1. Although much effort has been focused upon seeking available anti‐psoriatic drugs, there is currently no cure for psoriasis 2, 3. Generally, it has been accepted that the development and progression of psoriasis are mediated by the cross‐talk between keratinocytes and the host immune system, with many studies supporting a crucial role of the infiltration and cytokine production by pathogenic T cells in psoriatic skin lesions, which results in an excessively aberrant hyperproliferation and abnormal differentiation of keratinocytes 4, 5, 6. Thus, deciphering the biology of host pathogenic T cells is critical for understanding the pathophysiological dysregulation of psoriasis.
CD3+CD20+ T cells are a group of CD3+ T cells co‐expressing T cell antigen CD3 and B cell antigen CD20 7. However, these cells do not express other classical B cell markers such as CD19 and immunoglobulin molecules, whereas a high level of surface interleukin (IL)‐7 receptor, CD45RO, CD28 and CD27 and intracellular cytokines IL‐17A, tumour necrosis factor (TNF)‐α and interferon (IFN)‐γ expression could be observed. In addition, there is a rapid rise in the intracellular calcium concentration of these cells following treatment with anti‐CD3 antibody, but not anti‐immunoglobulin 8. Therefore, CD3+CD20+ T cells have been postulated to represent a subpopulation of CD3+ T cells. Recently, studies have shown that CD3+CD20+ T cells display a T helper type 17 (Th17) feature and are involved in the pathogenesis of autoimmune diseases, including rheumatoid arthritis and multiple sclerosis 9, 10, 11, 12. Nevertheless, another study has found that CD3+CD20+ T cells have a predominantly Tc1 effector memory phenotype and are expanded in the ascites of patients with ovarian cancer 13, suggesting a functional diversity of these cells in different diseases. However, the prevalence and contribution of CD3+CD20+ T cells in psoriasis remain unknown.
In this study, we analysed the nature, phenotype and functional features of CD3+CD20+ T cells in the peripheral blood of patients with psoriasis, evaluated their relevance with the severity of disease and attempted to elucidate the biological role of CD3+CD20+ T cells in patients with psoriasis.
Materials and methods
Subjects and samples
Peripheral blood samples were obtained from 30 patients with psoriasis (male/female = 19/11; average age 42·1 ± 13·1 years; disease duration 13·4 ± 8·5 years) in the Xinan Hospital of the Third Military Medical University. The patients had no other acute or chronic disease and did not take any medication for at least 1 month before enrolment into this study. Psoriasis Area and Severity Index (PASI) scores were used to measure the disease activity of patients, as described previously 14. The PASI scores of 30 patients ranged from 3·4 to 48·8. Thirty healthy donors (male/female = 18/12; average age 41·8 ± 12·7 years) were enrolled as the control group. The Ethics Committee of Xinan Hospital, Third Military Medical University approved this study. Written informed consent was obtained from each subject. The clinical characteristics of patients with psoriasis are presented in Supporting information, Table S1.
Antibodies and flow cytometry analysis
The following labelled antibodies were used to stain single‐cell suspensions: anti‐CD3 allophycocyanin (APC)‐cyanin 7 (Cy7) (UCHT1), anti‐CD20 peridinin chlorophyll (PerCP)‐Cy5·5 (2H7), anti‐CD4 fluorescein isothiocyanate (FITC) (RPA‐T4), anti‐CD45RA FITC (HI100), anti‐CD45RO FITC (UCHL1), anti‐CD62L FITC (DREG‐56), anti‐CD69 FITC (FN50), anti‐CD8 phycoerythrin (PE) (HIT8a), anti‐CCR7 PE (G043H7), anti‐CD38 PE (HB‐7), anti‐CD86 PE (IT2.2), anti‐CD40 PE (HB14), anti‐CD28 APC (CD28.2), anti‐CD19 APC (HIB19), anti‐human leucocyte antigen D‐related (HLA‐DR) APC (L243), anti‐CD27 PE‐Cy7 (O323), anti‐IL‐17A PE (BL168), anti‐IL‐4 APC (8D4–8) anti‐IFN‐γ PE‐Cy7 (4S.B3), anti‐IL‐21 APC (3A3‐N2) and anti‐TNF‐α PE (MAB11) (Biolegend, San Diego, CA, USA). Fresh peripheral whole blood was incubated with lysing solution (BD Biosciences, San Jose, CA, USA) to lyse red blood cells and washed twice with phosphate‐buffered saline (PBS), following incubation with surface antibodies for 30 min at 4°C, and then fixed and permeabilized for 20 min using Cytofix/Cytoperm reagent (BD Biosciences). Next, the cells were washed twice with PBS containing 1% fetal calf serum (FCS) and then collected by flow cytometry with a FACSCanto II (BD Biosciences). Isotype‐matched labelled antibodies were used to enable correct compensation and the antibodies specificity. For intracellular cytokine staining, peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood of patients with psoriasis and healthy donors by Ficoll density gradient centrifugation (GE Healthcare, Shanghai, China), and PBMCs were then stimulated for 4 h with leucocyte activation cocktail, with BD GolgiPlug (BD Biosciences) before staining.
Statistical analysis
The results were summarized as the mean ± standard error of the mean (s.e.m.) or median with interquartile range. Statistical analysis was performed using Prism version 5.0 software. Differences between the two groups of data were determined by two‐tailed Student's t‐test. When variance was detected, the Mann–Whitney U‐test was used to analyse the difference between two groups. Multiple groups were analysed by one‐way analysis of variance (ANOVA), as appropriate. Correlation analysis between the groups was evaluated by Spearman's correlation test. P < 0·05 was accepted as statistically significant.
Results
Detection of circulating CD3+CD20+ T cells in patients with psoriasis
Using flow cytometry, we first detected the prevalence of CD20+ lymphocytes in the peripheral blood of patients with psoriasis. As shown in Fig. 1a,b, we observed that total lymphocytes contained not only a substantial amount of CD3‐CD20+ B cells (10·1 ± 0·9%), but also a small fraction of CD3+CD20+ T cells (1·7 ± 0·3%). The expression of CD20 on CD3+ T cells was not due to doublets between B and T cells, as these cells expressed a lower level of CD20 than CD3‐CD20+ B cells and typically lacked B cell markers such as CD19 and CD40 (Fig. 2 and Supporting information, Fig. S1). We found further that the level of CD3+CD20+ T cells in patients with psoriasis was similar to that in healthy donors (Fig. 1c). However, compared with CD3+CD20– T cells, CD3+CD20+ T cells contained more CD4+ cells and correspondingly fewer CD8+ cells in patients with psoriasis, whereas more CD8+ cells and fewer CD4+ cells of CD3+CD20+ T cells were observed in healthy donors (Fig. 1d).
Figure 1.
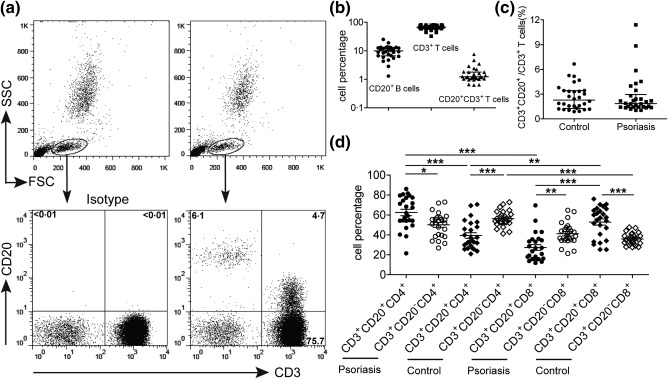
The identification of circulating CD3+CD20+ T cells in patients with psoriasis. (a) A representative flow cytometry analysis of CD3+CD20+ T cells in the peripheral blood of patients with psoriasis. Human peripheral whole blood from patients with psoriasis were stained with anti‐CD3 and anti‐CD20 antibodies or isotype controls. The level of CD3+CD20+ T cells was analysed from the lymphocyte gate as defined by a forward‐scatter (FSC) and side‐scatter (SSC) dot‐plot. (b) Statistical analysis of CD3–CD20+ B cell, CD3+ T cell and CD3+CD20+ T cell levels in the total lymphocyte of peripheral blood obtained from 30 patients with psoriasis. (c) The comparison of CD3+CD20+ T cell level in the CD3+ population between patients with psoriasis and healthy donors. (d) Statistical analysis of CD4+ and CD8+ subsets of CD3+CD20+ T cells and CD3+CD20– T cells in patients with psoriasis and healthy donors. Each plot represents a single donor. *P < 0·05; **P < 0·01; and ***P < 0·001.
Figure 2.
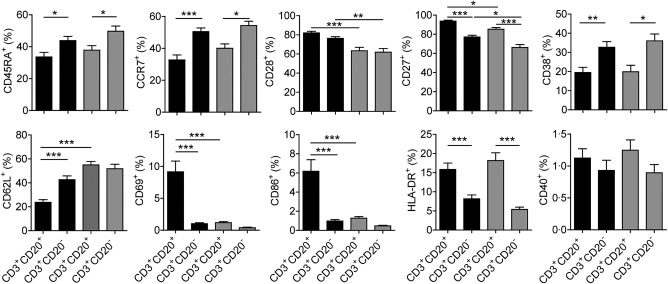
Phenotypical analysis of circulating CD3+CD20+ T cells in patients with psoriasis and healthy donors. Human peripheral whole blood from patients with psoriasis and healthy donors were stained with anti‐CD3, anti‐CD20, anti‐CD45RA, anti‐C‐C chemokine receptor type 7 (CCR7), anti‐CD28, anti‐CD27, anti‐CD38, anti‐CD62L, anti‐CD69, anti‐CD86, anti‐human leucocyte antigen D‐related (HLA‐DR) and anti‐CD40 antibodies or isotype controls. CD3+CD20+ and CD3+CD20– T cell subpopulations were gated, and then the levels of CD45RA+, CCR7+, CD28+, CD27+, CD38+, CD62L+, CD69+, CD86+, HLA‐DR+ and CD40+ cells in these two subpopulations were analysed (black: patients with psoriasis; grey: healthy donors). *P < 0·05; **P < 0·01; ***P < 0·001.
Circulating CD3+CD20+ T cells have an activation effector phenotype in patients with psoriasis
Next, we studied the phenotypical features of circulating CD3+CD20+ T cells in patients with psoriasis and healthy donors. As shown in Fig. 2, CD3+CD20+ T cells expressed lower levels of CD45RA and CCR7 but higher levels of CD27 and HLA‐DR than those on CD3+CD20– T cells in both patients with psoriasis and healthy donors, and more than half of CD3+CD20+ T cells belonged to CD45RA−CCR7− effector‐memory cells (Supporting information, Fig. S2). In addition, compared with healthy donors, CD3+CD20+ T cells in patients with psoriasis expressed higher levels of CD28 and CD27, suggesting that CD3+CD20+ T cells are a population of early‐differentiated effector‐memory cells in patients with psoriasis. Furthermore, CD3+CD20+ T cells in patients with psoriasis contained more CD69+ and CD86+ cells but fewer CD62L+ cells than those in healthy donors, implying that these cells displayed an activated phenotype. Nevertheless, CD3+CD20+ T cells expressed a lower level of CD38 than that on CD3+CD20‐ T cells in both patients with psoriasis and healthy donors, and an equivalent low level of CD40 expression was observed between them (Supporting information, Fig. S3).
Increased cytokine production by circulating CD3+CD20+ T cells in patients with psoriasis
To investigate further the function of circulating CD3+CD20+ T cells, PBMCs obtained from patients with psoriasis and healthy donors were stimulated with leucocyte activation cocktail for intracellular cytokine staining. As shown in Fig. 3a,b, we found that CD3+CD20+ T cells in both patients with psoriasis and healthy donors expressed higher levels of IL‐17A, IFN‐γ, TNF‐α and IL‐21 than those in CD3+CD20‐ T cells, but there was no significant difference between them for expression of IL‐4. Interestingly, CD3+CD20+ T cells in patients with psoriasis could produce more IL‐17A, TNF‐α and IL‐21 than those in healthy donors. However, there was no significant difference for the expression of IL‐4 and IFN‐γ in these cells between patients with psoriasis and healthy donors. We further analysed cytokine production by CD45RA+ or CD45RO+ and CD4+ or CD8+ subsets of CD3+CD20+ T cells in patients with psoriasis, and found that the majority of these cytokine‐production CD3+CD20+ T cells belonged to the CD45RO+ subset (Supporting information, Fig. S4). Additionally, the CD4+ subset produced higher levels of IL‐17A, TNF‐α and IL‐21 than the CD8+ subset, especially IL‐17A‐production CD3+CD20+ T cells almost belonged to the CD4+ subset (data not shown).
Figure 3.
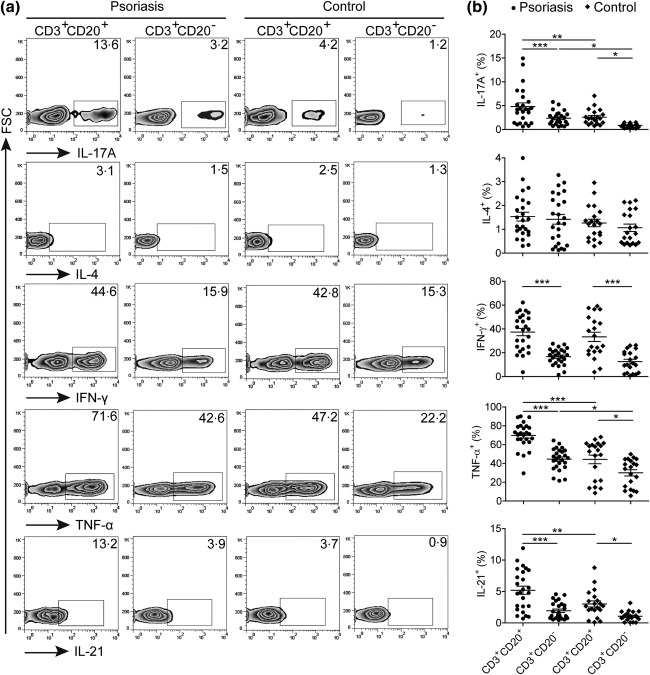
Functional characteristics of circulating CD3+CD20+ T cells in patients with psoriasis. (a) Representative dot‐plots of interleukin (IL)‐17A, IL‐4, interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α and IL‐21 expression levels in CD3+CD20+ and CD3+CD20– T cells from patients with psoriasis and healthy donors. (b) Statistical analysis of IL‐17A+, IL‐4+, IFN‐γ+, TNF‐α+ and IL‐21+ cell levels in CD3+CD20+ and CD3+CD20– T cells between patients with psoriasis and healthy donors. Symbols represent individual values from 25 patients and 20 healthy donors. Each plot represents a single donor. Data were expressed as the mean ± standard error of the mean. *P < 0·05; **P < 0·01; and ***P < 0·001.
Increased IL‐17A, TNF‐α and IL‐21‐production circulating CD3+CD20+ T cells correlated with disease severity in patients with psoriasis
To determine whether increased cytokine production of CD3+CD20+ T cells were associated with disease severity, the severity of disease was evaluated by PASI scores in patients with psoriasis. As shown in Fig. 4, the level of IL‐17A production of CD3+CD20+ T cells correlated positively with PASI scores, and a significant positive correlation was also observed between the levels of TNF‐α‐, IL‐21‐production CD3+CD20+ T cells and PASI scores. In addition, the levels of IL‐17A, TNF‐α and IL‐21 production of CD3+CD20– T cells were also shown to be correlated positively with PASI scores (Supporting information, Fig. S5). However, the levels of IL‐4‐, IFN‐γ‐production of CD3+CD20+ or CD3+CD20– T cells did not show a significant correlation with PASI scores, and similarly no correlation was observed between the level of CD3+CD20+ T cells and PASI scores.
Figure 4.
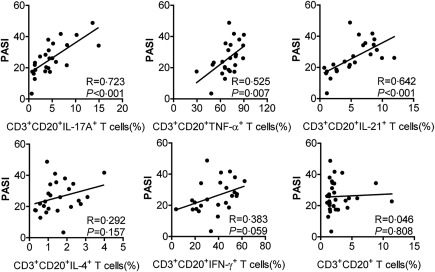
Correlation of circulating cytokine‐production CD3+CD20+ T cell levels as well as CD3+CD20+ T cell levels with disease severity in patients with psoriasis. The levels of circulating interleukin (IL)‐17A+ cells, tumour necrosis factor (TNF)‐α+ cells and IL‐21+ cells in CD3+CD20+ T cells correlated positively with the Psoriasis Area and Severity Index (PASI) scores. No correlation of circulating IL‐4+ cells and IFN‐γ+ cells in CD3+CD20+ T cells as well as CD3+CD20+ T cell levels with the PASI scores. P < 0·05 was considered significant for correlation between the two groups.
Discussion
Deciphering the function and clinical relevance of pathogenic T cells in psoriasis is critical for understanding how they affect the development and course of disease 15, 16. In the present study, we found that a subpopulation of CD3+CD20+ T cells were characterized as activated effector cells in the peripheral blood of patients with psoriasis, and these cells produced high levels of proinflammatory cytokines that correlated with disease severity, implying that CD3+CD20+ T cells might play a role in T cell‐mediated pathogenic immune response of psoriasis.
It has been acknowledged that CD20 is expressed on the surface of B cells but not other cell lineages. However, the low level of CD20 expression on a subpopulation of human CD3+ T cells was also reported 8, 17. Later, one study doubted that this subset might be an artefact of flow cytometry 18. Nevertheless, studies showed clearly that CD3+ T cells expressed CD20 at the mRNA and protein levels by real‐time polymerase chain reaction and confocal microscopy after sorting single cells, indicating the existence of the CD3+CD20+ T cell subpopulation 12, 19. Consistent with these reports, our study also showed that a subpopulation of CD3+ T cells expressed CD20 in the peripheral blood of patients with psoriasis and healthy donors at a similar level, and these cells lacked classical B cell markers CD19 and CD40. However, this subpopulation in patients with psoriasis contained more CD4+ cells and fewer CD8+ cells, whereas more CD8+ cells and fewer CD4+ cells of these cells were observed in healthy donors 7, suggesting that the constitution of a CD4+ and CD8+ subset in CD3+CD20+ T cells is altered in patients with psoriasis. In particular, previous studies also showed that CD3+CD20+ T cells were predominantly CD4+ cells in patients with multiple sclerosis and rheumatoid arthritis 11, 19; we speculated that this phenomenon might exist in a variety of diseases. In addition, we also observed that more than half of circulating CD3+CD20+ T cells in patients with psoriasis displayed an effector‐memory phenotype, as they showed a higher level of T cells that lacked CD45RA (a marker of naive cells) and C‐C chemokine receptor type 7 (CCR7) (a homing chemokine receptor of T cells to secondary lymphoid organs). However, these cells were still at the early‐differentiation stage, as most of them expressed CD28 and CD27.
We further analysed the activation status of circulating CD3+CD20+ T cells in patients with psoriasis. Our data revealed that CD3+CD20+ T cells expressed higher levels of CD69 and CD86, but a lower level of CD62L than those in healthy donors and CD3+ T cells lacking CD20, suggesting that these cells may be in an activated state. However, compared with CD3+CD20– T cells, the expression of CD38 was decreased significantly on CD3+CD20+ T cells. Although CD38 was also considered as one of the activation markers on T cells 20, studies on infectious diseases showed definitively that a subset of human T cells expressing HLA‐DR but not CD38 have a particularly high ability to suppress the virus 21, 22. Given the increased expression of HLA‐DR on CD3+CD20+ T cells, it was reasonable to assume that CD3+CD20+ T cells represented an activated subpopulation and had a potential effector function in patients with psoriasis. In fact, CD3+CD20+ T cells could produce more proinflammatory cytokines than those in CD3+CD20– T cells 7, 9, and a similar result was also observed in patients with psoriasis. Additionally, we found that CD3+CD20+ T cells in patients with psoriasis produced higher levels of IL‐17A, TNF‐α and IL‐21 than those in healthy donors, and these cytokine‐production cells showed a significantly positive correlation with the severity of disease. Because IL‐17A, TNF‐α and IL‐21 have been involved in the pathogenic process of psoriasis, and biological drugs targeting TNF‐α and IL‐17A in particular are used or undergoing clinical trials for the treatment of this disease 23, 24, 25. Therefore, these results would highlight further the involvement of CD3+CD20+ T cells in the pathogenesis of psoriasis.
In summary, our study identified a subpopulation of circulating CD3+CD20+ T cells in patients with psoriasis; their activated effector phenotype and a strong potential to cytokine production suggests a role of CD3+CD20+ T cells in the pathogenesis of psoriasis. Given their proinflammatory ability, further study to elucidate the precise role of CD3+CD20+ T cells in the pathogenic process of psoriasis is warranted.
Disclosure
The authors declare that they have no conflicts of interest.
Author contributions
J. N. designed and performed experiments, analysed the data and wrote the manuscript. Z. Z., Y. Z. and F. H. performed experiments and analysed the data. Z. S. and H. Z. designed experiments and wrote the manuscript.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. Expression of CD19 on circulating CD3+CD20+ T cells in patients with psoriasis. Human peripheral whole blood from patients with psoriasis was stained with anti‐CD3, anti‐CD20 and anti‐CD19 antibodies or isotype controls, and a representative flow cytometry analysis for CD20 versus CD3 expression after gating on lymphocytes; subsequently, the expression of CD19 on CD20+ B cells (Q1: green), CD3+CD20+ T cells (Q2: red) and CD3+CD20– T cells (Q3: blue) was analysed further.
Fig. S2. Expression of CD45RA and C‐C chemokine receptor type 7 (CCR7) on circulating CD3+CD20+ and CD3+CD20– T cells in patients with psoriasis and healthy donors (control). Human peripheral whole blood from patients with psoriasis was stained with anti‐CD3, anti‐CD20, anti‐CD45RA and anti‐CCR7 antibodies or isotype controls. CD3+CD20+ and CD3+CD20– T cell subpopulations were gated from the lymphocyte defined by a forward‐scatter (FSC) and side‐scatter (SSC) dot‐plot, and the expression of CD45RA and CCR7 in these two subpopulations was then analysed. **P < 0·01.
Fig. S3. Phenotypical feature of circulating CD3+CD20+ T cells and CD3+CD20– T cells in patients with psoriasis and healthy donors. Human peripheral whole blood from patients with psoriasis and healthy donors was stained with anti‐CD3, anti‐CD20, anti‐CD45RA, anti‐C‐C chemokine receptor type 7 (CCR7), anti‐CD28, anti‐CD27, anti‐CD38, anti‐CD62L, anti‐CD69, anti‐CD86, anti‐human leucocyte antigen D‐related (HLA‐DR) and anti‐CD40 antibodies or isotype controls. CD3+CD20+ and CD3+CD20– T cell subpopulations were gated from the lymphocyte defined by a forward‐scatter (FSC) and side‐scatter (SSC) dot‐plot, and the levels of CD45RA+, CCR7+, CD28+, CD27+, CD38+, CD62L+, CD69+, CD86+, HLA‐DR+ and CD40+ cells in these two subpopulations were then analysed.
Fig. S4. Cytokine production of CD45RA+ or CD45RO+ subsets of circulating CD3+CD20+ T cells in patients with psoriasis. (a) Representative dot‐plots of interleukin (IL)‐17A, IL‐4, interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α and IL‐21 expression levels in CD45RA+ or CD45RO+ subsets of CD3+CD20+ and CD3+CD20– T cells from patients with psoriasis. (b) Statistical analysis of IL‐17A+, IL‐4+, IFN‐γ+, TNF‐α+ and IL‐21+ cell levels in CD45RA+ or CD45RO+ subsets of CD3+CD20+ and CD3+CD20– T cells from 10 patients with psoriasis. Data are expressed as the mean ± standard error of the mean. *P < 0·05; **P < 0·01; ***P < 0·001.
Fig. S5. The relationship of circulating cytokines production CD3+CD20– T cell levels with disease severity in patients with psoriasis. The levels of circulating interleukin (IL)‐17A+ cells, tumour necrosis factor (TNF)‐α+ cells and IL‐21+ cells in CD3+CD20– T cells correlated positively with the Psoriasis Area and Severity Index (PASI) scores. No correlation of circulating IL‐4+ cells and IFN‐γ+ cells in CD3+CD20– T cells with the PASI scores. P < 0·05 was considered to be significant for correlation between the two groups.
Table S1. The clinical characteristics of patients with psoriasis
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (no. 81271754) and the Natural Science Foundation of Liaoning Province (no. 201602785).
Contributor Information
J. Niu, Email: niujun06@126.com.
Z. Song, Email: drsongzq@hotmail.com
H. Zhong, Email: zhonghua95@hotmail.com
References
- 1. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133:377–85. [DOI] [PubMed] [Google Scholar]
- 2. Hoffman MB, Hill D, Feldman SR. Current challenges and emerging drug delivery strategies for the treatment of psoriasis. Expert Opin Drug Deliv 2016; 13:1461–73. [DOI] [PubMed] [Google Scholar]
- 3. Sala M, Elaissari A, Fessi H. Advances in psoriasis physiopathology and treatments: up to date of mechanistic insights and perspectives of novel therapies based on innovative skin drug delivery systems (ISDDS). J Control Release 2016; 239:182–202. [DOI] [PubMed] [Google Scholar]
- 4. Eberle FC, Brück J, Holstein J, Hirahara K, Ghoreschi K. Recent advances in understanding psoriasis. F1000Res 2016; 5:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deng Y, Chang C, Lu Q. The inflammatory response in psoriasis: a comprehensive review. Clin Rev Allergy Immunol 2016; 50:377–89. [DOI] [PubMed] [Google Scholar]
- 6. Karczewski J, Dobrowolska A, Rychlewska‐Hańczewska A, Adamski Z. New insights into the role of T cells in pathogenesis of psoriasis and psoriatic arthritis. Autoimmunity 2016; 49:435–50. [DOI] [PubMed] [Google Scholar]
- 7. Schuh E, Berer K, Mulazzani M et al Features of human CD3+CD20+ T cells. J Immunol 2016; 197:1111–7. [DOI] [PubMed] [Google Scholar]
- 8. Hultin LE, Hausner MA, Hultin PM, Giorgi JV. CD20 (pan‐B cell) antigen is expressed at a low level on a subpopulation of human T lymphocytes. Cytometry 1993; 14:196–204. [DOI] [PubMed] [Google Scholar]
- 9. Eggleton P, Bremer E, Tarr JM et al Frequency of Th17 CD20+ cells in the peripheral blood of rheumatoid arthritis patients is higher compared to healthy subjects. Arthritis Res Ther 2011; 13:R208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schneider R. CD20+ T cells in multiple sclerosis. Mult Scler Relat Disord 2015; 4:58–9. [DOI] [PubMed] [Google Scholar]
- 11. Holley JE, Bremer E, Kendall AC et al CD20+inflammatory T‐cells are present in blood and brain of multiple sclerosis patients and can be selectively targeted for apoptotic elimination. Mult Scler Relat Disord 2014; 3:650–8. [DOI] [PubMed] [Google Scholar]
- 12. Palanichamy A, Jahn S, Nickles D et al Rituximab efficiently depletes increased CD20‐expressing T cells in multiple sclerosis patients. J Immunol 2014; 193:580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Bruyn M, Wiersma VR, Wouters MC et al CD20+ T cells have a predominantly Tc1 effector memory phenotype and are expanded in the ascites of patients with ovarian cancer. Oncoimmunology 2015; 4:e999536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica 1978; 157:238–44. [DOI] [PubMed] [Google Scholar]
- 15. Greb JE, Goldminz AM, Elder JT et al Psoriasis. Nat Rev Dis Primers 2016; 2:16082. [DOI] [PubMed] [Google Scholar]
- 16. Diani M, Altomare G, Reali E. T helper cell subsets in clinical manifestations of psoriasis. J Immunol Res 2016; 2016:7692024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Warzynski MJ, Graham DM, Axtell RA, Zakem MH, Rotman RK. Low level CD20 expression on T cell malignancies. Cytometry 1994; 18:88–92. [DOI] [PubMed] [Google Scholar]
- 18. Henry C, Ramadan A, Montcuquet N et al CD3+CD20+ cells may be an artifact of flow cytometry: comment on the article by Wilk et al . Arthritis Rheum 2010; 62:2561–3; author reply: 2563–2565. [DOI] [PubMed] [Google Scholar]
- 19. Wilk E, Witte T, Marquardt N et al Depletion of functionally active CD20+ T cells by rituximab treatment. Arthritis Rheum 2009; 60:3563–71. [DOI] [PubMed] [Google Scholar]
- 20. Quarona V, Zaccarello G, Chillemi A et al CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin Cytom 2013; 84B:207–17. [DOI] [PubMed] [Google Scholar]
- 21. Gonzalez SM, Taborda NA, Rugeles MT. Role of different subpopulations of CD8+ T cells during HIV exposure and infection. Front Immunol 2017; 8:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hua S, Lécuroux C, Sáez‐Cirión A et al Potential role for HIV‐specific CD38‐/HLA‐DR+ CD8+ T cells in viral suppression and cytotoxicity in HIV controllers. PLoS One 2014; 9:e101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Niu J, Song Z, Yang X, Zhai Z, Zhong H, Hao F. Increased circulating follicular helper T cells and activated B cells correlate with disease severity in patients with psoriasis. J Eur Acad Dermatol Venereol 2015; 29:1791–6. [DOI] [PubMed] [Google Scholar]
- 24. Dávila‐Seijo P, Dauden E, Carretero G et al Survival of classic and biological systemic drugs in psoriasis: results of the BIOBADADERM registry and critical analysis. J Eur Acad Dermatol Venereol 2016; 30:1942–50. [DOI] [PubMed] [Google Scholar]
- 25. Wcisło‐Dziadecka D, Zbiciak M, Brzezińska‐Wcisło L, Mazurek U. Anti‐cytokine therapy for psoriasis‐not only TNF‐α blockers. Overview of reports on the effectiveness of therapy with IL‐12/IL‐23 and T and B lymphocyte inhibitors. Postepy Hig Med Dosw (Online) 2016; 70:1198–205. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. Expression of CD19 on circulating CD3+CD20+ T cells in patients with psoriasis. Human peripheral whole blood from patients with psoriasis was stained with anti‐CD3, anti‐CD20 and anti‐CD19 antibodies or isotype controls, and a representative flow cytometry analysis for CD20 versus CD3 expression after gating on lymphocytes; subsequently, the expression of CD19 on CD20+ B cells (Q1: green), CD3+CD20+ T cells (Q2: red) and CD3+CD20– T cells (Q3: blue) was analysed further.
Fig. S2. Expression of CD45RA and C‐C chemokine receptor type 7 (CCR7) on circulating CD3+CD20+ and CD3+CD20– T cells in patients with psoriasis and healthy donors (control). Human peripheral whole blood from patients with psoriasis was stained with anti‐CD3, anti‐CD20, anti‐CD45RA and anti‐CCR7 antibodies or isotype controls. CD3+CD20+ and CD3+CD20– T cell subpopulations were gated from the lymphocyte defined by a forward‐scatter (FSC) and side‐scatter (SSC) dot‐plot, and the expression of CD45RA and CCR7 in these two subpopulations was then analysed. **P < 0·01.
Fig. S3. Phenotypical feature of circulating CD3+CD20+ T cells and CD3+CD20– T cells in patients with psoriasis and healthy donors. Human peripheral whole blood from patients with psoriasis and healthy donors was stained with anti‐CD3, anti‐CD20, anti‐CD45RA, anti‐C‐C chemokine receptor type 7 (CCR7), anti‐CD28, anti‐CD27, anti‐CD38, anti‐CD62L, anti‐CD69, anti‐CD86, anti‐human leucocyte antigen D‐related (HLA‐DR) and anti‐CD40 antibodies or isotype controls. CD3+CD20+ and CD3+CD20– T cell subpopulations were gated from the lymphocyte defined by a forward‐scatter (FSC) and side‐scatter (SSC) dot‐plot, and the levels of CD45RA+, CCR7+, CD28+, CD27+, CD38+, CD62L+, CD69+, CD86+, HLA‐DR+ and CD40+ cells in these two subpopulations were then analysed.
Fig. S4. Cytokine production of CD45RA+ or CD45RO+ subsets of circulating CD3+CD20+ T cells in patients with psoriasis. (a) Representative dot‐plots of interleukin (IL)‐17A, IL‐4, interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α and IL‐21 expression levels in CD45RA+ or CD45RO+ subsets of CD3+CD20+ and CD3+CD20– T cells from patients with psoriasis. (b) Statistical analysis of IL‐17A+, IL‐4+, IFN‐γ+, TNF‐α+ and IL‐21+ cell levels in CD45RA+ or CD45RO+ subsets of CD3+CD20+ and CD3+CD20– T cells from 10 patients with psoriasis. Data are expressed as the mean ± standard error of the mean. *P < 0·05; **P < 0·01; ***P < 0·001.
Fig. S5. The relationship of circulating cytokines production CD3+CD20– T cell levels with disease severity in patients with psoriasis. The levels of circulating interleukin (IL)‐17A+ cells, tumour necrosis factor (TNF)‐α+ cells and IL‐21+ cells in CD3+CD20– T cells correlated positively with the Psoriasis Area and Severity Index (PASI) scores. No correlation of circulating IL‐4+ cells and IFN‐γ+ cells in CD3+CD20– T cells with the PASI scores. P < 0·05 was considered to be significant for correlation between the two groups.
Table S1. The clinical characteristics of patients with psoriasis


