Abstract
After the controversial report by Carlsen et al. in 1992 showing a possible decline in human semen quality over the past 50 years, many laboratories investigated their own records of semen findings that had been kept for the past decades, and a significant decrease in sperm quality was reported from some laboratories, but not others. At the beginning of the 21st century, a definitive interpretation of this issue has not yet been offered; however, it seems plausible that there are large regional differences in semen quality. Decreases in semen quality have been reported from various regions around the world, and a concurrent rise in the incidence of other reproductive problems, such as testicular cancer and genital abnormalities, has been observed in many regions. However, most of the reports showing regional differences were from Western or Western‐derived countries, despite the fact that Asia is the region with the highest population on earth. Recently we undertook a cross‐sectional study on fertile men in Japan to describe the current status of semen quality of Japanese men. We took confounders into consideration to allow a comparison with a previous European study. Japanese fertile men proved to have a semen quality at the level of Danish men, who were reported to have the lowest level among the men examined in the European study. This low level of sperm concentration in fertile Japanese men may result from differences in lifestyle or other environmental factors, but we cannot rule out the possibility of ethnic differences caused by different genetic variation or combination. To address this issue we need more information on the reproductive function in Asian men, who have been reported to have certain differences in reproductive characteristics from Caucasian men. This article is an attempt to review our present knowledge concerning the current status of semen quality in healthy Asian men on the basis of the limited publications from Asia. (Reprod Med Biol 2007; 6: 185–193)
Keywords: Asia, ethnic differences, male reproductive function, semen quality, sperm concentration
INTRODUCTION
FIFTEEN YEARS HAVE already passed since a meta‐analysis by Carlsen et al. 1 published in 1992 showed a major decline in semen quality and indications of deteriorating male reproductive health since the 1940s. During that period, there has been increasing evidence that fetal exposure to endocrine‐disrupting chemicals potentially disrupts normal reproductive development, at least in animal experiments; however, the relationship between endocrine‐disrupting chemicals and human reproductive problems has not been well understood. Moreover, numerous reports based on a retrospective analysis of semen data have still not given a definitive answer to the question of whether semen quality has declined over the past few decades. Global time‐related trends are still inconclusive, but a demonstration of regional differences in semen quality seems to be plausible. A decrease in semen quality has been reported from different geographical regions, including the USA, 2 , 3 the UK, 4 France, 5 Scotland, 6 Norway, 7 Belgium, 8 Italy 9 and Finland; 10 no change has been reported from, for example, the USA, 11 France, 12 Finland 13 and Israel. 14 In contrast, we have only a limited number of semen studies from Asia and scarcely any available Asian data for a regional comparison. The number of published papers from Asian men included in the Carlsen et al. study was only five. 15 , 16 , 17 , 18 , 19
SEMEN QUALITY OF FERTILE MEN FROM KAWASAKI/YOKOHAMA
A NUMBER OF studies have indicated regional differences in semen quality. To examine the current status in Japan, we undertook a cross‐sectional study on the semen quality of fertile Japanese men from December 1997 who were participating in an international epidemiological study entitled ‘The reproductive function of normal men from different regions of Europe – A study of partners of pregnant women’. 20 These men were the only participants from Asia. In this study we investigated the semen parameters of 324 fertile men from the Kawasaki and Yokohama (Kawasaki/Yokohama) area. 21 The semen parameters of the Japanese men were compared with those published for fertile men from the four European cities: Copenhagen, Paris, Edinburgh and Turku. When adjusting for confounders, such as ejaculation abstinence period and age, the lowest sperm concentrations were detected in men from Kawasaki/Yokohama followed by men from Copenhagen, Paris, Edinburgh and Turku (Fig. 1), but only the differences between men from Kawasaki/Yokohama and men from Edinburgh and Turku were significant (P = 0.0008 and P < 0.0001, respectively). Total sperm count, percentage of motile spermatozoa and percentage of normal spermatozoa observed in Kawasaki/Yokohama were significantly lower than the percentages from all European centers, except for motile spermatozoa in men from Paris. Japanese fertile men had a semen quality at the level of Danish men, who have been reported to have the lowest level among investigated men in Europe. To our knowledge this is the first study describing the current semen quality of fertile Japanese men based on a regional comparison with different countries. The low level of semen quality in fertile Japanese men may be because of lifestyle or other environmental factors; however, ethnic differences caused by different genetic variation or combinations cannot be ruled out by this study.
Figure 1.
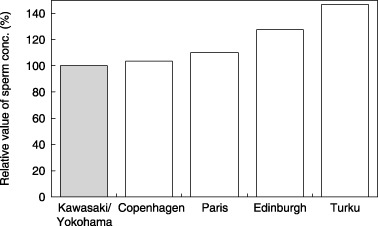
Relative differences in sperm concentration (conc.) of fertile men from five cities (Kawasaki/Yokohama, Copenhagen, Paris, Edinburgh, Turku). Results are corrected for the confounders of age and abstinence period.
TIME TREND OF SEMEN QUALITY IN ASIAN MEN
Asian populations included in the Carlsen et al. study
IN 1992 CARLSEN et al. 1 reported a significant global decline in sperm count over the past 50 years. The authors conducted a meta‐analysis of 61 studies published between 1938 and 1990, including a total of 14 947 men with proven or unknown fertility, and estimated the rate of change in mean sperm concentration as a function of publication year by fitting a simple regression model. The 61 studies included 28 studies from the USA, 17 from Europe, and 16 from non‐Western countries, including five from Africa, five from Asia, three from Latin America, two from the Middle East, and one from Australia. The five Asian studies included two Indian studies, two Hong Kong studies, and a Thai study. The mean sperm concentrations of the men from the Asian countries were 51.4 and 104.3 × 106/mL for India, 83.0 and 62.4 × 106/mL for Hong Kong, and 52.9 × 106/mL for Thailand. These values seem to be somewhat lower than those reported from other parts of the world. Subsequent to the publication of the Carlsen et al. meta‐analysis, 1 several re‐analyses of the studies included by them have been conducted. The conclusions of these analyses were either partial disapproval or confirmation of the conclusions made in the original meta‐analysis, and some authors pointed out the existence of regional differences in a time trend of sperm count after analyzing the data according to geographical region. 22 Using both a linear and non‐linear model, Swan et al. 23 showed that mean sperm concentrations were highest in Europe and lowest in non‐Western countries, and a significant decline in sperm concentration was seen in the USA and Europe, but not in non‐Western countries. The same conclusion was derived from an extended meta‐analysis of their own, including studies until 1996. 24 In non‐Western countries, where very few studies have been conducted and most are quite recent, there were insufficient data to evaluate this issue. In the Carlsen et al. meta‐analysis, only five Asian studies were included as part of the non‐Western countries. 1 To investigate any secular trend in semen quality in Asian men, we need more available data.
Healthy men with unknown fertility from Sapporo, Japan
A study group from the Sapporo Medical University compared semen quality between 253 (mean age: 24.2 ± 4.1 years) and 457 (mean age: 24.2 ± 54 years) normal, healthy volunteers who lived in Sapporo, the northernmost government‐designated city in Japan, in 1975–1980 and 1998, respectively. 25 The methods of recruiting volunteers were posters in 1975–1980 and handbills in 1998. Semen samples were collected by masturbation after 3 days or more of abstinence. There was no change in mean semen volume between 1975–1980 (2.8 ± 1.2 mL) and 1998 (2.9 ± 1.3 mL). The mean sperm concentrations were 70.9 ± 47.3 × 106/mL in 1975–1980 and 79.6 ± 49.3 × 106/mL in 1998. Although there may be various differences in the sources of populations between the two study times, the data demonstrated no significant change in sperm count between the terms of the two studies. No significant difference in the percentages of subjects with oligozoospermia or azoospermia was recognized between the two studies. The authors concluded that there was no evidence of deterioration in sperm quality of normal healthy men who lived in the Sapporo area over 20 years.
Chinese fertile men (meta‐analysis)
Zhang et al. 26 analyzed the change in mean sperm concentration in Chinese fertile men from 1983 to 1996. Semen data were collected from 114 published papers, including 256 set data from 9292 men and 11 726 semen assays from 39 cities and countries. The results of the analysis showed that the mean sperm concentration decreased from 103.02 × 106/mL in 1983 to 83.84 × 106/mL in 1996. Zhang et al. 26 stated that although the sperm quality of fertile men in China was better compared with reports from other countries for the same period, the speed of decline (coefficient of regression and decrease rate per year) was higher.
Artificial insemination by donors from university students in Tokyo, Japan (retrospective study)
A group from Keio University Hospital investigated the record of semen quality in artificial inseminations by donor (AID) (K. Sueoka et al. unpubl. data, 1998–2000). Inclusion criteria for AID candidates were as follows: age 20–25 years, semen volume 2 mL or more, sperm concentration 50 million/mL or more, and a sperm motility rate of 50% or more. These researchers compared semen data from the 1970s, 1980s and 1990s, 6048 samples in total. The mean sperm concentration was 65 × 106/mL in the 1970s and 57 × 106/mL after 1990. These researchers concluded that sperm count showed a tendency to decrease over the whole period, and this decrease was more significant in the period after 1990 than between 1970 and 1989. As for sperm motility, a slight but significant tendency to decrease was demonstrated in the period from 1970 to 1989, but no significant decrease was shown after 1990.
REVIEW OF REPRODUCTIVE PARAMETERS IN ASIAN HEALTHY MEN
IN THIS ARTICLE we limited ourselves to citing published papers dealing with male reproductive parameters in normal men (fertile men and healthy men with unknown fertility), and we excluded studies including infertile men. The papers were collected by a search of Medline.
Testicular size
As it has been reported that daily sperm production and sperm count are positively correlated with testicular size, a measurement of testicular size gives us important information on male reproductive function. Several studies have documented ethnic differences in testis size: smaller in Asian men than in Caucasian men. 27 , 28 Short 29 found smaller testes in Japanese and Korean men than in Caucasians. To compare testicular size among different Asian studies, we selected studies that used the Prader orchidometer, which is the most widely used instrument for measuring testicular volume. The mean testicular volumes (average of the left and right testis) of normal Korean men, 30 fertile Chinese men, 17 fertile Thai men 18 and normal Chinese men 17 were 19.4, 17.7, 17.2 and 17.0 mL, respectively. These values are smaller than those from a European study showing that the mean testicular volumes in fertile men from four European cities were 23.5 mL (Copenhagen), 23.0 mL (Edinburgh), 22.5 mL (Paris) and 23.0 mL (Turku). In our study of fertile Japanese men, the mean testicular volume was 21.5 mL. 21 This value is larger than any value found in the Asian studies discussed here, but it is still smaller than the values recorded from the four European cities. These results are consistent with those of previous studies on ethnic differences in testis size. Diamond 28 compared the testicular size of men from various countries around the world and reported that although measurements of testis size by orchidometry in living subjects were difficult to standardize, testis size in Japanese and Korean men was smaller than in Caucasians, and testis weight at autopsy was two‐fold lower in two Hong Kong Chinese samples compared with a Danish sample. However, some studies have questioned the reliability of comparative data on testicular size. Carlsen et al. 31 and Tatsunami et al. 32 have pointed out that inter‐investigator differences in the results of testicular measurements are considerable, and Hargreave 33 has documented that racial differences in testicular size are presumed to relate to differences in body weight. We should keep this problem in mind when data of testicular volume from different studies are compared.
Semen volume
At the time of ejaculation spermatozoa are admixed with secretions from the seminal vesicle and the prostate, which are both androgen target organs. Therefore, semen volume is an important parameter for evaluating male reproductive function.
Although the World Health Organization (WHO) manual 34 has recommended that semen volume be measured either with a graduated cylinder or by aspirating the entire sample into a graduated syringe or pipette, a European study 20 adopted weighing of all the semen together with the container for assessing volume as a more accurate measure. We compared the mean semen volumes assessed using two different methods, aspirating and weighing, and determined the difference between the two to be 0.49 mL, which was obtained from 102 semen samples of fertile Japanese men. 21 This discrepancy should be considered when comparing data of semen volumes between different laboratories. According to our comparison of the results from different Asian studies, the mean semen volumes of China (north/south/central), Thailand, Singapore and India were 3.0/2.9/2.4, 2.5, 2.4 and 2.5 mL, respectively. 35 In contrast, semen volume of the fertile Japanese men was 3.2 mL; 20 which is the highest value among the Asian men examined here. These values from Asian men appear to be lower than those from European men 20 (4.2, 4.1, 3.9 and 3.8 mL for French, Finnish, Scottish and Danish men, respectively). Semen volume might have regional or racial differences not only between Asian and Caucasian men, but also among Asian men. It is well known that semen volume increases as the abstinence period becomes longer. Therefore, we must consider the influence of this confounding factor when analyzing semen volume as a male reproductive parameter.
Sperm concentration
Many of the studies examining regional differences in sperm concentration have been carried out in the USA and Europe. Only a small number of studies have been conducted in Asia and other non‐Western countries. We must keep in mind that we should address data on sperm concentration carefully when comparing between various studies from different Asian countries because there are many confounding factors, such as age, sexual abstinence period, season, fertility status (proven or unknown), and specimen collection method; which are all known to have a significant influence on sperm concentration. For reference, we show the mean sperm concentration of normal Asian men from various geographical areas in Table 1, 17 , 18 , 25 , 36 , 37 , 38 , 39 , 40 , 41 , 42 and illustrate their geographical distribution in Fig. 2. Except for Japanese men in Kawasaki/Yokohama, Asian men seem to have a lower sperm concentration; all concentrations are below 100 × 106/mL. Even in the men from Kawasaki/Yokohama, a low level of sperm concentration was shown after the multivariate regression analyses were adjusted for known confounding factors. 20
Table 1.
Studies reporting sperm concentrations in different areas in Asia
| Fertility status | Authors (reference) | Year of publication | Location/Country | No. subjects | Mean sperm concentration (×106/mL) |
|---|---|---|---|---|---|
| Normal men (unknown fertility) | Ikegaki et al. 36 | 1984 | Sapporo/Japan | 254 | 71 |
| Wang et al. 17 | 1985 | Hong Kong | 1157 | 84 | |
| Ito et al. 25 | 2001 | Sapporo/Japan | 457 | 80 | |
| Junquing et al. 37 | 2002 | Seven areas†/China | 562 | 55 | |
| Danadevi et al. 38 | 2003 | South India/India | 57 | 63 | |
| Gao et al. 39 | 2007 | Six areas‡/China | 1191 | 65¶ | |
| Fertile men | Wang et al. 17 | 1985 | Hong Kong | 82 | 71 |
| Aribarg et al. 18 | 1986 | Bangkok/Thailand | 307 | 53 | |
| Chia et al. 40 | 1998 | Singapore | 243 | 45§ | |
| Baba et al. 41 | 2000 | Kawasaki & Yokohama/Japan | 255 | 108 | |
| Pal et al. 42 | 2007 | New Delhi/India | 368 | 68 |
Shanghai, Henen, Zhejiang, Shanxi, Shandon, Hebei and Guizhou.
Hebei, Henen, Shanxi, Zhejiang Qindao and Guizhou.
Geometric mean.
Median.
Figure 2.
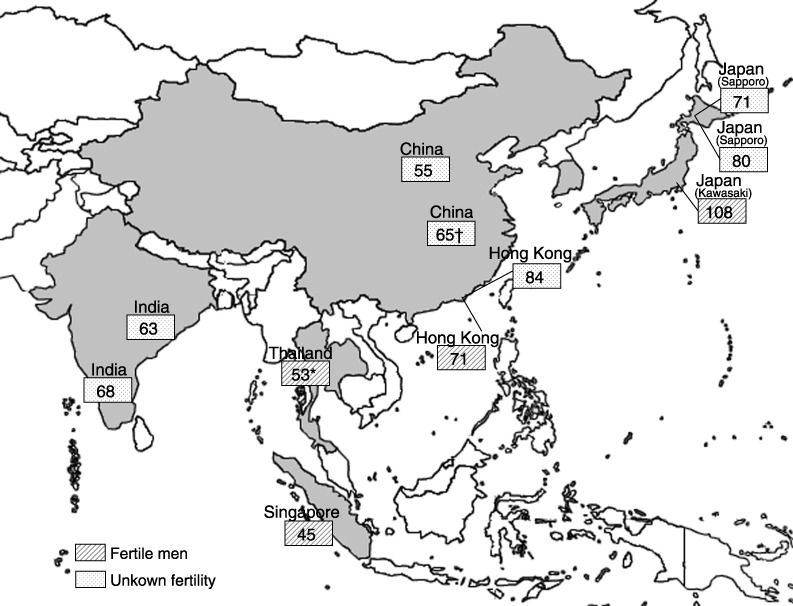
Mean sperm concentration (×106/mL) in healthy men from different areas in Asia. (Exception: *geometric mean; †median).
In Singapore it has been shown that there is no significant month‐to‐month fluctuation in semen volume and sperm concentration in fertile men who reside in the tropics. 35 The temperature in Singapore is relatively stable throughout the year because of its close proximately to the equator; average maximum and minimum temperatures are approximately 32°C and 25°C, respectively. 35 Variations in semen volume and sperm concentration from temperate climates were not observed in this study. These researchers concluded this result might be related to relatively constant temperatures and hours of light exposure in the tropics. Chia et al. 40 reported that there were no significant differences in the semen parameters among north, central and south Chinese, Malays, Indians and other races. Padungtod et al. 43 investigated the association between occupational pesticide exposure and semen quality in Chinese workers. In this study, the mean sperm concentration of 43 men from the control group, who had no history of exposure to organophosphate pesticides, was reported to be 62.8 × 106/mL.
Sperm motility
Mean percentage values of motile spermatozoa in Asian men reviewed in this article were all 50% or more (the normal range as defined in the WHO manual 34 ) and ranged from 55 to 60%. In the WHO manual, 34 a motility grading system is recommended, where the microscopic field is scanned systematically and the motility of each spermatozoon is graded ‘a’ (rapid progressive motility), ‘b’ (slow or sluggish progressive motility), ‘c’ (non‐progressive motility) or ‘d’ (immotility). Sperm motility is usually expressed as a percentage of the sum of spermatozoa in category ‘a’ and ‘b’. To compare the absolute values of sperm motility between different studies may be less meaningful because sperm motility assessment is difficult to standardize. Jorgensen et al. 20 have stated that despite attempts to standardize motility assessments between laboratories, inter‐technician variation might still be considerable.
Abstinence period
A number of studies have reported that a longer abstinence period has an increasing effect on semen volume and sperm concentration. 44 , 45 , 46 Figure 3 shows the relationship between abstinence period and sperm concentration. This figure is based on the semen data of 324 fertile Japanese men. 21 Our study also showed a significant influence of abstinence period on sperm concentration. We reported that the sperm concentration of 324 fertile men from Kawasaki/Yokohama in Japan was at the same low level as Danish men from Copenhagen based on multivariate regression analyses adjusted for known confounding factors. 21 In contrast, in a preliminary report of the ongoing study, we found that the first 255 included men from the Kawasaki/Yokohama area had a high sperm concentration as raw data unadjusted for confounding factors. 41 The opposite conclusion was drawn after the multivariate regression analyses adjusted for abstinence period as a major confounder. High sperm concentrations in raw data from the Japanese men included considerable increments caused by longer abstinence period; however, the Japanese men had lower sperm concentrations compared with the men from the four European cities once the abstinence effect was removed by the correction. 21 The Japanese men had a longer abstinence period (mean value 208 h) than the men from the European cities (mean value 81 h for the Danish, 109 h for the Finnish, 156 h for the Scottish and 157 h for the French). 20 , 21
Figure 3.
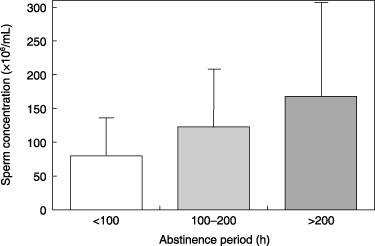
Relationship between abstinence period and sperm concentration. Result is based on the semen data of 324 fertile Japanese men. 21
The duration of the abstinence period may differ between age groups or groups with different fertility status. The mean abstinence periods of Japanese fertile men (mean age 32.3 ± 4.5 years) 21 and healthy young men with unknown fertility (20.4 ± 1.4 years; T. Iwamoto et al. unpubl. data, 1999–2000) were 208 h and 75 h, respectively. Differences in the abstinence period between the two groups could be explained by differences in lifestyle, including sexual activity. In contrast, in China, the opposite results were obtained: the mean abstinence periods of fertile men (43.0 ± 4.93 years) and healthy young men (28.7 ± 4.73 years) were 106.8 h and 158.9 h, respectively. 17
Despite the fact that abstinence period is a significant confounding factor for semen variables many studies have not specified the mean abstinence period of their subjects. Only a small number of Asian studies provided data on the abstinence period.
DISCUSSION
SKAKKEBÆK ET AL. AND other groups have pointed out that the significance of the detected regional differences in semen quality is associated with regional incidences of testicular cancer. 47 , 48 , 49 Denmark has been suggested to be the worst‐case scenario with respect to male reproductive health, with Danish men having a low semen quality and a high risk of testicular cancer. 50 The so‐called testicular dysgenesis syndrome (TDS) hypothesis implies that exogenous, environmental factors may interfere with normal testicular development, and lead not only to a reduced sperm count and increased risk of testicular cancer in adult life, but also at birth to an increased risk of hypospadias and undescended testes. 49 , 51 , 52 , 53 Therefore, we should pay attention to various indications relating to male reproductive health when considering circumstances in Asian men.
Our cross‐sectional study of Japanese men showed that fertile men from Kawasaki and Yokohama had a sperm concentration almost at the same level as the Danish men, who had the lowest count among the European men studied, whereas the Finnish men had the highest. 21 Although the Japanese men showed low levels in semen quality like Danish men, the incidence of testicular cancer is considerably lower, 46 , 47 , 48 approximately at the same low level as the Finnish men who were detected to have the best semen quality in the European study. 54 , 55
Comparatively low levels of sperm concentration in Asian men were suggested from various Asian studies. This may reflect ethnic differences rather than impaired male reproductive health. Although we have no available data from Japan and other Asian countries to elucidate the possible influence of ethnicity on semen quality, some reports have shown lower testis parenchymal weight, 56 lower 5 alpha‐dihydro testosterone levels despite similar serum testosterone levels, 57 , 58 lower testosterone production rate, 59 and longer CAG repeats in the androgen receptor gene 60 in Asian men compared with Hispanic or non‐Hispanic white men. The finding by Chia et al. 40 that Chinese, Malays and Indians have low sperm counts is in line with our findings, indicating low figures in Asian men. Wang et al. 17 investigated differences in reproductive functions in Asian men living in Asia and white men living in the USA. In consequence, the combination of smaller testes volumes, 17 elevated blood follicle stimulating hormone levels, 61 small decreases in daily sperm production and Sertoli cell numbers, together with an increased apoptotic germ cell rate in Asia, 62 have suggested that the spermatogenic reserve might be relatively reduced in Asian men compared with white men. Possible explanations for these observations may be differences in genetically and/or environmentally determined levels of peripheral testosterone metabolism. 63 Furthermore, dietary factors, and physical and sexual activity may also be related to the observed differences in reproductive parameters between Asian and Caucasian white men.
Asia is the region with the highest population on earth; however, only a small number of semen studies have been conducted. Except for our Japanese study, we have no epidemiological data on regional differences in semen quality from Asia. Comparable data of Asian men with different cultural, nutritional, lifestyle and genetic backgrounds are valuable to explore the factors responsible for possible adverse effects on male reproductive health. For this purpose it is necessary to conduct well‐designed international coordinated studies on male reproductive function, including a broader representation of Asian populations.
ACKNOWLEDGMENT
THIS STUDY WAS supported by the Ministry of Health and Welfare, Japan (Health Scientific Grant No. 1013201).
REFERENCES
- 1. Carlsen E, Giwercman A, Keiding N, Skakkebaek N. Evidence for decreasing quality of semen during past 50 years. BMJ 1992; 305: 609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leto S, Frensilli FJ. Changing parameters of donor semen. Fertil Steril 1981; 36: 766–770. [DOI] [PubMed] [Google Scholar]
- 3. Swan SH, Brazil C, Drobinis EZ et al Geographic differences in semen quality of fertile US males. Environ Health Perspect 2003; 111: 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ginsburg J, Okolo S, Prelevic G, Hardiman P. Residence in the London area and sperm density. Lancet 1994; 343: 230. [DOI] [PubMed] [Google Scholar]
- 5. Auger J, Kunstmann JM, Czyglik F et al Decline in semen quality among fertile men in Paris during the past 20 years. New Eng J Med 1995; 332: 281–285. [DOI] [PubMed] [Google Scholar]
- 6. Irvine RA, Cawood E, Richardson D, MacDonald E, Aitken J. Evidence of deteriorating semen quality in the United Kingdom: birth cohort study in 577 men in Scotland over 11 years. BMJ 1996; 24: 467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bendvold E. Semen quality in Norwegian men over a 20‐year period. Int J Fertl 1989; 34: 401–404. [PubMed] [Google Scholar]
- 8. Van Waeleghem K, De Clercq N, Vermeulen L, Shoonjans F, Comhaire F. Deterioration of sperm quality in young healthy Belgian men. Hum Reprod 1996; 11: 325–329. [DOI] [PubMed] [Google Scholar]
- 9. Bilotta P, Guglielmo R, Steffe M. Analysis of decline in seminal fluid in the Italian population during the past 15 years. Minerva Gineco 1999; 51: 223–231. [PubMed] [Google Scholar]
- 10. Horte A, Vierula M, Toppari J, Suominen J. Reassessment of sperm morphology of archival semen smears from the period 1980–94. Int J Androl 2001; 24: 120–124. [DOI] [PubMed] [Google Scholar]
- 11. Fisch H, Goluboff E, Olson J, Feldshuh J, Broder S, Barad D. Semen analyses in 1283 men from the United States over a 25‐year period: No decline in quality. Fert Steril 1996; 65: 1009–1014. [DOI] [PubMed] [Google Scholar]
- 12. Bujan L, Mansat A, Pontonnier F, Mieusset R. Time series analysis of sperm concentration in fertile men in Toulouse, France between 1977 and 1992. BMJ 1992; 312: 471–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suominen J, Vierula M. Semen quality of Finnish men. BMJ 1993; 306: 1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benshushan A, Shoshanai O, Paltiel O, Schenker JG, Lewin A. Is there really a decrease in sperm parameters among healthy young men? A survey of sperm donations during 15 years. J Assist Reprod Genet 1997; 14: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brushan S, Pandey RC, Singh SP, Pandey DN, Seth P. Some observations on human semen analysis. Indian J Physiol Pharmacol 1978; 22: 393–396. [PubMed] [Google Scholar]
- 16. Roy S, Chatterjee S. Studies with cyproterone acetate for male contraception. J Steroid Biochem 1979; 11: 675–680. [DOI] [PubMed] [Google Scholar]
- 17. Wang C, Chan SYW, Leung A. Cross‐sectional study of semen parameters in a large group of normal Chinese men. Int J Androl 1985; 8: 257–274. [DOI] [PubMed] [Google Scholar]
- 18. Aribarg A, Kenkeerati W, Vorapaiboonsak V, Leepipatpaiboon S, Farley TM. Testicular volume, semen profile and serum hormone levels in fertile Thai males. Int J Androl 1986; 9: 170–180. [DOI] [PubMed] [Google Scholar]
- 19. Chan SYW, Wang C. Correlation between semen adenosine triphosphate and sperm fertilizing capacity. Fertil Steril 1987; 47: 717–719. [PubMed] [Google Scholar]
- 20. Jørgensen N, Andersen AG, Eustache F et al Regional differences in semen quality in Europe. Hum Reprod 2001; 16: 1012–1019. [DOI] [PubMed] [Google Scholar]
- 21. Iwamoto T, Nozawa S, Yoshiike M et al Semen quality of 324 fertile Japanese men. Human Reprod 2006; 21: 760–765. [DOI] [PubMed] [Google Scholar]
- 22. Giwercman A, Bonde JP. Declining male fertility and environmental factors. Endocrinol Metab Clin North Am 1998; 27: 807–830. [DOI] [PubMed] [Google Scholar]
- 23. Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect 1997; 105: 1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–96. Environ Health Perspect 2000; 108: 961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Itoh N, Kayama F, Tatsuki J, Tsukamoto T. Have sperm counts deteriorated over the past 20 years in healthy, young Japanese men? Results from the Sapporo area. J Androl 2000; 22: 40–44. [PubMed] [Google Scholar]
- 26. Zhang SC, Wang HY, Wang JD. Analysis of change in sperm quality of Chinese fertile men during 1981–96. Reprod Contracept 1999; 10: 33–39. [PubMed] [Google Scholar]
- 27. Mittwoch U. Ethnic differences in testis size: a possible link with the cytogenetics of true hermaphroditism. Hum Reprod 1988; 3: 445–449. [DOI] [PubMed] [Google Scholar]
- 28. Diamond JM. Variation in human testis size. Nature 1989; 320: 488–489. [DOI] [PubMed] [Google Scholar]
- 29. Short RV. One Medicine. Berlin: Springer, 1984. [Google Scholar]
- 30. Kim DH, Lee HY. Clinical investigation of testicular size. I. Testis size of normal Korean male. J Korean Med Ass 1982; 25: 135. [Google Scholar]
- 31. Carlsen E, Andersen AG, Buchreitz L et al Inter‐observer variation in the results of the clinical andrological examination including estimation of testicular size. Int J Androl 2000; 23: 248–253. [DOI] [PubMed] [Google Scholar]
- 32. Tatsunami S, Matsumiya K, Tsujimura A et al Inter/intra investigator variation in orchidometric measurements of testicular volume by ten investigators from five institutions. Asian J Androl 2006; 8: 373–378. [DOI] [PubMed] [Google Scholar]
- 33. Hargreve TB. Male Infertility. Berlin: Springer‐Verlag, 1983. [Google Scholar]
- 34. World Health Organization . WHO Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction, 4th edn. Cambridge: Cambridge University Press, 1999. [Google Scholar]
- 35. Chia SE, Lim ST, Ho LM, Tay SK. Monthly variation in human semen quality in male partners of infertile women in the tropics. Hum Reprod 2001; 16: 277–281. [DOI] [PubMed] [Google Scholar]
- 36. Ikegaki S, Maruta H, Ohno K, Kumamoto Y. Studies on seminal characteristics obtained from 254 healthy young adults and effects of frequent ejaculation on seminal quality. Jpn J Fertil Steril 1984; 29: 301–309. [Google Scholar]
- 37. Junqing W, Qiuying Y, Jianguo T et al Reference value of semen quality in Chinese young men. Contraception 2002; 65: 365–368. [DOI] [PubMed] [Google Scholar]
- 38. Danadevi K, Rozati R, Reddy PP, Grover P. Semen quality of Indian welders occupationally exposed to nickel and chromium. Reprod Toxicol 2003; 17: 451–456. [DOI] [PubMed] [Google Scholar]
- 39. Gao J, Gao ES, Yang Q et al Semen quality in a residential, geographic and age representative sample of healthy Chinese men. Hum Reprod 2007; 22: 477–484. [DOI] [PubMed] [Google Scholar]
- 40. Chia SE, Tay SK, Lim ST. What constitutes a normal seminal analysis? Semen parameters of 243 fertile men. Hum Reprod 1998; 13: 3394–3398. [DOI] [PubMed] [Google Scholar]
- 41. Baba K, Nishida T, Yoshiike M, Nozawa S, Hoshino T, Iwamoto T. Current status of reproductive function in Japanese fertile men: international collaborative project on a study of partners of pregnant women. Int J Androl 2000; 23: 54–56. [DOI] [PubMed] [Google Scholar]
- 42. Pal PC, Rajalakshmi M, Manocha M, Sharma RS, Mittal S, Rao DN. Semen quality and sperm functional parameters in fertile Indian men. Andrologia 2006; 38: 20–25. [DOI] [PubMed] [Google Scholar]
- 43. Padungtod C, Savitz DA, Overstreet JW, Christina DC, Ryan LM, Xu X. Occupational pesticide exposure and semen quality among Chinese workers. J Occup Environ Med 2000; 42: 982–992. [DOI] [PubMed] [Google Scholar]
- 44. Pellestor F, Girardet A, Andreo B. Effect of long abstinence periods on human sperm quality. Int J Fertil Menopausal Stud 1994; 39: 278–282. [PubMed] [Google Scholar]
- 45. Blackwell JM, Zaneveld LJ. Effect of abstinence on sperm acrosin, hypoosmotic swelling, and other semen variables. Fertil Steril 1992; 58: 798–802. [DOI] [PubMed] [Google Scholar]
- 46. Schwartz D, Laplanche A, Jouannet P, David G. Within‐subject variability of human semen in regard to sperm count, volume, total number of spermatozoa and length of abstinence. J Reprod Fertil 1979; 57: 91–95. [DOI] [PubMed] [Google Scholar]
- 47. Skakkebaek NE, Berthelsen JG, Giwercman A, Muller J. Carcinoma‐in‐situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl 1987; 10: 19–28. [DOI] [PubMed] [Google Scholar]
- 48. Skakkebaek NE, Rajpert‐De Meyts E, Jørgensen N et al Germ cell cancer and disorders of spermatogenesis: an environmental connection? APMIS 1998; 106: 3–11. [DOI] [PubMed] [Google Scholar]
- 49. Skakkebaek NE, Rajpert‐De Meyts E, Main KM. Testicular dysgenesis syndrom (TDS): an increasingly common developmental disorder with environmental aspects. Hum Reprod 2001; 16: 972–978. [DOI] [PubMed] [Google Scholar]
- 50. Sharpe RM. The ‘oestrogen hypothesis’– where do we stand now? Int J Androl 2003; 26: 2–15. [DOI] [PubMed] [Google Scholar]
- 51. Adami HO, Bergstrom R, Mohner M et al Testicular cancer in nine northern European countries. Int J Cancer 1994; 59: 33–38. [DOI] [PubMed] [Google Scholar]
- 52. Toppari J, Larsen J, Christiansen P et al Male reproductive health and environmental xenoestrogens. Environ Health Perspect 1996; 104: 741–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oshima A, Kitagawa T, Ajiki W, Tsukuma H, Takenaka S, Iura A. Survival of testicular cancer patients in Osaka. Japan Jpn J Clin Oncol 2001; 31: 438–443. [DOI] [PubMed] [Google Scholar]
- 54. Boisen KA, Kaleva M, Main KM et al Difference in prevalence of congenital cryptorchidism in infant between two Nordic countries. Lancet 1994; 363: 1264–1269. [DOI] [PubMed] [Google Scholar]
- 55. Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. J Urol 2003; 170: 5–11. [DOI] [PubMed] [Google Scholar]
- 56. Johnson L, Barnard JJ, Rodriguez L et al Ethnic differences in testicular structure and spermatogenic potential may predispose testes of Asian men to a heightened sensitivity to steroidal contraceptives. J Androl 1998; 19: 348–357. [PubMed] [Google Scholar]
- 57. Ross RK, Bernstein L, Lobo RA, S et al 5‐alpha‐reductase activity and risk of prostate cancer among Japanese and US white and black males. Lancet 1992; 339: 887–889. [DOI] [PubMed] [Google Scholar]
- 58. Lookingbill DP, Demers LM, Wang C, Leung A, Rittmaster RS, Santen RJ. Clinical and biochemical parameters of androgen action in normal healthy Caucasian versus Chinese subjects. J Clin Endocrinol Metab 1991; 72: 1242–1248. [DOI] [PubMed] [Google Scholar]
- 59. Santner SJ, Albertson B, Zhang GY et al Comparative rates of androgen production and metabolism in Caucasian and Chinese subjects. J Clin Endocrinol Metab 1998; 83: 2104–2109. [DOI] [PubMed] [Google Scholar]
- 60. Irvine RA, Yu MC, Ross RK, Coetzee GA. The CAG and GGC microsatellites of the androgen receptor gene are in linkage disequilibrium in men with prostate cancer. Cancer Res 1995; 55: 937–940. [PubMed] [Google Scholar]
- 61. Wang C, Berman N, Veldhuis JD. Graded testosterone onfusions distinguish gonadotropin negative‐feedback responsiveness in Asian and white men – A clinical research center study. J Clin Endcri Metab 1998; 84: 870–876. [DOI] [PubMed] [Google Scholar]
- 62. Sinha‐Hikim AP, Lue YH, Wang C, Wang XH, Johnson L, Swerdloff RS. Spontaneous germ cell apoptosis in humans. J Clin Endocrinol Metab 1998; 83: 152–156. [DOI] [PubMed] [Google Scholar]
- 63. Van Houten ME, Gooren LJ. Differences in reproductive endocrinology between Asian men and Caucasian men. Asian J Androl 2000; 2: 13–20. [PubMed] [Google Scholar]


