Abstract
Purpose
To study assisted reproductive technology (ART) protocols including superovulation, in vitro fertilization (IVF) and in vitro development (IVD) for BALB/cJ mice in comparison with a common ART protocol for NMRI mice.
Methods
Adult NMRI and BALB/cJ mice were superovulated using a 48 h G‐interval. In order to find a more suitable G‐interval for the BALB/cJ strain, G‐intervals including 44, 46 and 50 h were also examined. Superovulation rates were recorded in all groups. IVF rate of BALB/c oocytes in T6 and mHTF media were compared. IVD rates of BALB/cJ zygotes in mHTF, T6 and G1V5/G2V5 media were compared. In addition, IVF and IVD rates of BALB/cJ and NMRI oocytes were compared in T6 medium during IVF–IVD procedures.
Results
In BALB/cJ mice the highest superovulation rates were observed with 44–46 h G‐intervals. However, with a 48 h G‐interval, superovulation rates were significantly lower in BALB/cJ compared to NMRI mice (p < 0.05). mHTF medium significantly increased in vitro fertilization of BALB/cJ oocytes compared to T6 medium (p < 0.05). Fertilization rate of NMRI oocytes was significantly higher than BALB/cJ oocytes in T6 medium (p < 0.05). The BALB/cJ embryo IVD was significantly higher in G1/G2 medium compared to mHTF and T6 media (p < 0.01).
Conclusions
Superovulation with 48 h G‐interval and using T6 during all in vitro procedures produces embryos more efficiently for NMRI mice than for BALB/cJ mice. For BALB/cJ mice, a protocol including superovulation with a 44–46 h G‐interval, using mHTF during IVF and G1V5/G2V5 medium during IVD, may improve in vitro embryo production.
Keywords: In vitro development (IVD), In vitro fertilization (IVF), Medium, Mouse strain, Superovulation
Introduction
The inbred BALB/c mouse strain was established in 1935 [1] and is widely used for genetic research [2] and genetic engineering [3]. This strain has also been used in tumor and immunologic research. BALB/c is also one of the most important inbred mouse strains for mutagenesis and transgenesis research [3]. This wide range of applications is related to its clear genetic background; the strain has 99 % homozygosity, resulting in depletion of the reproductive outcome [4].
Production of embryos following assisted reproductive technologies (ARTs) depends on efficient ovulation induction, efficient in vitro fertilization (IVF) and efficient in vitro culture [5]. Superovulation is a hormonal treatment that induces follicular growth and ovulation of mature oocytes. There are many factors affecting ovulation induction including age, species [6], strain [5, 7], hormone dosage [8] and G‐interval delivery [7]. Genetic composition has been shown to be the major effective parameter for superovulation response [9]. It was reported that superovulation of mice has a strong correlation with strain and with proper interval of gonadotropin injection [10]. It has been suggested that gonadotropin interval (G‐interval) delivery affects superovulation response and should be determined for each mouse strain [11, 12]. In addition to superovulation, culture medium is another important parameter that influences the success of experiments [13, 14]. It was reported that the BALB/c strain and its subtypes are difficult to use for IVF as evidenced by low IVF outcome [15]. This has been attributed to the high incidence of abnormal sperm [16] and suboptimal conditions for sperm capacitation and insemination [17].
The most common medium used for mouse IVF is the T6 medium [18]. Human tubular fluid (HTF) medium is used for human IVF [19], and mouse ova have been successfully fertilized in the HTF medium [20]. In addition, G1, which is a commercial and synthetic medium, has been used for mouse in vitro development (IVD) [21]. According to Bayers et al. [5], IVF and IVD vary within the same protocol for different mouse strains. Reports exist regarding the use of various species and strains of laboratory animals on superovulation [22, 23, 24, 25], IVF [26, 27, 28] and IVD [26, 29, 30] protocols.
Outbred stocks of mouse are often used erroneously in toxicology, pharmacology and basic research, though there there may not be exact information about genetic or phenotypic characteristics [31]. Use of inbred laboratory animal strains improves the repeatability of research results in different laboratories or within the same laboratory over a given period of time, due to the elimination of genetic sources of variance [32]. Because of the wide range of applications of the BALB/c mouse strain in different scientific fields, fertility preservation in this strain is necessary. Furthermore, using this inbred strain for reproductive research, including studies of ARTs, may improve the repeatability and accuracy of results. In this research, we evaluated the effect of G‐interval delivery on superovulation rate in addition to the effects of different media on in vitro embryo production during IVF and IVD for BALB/cJ mice. These evaluations were undertaken with the use of a common protocol in previous research [33, 34, 35, 36, 37] using Naval Medical Research Institute (NMRI) mice, which is an outbred mouse stock [31]. The common protocol included a 48 h G‐interval [11, 12, 34, 35] and used T6 as a medium during the IVF and IVD processes [34, 35].
Materials and methods
Unless indicated, all chemicals were purchased from Sigma (St Louis, MO, USA).
Animals
Male and female BALB/cJ and NMRI mice were purchased from the Pasteur Institute (Tehran, Iran). Animal experiments were carried out according to the Declaration of Helsinki and the Guiding Principles in the Care and Use of animals (DHEW publication, NIH, 80–23). Animals were kept in a 12 h light/12 h dark photoperiod at a controlled temperature (22 ± 2 °C) and humidity of 50 % with ad libitum access to both water and food. For all experiments during sperm and oocyte recovery, male and female mice were euthanized by cervical dislocation.
Experimental procedure
The experiment consists of three steps: Step I (superovulation), Step II (IVF) and Step III (IVD).
Step I: superovulation
154 BALB/cJ female mice and 30 NMRI female mice (6–8 weeks old, 20–25 g weight) were treated with peritoneal injections of 7.5 IU pregnant mare serum gonadotropin (PMSG) (Folligon, Intervet) and 7.5 IU human chorionic gonadotropin (hCG) (Pregnenlone, intervet). Gonadotropins were injected without regard to the estrus cycle [38]. Mice that released more than 8–15 oocytes were considered superovulated [12]. Female BALB/cJ and NMRI mice were superovulated according to standard protocol (7.5 IU gonadotropin with 48 h G‐interval delivery) and superovulation rates were compared between the two strains. In order to find a more suitable G‐interval, female BALB/cJ mice were divided into four groups and superovulated based on the time interval of delivering PMSG injection followed 44, 46, 48 and 50 h later by hCG injections. In all groups and both strains, hCG injection was at 5 p.m. and about 16 h after hCG injection, BALB/cJ and NMRI oocytes were recovered by rupturing oviducts using 26‐gauge needles. The percentage of superovulated mice and numbers of total ovulated oocytes were recorded immediately after oocyte recovery. After a gentle pipetting, oocytes were evaluated for presence of the first polar body by inverted microscope. The number of total ovulated oocytes and completely matured oocytes (indicated by the presence of a polar body), were recorded and the best G‐interval was determined.
Step II: in vitro fertilization
Female BALB/cJ mice were ovulated according to a suitable interval delivery which was determined from step I, and female NMRI mice were superovulated with a 48 h G‐interval (common protocol). Epididims were removed from adult BALB/cJ and NMRI males and transferred to mHTF or T6 media. Capacitated BALB/cJ sperm in T6 medium were added to a T6 medium droplet containing BALB/cJ oocytes (group A). Capacitated BALB/cJ sperm in mHTF medium were transferred to a mHTF medium droplet containing BALB/cJ oocytes (group B). NMRI capacitated sperm were transferred to a T6 medium droplet containing NMRI oocytes (group C). An incubation period of approximately 2 h was considered sufficient for sperm capacitation. About 2 × 106 Sperm/ml was added to each IVF droplet. After 4–6 h incubation of oocytes and sperm, fertilization rate was recorded. Sperm capacitation and IVF incubation was in 5 % CO2, 37 °C with saturated humidity. In order to evaluate the fertilization rate, sperm and granulosa cells were gently removed by pipetting and the number of ova with male and female pronuclei (2PNs) were counted. Composition of mHTF and T6 media are shown in Table 1. The composition of T6 medium is according to Uranga and Arechaga [30] and mHTF composition is based on a report by Kito and Ohta [28]. mHTF was partially modified by addition of HEPES to make a HEPES buffered culture medium, which has been used for prevention of pH fluctuation in room air during washing and transferring to developmental medium [39]. The PH of mHTF before incubation was 8 and after incubation it was reduced to 7.3 (in the physiological range). Both media were prepared by deionized water and filtered through 0.22 μm sterile filters. Media were stored serum‐free. Serum was added before incubation of media and each medium was incubated overnight in 5 % CO2, 37 °C with saturated humidity. For IVF, the mHTF medium was supplemented with 4 mg/ml bovine serum albumin (BSA) (EC 232‐936‐2) and the T6 medium was supplemented with 15 mg/ml BSA. The IVF media were applied as 100 μl droplets covered with mineral oil. IVF droplets were about 100 μl and covered with mineral oil.
Table 1.
Media composition (mM/ml)
| T6 | HTF | |
|---|---|---|
| NaCl | 80.77 | 101.61 |
| KCL | 1.48 | 4.69 |
| NaH2PO4 | 0.39 | – |
| KH2PO4 | – | 0.40 |
| MgCl2 | 0.49 | – |
| MgSO4 | – | 0.20 |
| CaCl2 | 1.77 | 5.14 |
| NaHCO3 | 25.00 | 25.00 |
| Na lactate | 23.29 | 18.36 |
| Na pyruvate | 0.27 | 0.34 |
| d‐glucose | 5.55 | 2.70 |
| Penicillin a | 62.80 | 62.80 |
| Streptomycin a | 50.00 | 50.00 |
| Phenol red | 0.01 | 0.01 |
| HEPES | – | 2.49 |
| BSA a | 15.00 | 4.00 |
| EDTA | 0.02 | 0.01 |
aMaterials are in mg/ml
Step III: in vitro development
All procedures of media preparation and incubation were the same as those in step II. In order to determine the best media among T6, mHTF and G1V5 for BALB/cJ embryos IVD, 2PNs were produced by media with the best results of IVF in step II. For group A, some 2PNs were allowed to remain in mHTF medium and others were transferred into T6 (group B) and G1V5/G2V5 (group C) media. In group D, T6 was used during all in vitro procedures (sperm capacitation, IVF and IVD). For NMRI mice, all 2PNs directed from fertilized oocytes in T6 medium were transferred to IVD droplets of T6 medium (group E). For IVD, both mHTF and T6 media were supplemented with 4 mg/ml BSA. The G1V5 medium (Vitrolife) was supplemented with 100 μl/ml human serum albumin (HSA) (Vitrolife). IVD droplets were 15 μl and covered by mineral oil. Embryo development was recorded by counting 2‐ and 4‐cell, morula, blastocyst and hatched blastocyst embryos, respectively, 24, 48, 72, 96 and 192 h after insemination. Finally, IVD rates in all groups were compared to each other and IVD rates in BALB/cJ and NMRI mice were compared when T6 medium was used during all in vitro procedures.
Statistical analysis
All data were expressed as mean ± SEM. When the number of basic cases in each rate was different, weighted ANOVA models were used. In order to evaluate equality of variances among groups and normality, Leven's test and Kolmogorov–Smirnov tests were used, respectively. Departures from normality were adjusted using Arcsin and Box‐Cox power transformations. p values <0.05 were considered statistically significant. Furthermore, the Tukey multiple comparison test was used to control the type I error rates (α). All data were analyzed by PROC ANOVA (SPSS V. 16.0, Inc).
Results
Superovulation
Comparison of superovulation rates between BALB/cJ and NMRI mice with a 48 h G‐interval are shown in Fig. 1. The percentage of superovulated females (Fig. 1a), the total number of ovulated oocytes (Fig. 1b) and the percentage of oocytes with polar bodies per mouse were significantly higher in NMRI mice compared to BALB/cJ mice (Fig. 1c) (p < 0.05).
Figure 1.
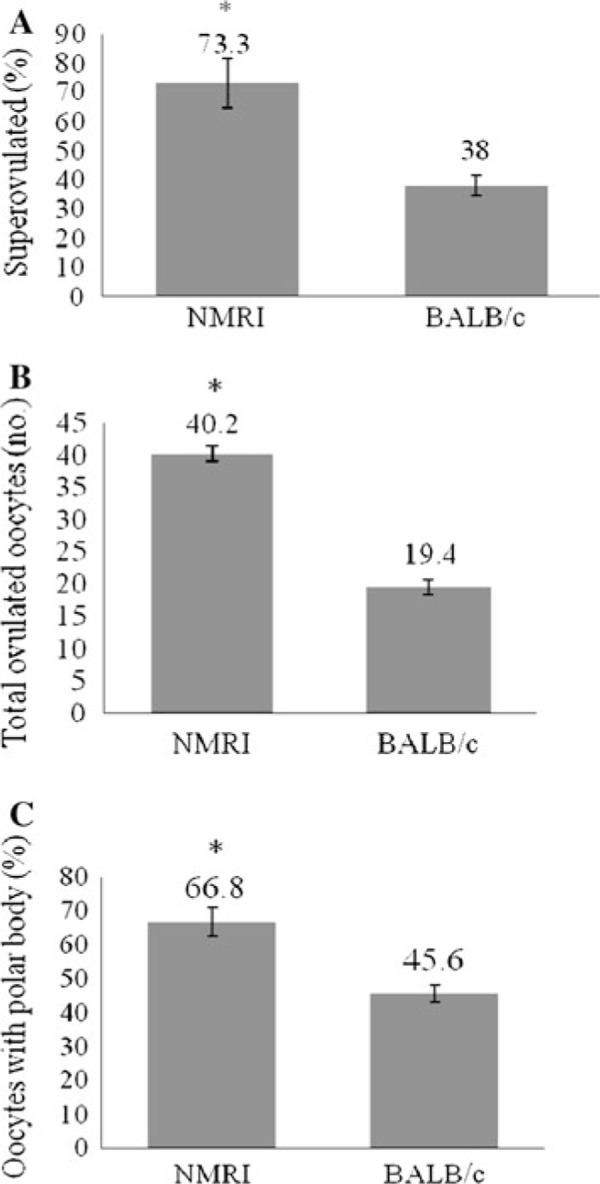
Superovulation of NMRI strain compared to BALB/c strain with a 48 h G‐interval. a Percentage of superovulated females (6–10 replications, 5 female mice per replication). b Total number of ovulated oocytes per mouse. c Percentage of ovulated oocytes with polar body extrusion per mouse. Values are mean ± SEM. *p < 0.05 versus BALB/c values
As shown in Fig. 2a, the highest numbers of superovulated mice were in the group with a 46 h G‐interval delivery. In this group, the percentage of superovulated mice was significantly higher when compared with the 48 and 50 h G‐intervals (p < 0.05). Total number of ovulated oocytes was significantly decreased in BALB/cJ mice with a 50 h G‐interval when compared to other groups (p < 0.05). The highest number of total ovulated oocytes (Fig. 2b) and the highest percentage of oocytes with polar body extrusion (completely matured oocytes) (Fig. 2c), were observed in the 46 h G‐interval delivery, and that was significantly higher than the 48 and 50 h G‐intervals.
Figure 2.
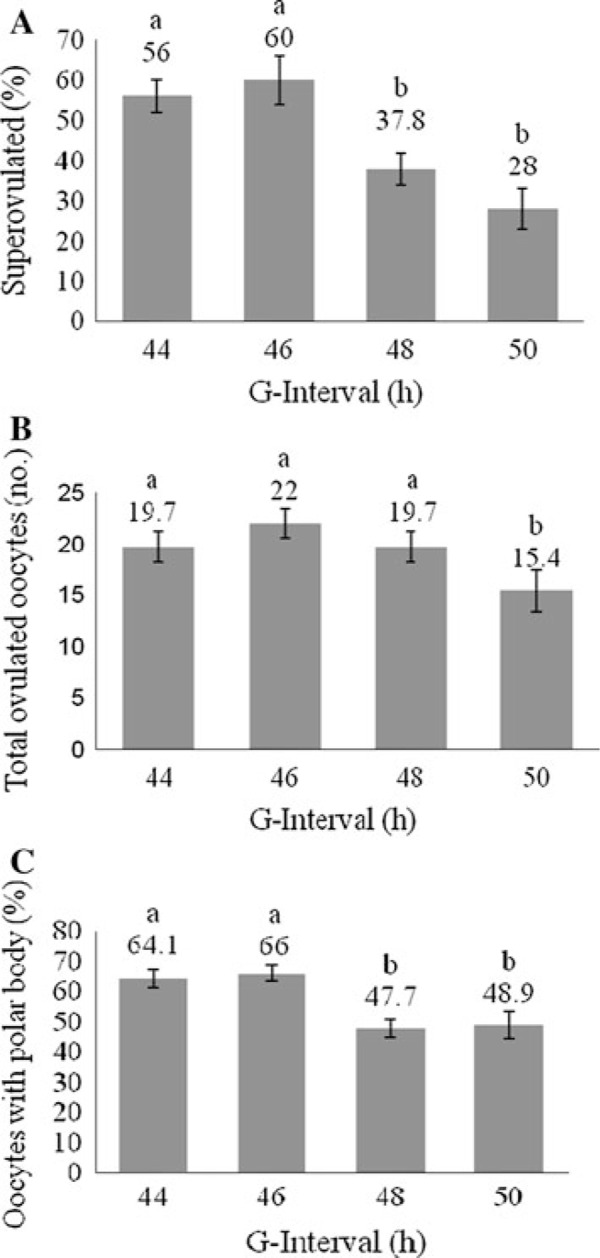
Results of superovulation with the effect of different gonadotropin intervals in BALB/cJ mice. a Percentages of superovulated mice (5–11 replications and 5 females per replication). b The number of total ovulated oocytes per mouse. c The percentage of oocytes without polar bodies per mouse. Values are expressed as (mean ± SEM). Columns with different superscripts are significantly different (p < 0.05)
IVF
The fertilization rate of BALB/cJ mouse oocytes was significantly higher when sperm capacitation and IVF were in the mHTF medium (group B) compared to sperm capacitation and IVF in the T6 medium (group A) (p < 0.05,). The percentage of 2PN formation was significantly higher in the NMRI (group C) strain compared to the BALB/cJ strain when T6 was used as the medium during sperm capacitation and IVF (p < 0.05). As shown in Table 2, the percentage of 2PN formation in BALB/cJ strain increased in the HTF medium and there was no significant difference between groups B and C.
Table 2.
BALB/cJ oocyte IVF rates in T6 and HTF media in comparison with NMRI oocyte IVF rates in T6 medium
| Groups | Strain | IVF medium | Total oocytes (no.) | Replication (no.) | 2PN formation (%) |
|---|---|---|---|---|---|
| A | BALB/cJ | T6 | 216 | 6 | 41.2 ± 2.4b |
| B | BALB/cJ | HTF | 211 | 5 | 65.2 ± 2.8a |
| C | NMRI | T6 | 104 | 6 | 77.5 ± 7.3a |
Percentages of 2PNs are expressed as mean ± SEM. Different superscripts within the same column are significantly different (p < 0.05)
IVD
In the BALB/c strain, the highest percentage of 2‐ and 4‐cell embryos was observed in group C (Table 3). The percentages of embryos that reached morula and hatched blastocyst stages in the BALB/cJ strain were significantly higher in group C (p < 0.01) compared to the other experimental groups (A, B and D). The lowest early embryo development until the 4‐cell stage was seen in group A, which was significantly lower than group C (p < 0.05). BALB/cJ embryos in all experimental groups could reach the 4‐cell stage, however only embryos in group C could reach blastocyst stage (Table 3). No significant differences were observed between the two strains in the T6 medium (group D vs. group E) in the rate of development to the 2‐cell stage (Table 3). However, the percentages of 4‐cell, morula and hatched blastocysts was significantly lower in group D compared to group E (p < 0.01). As shown in Table 3, BALB/cJ 2PN embryos in group C reached the blastocyst stage and the percentage of hatched blastocysts was not significantly different when compared to NMRI mice (group E).
Table 3.
IVD rates of BALB/cJ oocytes in different media combinations for IVF–IVD in comparison with IVD rates of NMRI oocytes in T6 medium
| Experimental groups | Strain | IVF medium | IVD medium | Replication (no.) | Total 2PNs | Embryo development | |||
|---|---|---|---|---|---|---|---|---|---|
| 2‐cell (%) | 4‐cell (%) | Morula (%) | Hatched blastocyst (%) | ||||||
| A | BALB/cJ | HTF | HTF | 5 | 110 | 39.3 ± 1.7b | 7.8 ± 2.3c | 00.9 ± 0.0b | 00.9 ± 0.0b |
| B | BALB/cJ | HTF | T6 | 6 | 109 | 47.5 ± 4.3ab | 14.2 ± 1.5c | 00.9 ± 0.0b | 00.9 ± 0.0b |
| C | BALB/cJ | HTF | G1/G2 | 7 | 270 | 56.3 ± 4.3ab | 30.0 ± 2.6ab | 27.3 ± 2.5a | 19.1 ± 2.3a |
| D | BALB/cJ | T6 | T6 | 5 | 87 | 51.8 ± 7.3ab | 24.0 ± 4.4b | 00.9 ± 0.0b | 00.9 ± 0.0b |
| E | NMRI | T6 | T6 | 6 | 82 | 60.2 ± 7.7a | 35.2 ± 5.2a | 33.2 ± 11.9a | 23.7 ± 5.2a |
Percentages of embryos are expressed as mean ± SEM. Different superscripts in each column show significant differences (p < 0.01)
Discussion
There are few reports regarding suitable media for IVF and sperm capacitation of the BALB/cJ strain for in vitro embryo production [17, 27, 28]. In addition, there is no comprehensive report regarding ART protocols, including superovulation and in vitro procedures, IVF and IVD, for in vitro embryo production in the BALB/cJ strain. The collection of a large number of MII oocytes or preimplantation embryos is necessary for ARTs and reproductive research [22]. In this research, the effect of a common superovulation protocol (48 h G‐interval delivery) was evaluated for superovulation of an outbred mouse strain (NMRI) and an inbred mouse strain (BALB/cJ). This comparison showed that superovulation with the same protocol exhibited different results, and superovulation rates were significantly lower, in BALB/cJ mice compared to NMRI mice (Fig. 1a, b, c). It has been shown that response to superovulation treatment varies among different inbred strains [5, 12]. In the BALB/cJ strain, the failure of some females to ovulate has been reported previously [11]. Earlier reports have indicated that the optimal G‐interval for complete oocyte maturation and subsequent superovulation is 40 to 50 h in mice [12, 38]. Lower results in the BALB/cJ strain indicate that a more suitable G‐interval should be determined. Therefore, other G‐intervals including 44, 46 and 50 h were examined and compared to the 48 h G‐interval delivery. Results showed that among the 44, 46, 48 and 50 h G‐intervals, 44–46 h G‐intervals increased the rate of hormone response for the inbred BALB/cJ strain compared to the standard 48 h G‐interval (Fig. 2a). Oocytes lacking first polar body emission were observed in both BALB/cJ and NMRI mice in all experimented G‐intervals. It was reported previously that superovulation may suppress polar body extrusion during oocyte maturation [40]. With 48 and 50 h G‐intervals, female BALB/cJ mice released the maximum percentage of oocytes without the first polar body. Nonetheless, with the 44 and 46 h intervals the percentages of matured oocytes with polar body extrusions were higher (Fig. 2c). Thus, the G‐interval may possibly effect complete oocyte maturation. Previous research on other inbred strains has also indicated that a G‐interval greater than 48 h did not exhibit a desirable superovulation outcome [41]. Extending the G‐interval up to 50 h for other outbred strains did not show a decline in the rate of superovulation [41]. Similarly, in this experiment, the results showed that the 48 h G‐interval in outbred NMRI mice exhibited higher superovulation rates compared to inbred BALB/cJ mice. The physiological effects of LH on oocyte maturation and its effects on meiotic resumption have been described [42]. These two mouse strains show different superovulation responses to the same G‐interval, which may be due to physiological effects of the different amounts of endogenous circulating gonadotropins, such as endogenous LH, in response to PMSG in different strains.
It has been shown that the mHTF medium was the most desirable medium for sperm capacitation [28], gamete interaction, sperm penetration through the zona pellucid and male pronucleus formation in the BALB/cA strain [17]. Similarly the results of this research obtained from the BALB/cJ strain indicate that the highest rate of 2PN formation was seen when both sperm capacitation and IVF were in the mHTF medium, rather than the T6 medium. As shown in Table 1, the concentration of calcium in the T6 medium is lower than in the mHTF medium. Kito and Ohta [28] have postulated that calcium concentrations over 5 mM enhanced zona penetration. Lower 2PN formation from BALB/cJ oocytes in T6 medium is possibly due to a lower calcium concentration. It was reported that higher concentrations of glucose in a media such as modified Krebs–Ringer medium TYH (more than 5.5 mM) increased the IVF rate [27]. However, the composition of HTF with a lower concentration of glucose (Table 1, 2.70 mM) exhibited higher IVF efficiency compared to T6 medium with a higher glucose concentration (5.55 mM). Lower rates of 2PN formation from BALB/cJ oocytes in T6 medium indicate that effects of medium composition on IVF are more visible in the BALB/cJ strain thanin the NMRI strain. Our study also indicates that effects of T6 medium during sperm capacitation and IVF are not as positive as effects of mHTF medium for IVF of BALB/cJ oocytes. Table 3 shows that T6 medium supports embryo development to 4‐cell stage, the same as G1V5 medium, however, in mHTF medium embryo development was significantly lower than G1V5. Previous research showed that media containing HEPES and HCO3 − support embryo development in room air [39, 43]. Also it was shown that 25 mmol/l HEPES with increasing CO2 has no adverse effect on embryo development [44]. Results of this research showed that IVF in a HEPES buffered medium incubated for 24 h in CO2 generated BALB/cJ 2PN embryos with the ability of developing to the blastocyst stage in G1V5 medium. It has been concluded that higher developmental competence of mouse models in T6 medium was probably due to lower concentrations of K+ in the T6 medium than in the mHTF medium [27]. The amount of NaCl in the T6 medium is lower than in the mHTF medium (Table 1).
It has been found that lower concentrations of NaCl in media such as simplex optimization medium (SOM), which is a previous version of potassium‐enrichment simplex optimization medium (KSOM) [45], resulted in higher rates of mRNA synthesis and greater mRNA stability, which partially improves developmental competence of mouse embryos [46]. Therefore, lower NaCl concentration in the T6 medium seems to be another reason for higher IVD rates in T6 medium compared to mHTF medium. Previous research by Erbach et al. [47] has shown that KSOM with a higher concentration of K+ and Na+ increases mouse embryo development, however, in another experiment by Uranga et al. [30], it has been shown that KSOM was not better than the T6 medium. Therefore, in this research a commercially available medium such as G1V5/G2V5 medium was used and compared with T6 and mHTF media in order to validate comparisons. The IVD rates of BALB/cJ 2PNs, which were generated in the mHTF medium, showed that those 2PN embryos were able to reach blastocyst stage in G1V5/G2V5 medium, while T6 and mHTF media could not support embryo development to the blastocyst stage in our laboratory conditions. This result indicates that in different laboratory conditions, G1V5/G2V5 seems to be a superior medium for BALB/cJ embryo IVD in comparison with T6 and mHTF media. Using T6 medium during sperm capacitation and IVF caused different IVF rates in NMRI and BALB/cJ mice, and the IVF rate in BALB/cJ was lower than in NMRI (Table 2). Data comparisons in Table 2 indicated that mHTF medium increased BALB/cJ IVF and there was no significant difference with NMRI oocyte IVF rates (Table 2), while the IVF rate in T6 medium was significantly lower than in group E. Table 3 shows that in the BALB/cJ strain (group C) the percentages were approximately the same as those of NMRI (group E). These results indicate that the type of medium during IVF–IVD and the medium combination in group C increased BALB/cJ oocyte IVD rates and reached the same level as NMRI oocytes IVF–IVD rates in T6 medium. With the consideration of superovulation and IVF results in two strains, we conclude that during ARTs, it is important to determine optimization of superovulation and IVF–IVD for each strain. It is suggested that, for, BALB/cJ mice, an ARTs protocol which includes a 44–46 h G‐interval and the use of mHTF medium during IVF and G1V5/G2V5 medium during IVD may improve in vitro embryo production.
Acknowledgments
This research supported by a Grant‐in‐Aid for scientific research from the Royan Institute.
References
- 1.Les E P. A brief history of the two substrains of BALB/c, BALB/cJ, and BALB/cByJ available from animal resources. JAX notes. 1990;443.
- 2. Wade CM, Kulbokas EJ 3rd, Kirby AW et al. The mosaic structure of variation in the laboratory mouse genome. Nature, 2002, 420, 574–578 10.1038/nature01252 [DOI] [PubMed] [Google Scholar]
- 3. Szczygiel MA, Kusakabe H, Yanagimachi R, Whittingham DG. Separation of motile populations of spermatozoa prior to freezing is beneficial for subsequent fertilization in vitro: a study with various mouse strains. Biol Reprod, 2002, 67, 287–292 10.1095/biolreprod67.1.287 [DOI] [PubMed] [Google Scholar]
- 4. Kishikawa H, Tateno H, Yanagimachi R. Chromosome analysis of BALB/c mouse spermatozoa with normal and abnormal head morphology. Biol Reprod, 1999, 61, 809–812 10.1095/biolreprod61.3.809 [DOI] [PubMed] [Google Scholar]
- 5. Byers SL, Payson SJ, Taft RA. Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs). Theriogenology, 2006, 65, 1716–1726 10.1016/j.theriogenology.2005.09.016 [DOI] [PubMed] [Google Scholar]
- 6. Wilson ED, Zarrow MX. Comparison of superovulation in the immature mouse and rat. J Reprod Fertil, 1962, 3, 148–158 10.1530/jrf.0.0030148 [DOI] [PubMed] [Google Scholar]
- 7. Zarrow MX, Caldwell AL Jr, Hafez ES, Pincus G. Superovulation in the immature rat as a possible assay for LH and HCG. Endocrinology, 1958, 63, 748–758 10.1210/endo‐63‐6‐748 [DOI] [PubMed] [Google Scholar]
- 8. Ozgunen KT, Erdogan S, Mazmanoglu N, Pamuk I, Logoglu G, Ozgunen T. Effect of gonadotrophin dose on oocyte retrieval in superovulated BALB/c mice. Theriogenology, 2001, 56, 435–445 10.1016/S0093‐691X(01)00575‐1 [DOI] [PubMed] [Google Scholar]
- 9. Spearow JL, Barkley M. Genetic control of hormone‐induced ovulation rate in mice. Biol Reprod, 1999, 61, 851–856 10.1095/biolreprod61.4.851 [DOI] [PubMed] [Google Scholar]
- 10. Hashlamoun LA, Killian GJ. Effects of timing of ovum recovery, cumulus cells, sperm preincubation time, and pH on in vitro fertilization in C57BL/6 mice. Arch Androl, 1985, 15, 159–171 10.3109/01485018508986906 [DOI] [PubMed] [Google Scholar]
- 11. Gates AH, Bozarth JL. Ovulation in the PMSG‐treated immature mouse: effect of dose, age, weight, puberty, season and strain (BALB/c, 129 and C129F1 hybrid). Biol Reprod, 1978, 18, 497–505 10.1095/biolreprod18.3.497 [DOI] [PubMed] [Google Scholar]
- 12. Nagy A, Gertsenstein M, Vintersten K, Behringer R Manipulating the mouse embryo: a laboratory manual, 2002. Cold Spring Harbor: Cold Spring Harbor Laboratory press; [Google Scholar]
- 13. Suzuki O, Asano T, Yamamoto Y, Takano K, Koura M. Development in vitro of preimplantation embryos from 55 mouse strains. Reprod Fertil Dev, 1996, 8, 975–980 10.1071/RD9960975 [DOI] [PubMed] [Google Scholar]
- 14. Menezo YJ, Herubel F. Mouse and bovine models for human IVF. Reprod Biomed Online, 2002, 4, 170–175 10.1016/S1472‐6483(10)61936‐0 [DOI] [PubMed] [Google Scholar]
- 15. Choi Y‐H, Seng S, Toyoda Y. Comparison of capacitation and fertilizing ability of BALB/c and ICR mice epididymal spermatozoa: analysis by in vitro fertilization with cumulus‐intact and zona‐free mouse eggs. J Mam Ova Res, 2000, 17, 9–14 10.1274/jmor.17.9 [Google Scholar]
- 16. Burruel VR, Yanagimachi R, Whitten WK. Normal mice develop from oocytes injected with spermatozoa with grossly misshapen heads. Biol Reprod, 1996, 55 (55) 709–714 10.1095/biolreprod55.3.709 [DOI] [PubMed] [Google Scholar]
- 17. Kito S, Hayao T, Noguchi‐Kawasaki Y, Ohta Y, Hideki U, Tateno S. Improved in vitro 249 fertilization and development by use of modified human tubal fluid and applicability of pronucleate embryos for cryopreservation by rapid freezing in inbred mice. Comp Med, 2004, 54, 564–570 [PubMed] [Google Scholar]
- 18. Sher G, Knutzen V, Stratton CJ et al. The development of a successful non‐university‐based ambulatory in vitro fertilization/embryo transfer program: phase I. Fertil Steril, 1984, 41, 511–518 [DOI] [PubMed] [Google Scholar]
- 19. Quinn P, Kerin JF, Warnes GM. Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertil Steril, 1985, 44, 493–498 [DOI] [PubMed] [Google Scholar]
- 20. Nakagata N. Use of cryopreservation techniques of embryos and spermatozoa for production of transgenic 257 (Tg) mice and for maintenance of Tg mouse lines. Lab Anim Sci, 1996, 46, 236–238 [PubMed] [Google Scholar]
- 21. Perin PM, Maluf M, Nicolosi Foltran Januario DA, Nascimento Saldiva PH. Comparison of the efficacy of two commercially available media for culturing one‐cell embryos in the in vitro fertilization mouse model. Fertil Steril, 2008, 90, 1503–1510 10.1016/j.fertnstert.2007.09.062 [DOI] [PubMed] [Google Scholar]
- 22. Martin‐Coello J, Gonzalez R, Crespo C, Gomendio M, Roldan ER. Superovulation and in vitro oocyte maturation in three species of mice (Mus musculus, Mus spretus and Mus spicilegus). Theriogenology, 2008, 70, 1004–1013 10.1016/j.theriogenology.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 23. Kameyama Y, Arai K, Ishijima Y. Interval between PMSG priming and hCG injection in superovulation of the mongolian Gerbil. J Mam Ova Res, 2004, 21, 105–109 10.1274/jmor.21.105 [Google Scholar]
- 24. Redina OE, Amstislavsky S, Maksimovsky LF. Induction of superovulation in DD mice at different stages of the oestrous cycle. J Reprod Fertil, 1994, 102, 263–267 10.1530/jrf.0.1020263 [DOI] [PubMed] [Google Scholar]
- 25. Mizoguchi H, Dukelow WR. Effect of timing of hCG injection on fertilization in superovulated hamsters. Biol Reprod, 1980, 23, 237–241 10.1095/biolreprod23.1.237 [DOI] [PubMed] [Google Scholar]
- 26. Summers MC, Bhatnagar PR, Lawitts JA, Biggers JD. Fertilization in vitro of mouse ova from inbred and outbred strains: complete preimplantation embryo development in glucose‐supplemented KSOM. Biol Reprod, 1995, 53, 431–437 10.1095/biolreprod53.2.431 [DOI] [PubMed] [Google Scholar]
- 27. Wu HT, Chou CK, Lin CS, Huang MC. Effects of glucose concentration on in vitro fertilization in BALB/c mice. Reprod Domest Anim, 2003, 38, 470–474 10.1046/j.0936‐6768.2003.00465.x [DOI] [PubMed] [Google Scholar]
- 28. Kito S, Ohta Y. In vitro fertilization in inbred BALB/c mice I: isotonic osmolarity and increased calcium enhanced sperm penetration through the zona pellucida and male pronuclear formation. Zygote, 2008, 16, 249–257 [DOI] [PubMed] [Google Scholar]
- 29. Nakazawa T, Ohashi K, Yamada M et al. Effect of different concentrations of amino acids in human serum and follicular fluid on the development of one‐cell mouse embryos in vitro. J Reprod Fertil, 1997, 111 (2) 327–332 10.1530/jrf.0.1110327 [DOI] [PubMed] [Google Scholar]
- 30. Uranga JA, Arechaga J. Comparative analysis of in vitro development of outbred mouse embryos cultured in Krebs‐Ringer or tyrode‐derived media. Reprod Nutr Dev, 1997, 37, 41–49 10.1051/rnd:19970105 [DOI] [PubMed] [Google Scholar]
- 31. Chia R, Achilli F, Festing MF, Fisher EM. The origins and uses of mouse outbred stocks. Nat Genet, 2005, 37, 1181–1186 10.1038/ng1665 [DOI] [PubMed] [Google Scholar]
- 32.Festing MF. Warning: the use of heterogeneous mice may seriously damage your research. Neurobiol Aging. 1999;20:237–44. [DOI] [PubMed]
- 33. Ghaemi SR, Salehnia M, Valojerdi MR. The effect of progesterone and exogenous gonadotropin on preimplantation mouse embryo development and implantation. Exp Anim, 2008, 57, 27–34 10.1538/expanim.57.27 [DOI] [PubMed] [Google Scholar]
- 34. Golkar‐Narenji A, Eimani H, Samadi F et al. Effect of Papaver rhoeas extract on in vitro maturation and developmental competence of immature mouse oocytes. Reprod Med Biol, 2010, 9, 1211–1215 10.1007/s12522‐010‐0059‐0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barekati Z, Gourabi H, Valojerdi MR, Yazdi PE. Previous maternal chemotherapy by cyclophosphamide (Cp) causes numerical chromosome abnormalities in preimplantation mouse embryos. Reprod Toxicol, 2008, 26, 278–281 10.1016/j.reprotox.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 36. Khalili MA, Anvari M. The effect of in vitro culture on cleavage rates and morphology of the in vivo291 developed embryos in mice. Iran J Reprod Med, 2007, 5, 17–22 [Google Scholar]
- 37. Zavareh S, Saberivand A, Salehnia M. The effect of progesterone on the in vitro maturation and developmental competence of mouse germinal vesicle oocytes. Int J Fert Steril, 2009, 3, 21–28 [Google Scholar]
- 38. Fowler RE, Edwards RG. Induction of superovulation and pregnancy in mature mice by gonadotrophins. J Endocrinol, 1957, 15, 374–384 10.1677/joe.0.0150374 [DOI] [PubMed] [Google Scholar]
- 39. Behr BR, Stratton CJ, Foote WD, Knutzen V, Sher G. In vitro fertilization (IVF) of mouse ova in HEPES297 buffered culture media. J In Vitro Fert Embryo Transf, 1990, 7, 9–15 10.1007/BF01133877 [DOI] [PubMed] [Google Scholar]
- 40. Takagi N, Sasaki M. Digynic triploidy after superovulation in mice. Nature, 1976, 264, 278–281 10.1038/264278a0 [DOI] [PubMed] [Google Scholar]
- 41. Vergara GJ, Irwin MH, Moffatt RJ, Pinkert CA. In vitro fertilization in mice: Strain differences in response to superovulation protocols and effect of cumulus cell removal. Theriogenology, 1997, 47, 1245–1252 10.1016/S0093‐691X(97)00104‐0 [DOI] [PubMed] [Google Scholar]
- 42. Rossant J, Pedersen RA Experimental approaches to mammalian embryonic development, 1987. Cambridge: Cambridge University Press; [Google Scholar]
- 43. Mahadevan MM, Fleetham J, Church RB, Taylor PJ. Growth of mouse embryos in 303 bicarbonate media buffered by carbon dioxide, hepes, or phosphate. J In Vitro Fert Embryo Transf, 1986, 3, 304–308 10.1007/BF01133390 [DOI] [PubMed] [Google Scholar]
- 44. Swain JE, Pool TB. New pH‐buffering system for media utilized during gamete and embryo manipulations for assisted reproduction. Reprod Biomed Online, 2009, 18, 799–810 10.1016/S1472‐6483(10)60029‐6 [DOI] [PubMed] [Google Scholar]
- 45. Lawitts JA, Biggers JD. Joint effects of sodium chloride, glutamine, and glucose in mouse preimplantation embryo culture media. Mol Reprod Dev, 1992, 31, 189–194 10.1002/mrd.1080310305 [DOI] [PubMed] [Google Scholar]
- 46. Ho Y, Doherty AS, Schultz RM. Mouse preimplantation embryo development in vitro: effect of sodium concentration in culture media on RNA synthesis and accumulation and gene expression. Mol Reprod Dev, 1994, 38, 131–141 10.1002/mrd.1080380203 [DOI] [PubMed] [Google Scholar]
- 47. Erbach GT, Lawitts JA, Papaioannou VE, Biggers JD. Differential growth of the mouse preimplantation embryo in chemically defined media. Biol Reprod, 1994, 50, 1027–1033 10.1095/biolreprod50.5.1027 [DOI] [PubMed] [Google Scholar]


