Abstract
INTRODUCTION
Human papillomavirus (HPV)-related cancers can be averted by type-specific vaccination (primary prevention) and/or through detection and ablation of precancerous cervical lesions (secondary prevention). This review presents current challenges to cervical cancer screening programs, focusing on recent molecular advances in HPV testing and potential improvements on risk stratification.
AREAS COVERED
High-risk (HR)-HPV DNA detection has been progressively incorporated into cervix cancer prevention programs based on its increased sensitivity. Advances in next-generation sequencing (NGS) are being rapidly applied to HPV typing. However, current HPV DNA tests lack specificity for identification of cervical precancer (CIN3). HPV typing methods were reviewed based on published literature, with a focus on these applications for screening and risk stratification in the emerging complex clinical scenario post-vaccine introduction. In addition, the potential for NGS technologies to increase specificity is discussed in regards to reflex testing of specimens for emerging biomarkers for cervix precancer/cancer.
EXPERT COMMENTARY
Integrative multi-disciplinary molecular tests accurately triaging exfoliated cervical specimens will improve cervical cancer prevention programs while simplifying healthcare procedures in HPV-infected women. Hence, the concept of a “liquid-biopsy” (i.e., “molecular” Pap test) highly specific for early identification of cervical precancerous lesions is of critical importance in the years to come.
Keywords: cervical cancer, screening, human papillomavirus, molecular testing, triage, NGS
1. INTRODUCTION
Human papillomaviruses (HPVs) through co-evolution have adapted to the human species and most infections are non-pathogenic and commensal. Rather than manifesting as highly infectious and symptomatic infections with rapid disease development, it is predominantly a silent and subclinical infection, replicating during cell differentiation of the cervical epithelium while remaining nearly imperceptible to the host’s natural mechanisms of defense. Cervical HPV infections have a long virtually unavoidable exposure period, where any form of intervention can have a major role in reducing one of the most preventable cancers worldwide. Here in this review, we present current cervical cancer prevention strategies and their challenges, focusing on recent advances in HPV molecular testing and how they can improve risk stratification.
2. Burden of HPV-associated Cancers
HPV is now recognized as an etiological agent for multiple anal-genital, head and neck, and possibly skin cancers [1,2]. Cervical cancer is the fourth most common cancer in women worldwide, and the fourth leading cause of death in females. However, due to healthcare disparities especially regarding the access to clinical care, high incidence and mortality rates are predominantly observed in developing countries where cervical cancer screening programs may be limited or even absent [3]. In 2015 in the United States, the number of new cases of HPV-related cancers reached 38,723 (11.7 per 100,000 persons), including 23,000 among women (13.5 per 100,000 persons). Amongst these, the most frequent malignancy was cervix cancer with 11,771 new cases per year (7.4 per 100,000) and, of these, 7,800 (66.3%) cervical cancer cases were attributable to HPV types 16 and 18 (http://www.cdc.gov/cancer/hpv/). Nevertheless, cervical cancer incidence has decreased more than 50% in the United States based on the success of cytological screening and treatment programs, with a similar reduction in the mortality rate. The 4,100 estimated number of HPV-related cervical cancer deaths for 2015 was mainly attributable to lack of individual participation in cervical cancer screening, disparities in regional or state screening programs, or inadequate follow-up care for abnormal cervical cytology and/or HR-HPV (http://www.cancer.org/).
The differences observed in the geographic variation of cervical cancer incidence are mainly driven by a combination of HPV infection prevalence rates and availability (or not) of prevention programs: Eastern Europe, Central Asia and Africa present the highest prevalence rates (17.4% or higher) as compared with Western and Southern Europe or North America and Eastern Asia (lower than 10.7%) [4]. However, HR-HPV prevalence rates alone are not sufficient to explain such disparities in incidence rates of cervix cancer (20.6/100,000 versus 7.9/100,0000) [3]. Thus, lack of effective organized screening programs contribute to higher cervical cancer incidence rates. Many countries are struggling with non-organized cervical cancer screening programs or, when implemented, with very low coverage of the targeted screening population or even an inadequate screening program structure [4,5]. Opportunistic screening like small local college- or company-based programs have had little effect on decreasing mortality rates, since often women at highest risk do not participate [5].
3. Cervical Cancer Prevention: Implications for Screening and Molecular Methods
Primary prevention through HPV vaccination is lacking in many regions of the world with high rates of cervical cancer due to financial constrains and/or absence of the appropriate healthcare infrastructure. Moreover, when instituted, HPV prophylactic vaccination is not being integrated into national immunization programs (e.g., newborn and childhood vaccination routines) and this impacts the public health measures to protect the population from HPV-related diseases [5]. Nevertheless, vaccination will eventually be the best solution for these countries with a goal of eliminating HR-HPVs in the population through strategies to provide herd immunity.
Dealing with these disparities currently will entail a multifaceted approach. First, since HPV vaccination is still too costly for many developing countries, few nationwide organized immunization programs are expected in the near future in many of these countries. Nonetheless, there are programs that provide financial support to make HPV immunization more affordable in these countries, but they require organization and a national will. Cytology-based screening has proven to effectively decrease cervical cancer incidence rates in many countries [6]; however, the relatively low sensitivity observed with a single Pap smear usually means either higher false-negative results and/or the requirement of multiple Pap tests, making the costs prohibitive for the early identification of precancerous lesions. More sensitive techniques are now utilizing HPV testing as a primary screening tool that presents advantages for early detection of the causative agent of cervical cancer, with improved identification of precancerous lesions compared to cytology-based programs [7]. In addition, the negative predictive value of a negative HR-HPV cervical sample allows longer screening intervals and saves healthcare resources [8,9]. More recently, there is mounting evidence from European randomized clinical trials showing that HPV testing as a primary screening tool will provide an additional 60–70% protection against invasive cervical cancer compared to cytology-based screening programs [10]. Moreover, HPV-based programs offer protection from glandular lesions, which cytology-based programs do not. This has been manifested in the lack of reduction in cervical adenocarcinomas by cervical cytology. Therefore, moving forward a combined approach of HPV vaccination and cervical cancer screening integrating HPV testing seems the most promising path to cervix cancer reduction [4,8].
Cervical cancer develops through a series of stages based predominantly on morphologic features of cervical dysplasia in lesions, nevertheless these lesions may persist and progress although a significant percentage regress and clear (see Figure 1) [11,12]. Currently, pathological features can identify cervical disease, but cannot absolutely attribute the underlying risk for progression of the identified cervical abnormality or lesion. Nevertheless, risk models based on cervical pathology are used in clinical treatment protocols. In contrast, HPV testing is suitable for identification of the HPV type present but cannot identify the cervical lesions: thus, presently an adjunct approach identifying cellular and/or molecular abnormalities predictive of cervical cancer development (e.g., through cytology and histology) is needed to distinguish women at high-risk of cervical cancer development and in need of lesion ablation [13,14].
Figure 1.
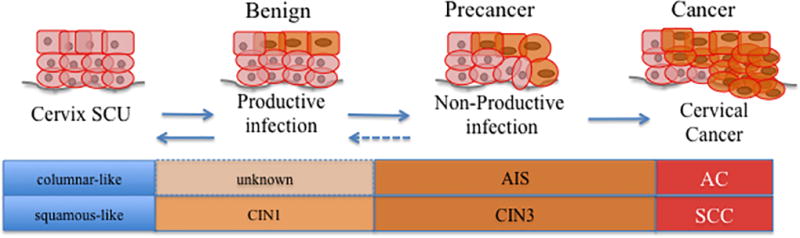
Simplified diagram of cervical carcinogenesis. The cell of origin infected initially by human papillomavirus (HPV) types influences the manifestation of HPV mediated carcinogenesis. Squamous-type lesions emerge from the cervix squamous-columnar junction (SCU) into low-grade cervical lesions (cervical intraepithelial neoplasia grade 1, CIN1), which may or may not progress to precancerous lesions (cervical intraepithelial neoplasia grade 3, CIN3); alternatively, glandular-type lesions have a poorly detected preinvasive stage (adenocarcinoma in situ, AIS). These lesions will evolve into either squamous cell carcinoma (SCC) or into adenocarcinoma (AC).
4. Purpose of Screening and HPV Typing for Risk Stratification: Identify Precancers for Treatment and Prevention of Cervix Cancer
The well-established causal relationship between cervical cancer development and HR-HPV infection [12] catapulted HPV science into both primary and secondary cervical cancer prevention strategies. The applicability of an HR-HPV test for screening can be used in a variety of settings: i) diagnosis of HR-HPV infection; ii) triage of low-grade cytological abnormalities based on HPV type-associated risk; iii) risk stratification by reflex cytology of an HR-HPV type-specific positive test; iv) assessment of HR-HPV persistent infection; v) follow-up after treatment of high-grade intraepithelial neoplasia; vi) self-sampling in low-resource settings for HPV detection; and, vii) epidemiological surveillance at the regional- or country-based level to provide baseline and follow-up data for global health planning [15,16]. Clinically, triaging low-grade cervical neoplasia by HPV testing has been documented to be more sensitive for detecting underlying precancerous cervical lesions through histology than repetitive liquid cytology analyses [17].
When cytologic and viral testing are used in a combined approach, sensitivity reaches approximately 90% while reducing the 5-year risk for precancerous cervical lesions to a negligible level following negative co-test results [18]. These improvements in cervix cancer prevention have been achieved through good screening coverage and high-quality treatment and follow-up methodologies, most of which are lacking in low-resource settings [19]. The high negative predictive value along with high sensitivity propelled HPV testing into primary screening, where downstream cytologic observations can help identify progressive HPV infections [14]. Furthermore, HPV tests are biochemical and readily standardized, whereas cytology is subjective and requires visual identification. Therefore, as cervix cancer prevention programs are increasingly utilizing HPV testing, enhanced methods for improved risk stratification of HPV-positive women are urgently needed [14].
5. HPV Screening and The Role of Molecular Tests
Major guidelines for cervix cancer screening programs vary worldwide due to the differences in availability of resources to finance adequate public healthcare measures. For example, in economically poor sub-Saharan Africa, cervix cancer remains one of the most common and lethal cancers among females, while affluent Finland has one of the lowest incidence rates of cervix cancer. Such situations are mainly linked to a poor or nonexistent cervical cancer-screening program in many African countries, even though fortunately HPV vaccination is on the rise in many areas [4]. Across Europe, several countries utilize a cytology-based organized cervical cancer-screening program. New recommendations continue to encourage the introduction of primary HPV testing into cervical cancer screening programs with estimates of reducing cervical cancer incidence more than 30% for women aged 25–64 years in England, as an example [20]. In the United States a co-testing approach has been adopted, but new recommendations favoring primary HPV testing are gaining momentum [14]. Strengths of utilizing HPV testing strategies are supported by objective qualitative and/or quantitative results, reproducibility, and high negative predictive values [21]. Additional increase in sensitivity by HPV testing has the potential to identify women with missed cervical precancerous lesions, particularly glandular lesions, following a HPV-positive/cytology negative result if adequately evaluated in a screening program [22]. However, “false-positive” detection of a HR-HPV infection without an identified precancer is a clinical problem since HPV natural history studies indicate that most HR-HPV infections clear within 2 years [10,14,23], whereas the few patients who might go on to cancer are unpredictable.
6. HPV negative test results
The high negative predictive value from HPV testing allows for better management of HPV-negative women who are unlikely to develop cervical cancer over the next 5 to 10 years. This also provides greater reassurance of a low risk for cervical intraepithelial neoplasia grade 3 or worse (CIN3+) development in contrast to a cytology-negative result [7,14,24,25]. Based on one HPV-negative test re-screening can be extended to an interval of 3 to 5 years [10,14]. This allows for longer intervals between screening evaluations and fewer tests [19]. Since HR-HPV is the established etiologic agent for cervical cancer development, identification of HR-HPV DNA should be an appropriate measure to identify women at risk to develop cervical cancer. Thus, presence of persistent HR-HPV DNA assumes the role of a surrogate or “intermediate endpoint” for cervical precancer and cancer.
Furthermore, HPV testing also allows women to return to routine cervical screening upon a HR-HPV negative result following previous cervical abnormalities or following treatment, which implies better management of follow-up visits for cervical cancer screening. In addition, the rate of identifiable cervical disease in subsequent rounds of primary HPV-based screening is lower compared to cytology-based screening [14].
7. High-Risk HPV Detection is Creating an Opportunity for Improved Risk Stratification
The introduction of HR-HPV testing has complemented screening programs with the ability to: i) identify those HR-HPV positive women (i.e., with HPV16/18) likely to have cervical precancer now and will benefit from appropriate treatment; ii) identify those HR-HPV positive who might develop disease in the next few years and require follow-up; iii) reduce false-positive cytology results found in HPV-negative women; or iv) identify those who test negative and therefore do not need to be screened over the next 3–5 years. Nevertheless, a major obstacle is the management of HR-HPV infection in the absence of cytological abnormalities, which constitutes the majority of HPV-positive women [26].
Over time, cervical cancer screening is designed to identify a subgroup of women that have an increased risk of cervical cancer development due to HR-HPV persistent infection. Clinical management of these women, represents a challenge to the resources of organized screening programs, such as access to colposcopy [26,27]. Programmatically, characterizing and managing an increased risk group currently includes identification of HR-HPV infection with or without cytology, referral for colposcopy and mandatory treatment of precancerous cervical lesions (identifiable as high-grade intraepithelial lesion (HSIL) or adenocarcinoma in situ (AIS) by histology). Within this framework, women with HR-HPV positive but low-grade cytological abnormalities (ASC-US, atypical squamous cell of undetermined significance or LSIL, low-grade squamous intraepithelial lesion) may be referred to colposcopy-biopsy evaluation or follow-up with yearly testing. Cytology tests such as ASC-US may correspond to cervical abnormalities not related to HPV, which could present an elevated burden on the number of colposcopies performed [10,19,21,22], so that HR-HPV identification in women with ASC-US diagnoses is widely utilized and only HR-HPV positive are deemed at high-risk [28,29].
8. Moving Past HPV-positivity: what is there to do?
There is compelling evidence that HPV infections clear in 90% of infected women, regardless of the presence or absence of abnormal cytology [19,30]. Moreover, the recognized limitation of cytology to identify glandular lesions, also contributes to the value of a HR-HPV positive test result, which has a high sensitivity for both squamous and glandular lesions [14]. The challenge is how to better manage an HPV-positive result at the initial stages of screening where fewer precancerous lesions are likely to be identified. An HPV-positive result may only send women for an additional triage test instead of automatic colposcopy evaluation. Cytology can in part fill that role since it has increased specificity in detecting cervical precancerous lesions; or a subsequent HPV test in 6 to12 months can identify persistent HR-HPV infections [31]. Based on current clinical management schemes, only after two or more HPV-positive tests and/or upon detection of cytological abnormalities, or detection of HPV16 and HPV18 are women recommended for colposcopy evaluation [18,21,32].
A step further on triaging HPV-positive results can be achieved by HPV-specific typing due to the well-known established risk stratification associated with individual HPV types, particularly with HPV16. While HPV typing techniques can predict an increased risk of cervical precancerous lesions, it does not differentiate transient from persistent infections and may increase the colposcopy referral. Nonetheless, screening vaccinated women will change the characteristics of risk stratification as HPV16 and HPV18 infections are reduced with effective vaccine uptake [26].
9. Historical Overview of HPV Typing
The identification of carcinogenic HPVs in cervical lesion biopsies and exfoliated cells have evolved from restriction endonuclease cleavage patterns and DNA-DNA hybridization techniques to PCR-based systems [33] and most recently next-generation sequencing (NGS) assays [34]. Initially, techniques such as in situ hybridization or Southern blotting used radioactive probe-labeled nucleic acid hybridization to detect the presence of HPV DNA, providing high-quality data although technically demanding, time-consuming, and with lower sensitivity. Current HPV genome typing is primarily based on identification of individual types by a variety of methods utilizing the highly conserved L1 gene and PCR-based methods employing consensus primers. These techniques remain the most validated methodology to identify and characterize clinically relevant papillomaviruses [16,35,36]. These methods utilize different primers that amplify different sized fragments such as 455bp with the MY09/11|PGMY system [37]; 150bp with the GP5+/6+ system [38]; or <100bp with SPF10 [39,40]. Further typing is then possible with type-specific probes or DNA sequencing (initially with Sanger sequencing and more recently with high-throughput NGS) [36,41]. Other types of assays target different regions of the viral genome and maybe type-specific with immediate discrimination and quantitation of specific HPV types in a “one tube” assay. These methods employ real-time (RT)-PCR that distinguishes individual HPV types directly using type-specific primer pairs and fluorescent probes, coupled with beta-globin detection for quality control utilizing specialized detection systems [42].
Cervical cancer malignant pathways are tightly correlated to the viral E6 and E7 oncoprotein activities where alteration of transcriptional control leads to persistent viral oncogene expression. This deregulation contributes to the accumulation of cellular genomic mutations that may also involve viral integration [41]. Theoretically, amplification of different HPV regions (e.g. L1 versus E6) might show some loss of sensitivity and specificity in identifying high risk-progressive HPV infections containing integrated HPV with deletions in the L1genomic region. One study has suggested an increased identification rate of CIN2+ using E6- versus L1-based PCR systems [43]. Additional viral load data also provides insight into risk assessment and “clearance versus persistence” outcomes [41]. Nevertheless, amplification product size, region of the genome targeted, amount of DNA tested, polymerase employed, and assay system utilized may all contribute to differences in sensitivity and specificity.
Epidemiological studies provide the basis for knowledge on HPV persistence, progression, regression and clearance through the molecular analysis of clinical samples. The most recent advances in DNA NGS sequencing technologies have the potential to contribute to a better and deeper knowledge of HPV biology; improve viral detection and identification; expand the characterization of mechanisms associated with cervical malignancy; and perhaps facilitate new therapy development [44–46]. Likewise, understanding the molecular interactions present in the unique viral-host milieu within a patient by novel assays should enhance development of treatments and/or therapies that will have beneficial implications in fighting cervical cancer worldwide [33]. Additionally, initial studies of HPV epigenetics using methylation-specific restriction endonuclease patterns showed a correlation between increased CpG site methylation levels and high-grade cervical lesions and have evolved into more quantitative assays of CpG methylation [47]. More recent studies indicate that NGS assays can provide single molecule CpG methylation levels to help unravel the physiological role of methylation in cervical cancer development [33,47], as discussed below.
10. HPV Testing Technologies
Molecular HPV testing is rapidly being introduced into cervical cancer screening and management of cytology-positive women [28,48] as it can provide both diagnostic and prognostic information for the clinical evaluation of at risk women. Several technical advances have begun to emerge for the molecular diagnosis of HPV DNA, although a number of these tests are in process of, or require, clinical validation for clinical screening purposes, essential in evaluating the characteristics and performance parameters including sensitivity and specificity [49], and/or the negative predictive value of an HPV test in high and changing HPV prevalence settings (i.e., due to HPV vaccines) [26].
A signal amplification semi-quantitative assay, Hybrid Capture II (HC2, Digene Corp, Gaithersburg, MD, USA) was the first licensed and approved methodology for screening purposes and ASC-US triage by the U.S. Food and Drug Administration (FDA). HC2 has similar analytic sensitivity compared to many PCR methods of HPV DNA detection and clinical performance evaluations of emerging commercial assays are usually conducted in comparison to HC2 [21,29,50–53]. Alternatively, real time PCR-based assays such as Cobas® 4800 HPV (Roche Molecular Diagnostics, Pleasanton, CA, USA) use automated sample preparation and concomitant amplification and identification of multiple HPV types in a single reaction. This system detects 13 HR-HPV types and identifies the presence of HPV16 and HPV18 individually. Cobas has been clinically validated and is FDA-approved for ASC-US triage and use in cervical cancer screening of women aged 30 years and above that can include co-testing with cytology [22], applications that have been recommended by numerous guidelines such as United States Preventive Services Task Force (USPSTF) [14]. Triage via identification of specific HR-HPV types, specifically HPV16 and HPV18, was also FDA-approved as a validated method for triage to colposcopy [14].
The classification of papillomaviruses is based on viral genome sequence information. A distinct HPV type is established when the viral DNA sequence of the L1 open reading frame differs from any other closely related type by at least 10% of this region of the viral genome [54]. Isolates of the same HPV type are referred to as variants with L1 DNA sequence differences less than 10% [33]. Major advances in HPV genome analyses are being made with next-generation sequencing (NGS) that constitutes a multiple highly parallel sequencing technique. MGS is a high-throughput methodology able to sequence individual molecules from small amounts of DNA. It has been progressively applied to HPV typing and has proven to be highly accurate, reproducible and with high sensitivity to detect and identify multiple HPV type infections [55]. By allowing multiplexed pooled samples, uniquely barcoded for bioinformatic analysis, NGS is suitable to process larger numbers of samples with virtually no loss of sensitivity (see Figure 2). Moreover, NGS is able to detect unknown and uncharacterized HPV types since the read out is a DNA sequence [46,56]. For phylogenetically related HPV types, NGS can reveal novel HPV types that are present avoiding false-positive results by mis-typing from other methodologies that cannot discriminate related HPV types [55]. As part of this complex technology, the NGS bioinformatic pipeline should be robust enough to detect artifacts such as “chimeric” sequences that occurred from merged sequences originating from different viruses and/or other DNA molecules [55], and this way fully characterize unknown HPV types for which there is no available DNA sequence [33,55].
Figure 2.
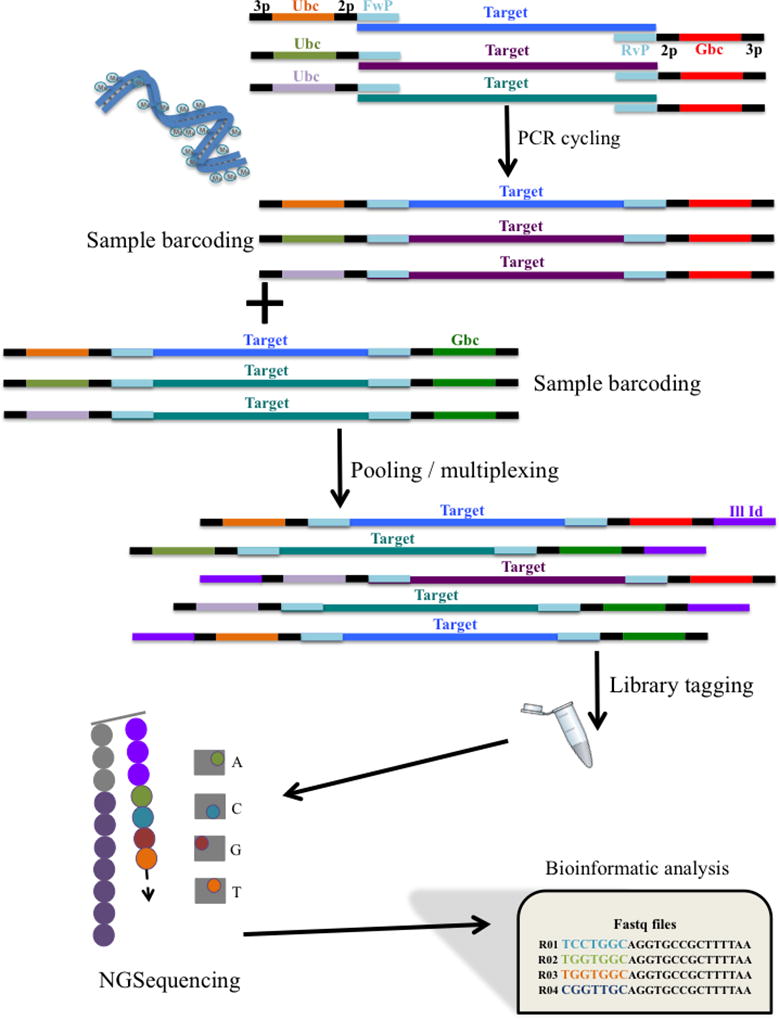
Simplified workflow for HPV typing using NGS methods. During PCR each sample is uniquely barcoded via the primer pair. The use of multiple barcodes allows for larger number of samples to be tested. NGS has its own chemistry that recognizes the presence of an Illumina Index (Ill Id) for sequencing purposes. During sequencing in the flow cell, as each nucleotide is incorporated releasing a flash of light, a picture is retained and analyzed by specialized software to identify the specific nucleotide incorporated. Raw data files comprise million of sequence reads from a pool of samples that need to be analyzed using bioinformatic tools (3p: 3bp-pad; Ubc: unique forward barcode sequence; 2p: 2bp-pad; FwP: forward primer sequence; HPV target: HPV amplicon of interest; RvP: reverse primer sequence; Gbc: general reverse barcode sequence).
The workflow of sample processing for NGS to identify HPV molecules (see Figure 2) involves use of special primer pairs during amplification, called “barcoded-primers” that incorporate 8bp- or 12bp-unique sequences into the target amplicon after the first cycle of PCR [57]. Thus, each sample becomes uniquely barcoded by the primer pair for post-sequencing identification and computer processing analysis. By maximizing the number of barcode combinations, post-PCR uniquely-barcoded samples can be multiplexed (i.e. the pooling of multiple samples into a single tube for NGS) increasing the number of samples processed per each single NGS run. In addition, this high-throughput technique requires preparation of a library, of the amplified samples (barcoded), by molecular manipulations that generate blunt ends and poly-A tails into the target amplicons for proper adapter indexing ligation (i.e., attachment of short nucleotide sequences that are recognized during sequencing by the NGS equipment). These adapters will facilitate the: i) binding of the fragments to the flow cell during sequencing; ii) enrichment of adapter-ligated fragments by PCR; and iii) possibility of multiplexing different libraries in the same flow cell. Depending on molecular size and concentration, multiple PCR assays can be combined during the NGS library-indexing step. After sequencing, the raw data files from the NGS machine are first filtered for low quality reads (minimum average read PHRED score of 25), and then demultiplexed based on the barcodes uniquely assigned to each sample (all the reads with the same unique barcode are put into one folder). Single or paired-end reads are subsequently aligned and mapped to the HPV reference sequences to determine the type present. Multiple assays (i.e., amplification of different regions of the HPV genome) can be performed in parallel enabling more accurate HPV typing for alpha-, beta- or gamma-HPVs and improved efficiency [46].
Overall, NGS combined with barcoding each sample is highly sensitive for the detection of HPV in multitudes of samples and even different types of samples, enlarging the general applicability of this technique. A NGS approach has demonstrated utility for the detection and identification of HPV types in clinical samples, as well as providing large amounts of data for use as a diagnostic, prognostic or risk stratification tool in clinical settings [34,44]. Various assays applicable to HPV testing and HPV typing are under development. This includes use of different primer pairs, different NGS platforms (e.g., Illumina) and different bioinformatic pipelines. Furthermore, small variations or point mutations are easily identified by NGS HPV typing methods, which have proven to be useful in epidemiological studies, HPV vaccination surveillance programs, and to monitor viral variants that may escape immunity [44,55]. Different variants of HR-HPV types may also influence cervical cancer progression stages by: i) differences in infectivity favoring acquisition of infection upon exposure; ii) duration of persistence; and iii) differences in oncogenicity favoring cervical cancer development. For instance, multiple studies have provided compelling data that HPV16 variant lineages B/C/D (previously called non-European) are considered more pathogenic than HPV16 lineage A (European) [44,58]. These features of an HPV infection can be readily obtained from NGS assays, but not as simply from RT-PCR fluorometric assays (e.g., Cobas). Thus, considering the trade off is between an HPV test that can be performed and completed in a single tube, in contrast to a NGS assay that can run thousands of samples simultaneously but requires transfer from a single tube reaction where the result is generated with the PCR.
11. Potential New Biomarkers to Further Stratify HPV-positive Women
Molecular biomarkers with the ability to identify HR-HPV positive women with precancer and/or at risk for progressive disease and in need of ablative treatment are of major importance to: i) serve as monitoring tools that can be applied to screening and risk stratification, and ii) elucidate the mechanisms driving HPV oncogenicity [26,59]. Risk stratification and triage methods should be able to provide higher specificity for detection of cervical precancers, and these tests need to be affordable. Currently available strategies to complement HR-HPV DNA testing include HPV typing, HPV mRNA testing, and/or histological markers such as p16/Ki67 [19,60]. Future considerations should take into account potential false-positive and/or false-negative features of such tests as they might apply to current guidelines and screening strategies to improve the quality and health management of HR-HPV infected women [26,61].
HPV RNA testing can add prognostic value and commonly uses reverse-transcription mediated amplification of viral mRNA with increased specificity to identify high-risk lesions [62], although there is some loss of sensitivity in comparison to HPV DNA testing. Therefore, the detection of abundant expression of HPV E6/E7 transcripts [62,63] is predictive of underlying cervical precancer since it detects viral transcriptional activity, representing action of the HR-HPV and not simply viral presence [26,61,62]. Additionally, RT-PCR based assays can provide quantitation of viral load although this has not yet been proven to be clinically useful, except perhaps for HPV16 [64,65]. At later stages of cervical disease, viral integration into the cellular genome may take place and emerging NGS sequencing methods are rapidly being developed to detect these events, as described below [59,66,67].
Advances in molecular genetics provide a better understanding of the natural history of HPV infection at the molecular level. One of these molecular “traits” is the ability of the HPV genome to be found integrated into the genome of the host cell as cervical disease progresses to cancer [66,68]. HPV integration is believed to act as a driver mutation in cervical carcinogenesis by facilitating persistent expression of E6 and E7 oncoproteins. However, it is unknown whether both chromosomal aberrations and cellular clonal expansion precedes and facilitates HPV integration, or whether viral integration triggers cascades of molecular events ultimately leading to the accumulation of host somatic changes [66,67,69]. The development of assays allowing the robust detection and mapping of HPV integrations has been slow and encountered technical challenges based on the identification of rare viral-cellular junctions in the presence of massive amounts of episomal viral and/or human genome DNA. Therefore, a PCR-based approach such as Amplification of Papillomavirus Oncogene Transcripts (APOT) distinguishes mRNAs derived from integrated versus episomal viral genomes [68], and is based on structural differences at the 3’ end of the viral transcripts; alternatively, other methods have been proposed: restriction site PCR [70], Southern blot and Detection of Integrated Papillomavirus Sequences (DIPS) [71], and RT-PCR for the determination of the HPV physical status [72]. However, these assays are usually time-consuming, laborious and often subjective to interpretation, with loss in sensitivity that may be greatly influenced by type-specific viral load, or frequency of integrated and episomal forms [66,67]. More recently, an innovative NGS assay can detect viral-cell junctions by capturing all viral containing molecules after hybridization with customized HPV probes and deep sequencing. This requires a specialized bioinformatic pipeline sensitive to the identification of viral-cell chimeric molecules, the definitive evidence of HPV integration. Additionally, the depth and resolution achieved in a NGS run allows for an accurate distinction of a discreet number of integrated forms of different HR-HPV types simultaneously, that can also map multiple viral-cell junctions with tens or hundreds of samples multiplexed [66,67]. HPV integration may disrupt or activate important pathways that promote tumor growth (e.g., RAS oncogenic pathway via RASGRF1). It appears that initial viral-induced chromosomal instability facilitates HPV integration with selection for E6/E7 oncoprotein expression that promotes clonal expansion, ultimately leading to cervical cancer with in many cases high complexity in the patterns of HPV integration [66,67,69]. However, false-positives due to discordant read-pairs may obscure HPV integration detection, which warrant ongoing improvements in this methodology. Nevertheless, the use of a capture NGS assay for evaluation of HPV integration looks very promising particularly as the costs of NGS fall and the technology is improved.
Cancer development represents a specific set of molecular changes within a dysregulated cell leading to abnormal proliferation where epigenetics plays a role by influencing changes in gene expression [73–77]. This is an expanding area where global cellular and/or viral changes including hypomethylation and hypermethylation are being investigated as diagnostic tools for detection of cervical precancerous lesions [47,78–81]. Determining methylation levels on HR-HPV genomes has the potential to detect precancerous lesions with higher specificity than HPV testing alone [26]. As HR-HPV infections remain widespread among females worldwide, triage of these same women should be based on risk stratification ideally using the clinical sample in which HR-HPV was detected in a sequential testing strategy [82]. Ongoing efforts will continue to require large prospective clinical studies to validate sensitivity, specificity, positive and negative predictive values for new techniques such as NGS that have been shown to be accurate and are becoming more cost-effective, where computational advances allow for increased sensitivity and depth of sequencing [83]. Further research on how changes in the viral and host methylome are associated with cervical cancer development should provide mechanistic insights facilitating prevention and treatment [74,81].
Methylation of CpG sites within viral and cellular gene promoter regions can lead to alterations in gene expression [84], as well as predicting risk of precancerous lesions. These markers provides value to distinguish women with benign HPV infection from those requiring potentially immediate management by increasing the specificity of molecular assays for high-grade disease detection and increased risk of cervical cancer development [85]. Furthermore, the fact that CpG methylation states appear stable makes them suitable for longitudinal studies [73,86]. Currently, there is wide variability in terms of assays performed, the viral/host genes targeted combined with the need for reproducibility studies amongst different methods and cohorts [47,73,78–82]. Thus, there is currently no consensus on which host gene methylation profiles should be evaluated and whether these will develop into molecule tests with sufficient predictive values. This precludes current recommendations or insights on how to use host methylation as a cervical cancer biomarker for early detection of precancerous lesions [85].
Identification of additional biomarkers in the era of very sensitive HR-HPV DNA tests will require higher specificity and increased positive predictive value for high-grade disease. These characteristics have not yet been achieved for HR-HPV women using methylation levels for host gene targets. Nonetheless, there is potential for use of methylation as a reflex panel of tests (viral and/or cellular) that would provide improved risk stratification for triage of women with HR-HPV infections [80,87]. As an example, there is increasing evidence towards CDKN2A (cyclin-dependent kinase inhibitor 2A, gene encoding p16) as a “candidate” showing differential levels of methylation between low-grade CIN and high-grade CIN [81]. These considerations still require proper evaluation and validation for future applications.
Nevertheless, quantitative individual CpG site methylation levels within the viral late/capsid genes have been repeatedly demonstrated to be associated with cervical cancer development in women infected by HPV16 [44,47,54,80,84]. Sequencing of small fragments of bisulfite-converted HPV DNA using NGS platforms provides highly informative quantitative measurements of single CpG site methylation levels that are associated with disease status [81,83,87]. The extension of this association to other HR-HPV types looks promising, but it has been difficult to study due to the lower prevalence of HR-HPV types other than HPV16 [88–90]. NGS techniques applied to the determination of single CpG site methylation levels leverages the high throughput potential and strengths of the method including accuracy, sequencing depth, normalization, scaling and reproducibility; also, it may be adapted to partial or full automation [83].
In summary, quantitation of CpG sites within HR-HPV DNA and/or host cellular regions provides a type of test that could be done on the same material used for HPV testing. Technical considerations include how the CpG sites are quantitated (e.g., pyrosequencing, NGS and/or quantitative PCR – methylation specific PCR), which platforms are used for each technique (e.g., Illumina or IonTorrent) and the underlying biology that specifies the relationship between CpG methylation levels and risk of high-grade neoplasia and cancer.
12. Screening a Vaccinated Population - what has and will change?
Over the last decade, primary prevention against HR-HPV infections has become preeminent with the commercialization of prophylactic HPV vaccines (bivalent, quadrivalent, and 9-valent) [91]; and HPV vaccination programs are widespread, but not universal. These HPV vaccines, regardless of specific guidelines, have been introduced in several countries mainly targeting young adolescents with some public health programs offering vaccines up through 26 years of age (http://www.cdc.gov/mmwr/). HPV vaccination is also recommended through age 26 years for men who have sex with men and for immunocompromised individuals (including those with HIV infection). All HPV vaccines are noninfectious, virus-like particle (VLP) vaccines with the most recent version (9-valent) approved by the FDA at the end of the year of 2014. In many countries including the US, the 9-valent is replacing the quadrivalent vaccine [22]; the 9-valent vaccine targets immunization against HPV types 16 and 18 which alone causes more than 60% of all HPV-associated cancers in the United States, and incorporates additional coverage against HPV types 31, 33, 45, 52 and 58 [91]. Unfortunately, it does not include HPV35, which is one of the most prevalent HPV types in many parts of the world, especially Africa [4]. Current recommendations for young adolescent immunization programs may provide coverage of about 90% of HPV infections based on the prevalence of HR-HPV types on cervical cancer globally [92]. More recently, in Europe, the World Health Organization (WHO) has proposed a two-dose schedule since 2014, based on data that indicated that a two-dose schedule would provide the same immunogenicity and safety compared to the previous three-dose schedule, when administered to young adolescents (i.e., below 15 years of age) [93,94].
Preventing HPV infection itself has the high potential to reduce the prevalence of cervical neoplasia and to eradicate cervical cancer by preventing the establishment of a HR-HPV infection. Countries where the coverage rates are high are already detecting dramatic decline in HPV-related disease [95], namely an impressive decrease in the number of new cases of anogenital warts [96]; or decreases close to 50% in the number of cervical abnormalities diagnosed [97]. Switching to primary HPV-based screening in the post-vaccination era should constitute an improved option due to the substantial decline in HR-HPV infections [22]. Additionally, an effective HPV vaccination program with high coverage has the power to disrupt the current balance between follow-up concerns, associated morbidity, and beneficial screening effects. In fact, decreases in prevalence of HPV-included types will dramatically reduce the positive predictive value of HPV testing; and reduce referral for colposcopy [98,99]. These changes in HPV prevalence and the transition period of decades may have serious implications on current cervical cancer screening guidelines including: i) starting screening at older ages; ii) longer screening intervals; and iii) increased reliance on biomarker testing for triage. Moreover, much of the knowledge on cervical cancer prevention has been based on the disproportionate role of HPV16 as the major cervical carcinogen. As HPV16 and other HR-HPVs are depleted from the population, new paradigms will be required, as cervical cancer will continue to afflict women throughout the world in potentially unpredictable ways as screening practices change and healthcare providers grapple with the most efficient strategy to prevent cervix cancer in a heterogeneous set of women. Thus, the medical system will need guidelines and assays relevant to women who never received a vaccine, women who received the HPV16/18 vaccine only, women infected with HR-HPV types not included in any vaccine or DNA assays (e.g., HPV73, 82) and various other clinical scenarios sure to arise.
13. CONCLUSION
Cervical cancer prevention is directed at preventing HR-HPV infection by type-specific vaccines and/or identification and removal of cervical precancerous lesions. Diagnostic screening for HR-HPV infection has or will become the major modality of cervical screening programs. In the transition period as many HR-HPV types will hopefully be eliminated from the population, public health experts face a challenge in how to manage HR-HPV infected women at risk and needing more intense screening than those vaccinated. Advanced HPV detection technologies identifying a set of HR-HPV types in a robust and economic manner with the ability to provide expanded and complementary information will be required. NGS technology has many of the attributes necessary and is predicted to play an increasing role in the future along with RT-PCR that can discriminate multiple HPV types. Nevertheless, novel diagnostic biomarkers with high specificity for cervical precancer will become critical to complement HPV typing. Increased knowledge of the molecular changes in cervical precancer and cancer such as genetic and/or epigenetic changes associated with cervical neoplastic progression combined with HR-HPV infection will be important for the next generation of screening programs. Extensive investigations and studies already provide evidence of an active role of DNA methylation particularly of the HR-HPV genome in L1/L2 and suggest its status as a future molecular biomarker in cervical cancer prevention programs. Comparative analyses with complementing data from HR-HPV testing, cytology, triage algorithms, colposcopy, follow-up visits, and cost assessments from such screening programs needs to be under constant evaluation and revision as we learn from the global experience.
14. EXPERT COMENTARY
The clinical landscape of disease diagnosis, especially cancer, has been transformed in the last decade by the availability of genome-wide diagnostic technologies. Thus, advances in NGS has enabled low-cost sequencing of customized or standardized gene panels, in addition to whole exome and whole genome sequencing that has uncovered genomic variations and relevant cancer specific “finger prints” [77]. Clinical and histological categorization has revealed a complex phenotypic disease, whereas genome sequencing has helped decipher underlying molecular changes leading to a more precise phenotype that should assist clinical management strategies. Handling NGS data however is a challenge since the amount of information generated and its complexity must be analyzed using specialized software and computational biology methods. NGS data requires pipeline development that includes demultiplexing, analysis, data management, data visualization and statistics. Millions of reads are compacted into gigabytes of raw data in encrypted software-specific files that are extracted in a hierarchical computer structure. Therefore, the analytical strategy needs to be directed according to the translational information needed for clinical practice. This requires a team approach with expertise in translational and precision medicine.
NGS-based published data combined with The Cancer Genome Atlas project (TCGA) data have revealed extensive complexity and heterogeneity in cervical cancers. Integrative multi-disciplinary molecular tests that can accurately triage HR-HPV infections and detect cervical disease most at risk for cancer development are under progress and evaluation. In this new triage scenario, avoiding unnecessary colposcopy and biopsies will reduce the burden of healthcare procedures for HPV-infected women. Moreover, obtaining a cervical sample suitable for HPV and other testing, including high-throughput NGS panels, for identification of women at risk of developing high-grade neoplasia is crucial for the standardization of triage strategies. Thus, the concept of a “liquid biopsy” (i.e. “molecular” Pap test) as a cervical sample providing these characteristics is the future. HPV DNA and RNA testing, NGS HPV genetic and epigenetic variation characterization are emerging technologies that fit this model, as a liquid-based sample is suitable for HR-HPV DNA enrichment assays and NGS sequencing. Current guidelines for biomarker development require a clinical assay that can provide improved risk stratification for triage. Hence, developing a reflex test on a HR-HPV positive individual, from the same exfoliated cervical sample, highly specific to discriminate high-risk neoplasias from CIN1 or benign infections is of critical importance in the years to come.
To better understand the relationship between multiple risk factors for cervical cancer development, such as HPV type, mRNA overexpression, methylation levels or integration sites, in conjunction with histological diagnoses, exploratory studies are ongoing for molecular classifiers of exfoliated cervical samples from “liquid biopsy” specimens. NGS alone may provide all these diagnostic measurements on just one single sample/processing step, which will decrease cost and time, and should increase method standardization. Current HPV genotyping NGS assays have recently been investigated with promising results, including identifying HPV variants and novel papillomaviruses; HPV type-specific methylation assays continue to provide encouraging data, as well as some host promoter regions with discriminatory performance for the identification of precancerous lesions. This multifaceted approach implies robust computational biology, specialized software and multivariate models with the ability to process large amounts of data, comprehensive data presentation and probabilistic analyses. The predictability of a biomarker panel should also be informative of the disease state per se but may also provide quantitative prediction of cervical cancer risk. Therefore, a future panel should integrate multiple data, including a set of reflex tests after detection of HR-HPV that has high predictive value for cervical precancer and/or risk for progression.
The target population that can benefit the most from triage methods improvements will be asymptomatic HPV+ women with cervical cancer precursors or invasive cervical cancer requiring therapeutic intervention. A collaborative review and recent guideline recommendations [100] from the ASCO Resource-Stratified Guidelines Advisory Group point to full implementation of HPV testing in all resource settings with differentiated stratified levels of screening aiming to maximize resources while assuring the best healthcare procedures. There are challenges moving forward to the post-vaccination era, where HPV detection will be less predictive of CIN3. This magnifies the continued need for biomarker research as addressed in this review.
15. FIVE-YEAR VIEW
Cervical cancer prevention programs are under rapid evolution based on emerging opportunities for primary prevention (i.e., HPV vaccines) and development of new technologies and knowledge for secondary prevention. As the prevalence of HR-HPV and cervix cancer decline through vaccination, utility of cervix cancer screening programs will change. It is envisioned, that in the near future, the introduction of a “liquid-biopsy” from exfoliated cells (i.e., “molecular” Pap test) will encompass a format that can be adapted to a platform with sequential tests enabling personalized risk stratification. For instance, technological advances in molecular HPV testing can incorporate findings from the cervix cancer TCGA data [77] that identify driver mutations found in cervix cancer tissues. In addition, both viral and cellular CpG methylation levels will provide cumulative risk scores for HPV infected women. Moreover, knowing that not all CIN3 are true cervical precancers (e.g., HPV51 is associated with a substantial number of CIN3 lesions that rarely progress to cancer) new molecular data will supplant the classical histologic diagnosis of a colposcopic biopsy, although it is likely that biopsies or at least a tissue sample may still be needed over this transition period. Thus, the next phase will encompass risk stratification beyond HPV type and move towards better and more personalized intervention and/or treatment strategies improving the effectiveness of cervix cancer screening/prevention programs, particularly in the face of decreasing precancer and cancer.
16. KEY ISSUES
Cervical cytology-based prevention programs are expensive, time-consuming and technically demanding with relatively low sensitivity for a single Pap smear, nevertheless they have been effective in high-resource organized settings;
HR-HPV DNA detection has been successfully incorporated into cervical cancer prevention programs based on its higher sensitivity and increased negative predictive value. Accumulating evidence from European randomized clinical trials has shown that HPV testing as a primary screening tool will provide an additional 60–70% protection against invasive cervical cancer compared to cytology-based screening programs;
Risk stratification and triage methods should be able to provide higher specificity for the detection of cervical precancers in an affordable manner;
Major advances in HPV genome analyses are being made with next-generation sequencing (NGS), a massively parallel single molecule high-throughput sequencing technique that is able to sequence individual molecules from small amounts of DNA. It has been progressively applied to HPV typing and has proven to be highly accurate, reproducible, with high sensitivity to detect and identify multiple HPV type infections, and/or detect uncharacterized HPV types;
Target enrichment combined with NGS allows the detection of integrated forms of HPV DNA with mapping of viral-cellular junctions to host chromosomes. Hence, NGS makes it possible to document and describe HPV integration events;
Cellular and viral epigenetic changes have been increasingly associated with cervical cancer development. Hypermethylation levels of viral and cellular DNA are being investigated as an adjunct molecular classifier for cervical cancer progression;
Convergence of the HPV vaccine, NGS, TCGA (tumor cancer genome atlas) and methylation data through advanced technology will change the landscape of cervix cancer prevention programs;
A “molecular” Pap test has the potential to transform the clinical arena of cervical disease diagnoses, while uncovering relevant cancer specific “finger prints”, towards an improved and personalized medicine.
Acknowledgments
Grandissimo is supported in part by a grant from the National Cancer Institute (CA78527), NIH. R.D. Burk is supported in part by grants from the National Cancer Institute (CA78527) and the National Institute of Allergy and infectious Diseases (AI072204), NIH.
Footnotes
Funding
This paper was not funded.
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Aldabagh B, Yu J, Arron ST. Role of human papillomavirus in cutaneous squamous cell carcinoma: a meta-analysis. J Am Acad Dermat. 2014;70(4):621–629. doi: 10.1016/j.jaad.2014.01.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Bosch FX, Broker TR, Forman D, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(Suppl 7):H1–31. doi: 10.1016/j.vaccine.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kesic V. Prevention of cervical cancer in Central and Eastern Europe and Central Asia: a challenge for the future. Vaccine. 2013;31(Suppl 7):vii–ix. doi: 10.1016/j.vaccine.2012.11.105. [DOI] [PubMed] [Google Scholar]
- 6.Anttila A, Nieminen P. Cervical cancer screening programme in Finland. Eur J Cancer. 2000;36(17):2209–2214. doi: 10.1016/s0959-8049(00)00311-7. [DOI] [PubMed] [Google Scholar]
- 7.Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Bosch FX, Robles C, Diaz M, et al. HPV-FASTER: broadening the scope for prevention of HPV-related cancer. Nat Rev Clin Oncol. 2016;13(2):119–132. doi: 10.1038/nrclinonc.2015.146. This paper describes an approach for cervix cancer prevention utilizing combined vaccination and screening, as a new paradigm. [DOI] [PubMed] [Google Scholar]
- 9.Ogilvie GS, Krajden M, van Niekerk D, et al. HPV for cervical cancer screening (HPV FOCAL): Complete Round 1 results of a randomized trial comparing HPV-based primary screening to liquid-based cytology for cervical cancer. Int J Cancer. 2017;140(2):440–448. doi: 10.1002/ijc.30454. [DOI] [PubMed] [Google Scholar]
- 10.Ronco G, Dillner J, Elfstrom KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 11*.McCredie MR, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–434. doi: 10.1016/S1470-2045(08)70103-7. This was a study that followed women with CIN3 of whom 31–50% not adequately treated went on to develop invasive cancer, providing an estimate of the risk of progression. [DOI] [PubMed] [Google Scholar]
- 12**.Schiffman M, Doorbar J, Wentzensen N, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016;2:16086. doi: 10.1038/nrdp.2016.86. This is an expansive review of the biology and epidemiology of oncogenic HPV in the human population. [DOI] [PubMed] [Google Scholar]
- 13.Cuzick J. Long-term cervical cancer prevention strategies across the globe. Gynecol Oncol. 2010;117(2 Suppl):S11–14. doi: 10.1016/j.ygyno.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 14*.Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182. doi: 10.1016/j.ygyno.2014.12.022. This guideline discusses the use of HPV DNA testing for prevention of cervix cancer in a screening program. [DOI] [PubMed] [Google Scholar]
- 15.Abreu AL, Souza RP, Gimenes F, Consolaro ME. A review of methods for detect human Papillomavirus infection. Virol J. 2012;9:262. doi: 10.1186/1743-422X-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15(2):172–183. doi: 10.1016/S1470-2045(13)70570-9. [DOI] [PubMed] [Google Scholar]
- 17.Kelly RS, Patnick J, Kitchener HC, Moss SM Group NHSI. HPV testing as a triage for borderline or mild dyskaryosis on cervical cytology: results from the Sentinel Sites study. Br J Cancer. 2011;105(7):983–988. doi: 10.1038/bjc.2011.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorincz A, Castanon A, Wey Lim AW, Sasieni P. New strategies for human papillomavirus-based cervical screening. Womens Health (Lond) 2013;9(5):443–452. doi: 10.2217/whe.13.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castanon A, Landy R, Sasieni P. By how much could screening by primary human papillomavirus testing reduce cervical cancer incidence in England? J Med Screen. 2016 doi: 10.1177/096914136654197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rijkaart DC, Berkhof J, van Kemenade FJ, et al. Evaluation of 14 triage strategies for HPV DNA-positive women in population-based cervical screening. Int J Cancer. 2012;130(3):602–610. doi: 10.1002/ijc.26056. [DOI] [PubMed] [Google Scholar]
- 22.Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197. doi: 10.1016/j.ygyno.2014.11.076. [DOI] [PubMed] [Google Scholar]
- 23.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338(7):423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 24.Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12(7):663–672. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitchener HC, Gilham C, Sargent A, et al. A comparison of HPV DNA testing and liquid based cytology over three rounds of primary cervical screening: extended follow up in the ARTISTIC trial. Eur J Cancer. 2011;47(6):864–871. doi: 10.1016/j.ejca.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 26*.Wentzensen N, Schiffman M, Palmer T, Arbyn M. Triage of HPV positive women in cervical cancer screening. J Clin Virol. 2016;76(Suppl 1):S49–55. doi: 10.1016/j.jcv.2015.11.015. This review gives an update on the triage of women identified with an HPV infection with and without cytological abnormalities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arbyn M, Ronco G, Cuzick J, Wentzensen N, Castle PE. How to evaluate emerging technologies in cervical cancer screening? Int J Cancer. 2009;125(11):2489–2496. doi: 10.1002/ijc.24774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(Suppl 5):F88–99. doi: 10.1016/j.vaccine.2012.06.095. [DOI] [PubMed] [Google Scholar]
- 29.Levi AW, Bernstein JI, Hui P, Duch K, Schofield K, Chhieng DC. A Comparison of the Roche Cobas HPV Test With the Hybrid Capture 2 Test for the Detection of High-Risk Human Papillomavirus Genotypes. Arch Pathol Lab Med. 2016;140(2):153–157. doi: 10.5858/arpa.2015-0027-OA. [DOI] [PubMed] [Google Scholar]
- 30.Mesher D, Szarewski A, Cadman L, et al. Long-term follow-up of cervical disease in women screened by cytology and HPV testing: results from the HART study. Br J Cancer. 2010;102(9):1405–1410. doi: 10.1038/sj.bjc.6605619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst. 2009;101(2):88–99. doi: 10.1093/jnci/djn444. [DOI] [PubMed] [Google Scholar]
- 32.Sasieni P, Cuzick J. Could HPV testing become the sole primary cervical screening test? J Med Screen. 2002;9(2):49–51. doi: 10.1136/jms.9.2.49. [DOI] [PubMed] [Google Scholar]
- 33.Harari A, Chen Z, Burk RD. Human papillomavirus genomics: past, present and future. Curr Probl Dermatol. 2014;45:1–18. doi: 10.1159/000355952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conway C, Chalkley R, High A, et al. Next-generation sequencing for simultaneous determination of human papillomavirus load, subtype, and associated genomic copy number changes in tumors. J Mol Diagn. 2012;14(2):104–111. doi: 10.1016/j.jmoldx.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Snijders PJ, van den Brule AJ, Schrijnemakers HF, Snow G, Meijer CJ, Walboomers JM. The use of general primers in the polymerase chain reaction permits the detection of a broad spectrum of human papillomavirus genotypes. J Gen Virol. 1990;71(Pt 1):173–181. doi: 10.1099/0022-1317-71-1-173. [DOI] [PubMed] [Google Scholar]
- 36.Qu W, Jiang G, Cruz Y, et al. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J Clin Microbiol. 1997;35(6):1304–1310. doi: 10.1128/jcm.35.6.1304-1310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Resnick RM, Cornelissen MT, Wright DK, et al. Detection and typing of human papillomavirus in archival cervical cancer specimens by DNA amplification with consensus primers. J Natl Cancer Inst. 1990;82(18):1477–1484. doi: 10.1093/jnci/82.18.1477. [DOI] [PubMed] [Google Scholar]
- 38.de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3' ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76(Pt 4):1057–1062. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- 39.Castle PE, Porras C, Quint WG, et al. Comparison of two PCR-based human papillomavirus genotyping methods. J Clin Microbiol. 2008;46(10):3437–3445. doi: 10.1128/JCM.00620-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koskinen WJ, Brondbo K, Mellin Dahlstrand H, et al. Alcohol, smoking and human papillomavirus in laryngeal carcinoma: a Nordic prospective multicenter study. J Cancer Res Clin Oncol. 2007;133(9):673–678. doi: 10.1007/s00432-007-0219-8. [DOI] [PubMed] [Google Scholar]
- 41.Tjalma WA, Depuydt CE. Cervical cancer screening: which HPV test should be used--L1 or E6/E7? Eur J Obstet Gynecol Reprod Biol. 2013;170(1):45–46. doi: 10.1016/j.ejogrb.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Kerr DA, Sweeney B, Arpin RN, 3rd, et al. Automated Extraction of Formalin-Fixed, Paraffin-Embedded Tissue for High-Risk Human Papillomavirus Testing of Head and Neck Squamous Cell Carcinomas Using the Roche Cobas 4800 System. Arch Pathol Lab Med. 2016;140(8):844–848. doi: 10.5858/arpa.2015-0272-OA. [DOI] [PubMed] [Google Scholar]
- 43.Depuydt CE, Boulet GA, Horvath CA, Benoy IH, Vereecken AJ, Bogers JJ. Comparison of MY09/11 consensus PCR and type-specific PCRs in the detection of oncogenic HPV types. J Cell Mol Med. 2007;11(4):881–891. doi: 10.1111/j.1582-4934.2007.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mirabello L, Yeager M, Cullen M, et al. HPV16 Sublineage Associations With Histology-Specific Cancer Risk Using HPV Whole-Genome Sequences in 3200 Women. J Natl Cancer Inst. 2016;108(9) doi: 10.1093/jnci/djw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jelen MM, Chen Z, Kocjan BJ, et al. Global Genomic Diversity of Human Papillomavirus 11 Based on 433 Isolates and 78 Complete Genome Sequences. J Virol. 2016;90(11):5503–5513. doi: 10.1128/JVI.03149-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Agalliu I, Gapstur S, Chen Z, et al. Associations of Oral alpha-, beta-, and gamma-Human Papillomavirus Types With Risk of Incident Head and Neck Cancer. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2015.5504. This is the first paper to utilize next-generation sequencing with barcoded primers to characterize the gammut of alpha-, beta- and gamma-HPVs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clarke MA, Wentzensen N, Mirabello L, et al. Human papillomavirus DNA methylation as a potential biomarker for cervical cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2125–2137. doi: 10.1158/1055-9965.EPI-12-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cuschieri K, Bhatia R, Cruickshank M, Hillemanns P, Arbyn M. HPV testing in the context of post-treatment follow up (test of cure) J Clin Virol. 2016;76(Suppl 1):S56–61. doi: 10.1016/j.jcv.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124(3):516–520. doi: 10.1002/ijc.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pista A, Verdasca N, Oliveira A. Clinical performance of the CLART human papillomavirus 2 assay compared with the hybrid capture 2 test. J Med Virol. 2011;83(2):272–276. doi: 10.1002/jmv.21952. [DOI] [PubMed] [Google Scholar]
- 51.Park Y, Lee E, Choi J, Jeong S, Kim HS. Comparison of the Abbott Real Time High-Risk Human Papillomavirus (HPV), Roche Cobas HPV, and Hybrid Capture 2 assays to direct sequencing and genotyping of HPV DNA. J Clin Microbiol. 2012;50(7):2359–2365. doi: 10.1128/JCM.00337-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuschieri K, Cubie H, Graham C, et al. Clinical performance of RNA and DNA based HPV testing in a colposcopy setting: Influence of assay target, cut off and age. J Clin Virol. 2014;59(2):104–108. doi: 10.1016/j.jcv.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Basu P, Banerjee D, Mittal S, et al. Sensitivity of APTIMA HPV E6/E7 mRNA test in comparison with hybrid capture 2 HPV DNA test for detection of high risk oncogenic human papillomavirus in 396 biopsy confirmed cervical cancers. J Med Virol. 2016;88(7):1271–1278. doi: 10.1002/jmv.24453. [DOI] [PubMed] [Google Scholar]
- 54.Bernard HU, Burk RD, Chen Z, van Doorslaer K, Hausen H, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401(1):70–79. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arroyo LS, Smelov V, Bzhalava D, Eklund C, Hultin E, Dillner J. Next generation sequencing for human papillomavirus genotyping. J Clin Virol. 2013;58(2):437–442. doi: 10.1016/j.jcv.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 56.Ekstrom J, Bzhalava D, Svenback D, Forslund O, Dillner J. High throughput sequencing reveals diversity of Human Papillomaviruses in cutaneous lesions. Int J Cancer. 2011;129(11):2643–2650. doi: 10.1002/ijc.26204. [DOI] [PubMed] [Google Scholar]
- 57.Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods. 2008;5(3):235–237. doi: 10.1038/nmeth.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burk RD, Harari A, Chen Z. Human papillomavirus genome variants. Virology. 2013;445(1–2):232–243. doi: 10.1016/j.virol.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holmes ALS, Jeannot E, Marie Y, Castera L, Sastre-Garau X, Nicolas A. Mechanistic signatures of HPV insertions in cervical carcinomas. npj Genomic Medicine. 2016;1:16004. doi: 10.1038/npjgenmed.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leeson SC, Alibegashvili T, Arbyn M, et al. HPV testing and vaccination in Europe. J Low Genit Tract Dis. 2014;18(1):61–69. doi: 10.1097/LGT.0b013e318286b8d3. [DOI] [PubMed] [Google Scholar]
- 61.Pedersen K, Sorbye SW, Kristiansen IS, Burger EA. Using novel biomarkers to triage young adult women with minor cervical lesions: a cost-effectiveness analysis. BJOG. 2017;124(3):474–484. doi: 10.1111/1471-0528.14135. [DOI] [PubMed] [Google Scholar]
- 62.Oliveira A, Verdasca N, Pista A. Use of the NucliSENS EasyQ HPV assay in the management of cervical intraepithelial neoplasia. J Med Virol. 2013;85(7):1235–1241. doi: 10.1002/jmv.23590. [DOI] [PubMed] [Google Scholar]
- 63.Iftner T, Becker S, Neis KJ, et al. Head-to-Head Comparison of the RNA-Based Aptima Human Papillomavirus (HPV) Assay and the DNA-Based Hybrid Capture 2 HPV Test in a Routine Screening Population of Women Aged 30 to 60 Years in Germany. J Clin Microbiol. 2015;53(8):2509–2516. doi: 10.1128/JCM.01013-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gravitt PE, Kovacic MB, Herrero R, et al. High load for most high risk human papillomavirus genotypes is associated with prevalent cervical cancer precursors but only HPV16 load predicts the development of incident disease. Int J Cancer. 2007;121(12):2787–2793. doi: 10.1002/ijc.23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Z, Qin Y, Yu L, et al. Association between human papillomavirus (HPV) 16, HPV18, and other HR-HPV viral load and the histological classification of cervical lesions: Results from a large-scale cross-sectional study. J Med Virol. 2017;89(3):535–541. doi: 10.1002/jmv.24645. [DOI] [PubMed] [Google Scholar]
- 66.Bodelon C, Vinokurova S, Sampson JN, et al. Chromosomal copy number alterations and HPV integration in cervical precancer and invasive cancer. Carcinogenesis. 2016;37(2):188–196. doi: 10.1093/carcin/bgv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67**.Liu Y, Lu Z, Xu R, Ke Y. Comprehensive mapping of the human papillomavirus (HPV) DNA integration sites in cervical carcinomas by HPV capture technology. Oncotarget. 2016;7(5):5852–5864. doi: 10.18632/oncotarget.6809. This paper describes the use of capturing HPV sequencing and next-generation sequencing to identify and map HPV-cellular chimeric sequences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klaes R, Woerner SM, Ridder R, et al. Detection of high-risk cervical intraepithelial neoplasia and cervical cancer by amplification of transcripts derived from integrated papillomavirus oncogenes. Cancer Res. 1999;59(24):6132–6136. [PubMed] [Google Scholar]
- 69.Melsheimer P, Vinokurova S, Wentzensen N, Bastert G, von Knebel Doeberitz M. DNA aneuploidy and integration of human papillomavirus type 16 e6/e7 oncogenes in intraepithelial neoplasia and invasive squamous cell carcinoma of the cervix uteri. Clin Cancer Res. 2004;10(9):3059–3063. doi: 10.1158/1078-0432.ccr-03-0565. [DOI] [PubMed] [Google Scholar]
- 70.Thorland EC, Myers SL, Gostout BS, Smith DI. Common fragile sites are preferential targets for HPV16 integrations in cervical tumors. Oncogene. 2003;22(8):1225–1237. doi: 10.1038/sj.onc.1206170. [DOI] [PubMed] [Google Scholar]
- 71.Luft F, Klaes R, Nees M, et al. Detection of integrated papillomavirus sequences by ligation-mediated PCR (DIPS-PCR) and molecular characterization in cervical cancer cells. Int J Cancer. 2001;92(1):9–17. [PubMed] [Google Scholar]
- 72.Gradíssimo Oliveira ADC, Verdasca N, Pista A. Prognostic value of human papillomavirus types 16 and 18 DNA physical status in cervical intraepithelial neoplasia. Clin Microbiol Infect. 2013;19(10):447–450. doi: 10.1111/1469-0691.12233. [DOI] [PubMed] [Google Scholar]
- 73.Lorincz AT. The Promise and the Problems of Epigenetics Biomarkers in Cancer. Expert Opin Med Diagn. 2011;5(5):375–379. doi: 10.1517/17530059.2011.590129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ojesina AI, Lichtenstein L, Freeman SS, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506(7488):371–375. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rusan M, Li YY, Hammerman PS. Genomic landscape of human papillomavirus-associated cancers. Clin Cancer Res. 2015;21(9):2009–2019. doi: 10.1158/1078-0432.CCR-14-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77**.Cancer Genome Atlas Research N. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017 doi: 10.1038/nature21386. This paper is the most definitive description of the molecular characterization of cervix cancer providing information on differences between squamous, glandular and other forms of cervix cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brandsma JL, Sun Y, Lizardi PM, et al. Distinct human papillomavirus type 16 methylomes in cervical cells at different stages of premalignancy. Virology. 2009;389(1–2):100–107. doi: 10.1016/j.virol.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mirabello L, Schiffman M, Ghosh A, et al. Elevated methylation of HPV16 DNA is associated with the development of high grade cervical intraepithelial neoplasia. Int J Cancer. 2013;132(6):1412–1422. doi: 10.1002/ijc.27750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lorincz AT, Brentnall AR, Scibior-Bentkowska D, et al. Validation of a DNA methylation HPV triage classifier in a screening sample. Int J Cancer. 2016;138(11):2745–2751. doi: 10.1002/ijc.30008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wijetunga NA, Belbin TJ, Burk RD, et al. Novel epigenetic changes in CDKN2A are associated with progression of cervical intraepithelial neoplasia. Gynecol Oncol. 2016;142(3):566–573. doi: 10.1016/j.ygyno.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lorincz A, Raison C. Scratching the surface of tomorrow's diagnostics: the Editor-in-Chief's opinion at the 15th year of Expert Review of Molecular Diagnostics. Expert Rev Mol Diagn. 2015;15(1):5–8. doi: 10.1586/14737159.2015.998959. [DOI] [PubMed] [Google Scholar]
- 83.Mirabello L, Frimer M, Harari A, et al. HPV16 methyl-haplotypes determined by a novel next-generation sequencing method are associated with cervical precancer. Int J Cancer. 2015;136(4):E146–153. doi: 10.1002/ijc.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shen-Gunther J, Wang CM, Poage GM, et al. Molecular Pap smear: HPV genotype and DNA methylation of ADCY8, CDH8, and ZNF582 as an integrated biomarker for high-grade cervical cytology. Clin Epigenetics. 2016;8:96. doi: 10.1186/s13148-016-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wentzensen N, Sherman ME, Schiffman M, Wang SS. Utility of methylation markers in cervical cancer early detection: appraisal of the state-of-the-science. Gynecol Oncol. 2009;112(2):293–299. doi: 10.1016/j.ygyno.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 87.Guerrero-Preston R, Valle BL, Jedlicka A, et al. Molecular Triage of Premalignant Lesions in Liquid-Based Cervical Cytology and Circulating Cell-Free DNA from Urine, Using a Panel of Methylated Human Papilloma Virus and Host Genes. Cancer Prev Res (Phila) 2016;9(12):915–924. doi: 10.1158/1940-6207.CAPR-16-0138. [DOI] [PubMed] [Google Scholar]
- 88.Wentzensen N, Sun C, Ghosh A, et al. Methylation of HPV18, HPV31, and HPV45 genomes and cervical intraepithelial neoplasia grade 3. J Natl Cancer Inst. 2012;104(22):1738–1749. doi: 10.1093/jnci/djs425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kalantari M, Osann K, Calleja-Macias IE, et al. Methylation of human papillomavirus 16, 18, 31, and 45 L2 and L1 genes and the cellular DAPK gene: Considerations for use as biomarkers of the progression of cervical neoplasia. Virology. 2014;448:314–321. doi: 10.1016/j.virol.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marongiu L, Godi A, Parry JV, Beddows S. Human Papillomavirus 16, 18, 31 and 45 viral load, integration and methylation status stratified by cervical disease stage. BMC Cancer. 2014;14:384. doi: 10.1186/1471-2407-14-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107(6):djv086. doi: 10.1093/jnci/djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Joura EA, Ault KA, Bosch FX, et al. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1997–2008. doi: 10.1158/1055-9965.EPI-14-0410. [DOI] [PubMed] [Google Scholar]
- 93.Kreimer AR, Struyf F, Del Rosario-Raymundo MR, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol. 2015;16(7):775–786. doi: 10.1016/S1470-2045(15)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lowy DR. HPV vaccination to prevent cervical cancer and other HPV-associated disease: from basic science to effective interventions. J Clin Invest. 2016;126(1):5–11. doi: 10.1172/JCI85446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 96.Ali H, Guy RJ, Wand H, et al. Decline in in-patient treatments of genital warts among young Australians following the national HPV vaccination program. BMC Infect Dis. 2013;13:140. doi: 10.1186/1471-2334-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim J, Bell C, Sun M, et al. Effect of human papillomavirus vaccination on cervical cancer screening in Alberta. CMAJ. 2016;188(12):E281–288. doi: 10.1503/cmaj.151528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chesson HW, Flagg EW, Koutsky L, et al. Modeling the impact of quadrivalent HPV vaccination on the incidence of Pap test abnormalities in the United States. Vaccine. 2013;31(29):3019–3024. doi: 10.1016/j.vaccine.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Malik T, Reimer J, Gumel A, Elbasha EH, Mahmud S. The impact of an imperfect vaccine and pap cytology screening on the transmission of human papillomavirus and occurrence of associated cervical dysplasia and cancer. Math Biosci Eng. 2013;10(4):1173–1205. doi: 10.3934/mbe.2013.10.1173. [DOI] [PubMed] [Google Scholar]
- 100.Jeronimo JCPE, Temin S, Denny L, Gupta V, Kim JJ, Luciani S, Murokora D, Ngoma T, Qiao Y, Quinn M, Sankaranarayanan R, Sasieni P, Schmeler KM, Shastri SS. Secondary Prevention of Cervical Cancer: ASCO Resource-Stratified Clinical Practice Guideline. J Global Oncol. 2016 doi: 10.1200/JGO.2016.006577. [DOI] [PMC free article] [PubMed] [Google Scholar]


