Abstract
The availability of a wide range of independent lines for the annual medic Medicago truncatula led us to search for natural variants in the symbiotic association with Sinorhizobium meliloti. Two homozygous lines, Jemalong 6 and DZA315.16, originating from an Australian cultivar and a natural Algerian population, respectively, were inoculated with two wild-type strains of S. meliloti, RCR2011 and A145. Both plant lines formed nitrogen-fixing (effective) nodules with the RCR2011 strain. However, the A145 strain revealed a nitrogen fixation polymorphism, establishing an effective symbiosis (Nod+Fix+) with DZA315.16, whereas only small, white, non-nitrogen fixing nodules (Nod+Fix−) were elicited on Jemalong 6. Cytological studies demonstrated that these non-fixing nodules are encircled by an endodermis at late stages of development, with no visible meristem, and contain hypertrophied and autofluorescent infection threads, suggesting the induction of plant defense reactions. The non-fixing phenotype is independent of growth conditions and determined by a single recessive allele (Mtsym6), which is located on linkage group 8.
Bacteria of the genera Rhizobium, Bradyrhizobium, Azorhizobium, Sinorhizobium, and Mesorhizobium (collectively known as rhizobia) induce the formation of nitrogen-fixing nodules on the roots of leguminous plants. This symbiotic association is initiated when flavonoids or other plant compounds stimulate the bacteria to produce molecular signals known as Nod factors, which trigger a variety of plant responses leading to bacterial invasion and nodulation (for review, see Dénarié et al., 1996). The bacteria enter the developing nodule via tubular structures called infection threads (ITs) and are then taken up by plant host cells in an endocytosis-like process before differentiating into bacteroids capable of reducing atmospheric dinitrogen to ammonia, thereby supporting the growth of the plant (for review, see Panagiota et al., 1995; Van Rhijn and Vanderleyden, 1995).
The Rhizobium-legume symbiosis involves specific expression of both bacterial and plant genes. Plant and bacterial symbiotic mutants are very useful tools for the genetic dissection of the complex interactions that occur between the symbionts at both early and late stages of nodule development. Plant symbiotic mutants have been isolated in several leguminous species: sweetclover (Kneen and LaRue, 1988), pea (Duc and Messager, 1989), fava bean (Haser et al., 1992; Duc, 1995), Medicago truncatula (Sagan et al., 1995; Penmetsa and Cook, 1997), Lotus (Szczyglowski et al., 1998), and soybean (Devine and Kuykendall, 1996). The progeny of plants following mutagenic treatments are generally screened for the loss of their ability to establish an effective symbiosis due to the disruption of gene function. However, screening natural populations often reveals a range of allelic forms (variants) for each symbiotic gene, potentially giving rise to different phenotypes. The natural symbiotic variant alleles observed are usually of two kinds: either alleles (Nod− or Fix−) accumulating in allogamous plants that are revealed after selfing plants (Peterson and Barnes, 1981; Vance and Johnson, 1983), or alleles involved in strain-specific interactions as described for pea (Gelin and Blixt, 1964; Degenhardt et al., 1976; Lie 1981, 1984; Kneen and La Rue, 1984), soybean (Caldwell, 1966), vetch (Duc and Picard, 1986), and clover (Nutman, 1954; Smith and Knight, 1984).
We have chosen M. truncatula to study molecular, physiological, and genetic aspects of the Rhizobium-legume symbiosis because it is a diploid and homozygous autogamous legume with a small genome size (Barker et al., 1990; Cook et al., 1997; Cook, 1999). Snyman and Strijdom (1980) previously identified a high level of polymorphism in nodulation and fixation by studying the symbiotic characteristics of lines and cultivars of M. truncatula inoculated with Sinorhizobium meliloti strains of diverse origins. Following this initial study, we have screened natural populations of M. truncatula inoculated with wild-type S. meliloti strains and have found extensive symbiotic polymorphisms (nodulation, nitrogen fixation, and nitrogen fixation efficiency; L. Tirichine, J.-M. Prosperi, and T. Huguet, unpublished data). In this work, we focus on a specific ineffective interaction characterized by a Fix− phenotype between M. truncatula Jemalong 6 and S. meliloti A145. We show that the Fix− trait is under monogenic and recessive control, and the Fix− phenotype has been characterized cytologically. We have also localized the corresponding gene, called Mtsym6, on the genetic map of M. truncatula.
RESULTS AND DISCUSSION
The A145 Strain Induces an Ineffective Symbiosis with M. truncatula Jemalong 6
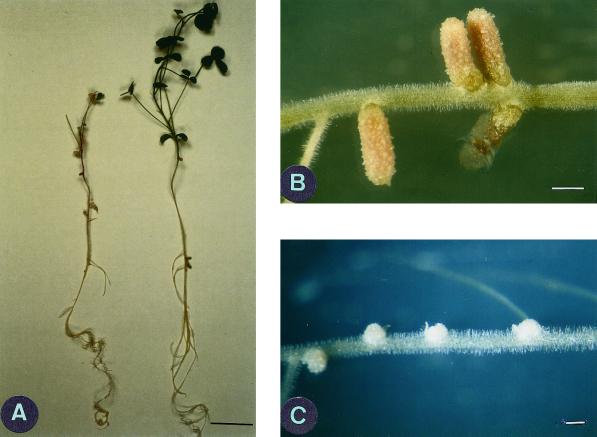
Sinorhizobium meliloti A145 is a wild-type strain isolated from alfalfa nodules (Rosenberg et al., 1981) that fixes nitrogen with the control alfalfa line Medicago sativa cv Gemini (Table I). However, inoculation of DZA315.16 and Jemalong 6 by S. meliloti A145 revealed a nitrogen fixation polymorphism. Whereas DZA315.16 is effectively nodulated, a non-nitrogen fixing phenotype is observed with Jemalong 6 (Fig. 1A). In addition to visual observations of the Fix− phenotype, dry weight measurements confirmed the nitrogen fixation polymorphism (Table I) and are in concordance with the absence of nitrogenase activity (data not shown). During the early stages of nodule development (10 and 20 d post-inoculation [DPI]), normal pink nodules were formed in the two plant lines. However, in the Jemalong 6/A145 interaction, nodules subsequently turned white and did not elongate (Fig. 1, B and C). This suggests that incompatibility occurs during late stages of Jemalong 6 nodule development when invaded by the A145 strain. It is interesting to note that non-fixing pea mutants, which develop some pink nodules without detectable nitrogenase activity, have been reported by Tsyganov et al. (1998).
Table I.
Shoot dry wt (mg/planta ± sd) of two M. truncatula lines and a M. sativa cultivar as a control, 39 DPI by A145 and RCR2011 strains of S. meliloti
| Medicago sp. |
S. meliloti Strains
|
||
|---|---|---|---|
| A145 | RCR2011 | Uninoculated | |
| M. truncatula DZA315.16 | 35.0a ± 4.0 | 25.0b ± 3.0 | 6.5d ± 1.0 |
| M. truncatula Jemalong 6 | 6.6d ± 1.0 | 16.0c ± 5.0 | 6.0d ± 1.0 |
| M. sativa cv Gemini | 24.7b ± 4.0 | 23.5b ± 2.8 | 3.7d ± 0.8 |
Mean values of 20 plants. Identical letters indicate that the dry wt averages are not significantly different at P = 5% using the Student's t test.
Figure 1.
DZA315.16 and Jemalong 6 lines, after inoculation by S. meliloti strain A145 (A), DZA315.16 (right) is green and well developed, whereas Jemalong 6 (left) shows nitrogen starvation symptoms, poor stem growth, and leaf chlorosis. Bar = 5 cm. Nodules of DZA315.16 are pink and elongated (B), whereas those of Jemalong 6 (C) are small and white. Bar = 1.5 mm. Photos were taken 39 DPI.
Differences in nodulation behavior depending upon nutrient solution, growth temperature, or the growth substrate have been reported in pea (Lie, 1981, 1984; Kneen and LaRue, 1988) and clover (Gibson, 1968). However, the Fix− phenotype for the Jemalong 6/A145 interaction was found to be identical under all conditions tested (pH, temperature, and growth medium), and furthermore, is heritable (Table II). This indicates that ineffectiveness of nitrogen fixation is unlikely to be an artifact due to specific growth conditions, but is indeed linked to the genetic background of Jemalong 6. Furthermore, Snyman and Stridjom (1980) have reported that the Jemalong cultivar, when planted in quartz sand in a Leonard jar and inoculated with S. meliloti A145 strain, showed a Fix− phenotype. Reciprocal grafting experiments, achieved for 30 plants, demonstrated that the expression of the Jemalong 6/A145 Fix− phenotype is controlled by the root.
Table II.
Segregation data of the Fix− phenotype in the F2 and F5 progenies of the cross Jemalong 6 × DZA315.16 inoculated by S. meliloti strain A145
| Generation | No. of Plants Observed
|
χ2 Values | |
|---|---|---|---|
| Fix+ | Fix− | ||
| DZA315.16 | 40 | 0 | |
| Jemalong 6 | 0 | 40 | |
| F1 | 20 | 0 | |
| F2 | 153 | 56 | χ2 = 0.40, P = 0.52 |
| F5 | 81 | 69 | χ2 = 0.049, P = 0.062 |
The χ2 value for the F2 and F5 segregation ratios indicate no significant deviation from the expected monogenic segregation. Tabulated χ2 for degrees of freedom = 1 is 3.84 at P = 0.95 level of significance.
Non-Nitrogen-Fixing Nodules on Jemalong 6 Plants Are Invaded and Have a Closed Endodermis
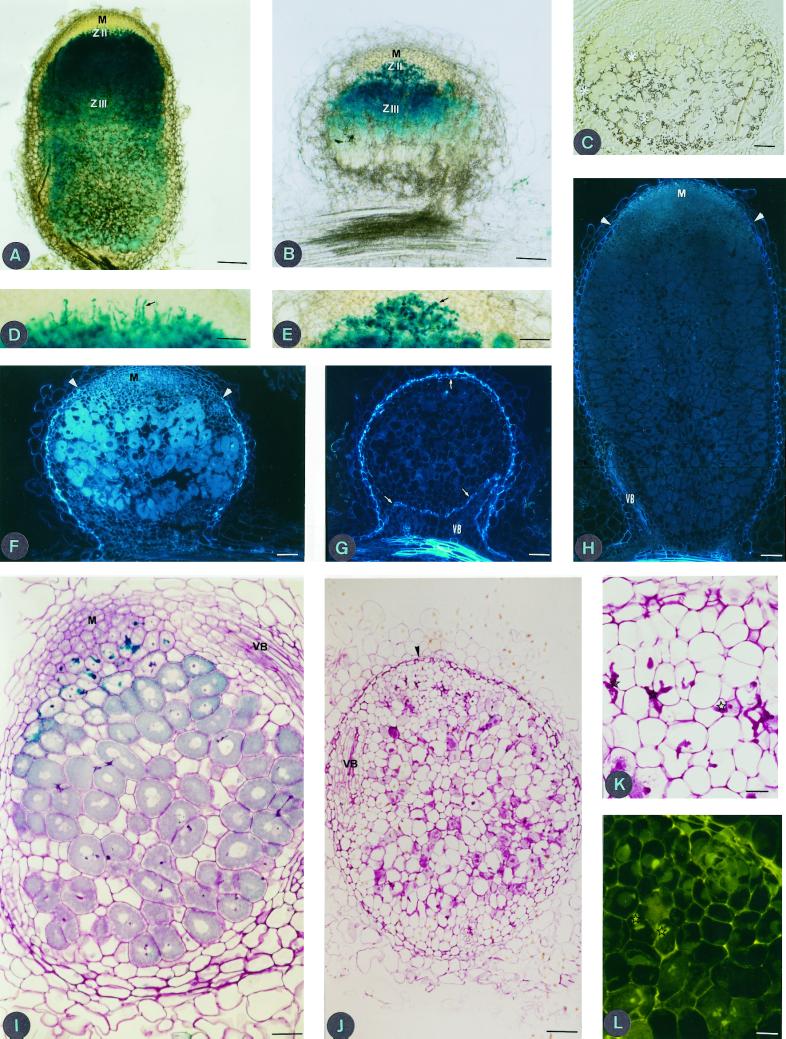
At 10 DPI, both plant lines inoculated by A145 showed curled, infected root hairs, and nodules whose central tissue is infected by the microsymbiont (Hac+ [hair curling], Inf+ [infection thread formation], and Nod+). At 20 DPI, although Jemalong 6 nodules resemble DZA315.16 nodules in zonation, they are significantly smaller in size (Fig. 2, A and B). Even though DZA315.16 nodules possess a characteristic amyloplast zone (data not shown; Vasse et al., 1990), this zone is not observed in Jemalong 6 nodules, where all invaded and non-invaded cells are filled with amyloplasts (Fig. 2C). ITs were seen to invade plant cells, and liberation of bacteria appeared to occur normally in both lines (Fig. 2, D and E). As in normal nodules, an active apical meristem and a peripheral endodermal cell layer was observed in thin serial sections of Jemalong 6 nodules at 20 DPI (Fig. 2F). Endodermal tissue also surrounds the nodule vascular bundles. However, by 30 DPI, thin serial sections showed that the meristem is no longer present and an atypical endodermal cell layer now enclosed the apical and proximal ends of the nodule and the vascular bundles (Fig. 2G).
Figure 2.
Longitudinal thick sections (80 μm) of DZA315.16 (A) and Jemalong 6 (B) nodules 20 DPI induced by S. meliloti strain A145 (pXGLD4), showing typical zonation of an effective M. truncatula nodule, with a meristematic zone (M), infection zone (ZII), and the nitrogen-fixing zone (ZIII). Bar = 100 μm. Section (4 μm thick) of a Technovit-embedded nodule of Jemalong 6 (C) 20 DPI after staining with iodine. Starch accumulation (asterisks) is observed in the whole nodule and not solely localized to an amyloplast-rich interzone II-III layer. Bar = 10 μm. Longitudinal sections (80 μm thick) of DZA315.16 (D) and Jemalong 6 (E) nodules 20 DPI. Note that DZA315.16 infection threads are thin and elongated, those of Jemalong 6 are short and hypertrophied. Infection threads are indicated by black arrows. Bar = 50 μm. Longitudinal and median thin sections (4 μm) of Jemalong 6 nodule 20 DPI (F) and 30 DPI (G) under UV illumination. Note the absence of meristematic zone and the autofluorescence of endodermal cells around the whole nodule and the vascular bundles (VB) in G compared to F. White arrows indicate endodermal cells at the distal part of nodule and around vascular bundles. Bar = 10 μm. Longitudinal and median thin section of DZA315.16 nodule 30 DPI (H); white arrowheads indicate the limit of endodermal cells. Bar = 10 μm. Thin section of Jemalong 6 nodule, viewed under light microscopy after staining with toluidine blue (I), at 20 DPI dividing meristematic cells (M) are observed. Bar = 50 μm. Thin section of Jemalong 6 nodule 30 DPI (J); note the presence of numerous empty cells indicating senescence of the nodule. Bar = 100 μm. Thin section (4 μm) through 30-d-old nodule of Jemalong 6 viewed under light microscopy (K), infection threads (stars) are thick and short and under blue light (L), note the autofluorescence of infection threads (stars). Bar = 20 μm.
In contrast, DZA315.16 nodules at 30 DPI have an active meristem and normal endodermal layers (Fig. 2H). We therefore conclude that, in Jemalong 6, cells of the nodule meristem stop dividing between 20 and 30 DPI, and that the endodermis subsequently encloses the entire nodule. In addition, we observed a precocious senescence of Jemalong 6 nodule cells. At 20 DPI, cells of Jemalong 6 nodules appear normal (Fig. 2I). However, at 30 DPI, most cells show symptoms of senescence and appear empty (Fig. 2J). Another difference between the two plant lines is related to infection thread morphology. At the early stage of nodule development (10 DPI), Jemalong 6 nodules induced by A145 have normal ITs. However, from 20 DPI and in later stages, they are thicker and shorter than those observed in DZA315.16 (Fig. 2K). UV illumination showed the Jemalong 6 ITs were autofluorescent (Fig. 2L), compared to those of DZA315.16 (data not shown).
Taken together, our observations show that normal nodule development occurs initially (nodule invasion, bacterial liberation, and leghemoglobin synthesis) during the Jemalong 6/A145 interaction, but without detectable nitrogen fixation and with an early senescence of cells in zone III. Four features characterize the Fix− phenotype: (a) IT morphology, (b) early senescence of the invaded zone, (c) the lack of meristematic activity at mature stages of nodule development, and (d) the closing of the nodule by the endodermis. Because early senescence of the invasion zone and nodule growth arrest appear at approximately the same stage, it is difficult to establish a causal relationship between meristem arrest and nodule senescence. The Jemalong 6 phenotype is thus Nop− (absence of nodule persistence). Nodules at 30 DPI appear to be physically isolated from the rest of the plant, encircled by a suberized endodermis at the basal and apical ends (Fig. 2G). To our knowledge, there are no prior reports of such a phenotype (enclosed endodermis) in a non-fixing interaction.
Because it has been proposed that suberin plays a role as a transport barrier for the uptake of water and ions (Schreiber et al., 1999), one possible hypothesis for the observed phenotype is that the deposition of lignin-like compounds or suberin in endodermal cells around vascular bundles prevents the exchange of nutrients between the vasculature and the nodule, thus arresting nodule growth. In any case, starch continues to accumulate in Jemalong 6-ineffective nodules. Another explanation for the Jemalong 6 phenotype could be related to the hypothetical synthesis, by the bacterial partner, of an activator for maintaining meristematic activity throughout nodule growth (Truchet et al., 1980; Grosjean and Huguet, 1997). In this scenario, the A145 activator would not be sufficient, qualitatively and/or quantitatively, to maintain Jemalong 6 nodular meristem activity. Finally, it is possible that the A145 strain synthesizes a substance that inhibits the division and growth of Jemalong 6 nodule meristematic cells. These two hypotheses speculate that specific signal exchange, and/or mutual recognition, is necessary during late stages of nodule development to maintain meristem activity and nodule growth.
Infection threads with thick walls have been previously reported in non-fixing mutants of pea (Novak et al., 1995; Tsyganov et al., 1998), M. truncatula (Bénaben et al., 1995), red clover (Chandler et al., 1973), fava bean (Haser et al., 1992), and in the ineffective nodules conditioned by the ie gene in a natural variant of red clover (Chandler et al., 1973). Premature degradation observed in invaded cells of Jemalong 6 nodules could be reminiscent of a host defense response, as reported for the Fix− mutants of pea, sym33, and sym40 (Tsyganov et al., 1998). However, it remains to be determined if the observed defense-like responses are the cause or the consequence of the activation of a senescence program. It is noteworthy that some of the features characterizing the Fix− phenotype can be observed when fix− bacterial mutants are inoculated on Medicago plants (Hirsch and Smith, 1987; Putnoky et al., 1988). In several cases, thick ITs were reported to be associated with modifications in bacterial surface components, and they have been correlated to plant defense reactions (Battisti et al., 1992; Perotto et al., 1994; Niehaus et al., 1998).
The Fix− Locus Is under Recessive Monogenic Control and Maps on Linkage Group 8
Analysis of the inheritance of the Fix− phenotype in F1, F2, and F5 generations indicates that a single recessive allele is involved in the expression of the non-fixing phenotype (Table II). The corresponding locus has been assigned the name Mtsym6 (M. truncatula symbiosis). The genetic analysis of naturally occurring non-fixing variants in other legume species also showed the respective allele to be recessive: in1, in2, and in3 in alfalfa (Peterson and Barnes, 1981), sym6 in pea (Lie and Timmermans, 1983), and i1 and ie in clover (Bergersen and Nutman, 1957). Finally, the analysis of F2 segregation data allowed us to map Mtsym6 on linkage group 8 of the M. truncatula map (data not shown).
The Fix− Phenotype of Jemalong 6 Results from a Strain x Cultivar Specificity
The two wild-type bacterial strains, RCR2011 and A145, used in this work are both fully effective when inoculated on M. sativa cv Gemini (Table I). However, although RCR2011 is effective with both Jemalong 6 and DZA315.16 M. truncatula lines, the A145 strain is effective with only DZA315.16 (Table I). Although we have not yet identified a bacterial gene(s) responsible for the symbiotic defect elicited by A145 on Jemalong 6, this strain x cultivar symbiotic specificity is reminiscent of gene-for-gene interactions observed in plant-pathogen relationships. Several examples of gene-for-gene interaction between a bacterial and a plant gene in symbiosis have been reported. For example, a single recessive plant gene rwt1 and the bacterial gene nodM condition strain-specific nodulation in subterranean clover (Lewis-Henderson and Djordjevic, 1991). In Afghanistan pea, an interaction between the sym2 plant gene and the nodX bacterial gene conditions nodulation ability (Kozik et al., 1995). In the case of the Jemalong 6/A145 interaction, the symbiotic defect concerns late nodule development and nitrogen fixation, and our findings favor the involvement of plant defense responses (enlarged IT and deposition of phenolic compounds) and/or senescence programs rather than a plant resistance response. Does mutual recognition break down during late stages of the interaction between the two wild-type symbionts A145 and Jemalong 6? In future research, it would be interesting, for example, to attempt to complement the Fix− phenotype of A145 with RCR2011 DNA, or to mutagenize the A145 strain to identify the gene(s) involved. Also, it would be useful to know if this observed strain x cultivar specificity is a general phenomenon of M. truncatula lines. Mapping the Mtsym6 gene now opens the way for map-based cloning of this gene and thereby to an improved molecular understanding of the basis of this symbiotic defect resulting in legume/Rhizobium incompatibility.
MATERIALS AND METHODS
Plant Material and Bacterial Strains
The Medicago truncatula line DZA315.16 was selected from a natural Algerian population DZA315 (collected by J.M. Prospéri, Institut National de la Recherche Agronomique, Montpellier, France). The Jemalong 6 line was selected from the registered Australian cultivar Jemalong (Oram, 1990). Medicago sativa cv Gemini was used as control for the nitrogen fixation capacity of the Sinorhizobium meliloti strains, RCR2011 and A145 (Rosenberg et al., 1981). The strains were grown on solid trypton yeast medium (Rosenberg et al., 1981) for 48 h before plant inoculation.
Plant Growth Conditions
M. truncatula seeds were surface-sterilized and germinated as described by Bénaben et al. (1995). Plantlets were inoculated 48 h after transfer to nitrogen-free Fahraeus agar slants (pH 7.5; Fahraeus, 1957) and grown in a controlled chamber at 22°C with a 16-h day/8-h night cycle. Other growth conditions also tested were pH (6.0 and 7.5), temperatures (varying from 22°C–28°C), and a growth pouch system (Mega International, Minneapolis) instead of agar medium. Nitrogen fixation efficiency was scored by measuring the dry weight of aerial parts of the plants 39 DPI. All data were analyzed by the Student's t test (Finney, 1978).
Histological and Cytological Studies
Plants were inoculated with the A145 strain containing the plasmid pXLGD4 carrying the constitutively expressed hemA::lacZ fusion (Leong et al., 1985). Nodules and roots were harvested 10, 20, and 30 DPI. Samples were fixed as described by Bénaben et al. (1995). Slices (80 μm) of Jemalong 6 and DZA315.16 nodules were made with a microcut H1200 (Bio-Rad Laboratories, Hercules, CA). Thin sections (4 μm) were prepared as described by Vernoud et al. (1999) and obtained from 10 nodules for each stage observed. Starch granules were visualized after staining with iodine solution (2% [w/v] KI and 0.2% [w/v] I2) for 30 s, rinsed with distilled water, and observed by bright-field microscopy. For observation of autofluorescence, unstained sections were viewed under UV or blue-light illumination. Microscopic observations and photographs were carried out with an Axiophot 2 light microscope (Carl Zeiss, Oberkochen, Germany).
Genetic Analysis and Mapping
The genetic analysis of the Fix− phenotype was performed in the F1, F2, and F5 progenies of the Jemalong 6 × DZA315.16 cross after inoculation by strain A145. For the genetic mapping, a set of 75 F2 individuals was typed by the analysis of their F3 progeny and MAPMAKER software (Lander et al., 1987) was used to localize the gene.
ACKNOWLEDGMENTS
We wish to thank Jean Marie Prospéri (Station de Génétique et d'Amélioration des Plantes, Institut National de la Recherche Agronomique, Domaine de Melgueil, 34130 Mauguio, France) for M. truncatula lines and Sandrine Cros-Arteil for technical advice. We also thank all of our colleagues, in particular D. Barker, J.V. Cullimore, C. Gough, T. Timmers, and J. Vasse for their critical comments during the preparation of the manuscript.
Footnotes
L.T. is supported by a Bourse du Gouvernement Français fellowship from the French Centre National des œuvres Universitaires et Sociales and the Ministère de l'Enseignement Supérieur et de la Recherche.
LITERATURE CITED
- Barker DG, Bianchi S, Blondon F, Dattée Y, Duc G, Essad S, Flament P, Gallusci P, Genier G, Guy P, Muel X, Tourneur J, Dénarié J, Huguet T. Medicago truncatula, a model plant for studying the molecular genetics of the Rhizobium-legume symbiosis. Plant Mol Biol Rep. 1990;8:40–49. [Google Scholar]
- Battisti L, Lara JC, Leigh JA. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc Natl Acad Sci USA. 1992;89:5625–5629. doi: 10.1073/pnas.89.12.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénaben V, Duc G, Lefebvre V, Huguet T. TE7, an inefficient mutant of Medicago truncatula Gaertn. cv Jemalong. Plant Physiol. 1995;107:53–62. doi: 10.1104/pp.107.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen FJ, Nutman PS. Symbiotic effectiveness in nodulated red clover: IV. The influence of host factors i1 and ie upon nodule structure and cytology. Heredity. 1957;11:175–184. [Google Scholar]
- Caldwell BE. Inheritance of a strain specific-ineffective nodulation in soybeans. Crop Sci. 1966;6:427–428. [Google Scholar]
- Chandler M, Dart PJ, Nutman PS. Annual Report Rothamsted Experimental Station, Part I. P.S. Rothamsted, UK: Nutman; 1973. The fine structure of hereditary ineffective red clover nodules; pp. 83–84. [Google Scholar]
- Cook DR. Medicago truncatula: a model in the making! Curr Opin Plant Biol. 1999;2:301–304. doi: 10.1016/s1369-5266(99)80053-3. [DOI] [PubMed] [Google Scholar]
- Cook DR, Vandenbosch K, de Bruijn FJ, Huguet T. Model legumes get the Nod. Plant Cell. 1997;9:275–281. [Google Scholar]
- Degenhardt TL, La Rue TA, Paul EA. Investigation of a non-nodulating cultivar of Pisum sativum. Can J Bot. 1976;54:1633–1636. [Google Scholar]
- Dénarié J, Debellé F, Promé JC. Rhizobium lipo-chitooligosaccharides nodulation factors signaling molecules mediating recognition and morphogenesis. Annu Rev Biochem. 1996;65:503–535. doi: 10.1146/annurev.bi.65.070196.002443. [DOI] [PubMed] [Google Scholar]
- Devine TE, Kuykendall LD. Host genetic control of symbiosis in soybean (Glycine max L.) Plant Soil. 1996;186:173–187. [Google Scholar]
- Duc G. Mutagenesis of faba bean (Vicia faba L.) and the identification of five different genes controlling no nodulation, ineffective nodulation or supernodulation. Euphytica. 1995;83:147–152. [Google Scholar]
- Duc G, Messager A. Mutagenesis of pea (Pisum sativum L.) and the isolation of mutants for nodulation and nitrogen fixation. Plant Sci. 1989;60:207–213. [Google Scholar]
- Duc G, Picard J. Note on the presence of the Sym-1 gene in Vicia faba hampering the symbiosis with Rhizobium leguminosarum. Euphytica. 1986;35:61–64. [Google Scholar]
- Fahraeus G. The infection of white clover root hairs by nodule bacteria studied by a simple slide technique. J Gen Microbiol. 1957;16:374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Statistical Method in Biological Assay. Ed 3. London: Charles Griffin and Company; 1978. [Google Scholar]
- Gelin O, Blixt S. Root nodulation in peas. Agric Hort Genet. 1964;22:149–159. [Google Scholar]
- Gibson A. Nodulation failure in Trifolium subterranean L. cv. Woogenellup (Syn. Marrar) Aust J Agric Sci. 1968;19:907–918. [Google Scholar]
- Grosjean C, Huguet T. A persistent meristem is formed in nodular structures elicited by Nod factor or by a Rhizobium meliloti exopolysaccharide mutant in alfalfa plants which nodulate spontaneously. Plant Sci. 1997;127:215–225. [Google Scholar]
- Haser A, Robinson DL, Duc G, Vance CP. A mutation in Vicia faba results in ineffective nodules with impaired bacteroid differentiation and reduced synthesis of late nodulin. J Exp Bot. 1992;43:1397–1407. [Google Scholar]
- Hirsch AM, Smith CA. Effects of Rhizobium meliloti nif and fix mutants on alfalfa root nodule development. J Bacteriol. 1987;169:1137–1146. doi: 10.1128/jb.169.3.1137-1146.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneen BE, La Rue TA. Peas (Pisum sativum L.) with strain specificity for Rhizobium leguminosarum. Heredity. 1984;52:383–389. [Google Scholar]
- Kneen BE, La Rue TA. Induced symbiosis mutants of pea (Pisum sativum) and sweetclover (Melilotus alba) Plant Sci. 1988;58:177–182. [Google Scholar]
- Kozik A, Heidstra R, Horvath B, Kulikova O, Tikhonovich I, Ellis THN, Van Kammen A, Lie TA, Bisseling T. Pea lines carrying sym1 or sym2 can be nodulated by Rhizobium strains containing nodX: syml and sym2 are allelic. Plant Sci. 1995;108:41–49. [Google Scholar]
- Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage map of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Leong S, Williams P, Ditta G. Analysis of the 5′ regulatory region of the gene for aminolevulinic acid of Rhizobium meliloti. Nucleic Acids Res. 1985;13:5967–5978. doi: 10.1093/nar/13.16.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Henderson WR, Djordjevic MA. A cultivar-specific interaction between Rhizobium leguminosarum bv. trifolii and subterranean clover is controlled by nodM, other bacterial cultivar specificity genes, and by a single recessive host gene. J Bacteriol. 1991;173:2791–2799. doi: 10.1128/jb.173.9.2791-2799.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie TA. Gene centers, a source for genetic variants in symbiotic nitrogen fixation: host induced ineffectivity in Pisum sativum ecotype fulvum. Plant Soil. 1981;61:125–134. [Google Scholar]
- Lie TA. Host genes in Pisum sativum L. conferring resistance to European Rhizobium leguminosarum strains. Plant Soil. 1984;82:415–425. [Google Scholar]
- Lie TA, Timmermans PCJM. Host-genetic control of nitrogen fixation in the legume-Rhizobium symbiosis: complication in the genetic analysis due to maternal effects. Plant Soil. 1983;75:449–453. [Google Scholar]
- Niehaus A, Lagares A, Pühler A. A Sinorhizobium meliloti lipopolysaccharide mutant induces effective nodules on the host plant Medicago sativa (alfalfa) but fails to establish a symbiosis with Medicago truncatula. Mol Plant-Microbe Interact. 1998;11:906–914. [Google Scholar]
- Novak K, Pesina K, Nebesarova J, Skrdleta V, Lisa L, Nasinec V. Symbiotic tissue degradation pattern in the ineffective nodules of three nodulation mutants of pea (Pisum sativum L.) Ann Bot. 1995;76:303–313. [Google Scholar]
- Nutman PS. Symbiotic effectiveness in nodulated red clover II: a major gene for ineffectiveness in the host. Heredity. 1954;8:47–60. [Google Scholar]
- Oram RN. Plant Cultivar. Ed 3. Canberra, Australia: Commonwealth Scientific and Industrial Research Organization; 1990. Register of Australian herbage. [Google Scholar]
- Panagiota M, Pawlowski K, Bisseling T. Symbiotic nitrogen fixation. Plant Cell. 1995;7:869–885. doi: 10.1105/tpc.7.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmetsa RV, Cook DR. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science. 1997;275:527–530. doi: 10.1126/science.275.5299.527. [DOI] [PubMed] [Google Scholar]
- Perotto S, Brewin NJ, Kennenberg EL. Cytological evidence for a host defense response that reduces cell and tissue invasion in pea nodules by lipolysaccharide-defective mutants of Rhizobium leguminosarum strain 3841. Mol Plant-Microbe Interact. 1994;7:99–112. [Google Scholar]
- Peterson MA, Barnes DK. Inheritance of ineffective nodulation and non-nodulation traits in alfalfa. Crop Sci. 1981;21:611–616. [Google Scholar]
- Putnoky P, Grosskopf E, Cam Ha DT, Kiss GB, Kondorosi A. Rhizobium fix genes mediate at least two communication steps in symbiotic nodule development. J Cell Biol. 1988;106:597–607. doi: 10.1083/jcb.106.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C, Boistard P, Dénarié J, Casse-Delbart F. Genes controlling early late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184:362–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Sagan M, Morandi D, Tarenghi E, Duc G. Selection of mycorrhizal mutants in the model plant Medicago truncatula (Gaertn.) after δ-ray mutagenesis. Plant Sci. 1995;111:63–71. [Google Scholar]
- Schreiber L, Hartmann K, Skrabs M, Zeier J. Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. J Exp Bot. 1999;50:1268–1280. [Google Scholar]
- Smith GR, Knight WE. Inheritance of ineffective nodulation in crimson clover. Crop Sci. 1984;24:601–604. [Google Scholar]
- Snyman CP, Strijdom BW. Symbiotic characteristics of lines and cultivars of Medicago truncatula inoculated with strains of Rhizobium meliloti. Phytophylatica. 1980;12:173–176. [Google Scholar]
- Szczyglowski K, Shaw RS, Wopereis J, Copeland S, Hamburger D, Kapranov P, Kasiborski B, Dazzo F, de Bruijn FJ. Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus. Mol Plant-Microbe Interact. 1998;11:684–697. [Google Scholar]
- Truchet G, Michel M, Dénarié J. Sequential analysis of the organogenesis of lucerne (Medicago sativa) root nodules using symbiotically defective mutants of Rhizobium meliloti. Differentiation. 1980;13:163–173. [Google Scholar]
- Tsyganov VE, Morzhina EV, Stefanov SY, Borisov AY, Lebsky VK, Tikhonovich IA. The pea (Pisum sativum L.) genes sym33 and sym40 control infection thread formation and root nodule function. Mol Gen Genet. 1998;259:491–503. doi: 10.1007/s004380050840. [DOI] [PubMed] [Google Scholar]
- Vance CP, Johnson EB. Plant determined ineffective nodules in alfalfa (Medicago sativa): structural and biochemical comparison. Can J Bot. 1983;61:93–106. [Google Scholar]
- Van Rhijn P, Vanderleyden J. The Rhizobium-plant symbiosis. Microbiol Rev. 1995;59:124–142. doi: 10.1128/mr.59.1.124-142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasse J, de Billy F, Camut S, Truchet G. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol. 1990;172:4295–4306. doi: 10.1128/jb.172.8.4295-4306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoud V, Journet EP, Barker D. MtENOD20, a Nod factor-inducible molecular marker for root cortical cell activation. Mol Plant-Microbe Interact. 1999;12:604–614. [Google Scholar]