Abstract
Mutations in the HGPRT1 gene, which encodes hypoxanthine-guanine phosphoribosyltransferase (HGprt), housekeeping enzyme responsible for recycling purines, lead to Lesch-Nyhan disease (LND). Clinical expression of LND indicates that HGprt deficiency has adverse effects on gastrointestinal motility. Therefore, we aimed to evaluate intestinal motility in HGprt knockout mice (HGprt−). Spontaneous and neurally evoked mechanical activity was recorded in vitro as changes in isometric tension in circular muscle strips of distal colon. HGprt− tissues showed a lower in amplitude spontaneous activity and atropine-sensitivity neural contraction compared to control mice. The responses to carbachol and to high KCl were reduced, demonstrating a widespread impairment of contractility. L-NAME was not able in the HGprt− tissues to restore the large amplitude contractile activity typical of control. In HGprt− colon, a reduced expression of dopaminergic D1 receptor was observed together with the loss of its tonic inhibitory activity present in control-mice. The analysis of inflammatory and oxidative stress in colonic tissue of HGprt− mice revealed a significant increase of lipid peroxidation associated with over production of oxygen free radicals. In conclusion, HGprt deficiency in mice is associated with a decrease in colon contractility, not dependent upon reduction of acetylcholine release from the myenteric plexus or hyperactivity of inhibitory signalling. By contrast the increased levels of oxidative stress could partially explain the reduced colon motility in HGprt− mice. Colonic dysmotility observed in HGprt− mice may mimic the gastrointestinal dysfunctions symptoms of human syndrome, providing a useful animal model to elucidate the pathophysiology of this problem in the LND.
Keywords: Lesch-Nyhan, HGprt enzyme, HGprt deficient mice, Gastrointestinal motility, Colon, Dopamine, Oxidative stress, Cytokines
1. Introduction
Hypoxanthine-guanine phosphoribosyltransferase (HGprt) is a housekeeping enzyme, widely expressed in many organisms from bacteria through mammals, responsible for recycling purines by converting free purine bases, hypoxanthine and guanine, into utilizable purine nucleotides (Jinnah et al., 2000). Deficient activity of this enzyme results in Lesch-Nyhan disease (LND), a rare inherited X-linked recessive disorder caused by mutations in the HGPRT1 gene (Fu et al., 2014). Mutations lead to overproduction of purine nucleotides via de novo synthesis and accumulation of uric acid, and to a variable spectrum of neurological and behavioral phenotypes. Patients show a severe motor handicap associated with generalized dystonia, sometimes accompanied by choreoathetosis or spasticity (Jinnah et al., 2006). Many patients also have cognitive disability, and exhibit an unusual tendency towards dangerous behaviours that include recurrent self-injury, with self-biting being particularly prominent (Preston, 2007; Schretlen et al., 2005). The neurobehavioral phenotype has been linked with dysfunctions of the basal ganglia, and particularly of dopaminergic pathways (Gottle et al., 2014; Nyhan, 2000; Saito and Takashima, 2000; Visser et al., 2000).
HGprt deficiency in both humans and mice (HGprt−) is associated with a marked loss of basal ganglia dopamine, dopamine-related enzymes and dopamine transporters (Egami et al., 2007; Ernst et al., 1996; Wong et al., 1996), these neurochemically abnormalities are selective (Hyland et al., 2004; Jinnah et al., 1999; Jinnah et al., 1994), and neuroanatomically confined, because other brain dopaminergic pathways are not affected (Jinnah et al., 1992; Jinnah et al., 1994). Altered dopamine receptor expression in LND patient brains and lymphocytes has been also reported (Garcia et al., 2012; Saito and Takashima, 2000).
HGprt deficiency has also adverse effects on somatic growth, maturation of bone marrow stem cells into erythrocytes, and gastrointestinal function. Common symptoms include dysphagia, unexplained vomiting, constipation and bloating. There is a marked discrepancy between the prevalence and variety of gastrointestinal symptoms occurring in LND and the lack of research in this area. This lack of data is quite surprising considering how critical gastrointestinal function for the patient’s quality of life.
The aim of the present study was to investigate the mechanical activity of intestinal muscle strips from control and HGprt− mice in an attempt to point out possible differences in the motor pattern and in the control mechanisms, taking as experimental model the distal colon. Gastrointestinal motility, as other gastrointestinal functions, is organized and integrated by the enteric nervous system (ENS). Nearly every mediator/modulator neurotransmitter found in the central nervous system is also found in ENS, making the enteric nervous system a good model of nervous system, simpler and more accessible than the central nervous system. Since, among the different neurotransmitters/modulator that are involved in the control of intestinal contractility, acetylcholine (ACh) is thought to be the principal neurotransmitter involved in intestinal smooth muscle contraction, via activation of muscarinic receptors, we aimed to investigate the involvement of enteric excitatory cholinergic pathways on intestinal contractility in HGprt− mice, also in consideration that, we have previously reported that guanine-based purines (guanosine and guanine) are able to modulate negatively the excitatory cholinergic control of mouse intestine leading to a decrease in contractility (Zizzo et al., 2013, 2011). Since HPRT-deficient cells fail to recycle hypoxanthine and guanine, resulting in an accumulation of the purines, we hypothesized that an increased effect of guanine-based purines on neural target could cause an alteration of neural function, in turn resulting in motor dysfunctions.
In addition, since the main neurochemical defect in HGprt-deficiency is linked to prevailing dysfunction of dopaminergic pathway in the basal ganglia, we aimed also to investigate whether changes in colonic motility of HGprt− mice can be related to an impairment of dopaminergic control, according to our recent data showing a role for dopamine in the regulation of intestinal motility in mice (Auteri et al., 2017; Zizzo et al., 2016, 2010). Finally, we also verified the presence of inflammatory status and oxidative stress in the HGprt− mice colon by examining respectively specific markers for inflammation, e.g. interleukin-1β (IL-1β) and interleukin-6 (IL-6), and for oxidative stress, such as reactive oxygen species (ROS) and malondialdehyde (MDA) levels, as end product of lipid peroxidation mediated by oxygen free radicals.
2. Methods
2.1. Animals
Experiments were performed using as control adult male mice (C57BL/6) obtained from Charles River Laboratories (Calco-Lecco, Italy) and HGprt-deficient male mice (kindly provided by Dr. Jinnah, Emory University, Atlanta, GA). Animals were housed in a specific pathogen-free environment and kept under environmentally controlled conditions, (ambient temperature 24 °C, humidity 40% and 12 h light/dark cycle) and housed four per cage and food and water were available ad libitum. Protocols were approved by the University of Palermo Bioethical Committee and the national Committee on Animal Use and Care. Procedures involving animals and their care were conducted in conformity the European directives (2010/63/EU) for animal experiments. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. No other methods to perform the described experiments (3Rs) were found.
2.2. Functional studies in vitro
Control and HGprt-deficient mice were randomly selected for experiments and euthanized using isoflurane anesthesia followed by cervical dislocation. After laparotomy the colon was rapidly excised, placed in Krebs solution (mM: NaCl 119; KCl 4.5; MgSO4 2.5; NaHCO3 25; KH2PO4 1.2, CaCl2 2.5, glucose 11.1). Then, a segment of distal colon (about 5 mm proximal to the anus) of approximately 3.5 cm length was obtained and the content of the excised segment was gently flushed out with Krebs solution.
The contractile activity of distal colonic circular smooth muscle was recorded as previously described by Auteri et al. (2017). Briefly the segment of distal colon was opened along the mesenteric border and pinned mucosa side up. The mucosa was removed by sharp dissection under a microscope, to avoid the influence of the substances released from the mucosa, and full-thickness circular muscle strips (10 mm in length) were suspended in a four-channel organ bath containing 10 ml of oxygenated (95% O2 and 5% CO2) Krebs solution maintained to 37 °C. The distal end of each strip was tied to an organ holder and the proximal end was secured with a silk thread to an isometric force transducer (FORT 25, Ugo Basile, Biological Research Apparatus, Comerio VA, Italy). Mechanical activity was amplified and digitized via an analogue/digital interface (Quad Bridge and PowerLab/400, AD Instruments, Ugo Basile, Biological Research Apparatus, Comerio VA, Italy), prior being acquired onto a personal computer. The preparations were subjected to an initial tension of 500 mg and were allowed to equilibrate for at least 30 min.
After the equilibration time, preparations were challenged either with 10 μM carbachol (CCh), KCl (60 mM), isoproterenol (Iso, 1 μM) or with sodium nitroprusside (100 μM) for 2 min until stable responses were obtained.
Electrical field stimulation (EFS) was applied from a Grass S88 electrical stimulator (Grass Instruments Co., Quincy, MS, USA) through a stimulus isolation unit (SIU5) using direct coupling. Stimuli (0.5 ms, 10 V for 10 s) were delivered, via a pair of platinum plate electrodes, in trains of 4 Hz frequency. Such parameters were chosen to specifically activate cholinergic neurons (Zizzo et al., 2011).
Agonists were left in contact with the tissue for 8 min whilst the antagonists for a period of 20–30 min before to analyse their effects.
2.3. Faecal output and water content in faeces
Faecal output was assessed in mice placed individually in grid-floor cages and left there to become acclimatized to their environment for 3 days before the experiment. During this period, the animals were fed normal chow and supplied water ad libitum. The day of the experiments, food was withdrawn, and faecal pellet output was then monitored. The pellets discharged by each animal during a period of 1 h were collected, counted and weighed immediately and after drying (24 h at 80 °C). The water content of faeces was calculated as: stool water content (%) = (wet weight − dry weight)/wet weight × 100 (Cil et al., 2016).
2.4. Drugs
The following drugs were used: atropine, carbamylcholine chloride (carbachol, CCh), 7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH-23390), domperidone, guanine, isoproterenol (Iso), KCl, Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME), Sodium nitroprusside (SNP), tetrodotoxin (TTX) (Sigma–Aldrich, Inc., St. Louis, US). Domperidone was dissolved in dimethyl sulfoxide, guanine in 30% solution of 1 N NaOH, all the other drugs were dissolved in distilled water. The working solutions were prepared fresh on the day of the experiment by diluting the stock solutions in Krebs. Drugs were added to the organ bath in volumes of < 1.0% of the bathing solution. The maximal final concentration of dimethyl sulphoxide in the organ bath was 0.5%, whilst the final concentration of NaOH in the organ bath was 0.3%. Control experiments using solvents alone showed that both of them had effect neither on the spontaneous nor on the evoked contractile activity.
2.5. Histomorphological examination
The segment of distal colon was excised and frozen in isopentane precooled in liquid nitrogen, and stored at −80 °C until use. Distal colon sections (7 μm) were cut at −20 °C and thawed onto 3-amino-propyl-ethoxysilane-coated slides. Frozen sections were fixed in 4% paraformaldehyde and stained with hematoxylin-eosin and then dehydrated with ethanol and xylene, mounted with Entellan (Merck, Darmstadt, Germany). Computerized image analysis were performed using a Leica microscope (DMRBE, Leica Microsystems GmbH, Germany), equipped with digital video camera (Spot-RT Slider, Diagnostic Instruments, Mi, USA). Circular muscular thickness and myofiber diameter were measured with ImageJ software (Rasband, W.S., ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2017) (Frinchi et al., 2014).
2.6. Western blotting analysis
Colonic samples were homogenized in cold buffer containing 50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% Triton, 0.5% SDS, H2O and protease inhibitor cocktail (P8340, Sigma-Aldrich S.r.l., Milan, Italy). Homogenate were left on ice for 30 min and then centrifuged at 13,000 rpm for 30 min at 4 °C. The supernatants were stored at −80 °C and aliquots were taken for protein determination. Western blot analysis was performed as described (Frinchi et al., 2014). Shortly, the samples with 30 μg of protein and molecular weight markers (161-0375, Bio-Rad Laboratories S.r.l., Segrate, Milan, Italy) were run on 8% and/or 12% polyacrylamide gel at 100 V and electrophoretically transferred onto nitrocellulose membrane (Hybond-C-extra, GE Healthcare, formerly Amersham, Europe GmbH–Milan, Italy). Following 1 h incubation in blocking buffer (1× TBS, 0.1% Tween-20, 5% w/v non-fat dry milk) the membrane was incubated with gentle shaking overnight at +4 °C in the same buffer with the dopaminergic receptor D1 affnity purified antibodies (1:200) (SC31478, Santa Cruz Biotechnology), rat monoclonal antibody anti-DAT (dopamine transporter,1:200; SC-32258, Santa Cruz Biotechnology) and rabbit-polyclonal antibody anti-TH (tyrosine hydroxylase, 1:5000; AB152, Chemicon) antibodies. Mouse monoclonal HRP conjugated antibody anti β-actin (1:10000; SC-47778 HRP, Santa Cruz Biotechnology) was used as loading internal standard. After washing, the membrane was incubated for 1 h at room temperature with anti-rabbit IgG horseradish peroxidase-conjugated diluted 1:8000 (Sc 2004, Santa Cruz Biotechnology), or anti-goat HRP-conjugated secondary antibody diluted 1:6600 (SC-2768, Santa Cruz Biotechnology) or anti-rat HRP-conjugated secondary antibody diluted 1:5000 (Sc2006, Santa Cruz Biotechnology) and bands were visualized by chemiluminescence (ECL, GE Healthcare, Amersham). The blot was exposed to autoradiography film (Amersham Hyperfilm ECL; 28-9068-36), developed in Kodak D19 developer and fixer, and the densitometric evaluation of bands was performed by measuring the optical density (O.D.) using NIH ImageJ software. Results were expressed as arbitrary units.
2.7. Assay of dopamine by high-performance liquid chromatography
Samples were processed and assayed for dopamine (DA) by reverse phase high-performance liquid chromatography with electrochemical detection. In short, tissue was weighed and then homogenized in ice-chilled 0.6 M perchloric acid containing 1.7 mg/ml ethylene glycol tetra acetic acid and 1.1 mg/ml reduced glutathione. After centrifugation for 15 min at 2500 ×g at 0 °C, 1.1 mg/ml of the supernatant was adjusted to pH 8.6 with 6 M potassium hydroxide and then processed for determination of DA concentration. 25 μl of 3 M potassium chloride were added to 200 μl of the 0.2 M perchloric acid eluate. After centrifugation, 50 μl of the supernatant were injected into the high-performance liquid chromatography system. The limit of sensitivity in the supernatant, defined as two times baseline noise, was 0.01 ng/ml for DA.
2.8. Measurement of pro-inflammatory cytokines by ELISA-assay
Colon tissue (10 mg) was homogenized in 10 ml of homogenization buffer (supplied in the ELISA kit) and after centrifugation at 14,000 rpm for 30 min at 4 °C, 100 μl of the supernatant was used for determination of interleukin-1β (IL-1β, Cloud-Clone Corp) and interleukin-6 (IL-6, Cloud-Clone Corp) levels, according to manufacturer’s instructions. At the end of protocol, the plate was read on iMark™ Microplate Absorbance Reader at 450 nm.
2.9. Reactive oxygen species (ROS) analysis
The production of ROS was evaluated using 2′, 7′-dichlorodiidro-fluorescineacetate (DCFH-DA) (Molecular Probes, Eugene, OR). Colon tissue (5 mg) was homogenized in 5 ml PBS buffer and after centrifugation at 14,000 rpm for 30 min at 4 °C at 100 μl of the supernatant was added 1 μl of DCFH-DA. Following 5 min incubation at 37 °C, the oxidation levels were evaluated using the GloMax® Discover System (Promega) at excitation wavelength of 475 nm and emission wavelength 555 nm.
2.10. Lipid peroxidation assay
The lipid peroxidation assay kit (Sigma Aldrich) was used to detect the concentration of malondialdehyde (MDA), an end product of lipids peroxidation. The amount of 10 mg of colon tissues was homogenized in 300 μl of MDA lysis buffer (supplied in the kit) and colorimetric reaction with thiobarbituric acid (TBA) was read on iMark™ Microplate Absorbance Reader at 532 nm, according to manufacturer’s instructions.
2.11. Data and statistical analysis
All data were analysed by operators, blinded to the protocol assignment. Data are given as means ± SEM; n in the result section refers to the number of animals on which observations were made. At least 5 individual experiments were carried out for each independent protocol and the exact number is reported in the figure legends. The amplitude of the spontaneous and evoked mechanical activity was reported in absolute value. Statistical analysis of dopamine tonic modulation of colonic contractility was performed by means of oneway ANOVA followed by the Bonferroni’s post hoc test. All the others statistical comparisons were performed by mean of Student’s t-test. A probability value of 0.05 was regarded as significant.
3. Results
3.1. Mice body weight and colonic circular muscle morphology
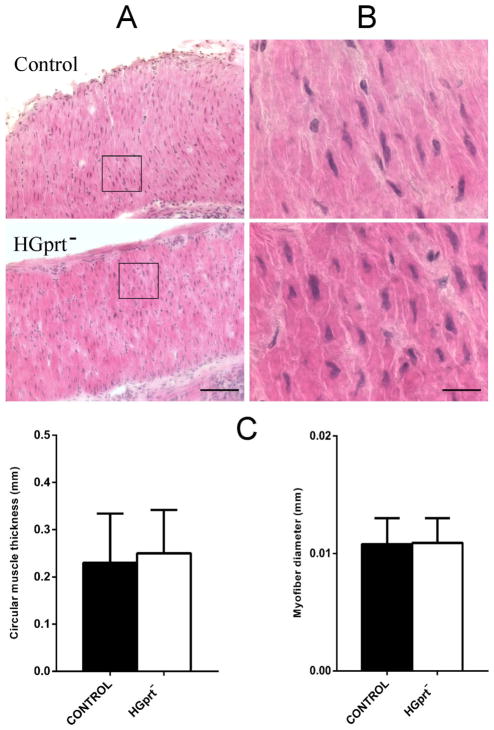
Animal body weight did not show significant difference between the two groups (34.2 ± 2.2 g in control vs 31.5 ± 1.8 g in HGprt− mice; n = 5 each, P > 0.05). In addition, the histomorphological analysis of the distal colon circular muscle did not reveal any trophic alteration, as evaluated by measuring circular muscle thickness and myofiber diameter in random sampled areas, in HGprt− mice vs control (Fig. 1).
Fig. 1.
Microphotographs from tissue sections of distal colon from control and HGprt− mice stained with hematoxylin eosin for morphological analysis. (A) low magnification (20×) showing circular muscle and (B) high magnification (100×) showing a sampled area in (A). In histograms, HGprt− and control mice are compared for circular muscle thickness (C) and myofibers diameter (D). Both circular muscle thickness and myofibers diameter did not show any significant differences. Square in (A) indicates a sampled area for myofibers diameter measurement. Scale bar: (A) 100 μm, (B) 20 μm.
3.2. Contractility of circular muscle strips from mouse colon
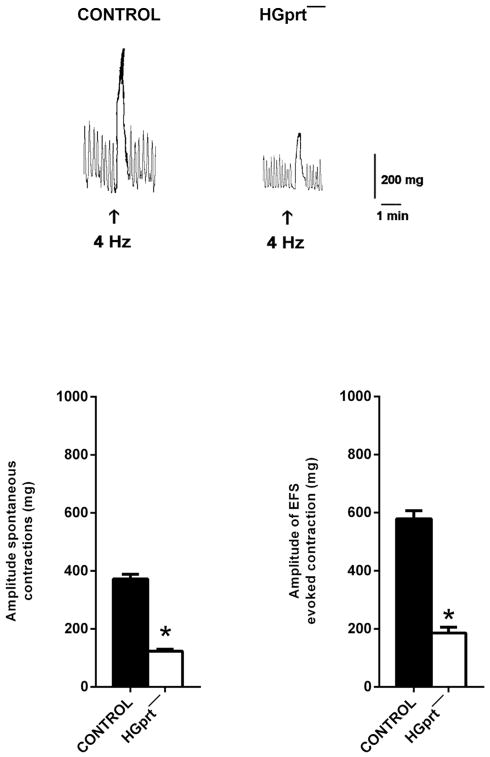
Strips of circular muscle from distal colon obtained from both control and HGprt− mice, once mounted in the organ bath, developed a spontaneous contractile activity consisting in ongoing phasic contractions. This spontaneous activity was approximately 50% lower in amplitude in HGprt− mice as compared to controls, even when normalized to the tissue dry weight (Fig. 2).
Fig. 2.
Upper panel - Original tracings showing the spontaneous phasic activity and the cholinergic response to EFS (0.5 ms, 4 Hz, 10 V for 10 s) in the circular muscle strips of mouse distal colon from control or in HGprt− mice. Lower panel - Histograms showing the amplitude of the spontaneous phasic activity and of the response to EFS (0.5 ms, 4 Hz, 10 V for 10 s) in the circular colonic muscle strips of mouse distal colon from control or HGprt− mice. Data are means ± S.E.M and are expressed in absolute value (n = 5 for each group). *P < 0.05.
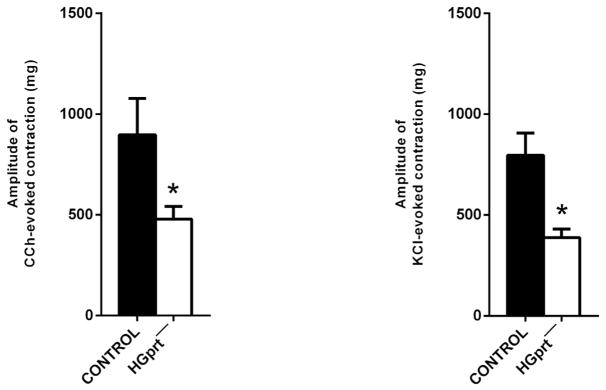
In control and HGprt− preparation, electrical field stimulation (EFS, 4 Hz) induced a TTX (1 μM) sensitive response, characterized by an early muscular inhibition followed by a cholinergic contraction. The cholinergic contraction was, once again, significantly lower in amplitude in HGprt− colon (decrease of the response in HGprt− vs control was 69.0 ± 6.2%, n = 5 each) (Fig. 2). The cholinergic receptor agonist, CCh (10 μM), induced TTX-insensitive sustained contractions that were smaller in HGprt− colon as well the contractions induced by high KCl (60 mM) (Fig. 3). The decrease of the amplitude of the evoked contractions was comparable in both circumstances (decrease of the response in HGprt− vs control was 48.7 ± 2.2% for carbachol (n = 5 each) and 53.7 ± 7.9% for KCl, n = 5 each, P > 0.05). The decrease in cholinergic nerve-mediated contractions to EFS was significantly higher than the decrease in the cholinergic myogenic contractions in response to carbachol (P < 0.05).
Fig. 3.
Histograms showing the amplitude of the contractile response evoked by CCh (10 μM) and KCl (60 mM) and the amplitude of the relaxant response evoked by Iso (1 μM) or SNP (1 mM) in the circular colonic muscle strips of mouse distal colon from control or HGprt− mice. Data are means ± S.E.M and are expressed in absolute value (n = 5 for each group). *P < 0.05.
Atropine did not have any effect on the spontaneous activity in both preparations (from 383.6 ± 11.2 mg to 364.1 ± 20.3 mg, n = 5, in the presence of 1 μM atropine in control mice and from 190.2 ± 8.2 mg to 179.3 ± 13.2 mg, n = 5, in the presence of 1 μM atropine in HGprt− mice).
To assess the magnitude of colonic smooth muscle tone, colon strips were exposed to 100 μM SNP, or 0.1 μM Iso, which at these doses elicited stable and reproducible relaxations. Both SNP and isoprotenerol caused a significant smaller relaxation in HGprt− colon (Fig. 3).
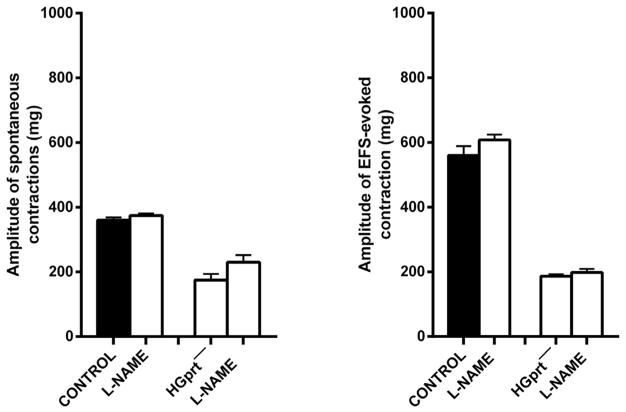
Since among the enteric inhibitory neurotransmitters, nitric oxide (NO) plays a major role, we attempted to reverse the strikingly decreased spontaneous contractions in colonic strips of HGprt− mice by adding L-NAME, a NO synthase inhibitor. L-NAME (100 μM) modified neither the amplitude of the spontaneous contractions nor the EFS-induced contractions in control mice, whilst in HGprt− mice, a slight increase was observed in the spontaneous activity, with the EFS-induced contractions being unchanged (Fig. 4). Indeed, L-NAME (100 μM), in both preparations, abolished the inhibitory muscular inhibition in response to EFS.
Fig. 4.
Histograms showing the effect induced by L-NAME (100 μM) on the amplitude of the spontaneous phasic activity and on the amplitude of the response to EFS (0.5 ms, 4 Hz, 10 V for 10 s) in the circular colonic muscle strips of mouse distal colon from control or HGprt− mice. Data are means ± S.E.M and are expressed as absolute value (n = 5 for each group).
As previously reported, guanine-based purines (guanosine and guanine) are able to modulate negatively the excitatory cholinergic control of mouse intestine (Zizzo et al., 2013, 2011). Guanine (1 mM) is still able to reduce by the same extent the neurally-evoked cholinergic contractile responses also in HGprt− mice (from 612.0 ± 10.6 mg to 373.5 ± 21.2 mg in control and from 306.5 ± 7.82 mg to 189.7 ± 19.7 mg ± 11.2 mg in HGprt− mice, in the presence of 1 mM guanine n = 5 each).
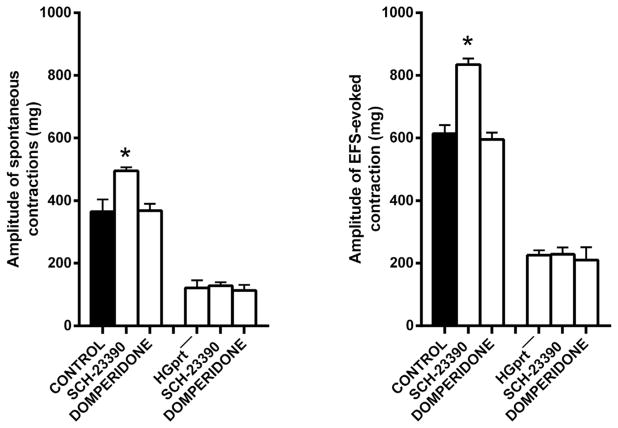
We recently reported that constitutively active D1-like receptors may be involved in a tonic inhibitory control of colonic contractility since the D1 receptor antagonist, SCH- 23390, caused a significant increase in the basal tone, in the amplitude of spontaneous and in EFS-evoked contractions (Auteri et al., 2017). Surprisingly, in HGprt− mice the effect of the D1 receptor antagonist SCH-23390 (3 μM) on the basal tone and on the spontaneous and neurally-evoked activity was lost, suggesting that in HGprt− mice there is an impairment of tonic dopamine modulation of colonic contractility, whilst domperidone (5 μM), a D2 receptor antagonist, had no effect in control or HGprt− mice (Fig. 5).
Fig. 5.
Histograms showing the effects induced by SCH-23390 (3 μM), D1 receptor antagonist, or by domperidone (5 μM) D2 receptor antagonist, on the amplitude of the spontaneous phasic activity and of the response to EFS (0.5 ms, 4 Hz, 10 V for 10 s) in the circular colonic muscle strips of mouse distal colon from control or HGprt− mice. Data are means ± SEM and are expressed in absolute value (n = 5 for each group). The graphed value for the control bars are the mean of the control data obtained before each treatment. *P < 0.05.
3.3. Faecal parameters measurements
Faecal output monitored by counting and weighing faecal pellets excreted over a period of 1 h by each animal, was significantly reduced in HGprt− mice. In fact, over 1 h, the faecal pellet number was 8.8 ± 1.6 and 6.3 ± 1.8 and the stool weight was 417.6 ± 30.1 mg and 255.8 ± 20 mg in control vs HGprt− mice (n = 5 each; P < 0.05). Furthermore the faeces produced by HGprt− mice had water contents lower than control mice (70.4 ± 3.6%) in control vs 56.7 ± 3.3% in HGprt− mice; n = 5 each; P < 0.05).
3.4. Dopamine-related markers
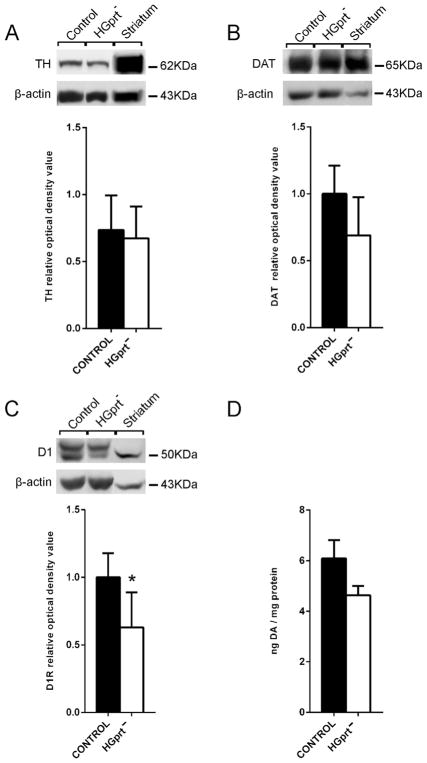
Western blot analysis of protein concentrations in the distal colon extract revealed a reduction of D1 receptor protein in the tissue from HGprt− mice, without significant differences in DAT or TH protein levels, as compared to tissue from control mice (Fig. 6).
Fig. 6.
Western blot analyses in distal colon extracts of dopamine receptor D1, tyrosine hydroxylase (TH) and dopamine transporter (DAT) protein levels (A–C) and HPLC analysis of dopamine (DA) concentration (D). TH, DAT and DA levels showed no significant change, whereas D1 receptor levels exhibited a significant reduction in colon preparations from HGprt− mice when compared to control mice. Striatum: tissue from brain region striatum, used as positive internal control. Data are means ± S.E.M. *P < 0.05.
HPLC analysis of samples from distal colon extracts showed no significant difference in dopamine concentration between control and HGprt− mice (Fig. 6).
3.5. Pro-inflammatory cytokines levels
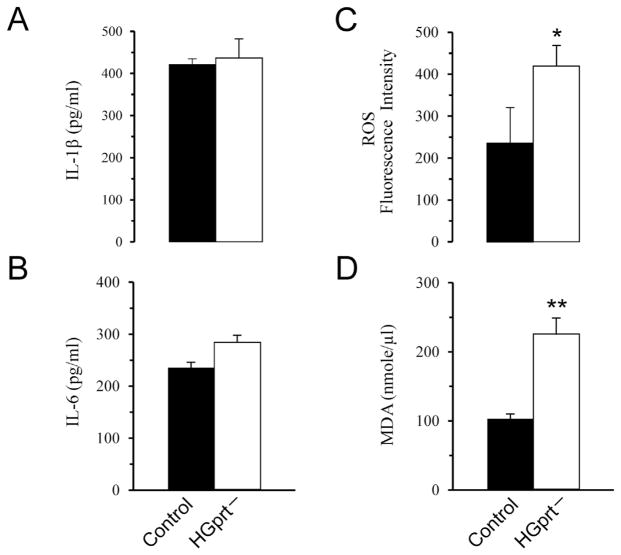
As shown in Fig. 7A–B we found that both IL-1β and IL-6 levels were unchanged in HGprt− mice as compared to control mice.
Fig. 7.
Pro-inflammatory cytokines, ROS and lipid peroxidation levels. The analysis of IL-1β (A) and IL-6 (B) protein levels showed that both cytokines were unchanged in HGprt− mice as compared to control mice. The analysis of ROS levels (C) revealed a significant increase of ROS levels in HGprt− mice as compared to control mice. Lipid peroxidation levels, detected by measuring the concentration of MDA (D), revealed a significant increase of MDA levels in HGprt− mice as compared to control mice. Data are means ± S.E.M. *P < 0.05, **P < 0.01.
3.6. ROS and lipid peroxidation levels
Oxidative stress, evaluated by detecting ROS, showed a significant increase in HGprt− mice as compared to control mice (Fig. 7C). Lipid peroxidation is the degradation of lipids that occurs as a result of oxidative damage, typically by reactive oxygen species, resulting in a well-defined chain reaction with the production of end products such as MDA. Therefore, in this work in order to measure lipid peroxidation levels we measured the concentration of MDA. The analysis revealed a significant increase of MDA levels in HGprt− mice as compared to control mice (Fig. 7D). This increase of MDA levels correlated with the increased levels of ROS.
4. Discussion
The present results demonstrate a significant decrease in intestinal contractility in HGprt deficient mice, which is not due to impairment of the excitatory cholinergic system or of the inhibitory nitrergic system. A widespread dysfunction of the contractile apparatus is suggested. Moreover, dysfunction in the enteric dopaminergic system with a reduction of D1 receptor expression has been observed. However, it remains to be solved the impact of dopaminergic dysfunction in the colonic motor alteration observed in HGprt− animals.
HGprt is an enzyme which plays a role in purine metabolism in virtually all cells of the body, recycling the purine bases, hypoxanthine and guanine, into the usable purine nucleotide pools. In the absence of HGprt hypoxanthine and guanine are degraded to uric acid. Secondary biochemical changes result in enhanced endogenous purine synthesis (Jinnah, 2009). Our results suggest that the failure of purine recycling owing to HGprt deficiency has a negative impact on the intestinal contractility in the circular muscle of mouse colon.
Our experiments showed that circular muscle preparations of colon from HGprt− mice, when compared to control mice, is characterized by a marked reduction of the amplitude of the spontaneous contractile activity, even when normalized to the tissue dry weight. We observed also a reduction in colonic contractility in response to carbachol and KCl. Moreover, the atropine-sensitive neurally-evoked contraction was lower in amplitude in HGprt− colon. The decrease in the cholinergic nerve-mediated contractions to EFS was significantly higher than the decrease in the cholinergic myogenic contractions to carbachol, suggesting a possible decrease in ACh release from the enteric nerves. However, a decrease in ACh release from the enteric nerves seems unlikely to be responsible to the reduced contractility of colonic muscle in HGprt− mice, since contractile activity was not affected by atropine indicating that it is not under a tonic excitatory control by cholinergic nerves. Since the contractile response to the muscarinic agonist itself was reduced in HGprt− animals, it is possible to speculate either that the circular muscle is less sensitive to ACh or that there is also widespread dysfunction of the contractile apparatus, which is also hinted by the reduced response to the depolarising agent KCl.
Recently, we have reported that guanine-based purines (guanosine and guanine) are able to modulate negatively gastrointestinal contractility in mouse, regulating the release of acetylcholine from the enteric excitatory cholinergic pathways intestine leading to a decrease in contractility (Zizzo et al., 2013, 2011). Data from the present work indicate that also in HGprt− mice exogenous administered guanine is still to modulate the prejuctional release of ACh by enteric nerves, indicating that cholinergic system is sensitive to guanine-based purines also in HGprt− mice, and then raising the possibility that cholinergic dysfunctions may derived from an increased effects of guanine-based purines on neural target.
Moreover, HGprt− colon displayed also a reduced muscle tone as assessed by the smaller relaxation in response to sodium nitroprusside or isoprotenerol. It is possible to hypothesize that the distal colon of HGprt− mice could be under a functional hyperactivity of inhibitory mediators and that this condition would therefore manifest as decreased smooth muscle tone and diminished amplitude of spontaneous phasic and EFS-induced contractions. However, hyperactivity of the NO system seems unlikely, since L-NAME, NO synthase inhibitor, was not able in the HGprt− tissue to restore the large amplitude spontaneous and nerve-evoked contractions characteristic of the control tissue. The observed reduced muscle tone, amplitude of spontaneous and EFS-induced contractions could indicate a muscle weakness. However, histological evaluation did not reveal trophic alteration of circular muscle mass. Further experiments are needed to solve this issue.
One puzzle result from our experiments was the observation that in HGprt− mice the excitatory effect of the D1-like antagonist SCH-23390 on the basal tone and on the spontaneous and evoked activity was lost. Western blot analysis showed a reduced expression of D1-like receptor in HGprt− colon that may explain the loss of the tonic inhibitory control of colonic contractility by constitutively active D1-like receptors, recently demonstrated in control animals (Auteri et al., 2017). However, this observation is difficult to reconcile with the hypomotility observed in HGprt− mice leaving opened the question of the role of constitutively active D1-like in the regulation of colonic contractility under physiological conditions. On the other hand, we did not observe change in the expression of TH and DAT and HPLC analysis revealed no significant difference in intestinal dopamine concentration between control and HGprt− colon. Therefore, enteric dopaminergic neurons seem not to be affected in HGprt deficiency, in agreement with several histopathological studies of autopsied brains, which have not revealed any consistent loss of neurons in the substantia nigra (Jinnah et al., 2006). As reported in the brain, more than one neurotransmitter system may be affected in the gut of HGprt− mice and the downregulation of D1 receptors may be an epiphenomenon resulting from the effects of other mediators. A suggestive hypothesis may be that the increase in hypoxanthine concentration interfering with adenosine transport, as reported in HGprt deficient peripheral blood lymphocytes from LND patients (Torres et al., 2004), increases extracellular adenosine levels and adenosine binding to its receptors, in turn leading to the reduction of D1 receptor expression, due to adenosine/dopamine receptor interaction (Fuxe et al., 2010). Further experiments will be done to explore this issue.
The conclusion of a decreased motor activity in the large intestine of HGprt− mice is also supported by the reduced faecal output and amount of water in the stools observed in these animals when compared to the controls.
The present study provided evidence for an increase in oxidative stress in the colon of HGprt− mice. We showed that in colonic tissue of HGprt− mice there is increased amounts of lipid peroxidation, associated with over production of oxygen free radicals. These findings are in agreement with previous data showing an induction of oxidative stress, particularly lipid peroxidation in the brain of HGprt− mice (Visser et al., 2002).
Indeed, these experimental findings are also consistent with human studies that have shown significant changes in free radical in the blood of patients with LND (Saugstad and Marklund, 1988). Thus, it is possible to speculate that the observed reduction of colon contractility in HGprt− mice could be partially dependent on increased oxidative stress. However, the inflammatory cytokines levels in the HGprt− colon were unchanged as compared to control mice, indicating absence of any inflammatory status.
In conclusion, our results reveal that, HGprt deficiency causes in mouse colon a decrease the contractility not dependent upon reduced ACh release from the myenteric plexus or hyperactivity of inhibitory signalling (nitrergic or dopaminergic system). By contrast, the observed reduction of colon contractility in HGprt− mice could be partially dependent on increased oxidative stress. In addition, since clinical observations of patients with LND indicate that the alteration of the purine recycling due to lack of HGprt enzyme activity is associated with dysfunction of intestinal motility, such as dysphagia, nausea-vomiting, constipation and bloating (Jinnah et al., 1999), results from the current study suggest that colonic dysmotility observed in HGprt− mice may mimic the gastrointestinal dysfunctions symptoms of LND. Therefore the HGprt− mice can be considered a viable model for elucidating disease pathogenesis in LND.
Acknowledgments
Grants
This work was supported by grants from “Fondi Finanziamento della Ricerca (FFR)”, University of Palermo. The work was supported in part by the National Institutes of Health in the USA (grant number R56 NS102980).
Abbreviations
- ACh
Acetylcholine
- CCh
Carbachol
- SCH-23390
7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride
- DA
Dopamine
- DAT
dopamine transporter
- HGprt
hypoxanthine-guanine phosphoribosyltransferase
- Iso
isoproterenol
- EFS
electrical field stimulation
- LND
Lesch-Nyhan disease
- ENS
enteric nervous system
- NO
nitric oxide
- L-NAME
Nω-nitro-L-arginine methyl ester hydrochloride
- SNP
sodium nitroprusside
- TTX
tetrodotoxin
- TH
tyrosine hydroxylase
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- ROS
reactive oxygen species
- MDA
malondialdehyde
Footnotes
Disclosure
The authors declare that they have no conflicts of interest.
Author contributions
Zizzo MG, Frinchi M, Nuzzo D performed experiments.
Jinnah HA provided HGprt knock out mice and contributed to write the text.
Mudò G, Mulè F processed data.
Condorelli DF, Caciagli F, Ciccarelli R, Di Iorio P contributed to write the text.
Belluardo N, Serio R conceived and planned the work and contributed to write the text.
References
- Auteri M, Zizzo MG, Amato A, Serio R. Dopamine induces inhibitory effects on the circular muscle contractility of mouse distal colon via D1- and D2-like receptors. J Physiol Biochem. 2017;73:395–404. doi: 10.1007/s13105-017-0566-0. [DOI] [PubMed] [Google Scholar]
- Cil O, Phuan PW, Lee S, Tan J, Haggie PM, Levin MH, Sun L, Thiagarajah JR, Ma T, Verkman AS. CFTR activator increases intestinal fluid secretion and normalizes stool output in a mouse model of constipation. Cell Mol Gastroenterol Hepatol. 2016;2:317–327. doi: 10.1016/j.jcmgh.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egami K, Yitta S, Kasim S, Lewers JC, Roberts RC, Lehar M, Jinnah HA. Basal ganglia dopamine loss due to defect in purine recycling. Neurobiol Dis. 2007;26:396–407. doi: 10.1016/j.nbd.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Hardy K, Hankerson JG, Doudet DJ, Cohen RM. Presynaptic dopaminergic deficits in Lesch-Nyhan disease. N Engl J Med. 1996;334:1568–1572. doi: 10.1056/NEJM199606133342403. [DOI] [PubMed] [Google Scholar]
- Frinchi M, Macaluso F, Licciardi A, Perciavalle V, Coco M, Belluardo N, Morici G, Mudo G. Recovery of damaged skeletal muscle in mdx mice through low-intensity endurance exercise. Int J Sports Med. 2014;35:19–27. doi: 10.1055/s-0033-1343405. [DOI] [PubMed] [Google Scholar]
- Fu R, Ceballos-Picot I, Torres RJ, Larovere LE, Yamada Y, Nguyen KV, Hegde M, Visser JE, Schretlen DJ, Nyhan WL, Puig JG, O’Neill PJ, Jinnah HA. Genotype-phenotype correlations in neurogenetics: Lesch-Nyhan disease as a model disorder. Brain. 2014;137:1282–1303. doi: 10.1093/brain/awt202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Borroto-Escuela DO, Guescini M, Fernandez-Duenas V, Tanganelli S, Rivera A, Ciruela F, Agnati LF. Adenosine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:e18–e42. doi: 10.1111/j.1755-5949.2009.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MG, Puig JG, Torres RJ. Adenosine, dopamine and serotonin receptors imbalance in lymphocytes of Lesch-Nyhan patients. J Inherit Metab Dis. 2012;35:1129–1135. doi: 10.1007/s10545-012-9470-5. [DOI] [PubMed] [Google Scholar]
- Gottle M, Prudente CN, Fu R, Sutcliffe D, Pang H, Cooper D, Veledar E, Glass JD, Gearing M, Visser JE, Jinnah HA. Loss of dopamine phenotype among midbrain neurons in Lesch-Nyhan disease. Ann Neurol. 2014;76:95–107. doi: 10.1002/ana.24191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland K, Kasim S, Egami K, Arnold LA, Jinnah HA. Tetrahydrobiopterin deficiency and dopamine loss in a genetic mouse model of Lesch-Nyhan disease. J Inherit Metab Dis. 2004;27:165–178. doi: 10.1023/B:BOLI.0000028728.93113.4d. [DOI] [PubMed] [Google Scholar]
- Jinnah HA. Lesch-Nyhan disease: from mechanism to model and back again. Dis Model Mech. 2009;2:116–121. doi: 10.1242/dmm.002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah HA, Langlais PJ, Friedmann T. Functional analysis of brain dopamine systems in a genetic mouse model of Lesch-Nyhan syndrome. J Pharmacol Exp Ther. 1992;263:596–607. [PubMed] [Google Scholar]
- Jinnah HA, Wojcik BE, Hunt M, Narang N, Lee KY, Goldstein M, Wamsley JK, Langlais PJ, Friedmann T. Dopamine deficiency in a genetic mouse model of Lesch-Nyhan disease. J Neurosci. 1994;14:1164–1175. doi: 10.1523/JNEUROSCI.14-03-01164.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah HA, Jones MD, Wojcik BE, Rothstein JD, Hess EJ, Friedmann T, Breese GR. Influence of age and strain on striatal dopamine loss in a genetic mouse model of Lesch-Nyhan disease. J Neurochem. 1999;72:225–229. doi: 10.1046/j.1471-4159.1999.0720225.x. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, De GL, Harris JC, Nyhan WL, O’Neill JP. The spectrum of inherited mutations causing HPRT deficiency: 75 new cases and a review of 196 previously reported cases. Mutat Res. 2000;463:309–326. doi: 10.1016/s1383-5742(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Visser JE, Harris JC, Verdu A, Larovere L, Ceballos-Picot I, Gonzalez-Alegre P, Neychev V, Torres RJ, Dulac O, Desguerre I, Schretlen DJ, Robey KL, Barabas G, Bloem BR, Nyhan W, De KR, Eddey GE, Puig JG, Reich SG. Delineation of the motor disorder of Lesch-Nyhan disease. Brain. 2006;129:1201–1217. doi: 10.1093/brain/awl056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhan WL. Dopamine function in Lesch-Nyhan disease. Environ Health Perspect. 2000;108(Suppl 3):409–411. doi: 10.1289/ehp.00108s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston R. The New Yorker. 2007. An error in the code: what can a rare disorder tell us about human behavior? pp. 30–36. [PubMed] [Google Scholar]
- Saito Y, Takashima S. Neurotransmitter changes in the pathophysiology of Lesch-Nyhan syndrome. Brain Dev. 2000;22(Suppl 1):S122–S131. doi: 10.1016/s0387-7604(00)00143-1. [DOI] [PubMed] [Google Scholar]
- Saugstad OD, Marklund SL. High activities of erythrocyte glutathione peroxidase in patients with the Lesch-Nyhan syndrome. Acta Med Scand. 1988;224:281–285. doi: 10.1111/j.0954-6820.1988.tb19374.x. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Ward J, Meyer SM, Yun J, Puig JG, Nyhan WL, Jinnah HA, Harris JC. Behavioral aspects of Lesch-Nyhan disease and its variants. Dev Med Child Neurol. 2005;47:673–677. doi: 10.1017/S0012162205001374. [DOI] [PubMed] [Google Scholar]
- Torres RJ, DeAntonio I, Prior C, Puig JG. Adenosine transport in HPRT deficient lymphocytes from Lesch-Nyhan disease patients. Nucleosides Nucleotides Nucleic Acids. 2004;23:1193–1196. doi: 10.1081/NCN-200027463. [DOI] [PubMed] [Google Scholar]
- Visser JE, Bar PR, Jinnah HA. Lesch-Nyhan disease and the basal ganglia. Brain Res Brain Res Rev. 2000;32:449–475. doi: 10.1016/s0165-0173(99)00094-6. [DOI] [PubMed] [Google Scholar]
- Visser JE, Smith DW, Moy SS, Breese GR, Friedmann T, Rothstein JD, Jinnah HA. Oxidative stress and dopamine deficiency in a genetic mouse model of Lesch-Nyhan disease. Brain Res Dev Brain Res. 2002;133:127–139. doi: 10.1016/s0165-3806(02)00280-8. [DOI] [PubMed] [Google Scholar]
- Wong DF, Harris JC, Naidu S, Yokoi F, Marenco S, Dannals RF, Ravert HT, Yaster M, Evans A, Rousset O, Bryan RN, Gjedde A, Kuhar MJ, Breese GR. Dopamine transporters are markedly reduced in Lesch-Nyhan disease in vivo. Proc Natl Acad Sci U S A. 1996;93:5539–5543. doi: 10.1073/pnas.93.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizzo MG, Mule F, Mastropaolo M, Serio R. D1 receptors play a major role in the dopamine modulation of mouse ileum contractility. Pharmacol Res. 2010;61:371–378. doi: 10.1016/j.phrs.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Zizzo MG, Mule F, Mastropaolo M, Condorelli DF, Belluardo N, Serio R. Can guanine-based purines be considered modulators of intestinal motility in rodents? Eur Aust J Pharm. 2011;650:350–355. doi: 10.1016/j.ejphar.2010.09.062. [DOI] [PubMed] [Google Scholar]
- Zizzo MG, Mule F, Amato A, Maiorana F, Mudo G, Belluardo N, Serio R. Guanosine negatively modulates the gastric motor function in mouse. Purinergic Signal. 2013;9:655–661. doi: 10.1007/s11302-013-9378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizzo MG, Cavallaro G, Auteri M, Caldara G, Amodeo I, Mastropaolo M, Nuzzo D, Di CM, Fumagalli M, Mosca F, Mule F, Serio R. Postnatal development of the dopaminergic signaling involved in the modulation of intestinal motility in mice. Pediatr Res. 2016;80:440–447. doi: 10.1038/pr.2016.91. [DOI] [PubMed] [Google Scholar]