Abstract
Mammalian oocytes grow and undergo meiosis within ovarian follicles. Oocytes are arrested at the first meiotic prophase, being controlled or influenced by follicular somatic cells. Under the influence of gonadotropins, immature oocytes resume meiosis. During meiotic progression, some cytoplasmic changes occur, so‐called cytoplasmic maturation. However, porcine follicular oocytes vary greatly in developmental competence. The present review summarizes recent studies highlighting the importance of cumulus cells in maintaining the developmental ability and in reorganizing the cytoskeleton and organelles of porcine oocytes. Factors affecting wide variation of the nuclear and cytoplasmic maturation observed in the porcine oocytes are discussed. (Reprod Med Biol 2006; 5: 183–194)
Keywords: cortical granules, cytoskeleton, mitochondria, pig oocyte
INTRODUCTION
FOLLICULAR OOCYTES RECOVERED from slaughtered animal ovaries are commonly used to study in vitro maturation (IVM) and fertilization (IVF) of the oocytes in current animal biotechnology of domestic animals. However, porcine follicular oocytes vary greatly in developmental competence. The quality of the oocytes from follicles of different sizes might be the main source of variation in IVM results in pigs, 1 , 2 , 3 , 4 as well as in cattle. 5 Furthermore, there is a large variation in the rate of meiotic progression of porcine oocytes in vitro. 2 , 3 , 6 The specific cause of such a wide variation is still unclear.
Several cytological and molecular events which are closely linked to nuclear maturation are associated with cytoplasmic maturation in mammalian oocytes. 7 These events are complex and well‐orchestrated processes, which are controlled or influenced by follicular somatic cells that are directly coupled to the oocyte by gap junctions. 8 It has been reported that cytoplasmic changes involve redistribution of chromosomes and organelles through reorganization of the cytoskeleton. 9 , 10 The present review will focus on discussing the fundamental effects of cumulus cells on the developmental ability and cytoskeletal distribution associated with the organelle distribution of porcine oocytes.
Cumulus cells on meiotic progression
The present study has shown the fundamental effects of cumulus cells on the maturation of porcine oocytes. Porcine ovaries were obtained from prepuberal gilts and the cumulus‐oocyte complexes (COC) were pooled and graded in four categories as described previously. 11 Grade 1 COC are mostly covered with more than three cumulus layers; grade 2 COC were covered with 2–3 layers; grade 3 COC were partially covered with clumped cumulus masses and the zona pellucida was partially exposed; and grade 4 COC were lacking cumulus cells and the oocyte was only enclosed by the zona pellucida. They were incubated for 44 h as reported previously. 12
The results obtained are summarized in Table 1. At the start of maturation, almost all of the oocytes were at the germinal vesicle (GV) stage but some of them were at diakinesis, showing GV break down (GVBD). After 44 h of incubation, the grade 1–2 oocytes showed significantly higher maturation rates than the grade 3–4 oocytes (87% and 76%vs 46% and 30%, respectively, P < 0.05). Lower‐grade oocytes showed an increased incidence of degeneration during incubation (13% for grade 4 oocytes).
Table 1.
Maturational stage of pig oocytes graded in four categories and cultured thereafter for 44 h with or without cumulus investment
| Grade† of COC | Time of culture (h) | No. oocytes examined | No. (%) oocytes at stage of: | No. (%) oocytes degenerated | |||
|---|---|---|---|---|---|---|---|
| GV | D | MI | MII | ||||
| 1 | 0 | 77 | 77 (100)* | ||||
| 44 | 91 | 1 (1)***** | 10 (11) | 79 (87)* | 1 (1)** | ||
| Denuded | 44‡ | 84 | 14 (17)**** | 14 (17) | 50 (60)** | 6 (7) | |
| 2 | 0 | 78 | 77 (99)* | 1 (1) | |||
| 44 | 93 | 1 (1)***** | 18 (20) | 71 (76)* | 3 (3) | ||
| Denuded | 44‡ | 80 | 21 (26)**** | 1 (1) | 15 (19) | 41 (51)** | 2 (3) |
| 3 | 0 | 63 | 60 (95)* | 3 (5) | |||
| 44 | 116 | 21 (18)**** | 37 (32) | 53 (46)** | 5 (4) | ||
| Denuded | 44‡ | 96 | 50 (52)*** | 3 (3) | 11 (12) | 28 (29)*** | 4 (4) |
| 4 | 0 | 69 | 63 (91)** | 6 (9) | |||
| 44 | 99 | 41 (41)*** | 15 (15) | 30 (30)*** | 13 (13)* | ||
COC, cumulus‐oocyte complexes; D, diakinesis stage; GV, germinal vesicle stage; MI, metaphase I stage; MII, metaphase II stage.
See text for grade 1–4 COC.
The oocytes incubated for 44 h after their cumulus cells were removed.
Values with different superscripts in the same column differ significantly (P < 0.05).
Oocytes that were cultured denuded of the cumulus cells (DO) showed a significant decrease in the metaphase II (MII) rate. However, oocytes assessed as grade 1 and 2 before denudation reached the MII stage at a higher rate than those assessed as grade 3 (60% and 51%vs 29%, respectively, P < 0.05). The inability of oocytes lacking cumulus cells to mature in vitro has been shown for porcine and bovine follicular oocytes. 13 , 14 It is believed that some regulating molecules act through mediation with the cumulus cells. 7
Cumulus cells and GV configurations
In porcine oocytes, chromatin configurations of the GV stages are also varied. 4 , 15 Thus, we carried out further experiments to classify the GV stage oocytes on the criterion described previously. 1 , 4 , 15 The results obtained are summarized in Table 2. At 0 h of incubation, more than half of the oocytes (57%) assessed as grade 1 were at the GVI, whereas mean percentage of GVI oocytes decreased significantly in lower‐grade oocytes (38, 27 and 25% for grades 2, 3 and 4, respectively). In contrast, the mean percentage of GVII–GVIV oocytes was 42% in grade 1 oocytes, but increased to 60, 62 and 71% for grade 2, 3 and 4, respectively. Furthermore, a few oocytes examined at 0 h (2–5%) had shown GVBD. These observations confirm the previous studies which reported a large variation in the process of meiotic progression of porcine oocytes, 2 , 3 suggesting that the progression from GVI to GVII or later might have some morphological correlation with the COC grading at recovery.
Table 2.
Maturational stage of pig oocytes graded in four categories and cultured thereafter for 12 or 24–28 h
| Grade† of COC | Time of culture (h) | No. oocytes examined | No. (%) oocytes at stage of: | No. (%) oocytes degenerated | |||||
|---|---|---|---|---|---|---|---|---|---|
| GV0 | GVI | GVII‐IV | D | MI | MII | ||||
| 1 | 0 | 53 | 30 (57)* | 22 (42)**,*** | 1 (2) | ||||
| 12 | 31 | 21 (68)* | 10 (32)***,**** | ||||||
| 24–28 | 40 | 1 (3)**** | 2 (5)***** | 30 (75)* | 6 (15) | 1 (3) | |||
| 2 | 0 | 47 | 1 (2) | 18 (38)** | 28 (60)*,** | ||||
| 12 | 32 | 1 (3) | 12 (38)** | 18 (56)*,** | 1 (3) | ||||
| 24–48 | 43 | 38 (88)* | 3 (7)** | 2 (5) | |||||
| 3 | 0 | 55 | 3 (5) | 15 (27)** | 34 (62)* | 3 (5) | |||
| 12 | 37 | 2 (5) | 5 (14)***,**** | 25 (68)* | 3 (8) | 2 (5)**** | |||
| 24–48 | 48 | 3 (6) | 9 (19)**** | 24 (50)** | 8 (17) | 4 (8) | |||
| 4 | 0 | 51 | 1 (2) | 13 (25)** | 36 (71)* | 1 (2) | |||
| 12 | 34 | 2 (6) | 6 (18)*** | 20 (59)*,** | 1 (3) | 3 (9)**** | 2 (6)** | ||
| 24–48 | 78 | 6 (8) | 16 (21)** | 22 (28)***,**** | 11 (14)*** | 17 (22)* | 6 (8) | ||
COC, cumulus‐oocyte complexes; D, diakinesis stage; GV, germinal vesicle stage; MI, metaphase I stage; MII, metaphase II stage.
See text for grade 1–4 COC.
Values with different superscripts in the same column differ significantly (P < 0.05).
In grades 1 and 2, no significant changes were noticed in the proportion of the GVI oocytes between 0 h and 12 h of culture. In grades 3 and 4, however, the proportion of the GVI oocytes decreased significantly compared with that at 0 h. There was a tendency for the percentage of the GVIII or GVIV oocytes to increase, unlike that of the GVII oocytes in lower‐grade groups (data not shown). Although they were only cultured for 12 h, 5% (2/37) of the grade 3 oocytes and 15% (5/34) of the grade 4 oocytes had developed to the MI stage or later, of which 6% (2/34) of the grade 4 eggs had already reached the MII stage. After 24–28 h of culture, more than 90% of oocytes in grades 1 and 2 proceeded to the MI stage or later. In grades 3 and 4, however, the oocytes beyond the MI stage decreased to 67 and 26%, respectively. The remaining eggs were arrested at GV (25 and 57%, respectively) or degenerated.
Grupen et al. reported that 27% of porcine oocytes had developed to the MII stage after 24 h of incubation and suggested that more advanced oocytes might have aged during the maturation period for 44 h. 3 On the basis of the present results, 15% of the grade 1 oocytes, which were at the MII stage after 24–28 h of culture, were aged for about 20 h at the end of culture period. Such advanced oocytes might be derived from the oocytes reaching GVII or later at recovery, because the same duration (18.7 h) is required to progress from GVI to GVII stages in pigs. 16
In summary, a higher proportion of the oocytes covered with fewer cumulus cells (the grade 2–3) might progress to the GVII stage or later compared with the oocytes covered compactly with cumulus (the grade 1), even if they are located in the ovarian follicles. The oocytes at advanced GV stages might develop much earlier to the MII stage than the GVI oocytes at the start of incubation, resulting in the occurrence of age‐related changes.
Cumulus cells and cytoskeletal distribution
The application of multiple fluorochroming techniques had made the analyses of the cytoskeletal elements during oocyte maturation possible. Typical fluorescence micrographs of the cytoskeleton in the porcine oocytes are shown in Figure 1. As observed previously, 11 most of the grade 1 oocytes (82%, 18/22) showed a dense and uniform distribution of the transzonal cumulus‐cell processes at 0 h of incubation (Fig. 1a). However, about 40% of the grade 2 oocytes (16/28) showed a partial loss of these processes and the majority of the oocytes at grade 3 and 4 (18/22 and 14/16, respectively) had already lost a greater part of their cumulus‐cell processes (Fig. 1b). Interestingly, in the oocytes cultured without cumulus cells, two sets of metaphase spindles were occasionally observed (1–5%), regardless of the COC grading (Fig. 2), suggesting that the factor deriving from the cumulus cells might be required for the normal reorganization of the cytoskeleton in the oocyte.
Figure 1.
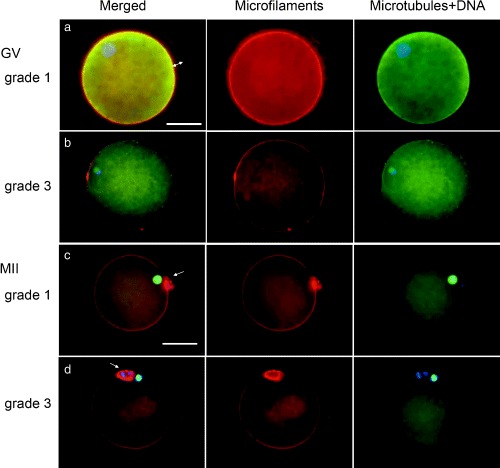
Fluorescent micrographs of germinal vesicle (GV)‐stage (a, grade 1; b, grade 3) and metaphase II (MII) stage (c, grade 1; d, grade 3) pig oocytes. Left panel: Three components; microtubules are green, microfilaments are red and nuclei/chromosomes are blue, yellow shows the overlap of microtubules and microfilaments. Middle panel: Microfilament labeling. Right panel: Microtubules and DNA labeling. (a) A GV‐stage oocyte assessed at grade 1 showing a dense and uniform distribution of cumulus‐cell processes consisting of microfilaments within the zona pellucida (double arrow). Microfilaments and microtubules in the ooplasm are strongly stained. (b) A GV‐stage oocyte assessed at grade 3. Oocyte showing partial loss of cumulus‐cell processes. Note decrease in density of microfilaments and microtubules in the ooplasm compared with (a). (c) A MII‐stage oocyte assessed at grade 1. Microfilaments are strongly stained in the cortex and on the contact surfaces of the oocyte and PBI (arrow). The MII spindle is located peripherally with a radial axis. (d) A MII‐stage oocyte assessed at grade 3 showing decreased density of cortical and cytoplasmic microfilaments. Bar represents 50 µm in (a) and (c).
Figure 2.
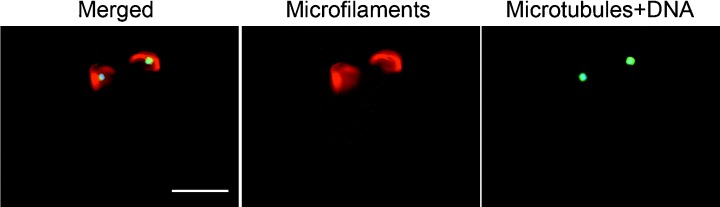
Fluorescent micrograph of an oocyte cultured and denuded of the cumulus cells showing two sets of metaphase spindles under each microfilament dome. Left panel: Three components; microtubules are green, microfilaments are red and nuclei/chromosomes are blue, yellow shows the overlap of microtubules and microfilaments. Middle panel: Microfilament labeling. Right panel: Microtubules and DNA labeling. Bar represents 50 µm in the left panel.
Fluorescence intensities of microfilaments and microtubules in the ooplasm are summarized in Table 3. As shown in Figure 1, matured grade 1 oocytes were characterized by the cortical microfilaments stained intensely and the cytoplasmic microfilaments stained weakly but distributed evenly (Fig. 1c). The cortical microfilaments had become strongly stained at 24 h of culture and then declined again at 44 h of culture. Microtubules were concentrated preferentially into the meiotic spindle at the MI and MII stages (24 and 44 h, respectively). The grade 2–4 oocytes showed significant decreases in fluorescence intensities of both microfilaments and microtubules compared with the grade 1 oocytes (P < 0.05, Fig. 1d). In contrast, cytoplasmic microtubules were higher in intensity in the grade 4 oocytes than in the grade 1–3 oocytes, although no significant differences were found in the intensity of the spindle microtubules. Interestingly, DO displayed higher intensities of microfilaments and microtubules in the ooplasm after incubation than the cumulus‐enclosed oocytes, regardless of the COC grading (Table 3).
Table 3.
Fluorescence intensities of microfilaments and microtubules in pig oocytes graded in four categories and cultured thereafter for 24–28 or 44 h with or without cumulus investment
| Cumulus layer | Grade† of COC | Time of culture (h) | No. oocytes examined | Fluorescence intensities‡ of | |||
|---|---|---|---|---|---|---|---|
| Microfilaments | Microtubules | ||||||
| Cytoplasm | Cortex | Cytoplasm | Spindle | ||||
| 1 | 0 | 22 | 100*,** | 136*,** | 100* | – | |
| 24–28 | 14 | 90*,**,*** | 148* | 39**** | 104 | ||
| 44 | 26 | 84**,*** | 108*** | 41**** | 114 | ||
| 2 | 0 | 28 | 80*** | 121**,*** | 99* | – | |
| Intact | 24–28 | 25 | 93**,*** | 144* | 56***,**** | 108 | |
| 44 | 31 | 86**,*** | 104*** | 51***,**** | 109 | ||
| 3 | 0 | 22 | 75*** | 115**,*** | 79**,*** | – | |
| 24–28 | 15 | 94**,*** | 151* | 54***,**** | 105 | ||
| 44 | 15 | 92*,**,*** | 103*** | 49***,**** | 108 | ||
| 4 | 0 | 16 | 74*** | 97*** | 92*,** | – | |
| 24–28 | 7 | 114* | 140*,** | 39***,**** | 108 | ||
| 44 | 11 | 78**,*** | 107*** | 63*** | 110 | ||
| Pooled SE | 1.4 (n = 232) | 1.9 (n = 232) | 1.8 (n = 232) | 1.1 (n = 82) | |||
| 1 | 24–28 | 14 | 119 | 151* | 41*** | 105 | |
| 44 | 18 | 109 | 122** | 69*,** | 125 | ||
| Denuded | 2 | 24–28 | 14 | 123 | 141 | 41*** | 98** |
| 44 | 16 | 120 | 138 | 75*,** | 118 | ||
| 3 | 24–28 | 8 | 118 | 147 | 46**,*** | 105 | |
| 44 | 13 | 105 | 120** | 87* | 128* | ||
| Pooled SE | 3.2 (n = 83) | 3.1 (n = 83) | 3.2 (n = 83) | 3.1 (n = 19) | |||
COC, cumulus‐oocyte complexes.
See text for grade 1–4 COC.
Values were calculated mean intensities of microfilaments and microtubules in the germinal vesicle cytoplasm of grade‐1 oocytes as 100%, respectively.
Values with different superscripts in the same column differ significantly (P < 0.05).
Table 4 shows the size of MI and MII spindles of the oocytes cultured with or without cumulus investment. The overall mean of pole distances of the MI spindle was 10.9 µm and the width of the equatorial plane was 12.5 µm. At the MII stage, however, the size decreased significantly to 8.6 µm in length and 9.9 µm in width (P < 0.05), although no remarkable differences were noted among those of the grade 1–3 oocytes. On the contrary, DO that reached the MI and MII stage showed extended distances of the spindle poles than the cumulus‐enclosed oocytes. The distance between the spindle poles in DO was increased by 17% (12.7/10.9 µm) for the MI spindle and profoundly increased by 53% (13.2/8.6 µm) for the MII spindle compared with those in the cumulus‐enclosed oocytes. In the grade 4 oocytes, which had lost their cumulus at recovery, the MII spindle was intermediate in length (10.6 µm) between COC and DO.
Table 4.
Size of meiotic apparatus in pig oocytes graded in four categories and cultured thereafter for 24–28 or 44 h with or without cumulus investment
| Grade† of COC | Cumulus layer | Stage & culture(h) of oocytes | No. oocytes examined | Distance (µm) of: | Denuded/Intact ratio of pole distance | |
|---|---|---|---|---|---|---|
| spindle poles | equatorial plane | |||||
| 1–3 | Intact | MI (24–28) | 35 | 10.9 ± 0.2** | 12.5 ± 0.3* | – |
| 1–3 | Denuded | MI (24–28) | 9 | 12.7 ± 1.0* | 12.1 ± 0.3* | 117% |
| 1–3 | Intact | MII (44) | 26 | 8.6 ± 0.2*** | 9.9 ± 0.2** | – |
| 1–3 | Denuded | MII (44) | 10 | 13.2 ± 0.6* | 9.5 ± 0.4** | 153% |
| 4 | No‡ | MII (44) | 7 | 10.6 ± 0.5** | 9.3 ± 0.4** | – |
COC, cumulus‐oocyte complexes.
See text for grade 1–4 COC. Group 1–3 shows pooled data of three grades, because of no significant differences among them.
The oocytes lacking cumulus cells at recovery.
Values with different superscripts in the same column differ significantly (P < 0.05).
Cumulus cells and cortical granule distribution
Pig cortical granules (CG) are able to be seen using fluorescein isothiocyanate (FITC)‐conjugated lectins. 17 By labeling with FITC‐peanut agglutinin, distribution of the CG was classified into three patterns according to the criterion by Yoshida et al. 17 with minor modifications: (i) located throughout the cortical cytoplasm (referred to as ‘cortical’); (ii) located next to the oolemma (referred to as ‘next to oolemma’); and (iii) ‘intermediate’ between both fashions described above, which is the third group we added in the present experiment (Fig. 3). In the ‘cortical’ pattern, the fluorescence spots are distributed sparsely in the range of approximately 20 µm inside the oolemma, whereas in the ‘next to oolemma’ pattern the stained spots were located within 4 µm beneath the oolemma in a linear arrangement.
Figure 3.
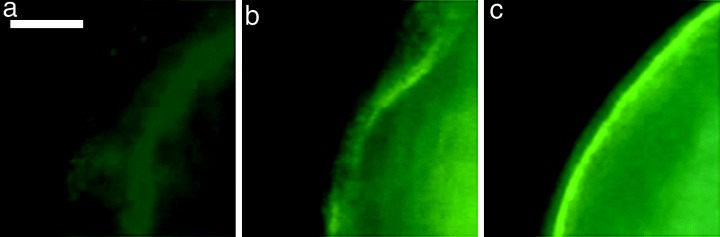
Fluorescent micrographs showing three distribution patterns of the cortical granules by fluorescein isothiocyanate‐conjugated peanut agglutinin (FITC‐PNA). (a) ‘Cortical’; (b) ‘intermediate’; (c) ‘next to oolemma’. See text for the explanation. Bar represents 50 µm in (a).
Table 5 shows distribution of the CG in pig oocytes graded into four categories and cultured for 12, 24 or 44 h. At 0 h of incubation, the majority (89–94%) of oocytes of grades 1–3 showed a ‘cortical’ pattern, whereas the grade 4 oocytes showed a significant decrease in the proportion (P < 0.05), which were either ‘next to oolemma’ or ‘intermediate’ patterns. After 12 h, no remarkable changes were noted in the grade 1 oocytes but in the grades 2 and 3, the percentage of oocytes showing a ‘cortical’ pattern fell to 62 and 69%, respectively. It was after 24 h that the grade 1 oocytes switched over from ‘cortical’ to the other two patterns. However, 70% of the grade 4 oocytes were still showing a ‘cortical’ pattern at this time. After 44 h of incubation (reaching the MII oocytes), almost all oocytes (93–100%) showed ‘next to oolemma’ and ‘intermediate’ patterns regardless of the COC grading.
Table 5.
Distribution of cortical granules in pig oocytes graded in four categories and cultured thereafter for 12, 24 or 44 h
| Grade† of COC | Time of culture (h) | No. oocytes examined | No. (%) oocytes showing distribution pattern‡ of: | ||
|---|---|---|---|---|---|
| Cortical | Intermediate | Next to oolemma | |||
| 1 | 0 | 35 | 33 (94)* | 2 (6)***,**** | 0 (0) |
| 12 | 17 | 15 (88)*,** | 1 (6)***,**** | 1 (6)**** | |
| 24 | 18 | 5 (28)***,**** | 6 (33)* | 7 (39)**,*** | |
| 44 | 28 | 2 (7)***** | 5 (18)*,**,*** | 21 (75)* | |
| 2 | 0 | 35 | 31 (89)* | 1 (3)**** | 3 (9)**** |
| 12 | 21 | 13 (62)** | 8 (38)* | 0 (0) | |
| 24 | 19 | 2 (10)***,**** | 7 (37)* | 10 (53)*,** | |
| 44 | 24 | 0 (0) | 5 (21)*,**,*** | 19 (79)* | |
| 3 | 0 | 29 | 26 (90)* | 2 (7)**,***,**** | 1 (3)**** |
| 12 | 16 | 11 (69)** | 4 (25)*,** | 1 (6)**** | |
| 24 | 14 | 5 (36)**,*** | 2 (14)*,**,***,**** | 7 (50)*,** | |
| 44 | 13 | 0 (0) | 3 (23)*,**,*** | 10 (77)* | |
| 4 | 0 | 30 | 17 (57)**,*** | 10 (33)* | 3 (10)**** |
| 12 | 15 | 11 (73)** | 2 (13)*,**,***,**** | 2 (13)*** | |
| 24 | 20 | 14 (70)** | 1 (5)***,**** | 5 (25)**,*** | |
| 44 | 7 | 0 (0) | 1 (14)*,**,***,**** | 6 (86)* | |
COC, cumulus‐oocyte complexes.
See text for grade 1–4 COC and distribution pattern of cortical granules, respectively.
Values with different superscripts in the same column differ significantly (P < 0.05).
In pig oocytes, migration of the CG occurred between 20 and 30 h post hCG in vivo. 18 This is consistent with our results on grade 1 oocytes. In contrast, 70% of the grade 4 oocytes showed a ‘cortical’ pattern even after 24 h of culture. Furthermore, the proportion of the oocytes showing ‘intermediate’ and ‘next to oolemma’ patterns at 0 h was higher in the grade 4 oocytes than in the grades 1–3 oocytes. These results suggest that migration of the CG through cytoskeletal reorganization might not proceed normally in oocytes lacking cumulus cells. The interaction between the cumulus cells and the oocyte is required to stabilize the distribution of CG. 19
Microfilaments play a role in the migration of CG. 20 Cortical ooplasm is rich in microfilaments in mice, 21 , 22 rats, 23 hamsters, 24 , 25 sheep 26 and pigs. 12 , 27 , 28 However, porcine oocytes without cumulus cells or aged after IVM showed a decreased density of the cortical microfilaments. 28 Altered distribution of the cortical microfilaments might affect localization of the CG. Displacement of the CG during IVM might lead to a disturbance in establishing the block to polyspermy, otherwise premature exocytosis of the CG might lead to an irreversible modification of the zona characteristics.
Cumulus cells and mitochondrial distribution
During oocyte maturation, mitochondria are required to provide energy/metabolites to specific regions in oocytes 29 , 30 , 31 which might ensure subsequent embryo development. 31 , 32 , 33 , 34 , 35 Translocation of mitochondria during oocyte maturation has been reported in cows, 36 pigs, 37 mice, 29 , 30 , 38 hamsters 39 and humans. 40 , 41 , 42 In the present study, we examined changes in the distribution and fluorescence intensity of the mitochondria by MitoTracker Red (0.1 µg/mL, Molecular Probes, Eugene, OR, USA), which are accumulated in active mitochondria of live cells depending on the membrane potential.
A typical fluorescence micrograph is shown in Figure 4. Mitochondrial distribution was classified into two patterns; more even distribution throughout the cytoplasm (‘even’ pattern) and less distribution in the peripheral ooplasm (‘peripheral free’ pattern). Table 6 shows the mitochondrial distribution in the oocytes of each grade. At 0 h of culture, the majority of oocytes in grades 1 and 2 were ‘even’ pattern (83 and 82%, respectively). However, in grades 3 and 4, the proportion of ‘even’ oocytes decreased (69 and 50%, respectively).
Figure 4.
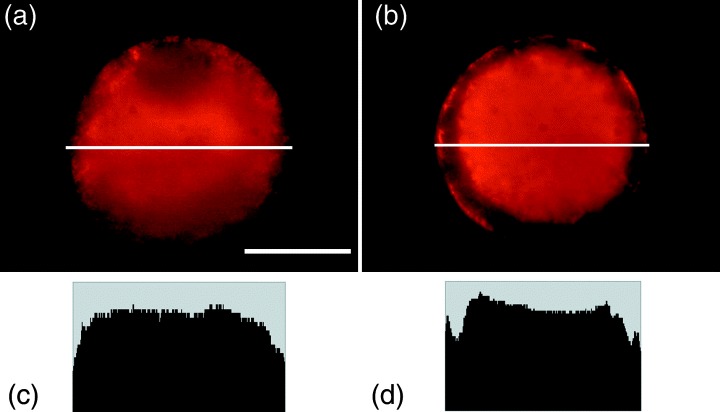
Fluorescent micrographs showing two distribution patterns of mitochondria by MitoTracker Red: (a) ‘Even’ and (b) ‘peripheral free’. Histograma show pixel strength of the equatorial plane of the oocyte (a white line in the figure). (c) ‘Even’ and (d) ‘peripheral free’. See text for the explanation. Bar represents 50 µm in (a).
Table 6.
Distribution of mitochondria in pig oocytes graded in four categories and cultured thereafter for 44 h
| Grade† of COC | Time of culture (h) | No. oocytes examined | No. (%) oocytes showing distribution pattern‡ of: | |
|---|---|---|---|---|
| Even | Peripheral free | |||
| 1 | 0 | 35 | 29 (83)* | 6 (17)*** |
| 44 | 35 | 12 (34)*** | 23 (66)* | |
| 2 | 0 | 34 | 28 (82)* | 6 (18)*** |
| 44 | 39 | 14 (36)*** | 25 (64)* | |
| 3 | 0 | 26 | 18 (69)*,** | 8 (31)**,*** |
| 44 | 25 | 9 (36)*** | 16 (64)* | |
| 4 | 0 | 30 | 15 (50)**,*** | 15 (50)*,** |
| 44 | 18 | 7 (39)*** | 11 (61)* | |
COC, cumulus‐oocyte complexes.
,
See text for grade 1–4 COC and distribution pattern of mitochondria, respectively.
Values with different superscripts in the same column differ significantly (P < 0.05).
We next examined whether the fluorescence intensity of the GV oocytes differ depending on the presence of a nucleus, the mean pixel intensity of 10 × 10 pixel boxes from the perinuclear and the cytoplasmic cortical regions in four subregions along radii (see Fig. 5) was quantitated from the raw digital images. The fluorescence intensity of mitochondria around the GV was approximately 20% lower in grade 4 oocytes than in grade 1–3 oocytes (P < 0.05), although no remarkable changes were found in the overall means of the fluorescence intensity in the ooplasm (Table 7). These observations suggest that mitochondrial transition from ‘even’ to ‘peripheral free’ patterns might begin in lower‐grade oocytes before the time of culture, thereby the number and activity of mitochondria around the GV might diminish in these oocytes. After 44 h of culture, more than half of the MII oocytes (61–66%) showed the ‘peripheral free’ pattern, regardless of the COC grading (Table 6).
Figure 5.
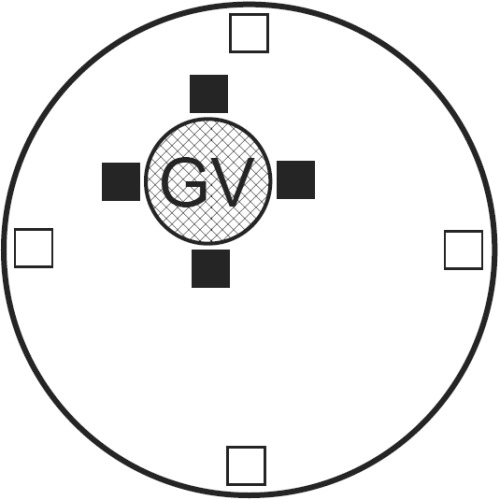
Schematic diagram illustrating areas for analysis of pixel intensity for quantification of mitochondrial distribution within a germinal vesicle (GV)‐stage oocyte. Solid boxes (10 × 10 pixels) indicate areas measured within the perinuclear areas of the oocyte and were placed adjacent to the GV. Open boxes indicate areas measured within the peripheral area of the oocyte and were placed four pixels from the cell membrane.
Table 7.
Fluorescence intensities of mitochondria in pig GV‐oocytes graded in four categories
| Grade† of COC | No. oocytes examined | Fluorescence intensities of mitochondria‡ | |
|---|---|---|---|
| Cortical cytoplasm | Around GV | ||
| 1 | 25 | 100 ± 3 | 131 ± 3* |
| 2 | 22 | 102 ± 3 | 131 ± 3* |
| 3 | 18 | 102 ± 4 | 134 ± 4* |
| 4 | 12 | 97 ± 5 | 109 ± 4** |
COC, cumulus‐oocyte complexes; GV, germinal vesicle.
See text for grade 1–4 COC.
Values were calculated the mean intensities of cortical cytoplasm in the grade‐1 oocytes as 100%.
Values with different superscripts in the same column differ significantly (P < 0.05).
What happens in pig oocytes within the ovarian follicles?
Cumulus and granulosa cells synthesize some meiosis‐inhibitory factors, including cyclic adenosine monophosphate or other molecules, that are transported into oocytes through numerous gap junctions and thereby mammalian follicular oocytes are arrested at the first meiotic prophase. 9 , 43 , 44 , 45 , 46 , 47 , 48 Disruption of the gap junctions between the cumulus cells and the oocyte is associated with the reinitiation of meiosis. 8 , 9 , 49 , 50 , 51 Alternatively, the resumption of meiosis might be mediated by positive factor(s) able to override the arrest imposed by the follicular environment. 9 , 52 , 53 , 54 , 55 A positive hormonal stimulus resulting from FSH interactions with the cumulus cells can override the meiotic arrest mechanism. The present observations showed lower maturation rates in porcine DO and in lower‐grade oocytes in the same culture condition. These results suggest that the oocyte must be responding to positive factor(s) produced by the cumulus cells surrounding it. The putative positive factor seemed to reach the oocyte through the gap junctions of the cumulus cell processes. 8 The positive factor has not been identified yet, but one possible candidate is calcium. 55 , 56 Calcium ions are essential second messengers in eukaryotic cells. A large variety of vital cell functions such as actin dependent motion and contraction, cell proliferation and secretion, gene expression and synaptic transmission depend on calcium concentrations. 57 Recent studies have emphasized the role of calcium on cytoskeleton assembly and mitochondrial distribution in the somatic cells. 58 The present study has shown that the transition of organelles, such as CG and mitochondria, might begin or proceed earlier in lower‐grade oocytes than the cumulus‐enclosed oocytes before the time of culture. Several studies of cytoskeletal dynamics during oocyte maturation and fertilization show that microfilaments play a role in specific processes, including spindle rotation (in rodent eggs), polar body emission (cytokinesis), CG exocytosis, sperm tail incorporation, and pronuclear migration in mouse, hamster, ovine, porcine and bovine eggs. 9 , 10 , 11 , 12 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 Microtubules as well as microfilaments might be involved in the mechanism of mitochondrial translocation. 29 , 31 , 34 , 38 Cumulus and granulosa cells alter the signal transduction pathways in the oocyte and influence the expression of maturation promoting factor and cytostatic factor activity through a cytoskeletal‐dependent mechanism. 9 , 71 , 72 Further studies will be necessary to show whether calcium mobilization that the cumulus cells stimulate can affect reorganization of the cytoskeleton and localization of the organelles in mammalian eggs, in addition to exploration of other molecules acting as a positive factor and definition of the signal transduction pathways that link follicle cells to the oocyte to regulate meiosis.
Characteristics of oocytes from atretic follicles
Most porcine IVM‐IVF systems utilized oocytes obtained from the ovaries of prepuberal gilts. Oocyte meiosis is arrested at the dictyate stage of the prophase before the onset of puberty. 43 Approximately half of the antral follicles (3–6 mm in diameter) present in prepuberal gilt ovaries undergo atresia, determined by pyknotic nuclei in the granulosa cells, in pre‐ and postpubertal gilts. 4 , 73 , 74 During early stages of oogenesis and folliculogenesis, granulosa cell apoptosis might play the primary role in oocyte degeneration. 75 Garrett and Guthrie reported that an antiapoptotic protein, such as Bcl‐2, was not expressed in oocytes and that the expression was greatest in the granulosa cells of the primordial and preantral follicles, as well as in the stromal cells. 76 Shimada et al. emphasized that the outer layers of cumulus cells, through the synthesis of connexin‐43, function to support the communication between the cumulus cells or between the cumulus cells and the oocyte. 77 It is suggested, therefore, that cumulus/granulosa cells might mediate a certain critical factor between the stromal cells and the oocyte.
It is reasonable that porcine oocytes with few or no cumulus cells are assumed to be derived from the atretic follicles and the characteristics of the oocytes classified as low grade might reflect the modification in the oocyte during the early stages of apoptosis and follicular atresia. They include modifications in the location and density of microfilaments, microtubules and mitochondria, asynchronous migration of CG and mitochondria with nuclear configurations, and changes in spindle morphology.
There were wider variations in the progression of the prophase in grade 2–4 oocytes than in grade 1 oocytes. It was found that up to 70% of lower‐grade oocytes in 3–6 mm diameter follicles had shifted to the GVII or later stages in prepubertal gilts. In pigs, follicles containing the oocytes undergoing GVBD were eliminated before the preovulatory LH surge. 75 In the IVM‐IVF protocols, however, it is very difficult to distinguish the oocytes derived from either atretic or nonatretic follicles. To develop the technique, retrieving the oocytes from atretic follicles might be required for an improvement in the efficiency of current animal biotechnology.
ACKNOWLEDGMENTS
THE AUTHORS THANK the staff of the Gene Research Center at Hirosaki University for use of the image analyzing system and the staff of the Inakadate Meat Inspection Office (Aomori, Japan) for supplying pig ovaries. The present work was supported by a Grant‐in‐Aid for Scientific Research (C) (No. 17580243) from the Ministry of Education, Science, Sports and Culture of Japan, and by a Grant‐in‐Aid from the Morinaga Houshikai.
REFERENCES
- 1. Christmann L, Jung T, Moor RM. MPF components and meiotic competence in growing pig oocytes. Mol Reprod Dev 1994; 38: 85–90. [DOI] [PubMed] [Google Scholar]
- 2. Hirao Y, Tsuji Y, Miyano T et al. Association between p34cdc2 levels and meiotic arrest in pig oocytes during early growth. Zygote 1995; 3: 325–332. [DOI] [PubMed] [Google Scholar]
- 3. Grupen CG, Nagashima H, Nottle MB. Asynchronous meiotic progression in porcine oocytes matured in vitro: a cause of polyspermic fertilization? Reprod Fertil Dev 1997; 9: 187–191. [DOI] [PubMed] [Google Scholar]
- 4. Lucas X, Martineza EA, Rocaa J et al. Relationship between antral follicle size, oocyte diameters and nuclear maturation of immature oocytes in pigs. Theriogenology 2002; 58: 871–885. [DOI] [PubMed] [Google Scholar]
- 5. Fair T, Hyttel P, Greve T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol Reprod Dev 1995; 42: 437–442. [DOI] [PubMed] [Google Scholar]
- 6. Funahashi H, Cantley T, Day BN. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol Reprod 1997; 57: 49–53. [DOI] [PubMed] [Google Scholar]
- 7. Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev 1996; 8: 485–489. [DOI] [PubMed] [Google Scholar]
- 8. Anderson E, Albertini DF. Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol 1976; 71: 680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albertini DF. Regulation of meiotic maturation in the mammalian oocyte: Interplay between exogenous cues and the microtubule cytoskeleton. BioEssays 1992; 14: 97–103. [DOI] [PubMed] [Google Scholar]
- 10. Terada Y, Morito Y, Tachibana M et al. Cytoskeletal dynamics during mammalian gametogenesis and fertilization: Implications for human reproduction. Reprod Med Biol 2005; 4: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suzuki H, Saito Y, Kagawa N, Yang X. In vitro fertilization and polyspermy in the pig. Factors affecting fertilization and cytoskeletal organization of the oocytes. Microsc Res Tech 2003; 61: 327–334. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki H, Jeong B‐S, Yang X. Dynamic changes of cumulus‐oocyte cell communication during in vitro maturation of the porcine oocytes. Biol Reprod 2000; 63: 723–729. [DOI] [PubMed] [Google Scholar]
- 13. Tsafriri A, Channing CP. Influence of follicular maturation and culture conditions on the meiosis of pig oocytes in vitro. J Reprod Fertili 1975; 43: 149–152. [DOI] [PubMed] [Google Scholar]
- 14. Leibfried L, First NL. Characterization of bovine follicular oocytes and their ability to mature in vitro. J Anim Sci 1979; 48: 76–86. [DOI] [PubMed] [Google Scholar]
- 15. Motlik J, Fulka J. Breakdown of the germinal vesicle in pig oocytes in vivo and in vitro. J Exp Zool 1976; 198: 155–162. [DOI] [PubMed] [Google Scholar]
- 16. Wehrend A, Meinecke B. Kinetics of meiotic progression, M‐phase promoting factor (MPF) and mitogen‐activated protein kinase (MAP kinase) activities during in vitro maturation of porcine and bovine oocytes: species specific differences in the length of the meiotic stages. Anim Reprod Sci 2001; 66: 175–184. [DOI] [PubMed] [Google Scholar]
- 17. Yoshida M, Cran DG, Pursel VG. Confocal and fluorescence microscopic study using lectins of the distribution of cortical granules during the maturation and fertilization of pig oocytes. Mol Reprod Dev 1993; 36: 462–468. [DOI] [PubMed] [Google Scholar]
- 18. Cran DG, Cheng WTK. Changes in cortical granules during porcine oocyte maturation. Gamete Res 1985; 11: 311–319. [Google Scholar]
- 19. Galeati G, Mondina S, Lauria A, Mattioli M. Follicle somatic cells influence pig oocyte penetrability and cortical granule distribution. Mol Reprod Dev 1991; 29: 40–46. [DOI] [PubMed] [Google Scholar]
- 20. DiMaggio AJ Jr, Longergan TA, Stewart‐Savage J. Cortical granule exocytosis in hamster eggs requires microfilaments. Mol Reprod Dev 1997; 47: 334–340. [DOI] [PubMed] [Google Scholar]
- 21. Maro B, Johnson MH, Pickering SJ, Flach G. Changes in actin distribution during fertilization of the mouse egg. J Embryol Exp Morph 1984; 81: 211–237. [PubMed] [Google Scholar]
- 22. Longo FJ, Chen DY. Development of cortical polarity in mouse eggs: involvement of the meiotic apparatus. Dev Biol 1985; 107: 382–394. [DOI] [PubMed] [Google Scholar]
- 23. Battaglia DE, Gaddum‐Rosse P. Influence of the calcium ionophore A23187 on rat egg behavior and cortical F‐actin. Gamete Res 1987; 18: 141–152. [DOI] [PubMed] [Google Scholar]
- 24. Webster SD, McGaughey RW. The cortical cytoskeleton and its role in sperm penetration of the mammalian egg. Dev Biol 1990; 142: 61–74. [DOI] [PubMed] [Google Scholar]
- 25. Terada Y, Fukaya T, Yajima A. Localization of microfilaments during oocyte maturation in golden hamster. Mol Reprod Dev 1995; 41: 486–492. [DOI] [PubMed] [Google Scholar]
- 26. Le Guen P, Crozet N, Huneau D, Gall L. Distribution and role of microfilaments during early events of sheep fertilization. Gamete Res 1989; 22: 411–425. [DOI] [PubMed] [Google Scholar]
- 27. Suzuki H, Takashima Y, Toyokawa K. Influence of incubation temperature on meiotic progression of porcine oocytes matured in vitro. J Mamm Ova Res 2001; 18: 8–13. [Google Scholar]
- 28. Suzuki H, Takashima Y, Toyokawa K. Cytoskeletal organization of porcine oocytes aged and activated electrically or by sperm. J Reprod Dev 2002; 48: 293–301. [Google Scholar]
- 29. Van Blerkom J, Runner MN. Mitochondrial reorganization during resumption of arrested meiosis in the mouse oocyte. Am J Anat 1984; 171: 335–355. [DOI] [PubMed] [Google Scholar]
- 30. Van Blerkom J. Microtubule mediation of cytoplasmic and nuclear maturation during the early stages of resumed meiosis in cultured mouse oocytes. Proc Natl Acad Sci USA 1991; 88: 5031–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tokura T, Noda Y, Goto Y, Mori T. Sequential observation of mitochondrial distribution in mouse oocytes and embryos. J Assisted Reprod General 1993; 10: 417–426. [DOI] [PubMed] [Google Scholar]
- 32. Barnett DK, Kimura J, Bavister BD. Translocation of active mitochondria during hamster preimplantation embryo development studied by confocal laser microscopy. Dev Dyn 1996; 205: 64–72. [DOI] [PubMed] [Google Scholar]
- 33. Barnett DK, Clayton MK, Kimura J, Bavister BD. Glucose and phosphate toxicity in hamster preimplantation embryos involves disruption of cellular organization, including distribution of active mitochondria. Mol Reprod Dev 1997; 48: 227–237. [DOI] [PubMed] [Google Scholar]
- 34. Bavister BD, Squirrell JM. Mitochondrial distribution and function in oocytes and early embryos. Hum Reprod 2000; 15 (Suppl 2): 189–198. [DOI] [PubMed] [Google Scholar]
- 35. Van Blerkom J, Davis P, Alexander S. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: relationship to microtubular organization, ATP content and competence. Hum Reprod 2000; 15: 2621–2633. [DOI] [PubMed] [Google Scholar]
- 36. Stojkovic M, Machado SA, Stojkovic P et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod 2001; 64: 904–909. [DOI] [PubMed] [Google Scholar]
- 37. Sun QY, Wu GM, Lai L et al. Translocation of active mitochondria during pig oocyte maturation, fertilization and early embryo development in vitro. Reproduction 2001; 122: 155–163. [PubMed] [Google Scholar]
- 38. Calarco PG. Polarization of mitochondria in the unfertilized mouse oocytes. Dev Gene 1995; 16: 36–43. [DOI] [PubMed] [Google Scholar]
- 39. Suzuki H, Satoh M, Toyokawa K. Changes in distribution of active mitochondria during oocyte maturation and fertilization in the hamster. J Mamm Ova Res 2005; 22: 163–169. [Google Scholar]
- 40. Sathananthan AH, Trounson AO. Mitochondrial morphology during preimplantational human embryogenesis. Hum Reprod 2000; 15 (Suppl 2): 148–159. [DOI] [PubMed] [Google Scholar]
- 41. Wilding M, Dale B, Placido GD. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod 2001; 16: 909–917. [DOI] [PubMed] [Google Scholar]
- 42. Van Blerkom J, Davis P. Domains of high‐polarized and low‐polarized mitochondria may occur in mouse and human oocytes and early embryos. Hum Reprod 2002; 17: 393–406. [DOI] [PubMed] [Google Scholar]
- 43. Tsafriri A, Channing CP. An inhibitory influence of granulosa cells and follicular fluid upon porcine oocyte meiosis in vitro. Endocrinology 1975; 96: 922–927. [DOI] [PubMed] [Google Scholar]
- 44. Sato E, Ishibashi T. Meiotic arresting action of the substance obtained from the cell surface of porcine oocyte granulosa cells. Jpn J Zootech Sci 1977; 48: 22–26. [Google Scholar]
- 45. Leibfried‐Rutledge ML, Crister ES, Parrish JJ, First NL. In vitor maturation and fertilization of bovine oocytes. Theriogenology 1989; 31: 61–74. [Google Scholar]
- 46. Sirard MA, Bilodeau S. Granurosa cells inhibit the resumption of meiosis in bovine oocytes in vitro. Biol Reprod 1990; 43: 777–783. [DOI] [PubMed] [Google Scholar]
- 47. Downs SM, Daniel SAJ, Eppig JJ. Induction of maturation in cumulus cell‐enclosed mouse oocytes by follicle stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool 1988; 245: 86–96. [DOI] [PubMed] [Google Scholar]
- 48. Buccione R, Schroeder AC, Eppig JJ. Interactions between somatic cells and germ cells throughout mammalian oogenesis. Biol Reprod 1990; 43: 543–547. [DOI] [PubMed] [Google Scholar]
- 49. Larsen WJ, Wert S, Brunner GD. A dynamic loss of cumulus cell gap junctions is correlated with germinal vesicle breakdown in rat oocytes. Dev Biol 1986; 113: 517–521. [DOI] [PubMed] [Google Scholar]
- 50. Racowsky C, Baldwin KV, Larabell CA, DeMarais A, Kazilek CJ. Down‐regulation of membrane granulosa cell gap junctions is correlated with irreversible commitment to resume meiosis in golden Syrian hamster oocytes. Eur J Cell Biol 1989; 49: 244–251. [PubMed] [Google Scholar]
- 51. Isobe N, Maeda T, Terada T. Involvement of meiotic resumption in the disruption of gap junctions between cumulus cells attached to pig oocytes. J Reprod Fertil 1998; 113: 167–172. [DOI] [PubMed] [Google Scholar]
- 52. Downs SM, Daniel SAJ, Eppig JJ. Induction of maturation in cumulus cell‐enclosed mouse oocytes by follicle stimulation hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool 1988; 245: 86–96. [DOI] [PubMed] [Google Scholar]
- 53. Fagbohun CF, Downs SM. Metabolic coupling and ligand‐stimulated meiotic maturation in the mouse oocyte‐cumulus cell complex. Biol Reprod 1991; 45: 851–859. [DOI] [PubMed] [Google Scholar]
- 54. Eppig JJ. Intercommunication between mammalian oocytes and companion somatic cells. BioEssays 1991; 13: 569–574. [DOI] [PubMed] [Google Scholar]
- 55. Downs SM. Factors affecting the resumption of meiotic maturation in mammalian oocytes. Theriogenology 1993; 39: 65–79. [Google Scholar]
- 56. Homa ST. Calcium and meiotic maturation of the mammalian oocyte. Mol Reprod Dev 1995; 40: 122–134. [DOI] [PubMed] [Google Scholar]
- 57. Rizzuto R, Pozzan T. When calcium goes wrong: genetic alterations of a ubiquitous signaling route. Nat Genet 2003; 34: 135–141. [DOI] [PubMed] [Google Scholar]
- 58. Saoudi Y, Rousseau B, Doussiere J et al. Calcium‐independent cytoskeleton disassembly induced by BAPTA. Eur J Biochem 2004; 271: 3255–3264. [DOI] [PubMed] [Google Scholar]
- 59. Longo FJ. Effects of cytochalasin B on sperm–egg interactions. Dev Biol 1978; 67: 249–265. [DOI] [PubMed] [Google Scholar]
- 60. Maro B, Johnson MH, Pickering SJ, Flach G. Changes in actin distribution during fertilization of the mouse egg. J Embryol Exp Morph 1984; 81: 211–237. [PubMed] [Google Scholar]
- 61. Schatten G, Schatten H, Spector I et al. Latrunculin inhibits the microfilament‐mediated processes during fertilization, cleavage and early development in sea urchins and mice. Exp Cell Res 1986; 166: 191–208. [DOI] [PubMed] [Google Scholar]
- 62. Schatten H, Simerly C, Maul G, Schatten G. Microtubule assembly is required for the formation of the pronuclei, nuclear lamin acquisition, and DNA synthesis during mouse, but not sea urchin, fertilization. Gamete Res 1989; 23: 309–322. [DOI] [PubMed] [Google Scholar]
- 63. Sutkovsky P, Navara CS, Schatten G. Fate of the sperm mitochondria, and the incorporation, conversion, and disassembly of the sperm tail structures during bovine fertilization. Biol Reprod 1996; 55: 1195–1205. [DOI] [PubMed] [Google Scholar]
- 64. Terada Y, Simerly C, Schatten G. Microfilament stabilization by jasplakinolide arrests oocyte maturation, cortical granule exocytosis, sperm incorporation cone resorption, and cell‐cycle progression, but not DNA replication, during fertilization in mice. Mol Reprod Dev 2000; 56: 89–98. [DOI] [PubMed] [Google Scholar]
- 65. Sun QY, Lai L, Park KW, Kuhholzer B, Prather RS, Schatten H. Dynamic events are differently mediated by microfilaments, microtubules, and mitogen‐activated protein kinase during porcine oocyte maturation and fertilization in vitro. Biol Reprod 2001; 64: 879–889. [DOI] [PubMed] [Google Scholar]
- 66. Tahara M, Tasaka K, Masumoto N, Mammoto A, Ikebuchi Y, Miyake A. Dynamics of cortical granule exocytosis at fertilization in living mouse eggs. Am J Physiol 1996; 270: C1354–C1361. [DOI] [PubMed] [Google Scholar]
- 67. McAveya BA, Wortzmana GB, Williamsb CJ, Evans JP. Involvement of calcium signaling and the actin cytoskeleton in the membrane block to polyspermy in mouse eggs. Biol Reprod 2002; 67: 1342–1352. [DOI] [PubMed] [Google Scholar]
- 68. Suzuki H, Takashima Y, Toyokawa K. Parthenogenetic development and cytoskeletal distribution of porcine oocytes treated by means of electrical pulses and cytochalasin D. J Mamm Ova Res 2002; 19: 6–11. [Google Scholar]
- 69. Suzuki H, Kagawa N, Toyokawa K. Pronuclear migration and cytoskeletal organization of porcine oocytes activated by various stimuli. J Mamm Ova Res 2002; 19: 96–103. [Google Scholar]
- 70. Calarco PG. The role of microfilaments in early meiotic maturation of mouse oocytes. Microsc Microanal 2005; 11: 146–153. [DOI] [PubMed] [Google Scholar]
- 71. Fan HY, Sun QY. Involvement of mitogen‐activated protein kinase cascade during oocyte maturation and fertilization in mammals. Biol Reprod 2004; 70: 535–547. [DOI] [PubMed] [Google Scholar]
- 72. Miao Y‐L, Liu X‐Y, Qiao T‐W, Maio D‐Q, Luo M‐J, Tan J‐H. Cumulus cells accelerate aging of mouse oocytes. Biol Reprod 2005; 73: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 73. Erickson BH. Radioresponse of the pre‐puberal porcine ovary. Int J Rad Biol 1967; 13: 57–67. [DOI] [PubMed] [Google Scholar]
- 74. Dalin AM. Ovarian follicular activity during the luteal phase in gilts. J Vet Series A 1987; 34: 592–601. [DOI] [PubMed] [Google Scholar]
- 75. Guthrie HD, Garrett WM. Apoptosis during folliculogenesis in pigs. Reprod 2001; 58: 17–29. [PubMed] [Google Scholar]
- 76. Garrett WM, Guthrie HD. Expression of Bcl‐2 and 3‐hydroxysteroid dehydrogenase protein during oocyte and follicle development in foetal and post‐natal pig ovaries. Reprod Fertil Dev 1999; 11: 463–470. [DOI] [PubMed] [Google Scholar]
- 77. Shimada M, Maeda T, Terada T. Dynamic changes of connexin‐43, gap junctional protein, in outer layers of cumulus cells are regulated by PKC and PI3‐kinase during meiotic resumption in porcine oocytes. Biol Reprod 2001; 64: 1255–1263. [DOI] [PubMed] [Google Scholar]


