Abstract
Background
We conducted a meta-analysis from randomized controlled trials (RCTs) and non-RCTs to assess the efficacy of aminocaproic acid in cases of primary total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Methods
Potentially relevant academic articles were identified from the Cochrane Library, MEDLINE (1966–2017 October 31), PubMed (1966–2017 October 31), EMBASE (1980–2017 October 31), and ScienceDirect (1985–2017 October 31). Secondary sources were identified from the references of the included literature. The pooled data were analyzed using RevMan 5.1.
Results
Three RCTs and four non-RCTs met the inclusion criteria. There were significant differences in total blood loss (mean difference (MD) = − 495.80, 95% CI − 837.29 to − 154.32, P = 0.004), drainage volume (MD = − 249.43, 95% CI − 286.78 to − 212.08, P < 0.00001), postoperative hemoglobin level (MD = 0.90, 95% CI 0.78 to 1.02, P < 0.00001), hemoglobin reduction (MD = − 0.75, 95% CI − 0.93 to − 0.57, P < 0.00001), transfusion rates (risk difference (RD) = − 0.17, 95% CI − 0.25 to − 0.09, P < 0.0001), average transfusion units (MD = − 0.28, 95% CI − 0.48 to − 0.09, P = 0.004), and length of hospital stay (MD = − 0.33, 95% CI − 0.43 to − 0.24, P < 0.00001) between the two groups. No significant differences were found regarding deep vein thrombosis (DVT) (RD = − 0.00, 95% CI − 0.01 to 0.00, P = 0.36) between the two groups.
Conclusions
The present meta-analysis indicated that the application of aminocaproic acid in THA or TKA decreases the total blood loss, drainage volume, transfusion rate, transfusion units per patient, and length of hospital stay and does not increase the risk of DVT.
Keywords: Aminocaproic acid, Arthroplasty, Blood loss, Transfusion, Meta-analysis
Background
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are common treatment for treating the patients suffering end-stage joint disease [1, 2]. It is known that significant blood loss may lead to acute anemia following a THA, and blood transfusions are often required for patients suffering from acute anemia and carry their own risks, such as inducing infectious disease, hemolysis, and anaphylactic reactions and increasing the economic burden [3, 4]. Multiple strategies have been utilized to minimize the blood loss, such as blood salvage, normovolemic hemodilution, electrocautery, hypotensive anesthesia, and hemostatic agents [5]. However, many patients still require blood transfusions.
Various studies have reported that aminocaproic acid reduces blood loss and allogenic blood transfusions in primary THA and TKA [6–12]. However, the results are not consistent. Moreover, some limitations exist in previous studies such as small sample size, inconclusive results, and inaccurate evaluations. Therefore, we conducted a large sample meta-analysis to evaluate the efficacy of aminocaproic acid in primary THA and TKA from randomized controlled trials (RCTs) and non-RCTs.
Methods
Search strategy
Electronic databases were searched, including Cochrane Library, MEDLINE (1966–2017 October 31), PubMed (1966–2017 October 31), EMBASE (1980–2017 October 31), and ScienceDirect (1985–2017 October 31). We then manually searched the reference lists of all included studies, relevant books, review articles, and meeting proceedings to identify trials that might have been missed in the electronic search. The search process was conducted as follows in Fig. 1. The key words “aminocaproic acid” and “replacement OR arthroplasty” were used in combination with the Boolean operators AND or. We made no restrictions on the language of the publication. This study is a meta-analysis and did not need approval from the ethics committee or institutional review board.
Fig. 1.
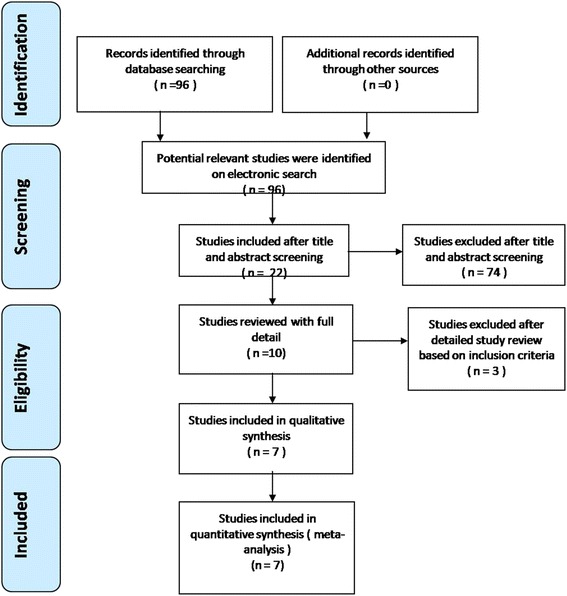
Flow chart of the study selection and inclusion process
Inclusion criteria
Studies were considered eligible for inclusion if they met the following criteria: (1) patients undergoing a primary THA or TKA; (2) the intervention used aminocaproic acid and studies contained a control group with placebo or null; (3) the outcomes included blood loss, operative time, blood transfusion rate, blood transfusion unit, perioperative outcomes, and complications; and (4) the study was a published or unpublished comparative trial (RCTs or non-RCTs).
Exclusive criteria
We excluded articles that were (1) studies without controlled groups, (2) articles without available full-text versions, and (3) no available outcomes data.
Selection criteria
Two reviewers independently screened the titles and abstracts for eligibility criteria. Subsequently, the full text of the studies that potentially met the inclusion criteria were read, and the literature was reviewed to determine final inclusion. Disagreement was resolved by consulting a third reviewer.
Quality assessment
According to whether the study is a randomized or non-randomized trial, the methodological Index for Non-randomized Studies (MINORS) form was used to assess retrospective controlled trials [13]. Quality assessment for RCT was conducted according to a modification of the generic evaluation tool used by the Cochrane Bone, Joint and Muscle Trauma Group [14]. Disagreements were resolved by consensus or consultation with the senior reviewer.
Data extraction
Two researchers independently extracted the data from the included literature. The corresponding author was consulted for details in the case of incomplete data. The following information was extracted: first author name, year of publication, intervening measures, comparable baseline, sample size, and outcome measures. We contacted the authors of the studies for further information. Other relevant parameters were also extracted from individual studies.
Data analysis and statistical methods
Pooling of data was analyzed by RevMan 5.1 (The Cochrane Collaboration, Oxford, UK). Heterogeneity was estimated depending on the value of P and I2 using the standard chi-square test. When I2 > 50%, P < 0.1 was considered to be significant heterogeneity. Therefore, a random effects model was applied for data analysis. A fixed effects model was used when no significant heterogeneity was found. Subgroup analysis was performed to investigate sources in the case of significant heterogeneity. Mean difference (MD) and 95% confidence interval (CI) were presented for continuous outcomes. Risk difference (RD) and 95% CIs were calculated for dichotomous data.
Results
Search results
A total of 96 studies were identified as potentially relevant literature reports. No additional studies were obtained after the reference review. Seventy-four studies were excluded after title and abstract screening, 22 studies reviewed with full detail, and 15 of studies excluded after detailed study review based on inclusion criteria. Ultimately, three RCTs and four non-RCTs were eligible for data extraction and meta-analysis. The search process is shown in Fig. 1.
Risk of bias assessment
RCT quality was assessed based on the Cochrane Handbook for Systematic Review of Interventions (Fig. 2). The RCT stated clear inclusion and exclusion criteria. Included RCT performed adequate methodology of randomization, concealment of allocation, blinding, and intent-to-treatment analysis. No unclear bias was reported due to incomplete outcome data or selective outcomes. For the non-RCTs, the MINORS scores were 18–20 for the retrospectively controlled trials. The methodological quality assessment is illustrated in Table 1.
Fig. 2.

The summary of bias risk of randomized controlled trials
Table 2.
Characteristics of included studies
| Study | Operation | Cases (A/C) | Mean age (A/C) | Gender (F) | Dosage | Prophylactic anticoagulant | Transfusion trigger |
|---|---|---|---|---|---|---|---|
| Camarasa et al. 2006 | TKA | 32/60 | 73/72 | 28/48 | 100 mg/kg administered intravenously in 30 min (before tourniquet release) + 3 g for 3 h following first dose | LMWH | Hb < 8 g/dl or 10 g/dl with clinical symptoms |
| Churchill et al. 2017 | TKA | 820/1492 | 63.9/63.9 | 527/956 | 5 g (BW < 50 kg); 10 g (BW > 50 kg) administered intravenously near the time of tourniquet release | Surgeon’s discretion | NS |
| Churchill et al. 2016 (THA) | THA | 911/643 | 65.1/65.4 | 392/377 | 5 g (BW < 50 kg); 10 g (BW > 50 kg) administered intravenously near the time of incision | Surgeon’s discretion | Surgeon’s discretion |
| Churchill et al. 2016 (TKA) | TKA | 25/25 | 65.2/66.6 | 21/15 | 10 g administered intravenously over 10 min and was completely infused before tourniquet deflation | Warfarin | Hb < 7 g/dl or 9 g/dl with clinical symptoms |
| Harley et al. 2002 | THA | 26/29 | 69/69 | 16/18 | 150 mg/kg administered intravenously over 20 min on the patient’s arrival in the operating room + 12.5 mg/kg/h for an additional 5 h | Heparin | Hb < 80 g/L or HCT < 0.24 or patients having anemia symptoms |
| Hobbs et al. 2017 | THA and TKA | 184/185 | 62.1/63.1 | 14/14 | 5 g administered intravenously over 20 min before incision + 5 g again during closure | Asprin, LMWH | Surgeon’s discretion |
| Ray et al. 2005 | THA | 15/15 | 72/69 | NS | 10 g administered intravenously over 30 min after the induction of anesthesia + 5 g over 3 h | Aspirin | NS |
THA total hip arthroplasty, TKA total knee arthroplasty, A aminocaproic acid, C control, F female, BW body weight, NS not state, M male, LMWH low molecular weight heparin, HCT hematocrit, Hb hemoglobin
Study characteristics
Demographic characteristics and details concerning the literature type of the included studies are summarized in Table 2. Statistically similar baseline characteristics were observed between both groups.
Table 1.
Quality assessment for non-randomized trials
| Quality assessment for non-randomized trials | Churchill et al. 2016 (THA) | Churchill et al. 2016 (TKA) | Churchill et al. 2017 | Hobbs et al. 2017 |
|---|---|---|---|---|
| A clearly stated aim | 2 | 2 | 2 | 2 |
| Inclusion of consecutive patients | 2 | 2 | 2 | 2 |
| Prospective data collection | 0 | 0 | 0 | 0 |
| Endpoints appropriate to the aim of the study | 2 | 2 | 2 | 2 |
| Unbiased assessment of the study endpoint | 2 | 2 | 2 | 2 |
| A follow-up period appropriate to the aims of study | 2 | 2 | 2 | 2 |
| Less than 5% loss to follow-up | 2 | 2 | 2 | 2 |
| Prospective calculation of the sample size | 0 | 0 | 0 | 0 |
| An adequate control group | 2 | 2 | 2 | 2 |
| Contemporary groups | 2 | 2 | 2 | 0 |
| Baseline equivalence of groups | 2 | 2 | 2 | 2 |
| Adequate statistical analyses | 2 | 2 | 2 | 2 |
| Total score | 20 | 20 | 20 | 18 |
Outcomes of meta-analysis
Total blood loss
Two of the included articles reported the outcomes for total blood loss [10, 12]. There was significant heterogeneity (χ2 = 4.00, df = 1, I2 = 75%, P = 0.05); as a result, a random model was applied. The pooled results demonstrated the total blood loss in the aminocaproic acid group was significantly lower than that in the control group (MD = − 495.80, 95% CI − 837.29 to − 154.32, P = 0.004; Fig. 3).
Fig. 3.
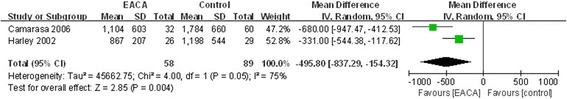
Forest plot of total blood loss
Drainage volume
Drainage volume was reported in four included studies [8, 10–12]. No significant heterogeneity was found, a fixed model was applied (χ2 = 3.34, df = 3, I2 = 10%, P = 0.34). The differences between the two groups was statistically significant (MD = − 249.43, 95% CI − 286.78 to − 212.08, P < 0.00001; Fig. 4).
Fig. 4.
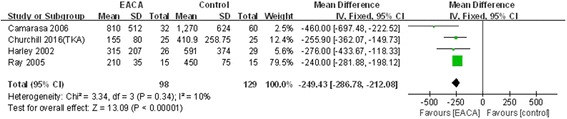
Forest plot of drainage volume
Postoperative hemoglobin level
Three included studies reported postoperative hemoglobin level [6, 8, 9]. There was no significant heterogeneity (χ2 = 0.17, df = 2, I2 = 0%, P = 0.92); as a result, a fixed model was applied. The pooled results demonstrated the postoperative hemoglobin level in the aminocaproic acid group was significantly higher than in the control group (MD = 0.90, 95% CI 0.78 to 1.02, P < 0.00001; Fig. 5).
Fig. 5.
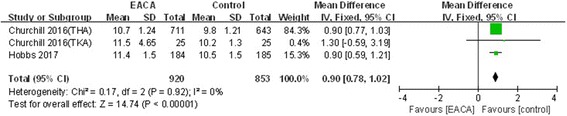
Forest plot of postoperative hemoglobin level
Hemoglobin reduction
Hemoglobin reduction was reported in two included studies [6, 10]. No significant heterogeneity was found, a fixed model was applied (χ2 = 1.38, df = 1, I2 = 27%, P = 0.24). The differences between the two groups was statistically significant (MD = − 0.75, 95% CI − 0.93 to − 0.57, P < 0.00001; Fig. 6).
Fig. 6.
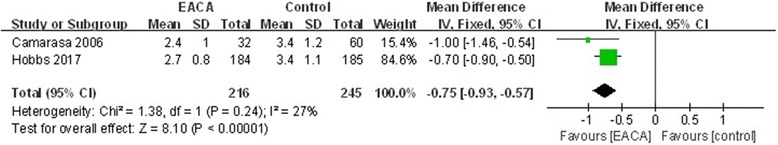
Forest plot of hemoglobin reduction
Blood transfusion rate
The blood transfusion rate was reported in seven included studies [6–12]. A random model was employed, which significant heterogeneity was found (χ2 = 52.02, df = 6, I2 = 88%, P < 0.00001). The difference between the two groups in regard to the blood transfusion rate was statistically significant (RD = − 0.17, 95% CI − 0.25 to − 0.09, P < 0.0001; Fig. 7).
Fig. 7.
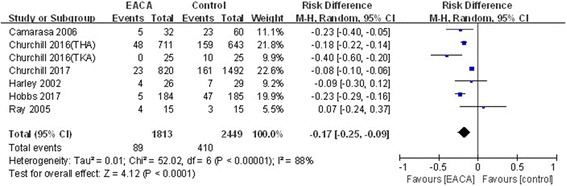
Forest plot of blood transfusion rate
A subgroup analysis was performed for the blood transfusion rate, showing that this positive effect persisted regardless of the delivered dosage, whether the patient had received TKA or THA, and whether a transfusion protocol existed (Table 3).
Table 3.
Subgroup analysis of blood transfusion rate
| Outcome of subgroup | Studies | Effect estimate | ||||
|---|---|---|---|---|---|---|
| χ 2 | I2 (%) | RD | 95% CI | P value | ||
| TKA | 3 | 13.34 | 88 | − 0.22 | [− 0.42, − 0.02] | 0.03 |
| THA | 3 | 3.20 | 38 | − 0.17 | [− 0.21, − 0.13] | 0.00001 |
| Transfusion trigger | 3 | 4.47 | 57 | − 0.23 | [− 0.35, − 0.12] | 0.0001 |
| Continuous application | 4 | 4.88 | 39 | − 0.20 | [− 0.26, − 0.14] | 0.00001 |
TKA total knee arthroplasty, THA total hip arthroplasty, CI confidence interval, MD mean difference
Transfusion units per patient
Three included studies reported the outcome of the transfusion units per patient [7, 9, 10]. The random model was employed according to a significant heterogeneity (χ2 = 26.32, df = 2, I2 = 92%, P < 0.00001). There were statistically significant differences between the two groups (MD = − 0.28, 95% CI − 0.48 to − 0.09, P = 0.004; Fig. 8).
Fig. 8.
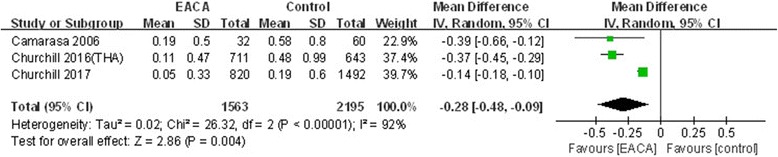
Forest plot of transfusion units per patient
Deep vein thrombosis (DVT)
The incidence of DVT had been reported in six studies [6, 7, 9–12]. The low significant heterogeneity was found, a fixed model was applied (χ2 = 1.17, df = 5, I2 = 0%, P = 0.95). No significant differences between the groups were found (RD = − 0.00, 95% CI − 0.01 to 0.00, P = 0.36; Fig. 9).
Fig. 9.
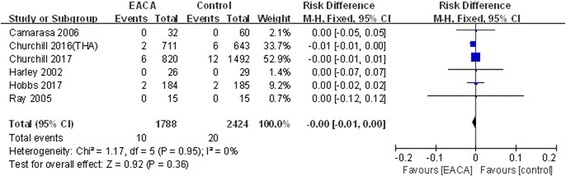
Forest plot of deep vein thrombosis
Length of hospital stay
Two studies reported the length of hospital stay [7, 9]. There was significant heterogeneity shown between the pooled results; therefore, a random model was applied (χ2 = 25.91, df = 1, I2 = 96%, P < 0.00001). There was a significant difference of length of hospital stay between the groups (MD = − 0.33, 95% CI − 0.43 to − 0.24, P < 0.00001; Fig. 10).
Fig. 10.
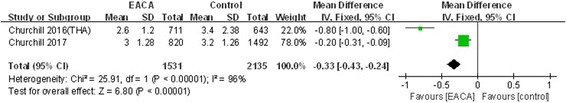
Forest plot of length of hospital stay
Discussion
The most important results of the present meta-analysis were that the application of aminocaproic acid during a THA and TKA decreased total blood loss, drainage volume, transfusion rate, transfusion units per patient, and length of hospital stay and does not increase the risk of DVT. Moreover, the length of hospital stay was shortened when aminocaproic acid was administered intravenously.
Aminocaproic acid, an antifibrinolytic drug, competitively block the lysine-binding site of plasminogen and has been used to reduce blood loss in surgery for many years [15]. The effectiveness of aminocaproic acid for decreasing perioperative blood loss during THA and TKA is widely reported. Present meta-analysis indicated that the intravenous application of aminocaproic acid could significantly decrease total blood loss and drainage volume. These results are similar to those of RCTs [10–12].
The indications for blood transfusion were based on hemoglobin levels and clinical symptoms of anemia [16]. Several studies have demonstrated that aminocaproic acid could reduce postoperative Hb reduction [6, 10]. Our meta-analysis was consistent with these results. Pooled result also showed that postoperative hemoglobin level in the aminocaproic acid group was significantly higher than that in the control group. Although transfusion trigger varied from included studies, present meta-analysis indicates that the application of aminocaproic acid significantly decrease the blood transfusion rate and the average transfusion units. An RCT reported by Ray et al. [11] showed that aminocaproic acid does not reduce transfusion requirements. Their studies did not report the transfusion trigger.
Theoretically, antifibrinolytic agents may increase the risk of thrombotic events [17]. DVT is a common complication in orthopedic surgery, especially in arthroplasty, and may progress to pulmonary embolism and even death [18, 19]. All included studies reported the use of an anticoagulant therapy after surgery. The meta-analysis showed that the use of aminocaproic acid did not increase the risk of DVT, which was 0.55% with the aminocaproic acid and 0.83% in the controls.
Several potential limitations should be noted. (1) Only seven studies were included, all of which had a relatively small sample size; (2) methodological weaknesses exist in all included studies, and some outcome parameters were not fully described so that we failed to perform a meta-analysis; and (3) subgroup analysis was not performed because of the limited number of included studies, and we could not determine the source of heterogeneity.
Conclusions
The present meta-analysis indicated that the application of aminocaproic acid in THA and TKA decreases the total blood loss, drainage volume, transfusion rate, transfusion units per patient, and length of hospital stay and does not increase the risk of DVT or other complications.
Acknowledgements
We thank the authors of the included studies.
Availability of data and materials
As this paper is a meta-analysis, there are no patient data sets. The search strategy for the study selection supports the conclusion of the meta-analysis.
Abbreviations
- CI
Confidence interval
- DVT
Deep vein thrombosis
- MD
Mean difference
- MINORS
Methodological Index for Non-randomized Studies
- RCTs
Randomized controlled trials
- RD
Risk difference
- THA
Total hip arthroplasty
- TKA
Total knee arthroplasty
Authors’ contributions
YJL and BSX conceived the study. YJL and SPB searched the literature and collected the data. YJL, XJG, BSX, and XYY performed the statistical analysis. YJL and BSX drafted the manuscript. XJG reviewed the manuscript. All authors have read and approved the final paper.
Authors’ information
The author information can be found in the title page.
Ethics approval and consent to participate
Not applicable, this meta-analysis does not involve research on humans.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yong-jiang Li, Phone: +86-728-5237400, Email: 18696368115@163.com.
Bi-sheng Xu, Email: tianmenxbs@163.com.
Sun-peng Bai, Email: tianmenbsp@163.com.
Xiao-jun Guo, Email: tianmengxj@163.com.
Xiang-yuan Yan, Email: tianmenyxy@163.com.
References
- 1.Finney A, Healey E, Jordan JL, Ryan S, Dziedzic KS. Multidisciplinary approaches to managing osteoarthritis in multiple joint sites: a systematic review. BMC Musculoskelet Disord. 2016;17:266. doi: 10.1186/s12891-016-1125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harato K, Kobayashi S, Kojima I, Sakurai A, Tanikawa H, Niki Y. Factors affecting one-leg standing time in patients with end-stage knee osteoarthritis and the age-related recovery process following total knee arthroplasty. J Orthop Surg Res. 2017;12(1):21. doi: 10.1186/s13018-017-0522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sizer SC, Cherian JJ, Elmallah RD, Pierce TP, Beaver WB, Mont MA. Predicting blood loss in total knee and hip arthroplasty. Orthop Clin North Am. 2015;46(4):445–459. doi: 10.1016/j.ocl.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen AB, Mehnert F, Overgaard S, Johnsen SP. Allogeneic blood transfusion and prognosis following total hip replacement: a population-based follow up study. BMC Musculoskelet Disord. 2009;10:167. doi: 10.1186/1471-2474-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerman DM, Rapp TB. Minimizing blood loss in orthopaedic surgery the role of antifibrinolytics. Bull Hosp Jt Dis (2013) 2015;73(2):83–89. [PubMed] [Google Scholar]
- 6.Hobbs JC, Welsby IJ, Green CL, Dhakal IB, Wellman SS. Epsilon aminocaproic acid to reduce blood loss and transfusion after total hip and Total knee arthroplasty. J Arthroplast. 2018;33(1):55-60. [DOI] [PubMed]
- 7.Churchill JL, Puca KE, Meyer E, Carleton M, Anderson MJ. Comparing epsilon-aminocaproic acid and tranexamic acid in reducing postoperative transfusions in total knee arthroplasty. J Knee Surg. 2017;30(5):460–466. doi: 10.1055/s-0036-1593362. [DOI] [PubMed] [Google Scholar]
- 8.Churchill JL, Toney VA, Truchan S, Anderson MJ. Using aminocaproic acid to reduce blood loss after primary unilateral total knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016;45(5):E245–E248. [PubMed] [Google Scholar]
- 9.Churchill JL, Puca KE, Meyer ES, Carleton MC, Truchan SL, Anderson MJ. Comparison of epsilon-aminocaproic acid and tranexamic acid in reducing postoperative transfusions in total hip arthroplasty. J Arthroplast. 2016;31(12):2795–2799. doi: 10.1016/j.arth.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Camarasa MA, Olle G, Serra-Prat M, Martin A, Sanchez M, Ricos P, Perez A, Opisso L. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576–582. doi: 10.1093/bja/ael057. [DOI] [PubMed] [Google Scholar]
- 11.Ray M, Hatcher S, Whitehouse SL, Crawford S, Crawford R. Aprotinin and epsilon aminocaproic acid are effective in reducing blood loss after primary total hip arthroplasty—a prospective randomized double-blind placebo-controlled study. J Thromb Haemost. 2005;3(7):1421–1427. doi: 10.1111/j.1538-7836.2005.01457.x. [DOI] [PubMed] [Google Scholar]
- 12.Harley BJ, Beaupre LA, Jones CA, Cinats JG, Guenther CR. The effect of epsilon aminocaproic acid on blood loss in patients who undergo primary total hip replacement: a pilot study. Can J Surg. 2002;45(3):185–190. [PMC free article] [PubMed] [Google Scholar]
- 13.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 14.Handoll HH, Gillespie WJ, Gillespie LD, Madhok R. The Cochrane Collaboration: a leading role in producing reliable evidence to inform healthcare decisions in musculoskeletal trauma and disorders. Indian J Orthop. 2008;42(3):247–251. doi: 10.4103/0019-5413.41849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy JH, Tanaka KA. Management of surgical hemostasis: systemic agents. Vascular. 2008;16(Suppl 1):S14–S21. [PubMed] [Google Scholar]
- 16.Ponnusamy KE, Kim TJ, Khanuja HS. Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg Am. 2014;96(21):1836–1844. doi: 10.2106/JBJS.N.00128. [DOI] [PubMed] [Google Scholar]
- 17.Foreman PM, Chua M, Harrigan MR, Fisher WS, 3rd, Tubbs RS, Shoja MM, Griessenauer CJ. Antifibrinolytic therapy in aneurysmal subarachnoid hemorrhage increases the risk for deep venous thrombosis: a case-control study. Clin Neurol Neurosurg. 2015;139:66–69. doi: 10.1016/j.clineuro.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Mitani G, Takagaki T, Hamahashi K, Serigano K, Nakamura Y, Sato M, Mochida J. Associations between venous thromboembolism onset, D-dimer, and soluble fibrin monomer complex after total knee arthroplasty. J Orthop Surg Res. 2015;10:172. doi: 10.1186/s13018-015-0315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Chen Z, Zheng J, Breusch SJ, Tian J. Risk factors for venous thromboembolism after total hip and total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2015;135(6):759–772. doi: 10.1007/s00402-015-2208-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As this paper is a meta-analysis, there are no patient data sets. The search strategy for the study selection supports the conclusion of the meta-analysis.


