Abstract
>The goal of the present study was to investigate anticancer effect of amentoflavone on glioblastoma cells in vitro. Our results demonstrated that amentoflavone not only significantly reduced cell viability, nuclear factor-ĸappa B (NF-ĸB) activation, and protein expression of cellular Fas-associated protein with death domain-like interleukin 1 beta-converting enzyme inhibitory protein (C-FLIP) and myeloid cell leukemia 1 (MCL1), but significantly triggered cell accumulation at the sub-G 1 phase, loss of mitochondrial membrane potential, and expression of active caspase-3 and -8. In order to verify the effect of NF-ĸB inhibitor on expression of anti-apoptotic proteins, we performed western blotting. We found that the of NF-ĸB inhibitor or amentoflavone markedly diminished protein levels of MCL1 and C-FLIP. Taken all together, our findings show that amentoflavone induces intrinsic and extrinsic apoptosis and inhibits NF-ĸB-modulated anti-apoptotic signaling in U-87 MG cells in vitro.
Keywords: Glioblastoma, amentoflavone, and NF-ĸB, apoptosis
Glioblastoma is the most common primary malignancy of the adult central nervous system. The 5-year survival rate for patients with glioblastoma is only approximately 4.5% after diagnosis. Patients with glioblastoma have unsatisfactory response to current standard treatments and median survival less than 15 months (1,2). Therefore, development of adjuvant anticancer therapy may offer benefits for patients with glioblastoma.
Apoptosis, i.e. programmed cell death, can be initiated by anticancer agents through extrinsic and intrinsic pathways (3). Nuclear factor-ĸappa B (NF-ĸB), a transcription factor, modulates the hallmarks of cancer by inducing expression of its target genes. Expression of anti-apoptotic proteins such as cellular Fas-associated protein with death domain-like interleukin 1 beta-converting enzyme inhibitory protein(C-FLIP) and myeloid cell leukemia-1 (MCL1) are linked to NF-ĸB activation and associated with resistance to apoptosis induced by anticancer agents in glioblastoma cells (4-6).
Amentoflavone, a flavonoid isolated from Selaginella tamariscina, is able to cross the blood–brain barrier and afford neuroprotection against neonatal hypoxic-ischemic brain injury (7). In our previous studies, we demonstrated amentoflavone-induced apoptosis and reduced NF-ĸB activation in MCF-7 breast cancer cells (8,9). However, the anticancer effect of amentoflavone on glioblastoma cells is ambiguous. The aim of the present study was to investigate the effect of amentoflavone on tumor cell growth and NF-ĸB-modulated anti-apoptotic mechanism by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, flow cytometry, western blotting, and NF-ĸB reporter gene assay in glioblastoma cells in vitro. We also used NF-ĸB inhibitor (QNZ) to verify the effect of NF-ĸB inactivation on expression of anti-apoptotic-related proteins.
Materials and Methods
Drugs. Amentoflavone was purchased from Sigma-Aldrich (St. Louis, MO, USA). Amentoflavone was dissolved using dimethyl sulfoxide (0.1% DMSO) as 10 mM stock solution. NF ĸB inhibitor (QNZ) was purchased from Apexbio Technology LLC (Houston, TX, USA) and prepared as 1 mM stock solution in 0.1% DMSO.
Cell culture. Human U-87 MG glioblastoma cells were kindly provided by Professor Ruei-Ming Chen, Graduate Institute of Medical Sciences, College of Medicine, Taipei Medical University and used for this study. Cells were cultured in Minimum Essential Medium Eagle’s medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin (10,000 U/ml) and 1% sodium pyruvate (100 mM) at 37˚C in a humidified incubator with 5% CO2 (10).
MTT assay. MTT was obtained from Sigma-Aldrich. U-87 MG cells were cultured in 96-well plates at 2×104 cells/well overnight. Cells were treated with different concentration of amentoflavone (0-200 μM) for 48 h, and then cell viability was analyzed with MTT assay (11). The absorbance in each well, including the blanks, was measured at 570 nm by Multiskan™ GO Microplate Spectrophotometer (Thermo Fisher Scientific, Rockford, IL, USA).
Detection of the sub-G1 population. This is a method used to detect cells in the late stage of apoptosis. Propidium iodide (PI) and RNase were purchased from Biovision (Mountain view, CA, USA) and Thermo Fisher Scientific, respectively. U-87 MG cells (5×105) were seeded in 6-well plates and treated with 50 or 100 μM amentoflavone for 48 h before harvesting. Cells were then fixed with 70% ethanol overnight and stained with PI/RNase solution (40 μg/ml PI, 100 μg/ml RNase and 1% Triton X-100 in phosphate-buffered saline (PBS) for 30 min. Cell samples in PI/RNase solution were analyzed by flow cytometry and at least 10,000 single cells for each group were saved (12). The percentage of subG1 phase cells was measured by FlowJo 7.6.1 software (FlowJo LLC, Ashland, OR, USA).
Detection of active caspase-3 and -8. U-87 MG cells were cultured in 6-well plates at 5×105 cells/well overnight. Cells were treated with 0, 50 and 100 μM of amentoflavone for 48 h. Afterwards, cells were harvested by centrifugation and washed twice with PBS, and then stained with fluorescein isothiocyanate-Asp(OCH3)-Glu(OCH3)-Val-Asp(OCH3)-fluoromethyl ketone (FITC-DEVD-FMK) working solution (1 μl FITC-DEVD-FMK in 300 μl PBS) or with sulforhodamine-Ile-Glu-Thr-Asp-fluoromethyl ketone (Red-IETD-FMK) working solution (1 μl Red-IETD-FMK in 300 μl PBS) according to the manufacturer’s instructions (CaspGLOW™ Fluorescein Active Caspase-3 and Caspase-8 assay kits, respectively; BioVision Inc., Milpitas, CA, USA). Expression of active caspase-3 was evaluated with flow cytometry (FACSCalibur™; Becton-Dickinson, Franklin Lakes, NJ, USA) as described by Chiang et al. (3).
Detection of mitochondrial membrane potential (Ψm). 3,3’-Dihexyloxacarbocyanine iodide [DiOC6(3)] was bought from Enzo Life Sciences (Farmingdale, NY, USA). U-87 MG cells were cultured in 6-well plates at 5×105 cells/well overnight. Cells were treated with 0, 50 and 100 μM of amentoflavone for 48 h. Afterwards, cells were harvested by centrifugation and washed twice with PBS, and then stained with DiOC6(3) working solution (4 μM DiOC6(3) in 500 μl PBS) for 30 min in the dark. Detection of ∆Ψm was performed by using flow cytometry (FACSCalibur™) as described by Wang et al. (13).
Plasmid transfection. NF-ĸB-luciferrase2 vector (pNF-ĸB/luc2) was purchased from Promega (Madison, WI, USA). One million U-87 MG cells were seeded into 10 cm dishes and incubated overnight. Cells were then transfected with pNF-ĸB/luc2 by using jetPEI™ kit (Polyplus transfection, New York, NY, USA) as previously described (12).
NF-ĸB luciferase reporter gene assay. U-87 MG cells transfected with pNF-ĸB/luc2 were cultured in 96-well plates at 2×104 cells/well overnight and then treated with amentoflavone 0-200 μM or 3 μM QNZ followed by incubation for 48 h. Afterwards, 100 μl of 15 mg/ml D-luciferin was added to each well and plates were incubated for a further 5 min. The signal intensity of each well was acquired by IVIS200 Imaging System (Xenogen, Alameda, CA, USA) for 1 min and quantified into photons per second using Living Image software (Xenogen). D-luciferin was obtained from Gold Biotechnology (St. Louis, MO, USA). Relative NF-ĸB activity was corrected for cell viability which was analyzed using MTT assay as described previously (13).
Western blotting assay. U-87 MG cells (3×106) were seeded into 10 cm diameter dishes and grown overnight. Cells were treated with 0, 50 and 100 μM of amentoflavone or 0.3 μM QNZ for 48 h. Total protein cell lysis buffer (120 mM NaCl, 0.5% NP-40, 50 mM Tris-HCl pH 8.0, and 1 mM phenylmethanesulfonyl fluoride) was used to extract total protein from cells. Protein expression of MCL1 and C-FLIP was determined with western blotting assay as previously described (12). C FLIP (catalog no. O15519; anti rabbit) was bought from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibody to MCL1 (anti rabbit) was obtained from BioVision Inc. (Milpitas, CA, USA) al that to β-actin (anti rabbit) was purchased from Thermo Fisher Scientific. Protein bands were quantified with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis. All statistical analyses were performed using SigmaPlot 10.0 (Systat Software Inc., San Jose, CA, USA). Parametric data are shown as the mean±standard deviation. To compare values between two groups, Student’s t-test was performed. Differences with a p-value less than 0.05 was considered statistically significant.
Results
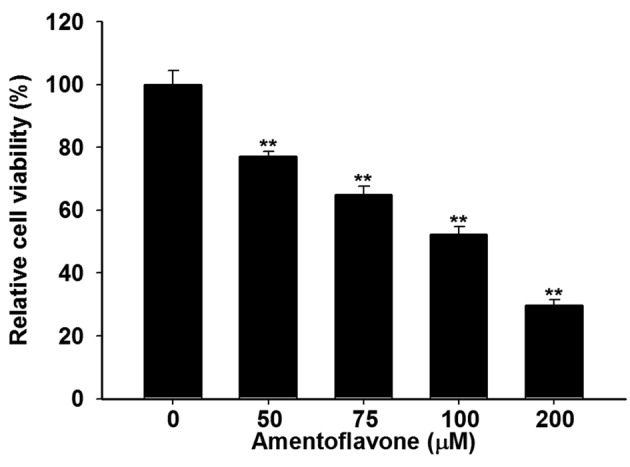
Amentoflavone induced cytotoxicity towards U-87 MG glioblastoma cells. We used MTT assay to evaluate effect of amentoflavone on cell viability in U-87 MG cells. Figure 1 indicates that amentoflavone treatment (50-200 μM) significantly inhibited the viability of U-87 MG cells by 23-71% at 48 h as compared to controls. The half-maximal inhibitory concentration (IC50) of amentoflavone was 100 μM under the 48 h treatment schedule.
Figure 1. U-87 MG cells (2×104) were seeded in 96-well plates and treated with 0, 50, 100, 150, 200 μM amentoflavone in 0.1% dimethylsulfoxide for 48 h. The viability was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay. **Significantly different at p<0.01 vs.0 μM.
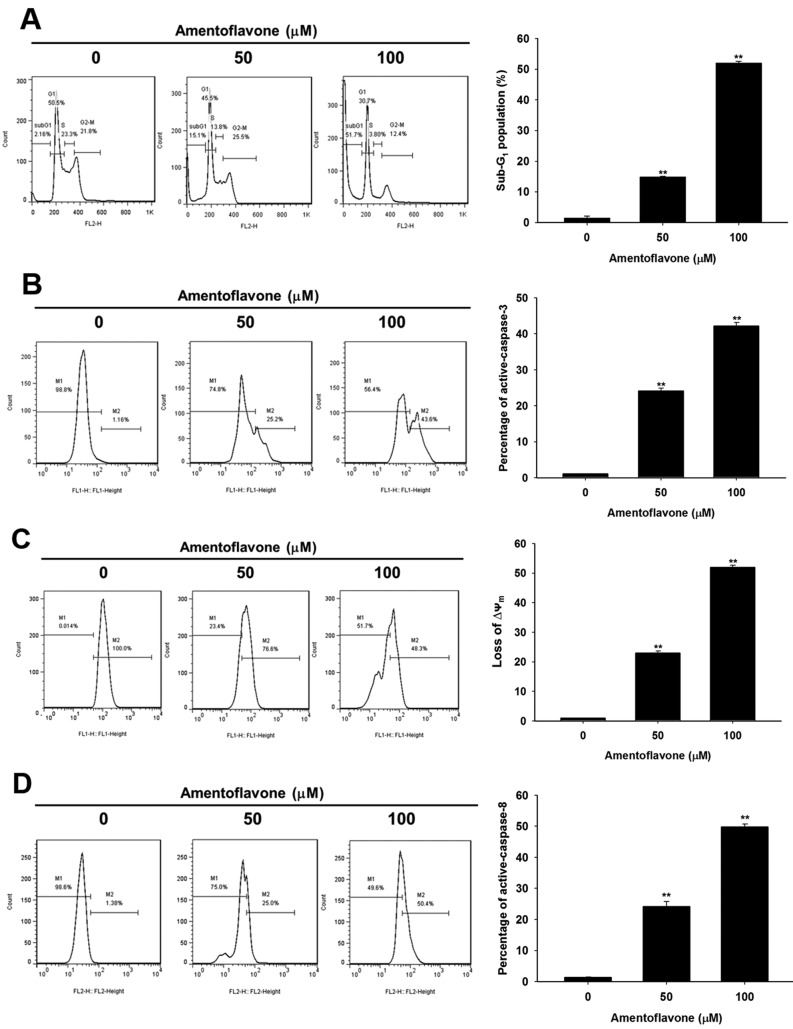
Amentoflavone-induced apoptosis is dependent on intrinsic and extrinsic apoptotic signaling pathways in U-87 MG glioblastoma cells. Amentoflavone-induced apoptotic signaling was analyzed using flow cytometric apoptosis assays. Figure 2A and B show that amentoflavone significantly induced the accumulation of cells in the sub-G1 population and increased the level of active caspase-3 by 14-52% and 24-42%, respectively, at 48 h as compared to controls. We also found amentoflavone significantly triggered the loss of Ψm and the expression of active caspase-8 by 23-53% and 25-50%, respectively, at 48 h as compared to controls (Figure 2C and D).
Figure 2. U-87 MG cells were treated with 0, 50, 100 μM amentoflavone for 48 h and prepared for cell cycle and caspase-3 flow cytometry (A, B),or analyzed for mitochondrial membrane potential and caspase-8 (C, D). **Significantly different at p<0.01 vs. 0 μM.
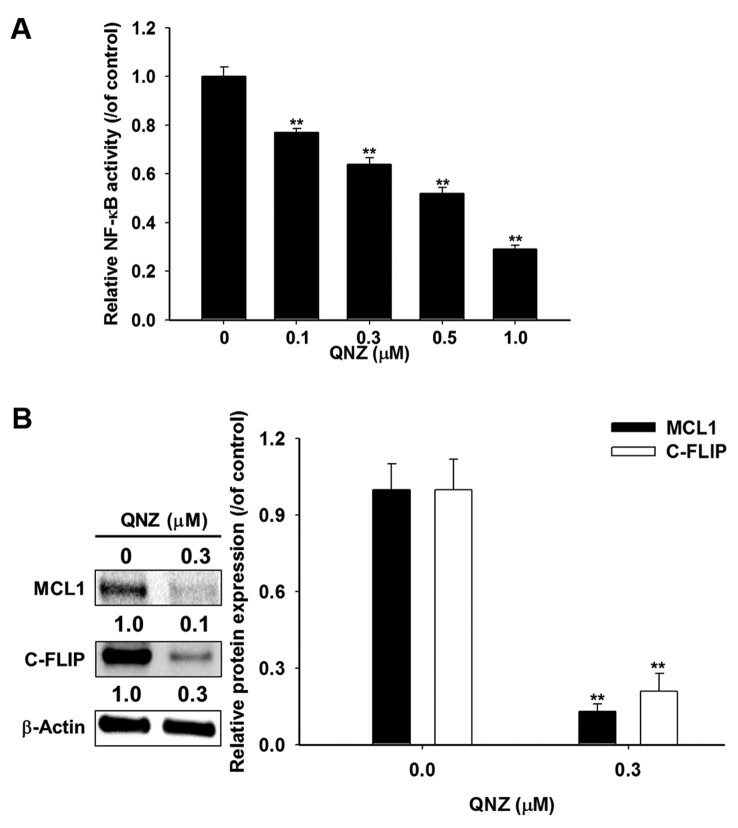
Blockage of NF-ĸB activation reduces expression of anti-apoptotic proteins MCL1 and C-FLIP in U-87 MG glioblastoma cells. We used QNZ, a specific NF-ĸB inhibitor, to verify the effect of NF-ĸB inactivation on expression of anti-apoptotic proteins MCL1 and C-FLIP in U-87 MG cells. NF-ĸB reporter gene assay was performed to verify the effect of QNZ on NF-ĸB activation. Relative NF-ĸB activity was corrected with cell viability. Figure 3A indicates that amentoflavone significantly suppressed NF-ĸB activation in a dose-dependent manner by 23-71% at 48 h as compared to the control. We also found 0.3 μM QNZ significantly reduced protein levels of MCL1 and C-FLIP by 87% and 79%, respectively, at 48 h as compared to controls (Figure 3B).
Figure 3. U-87 MG cells were treated with 0-1 μM of the nuclear factor-ĸappa B (NF-ĸB) inhibitor QNZ for 48 h and NF-ĸB activation was evaluated by bioluminescence imaging. B: U-87 MG cells were treated with 0 and 0.3 μM QNZ for 48 h and harvested for western blotting assay using Image J system to quantify the protein level of myeloid cell leukemia 1 (MCL1) and cellular Fas-associated protein with death domain-like interleukin 1 beta-converting enzyme inhibitory protein (C-FLIP). **Significantly different at p<0.01 vs. 0 μM.
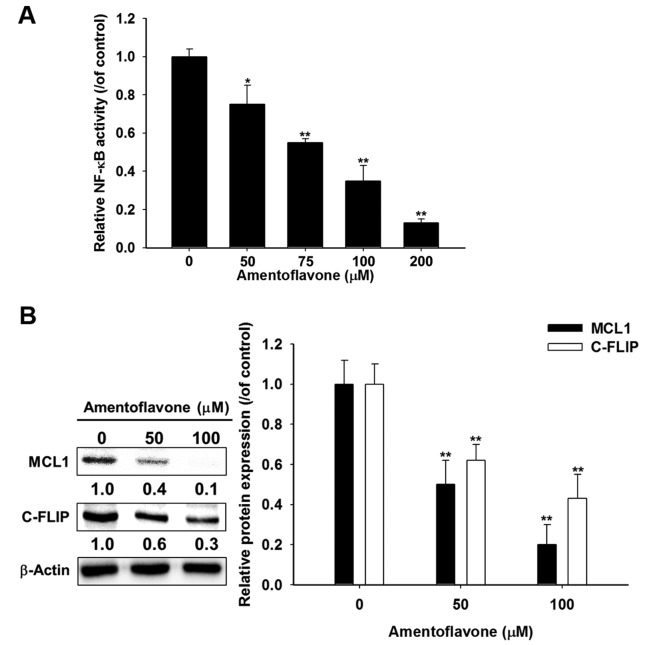
Amentoflavone diminishes protein expression of MCL1 and C-FLIP through suppression of NF-ĸB activation in U-87 MG glioblastoma cells. Figure 4A shows amentoflavone significantly reduced NF-ĸB activation in a dose-dependent manner by 25-87% at 48 h as compared to the control. Amentoflavone significantly reduced protein expression of MCL1 and C-FLIP by 50-80% and 38-57%, respectively, at 48 h as compared to controls (Figure 4B).
Figure 4. U-87 MG cells (2×104) were seeded in 96-well plates and treated with 0-200 μM amentoflavone in 0.1% dimethylsulfoxide (DMSO) for 48 h. Nuclear factor-ĸappa B (NF-ĸB) activation was evaluated by reporter gene assay. Relative NF-ĸB activity of each group was normalized with viability and calculated through dividing by the value for the control group treated with 0.1% DMSO. B: Cells were treated with 0, 50, 100 μM amentoflavone for 48h and harvested for western blotting assay. The quantification of protein expression of myeloid cell leukemia 1 (MCL1) and cellular Fas-associated protein with death domain-like interleukin 1 beta-converting enzyme inhibitory protein (C-FLIP) was measured by Image J. **Significantly different at p<0.01 vs. 0 μM.
Discussion
Amentoflavone has been reported to trigger apoptosis and reduce NF-ĸB activation in breast cancer and melanoma cells (8,9,14). The effects of amentoflavone on glioblastoma cells has not been elucidated. Therefore, we evaluated the effect of amentoflavone on tumor cell growth and NF-ĸB-modulated anti-apoptotic signaling in U-87 MG cells in vitro.
Many cytotoxic agents inhibit growth of glioblastoma cells by inducing apoptosis (15). Apoptotic signaling pathways are divided into intrinsic (mitochondria) and extrinsic (death receptors) pathways (16). Temozolomide (TMZ), an oral O6-methylating agent used for treatment of glioblastoma, has been shown to trigger intrinsic and extrinsic apoptosis by inducing DNA lesion in O6-methylguanine. Active caspase-8 is key mediator of the extrinsic apoptotic signaling pathway (17). DNA fragmentation initiated by active caspase-3 can be triggered via intrinsic and extrinsic apoptotic signaling pathways. High expression of cleaved caspase-8 and active caspase-3 are positive prognostic indicators for patients with glioma (1,18). Figure 2B and D indicate amentoflavone significantly induced expression of active caspase-3 and -8, while Figure 2C shows amentoflavone significantly induced loss of Ψm, all associated with apoptosis.
Constitutive activation of NF-ĸB was observed in glioma and correlated with glioma grade (19). High expression of NF-ĸB contributes tumor progression and modulates acquired resistance to treatment in glioblastoma (20). TMZ not only induces apoptosis but also triggers NF-ĸB activity in glioblastoma cells. Therapeutic efficacy of TMZ is limited because of overexpression of NF-ĸB-modulated anti-apoptotic proteins; inhibition of NF-ĸB activation sensitizes glioblastoma cells to TMZ (21,22). Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a potential anticancer agent because it induces apoptosis leading to tumor growth inhibition in cancer (23). MCL1 and C-FLIP are disruptors of intrinsic and extrinsic apoptotic pathways, respectively. Protein expression of MCL1 and C-FLIP are modulated by NF-ĸB activation in cancer (12). Han et al. showed that silencing NF-ĸB reduced C-FLIP expression and enhanced TRAIL-induced apoptosis in glioblastoma cells (24). Murphy et al. also demonstrated that silencing MCL1 increased sensitivity to TRAIL in glioblastoma cells (6). We confirmed such effects by finding that NF-ĸB inhibitor reduced NF-ĸB activation and protein expression of MCL1 and C-FLIP in U-87 MG cells (Figure 3). We also found amentoflavone, as an inhibitor of NF-ĸB signaling, reduced expression of NF-ĸB-modulated anti-apoptotic proteins MCL1 and C-FLIP (Figure 4A and B).
Conclusion
In conclusion, the present study demonstrated that amentoflavone not only induced intrinsic and extrinsic apoptosis, but also reduced NF-ĸB-modulated anti-apoptosis signaling in glioblastoma cells in vitro. We suggest amentoflavone as a potential adjuvant which may provide benefit for treatment of patients with glioblastoma.
Financial - Competing Interests Disclosure
The Authors declare no competing financial interests.
Acknowledgements
This study was supported by Taipei Medical University/Taipei Medical University Hospital (Grant no. 105TMU-TMUH-23, TMU105-AE1-B49 and 106TMU-TMUH-27). This study was also supported by Grants RD2017-023, CTU105-CCGH-001, and CTU105-P-16 from National Yang-Ming University Hospital, Yilan, Taiwan, Cheng Ching General Hospital, Taichung, Taiwan and Central-Taiwan University of Science and Technology, Taichung, Taiwan.
References
- 1.Cahill KE, Morshed RA, Yamini B. Nuclear factor-kappaB in glioblastoma: insights into regulators and targeted therapy. Neuro Oncol. 2016;18:329–339. doi: 10.1093/neuonc/nov265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen JH, Chen WL, Liu YC. Amentoflavone Induces Anti-angiogenic and Anti-metastatic Effects Through Suppression of NF-kappaB Activation in MCF-7 cells. Anticancer Res. 2015;35:6685–6693. [PubMed] [Google Scholar]
- 3.Chiang IT, Chen WT, Tseng CW, Chen YC, Kuo YC, Chen BJ, Weng MC, Lin HJ, Wang WS. Hyperforin inhibits cell growth by inducing intrinsic and extrinsic apoptotic pathways in hepatocellular carcinoma cells. Anticancer Res. 2017;37:161–167. doi: 10.21873/anticanres.11301. [DOI] [PubMed] [Google Scholar]
- 4.Chiang IT, Liu YC, Wang WH, Hsu FT, Chen HW, Lin WJ, Chang WY, Hwang JJ. Sorafenib inhibits TPA-induced MMP-9 and VEGF expression via suppression of ERK/NF-kappaB pathway in hepatocellular carcinoma cells. In Vivo. 2012;26:671–681. [PubMed] [Google Scholar]
- 5.Guruvayoorappan C, Kuttan G. Effect of amentoflavone on the inhibition of pulmonary metastasis induced by B16F-10 melanoma cells in C57BL/6 mice. Integr Cancer Ther. 2007;6:185–197. doi: 10.1177/1534735407302345. [DOI] [PubMed] [Google Scholar]
- 6.Hamsa TP, Kuttan G. Harmine activates intrinsic and extrinsic pathways of apoptosis in B16F-10 melanoma. Chin Med. 2011;6:11. doi: 10.1186/1749-8546-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han H, Xu B, Hou P, Jiang C, Liu L, Tang M, Yang X, Zhang Y, Liu Y. Icaritin sensitizes human glioblastoma cells to TRAIL-induced apoptosis. Cell Biochem Biophys. 2015;72:533–542. doi: 10.1007/s12013-014-0499-y. [DOI] [PubMed] [Google Scholar]
- 8.Jane EP, Premkumar DR, Pollack IF. Bortezomib sensitizes malignant human glioma cells to TRAIL, mediated by inhibition of the NF-{kappa}B signaling pathway. Mol Cancer Ther. 2011;10:198–208. doi: 10.1158/1535-7163.MCT-10-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi T, Masumoto J, Tada T, Nomiyama T, Hongo K, Nakayama J. Prognostic significance of the immunohisto-chemical staining of cleaved caspase-3, an activated form of caspase-3, in gliomas. Clin Cancer Res. 2007;13:3868–3874. doi: 10.1158/1078-0432.CCR-06-2730. [DOI] [PubMed] [Google Scholar]
- 10.Lin CJ, Lin YL, Luh F, Yen Y, Chen RM. Preclinical effects of CRLX101, an investigational camptothecin-containing nanoparticle drug conjugate, on treating glioblastoma multiforme via apoptosis and antiangiogenesis. Oncotarget. 2016;7:42408–42421. doi: 10.18632/oncotarget.9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu YC, Chiang IT, Hsu FT, Hwang JJ. Using NF-kappaB as a molecular target for theranostics in radiation oncology research. Expert Rev Mol Diagn. 2012;12:139–146. doi: 10.1586/erm.12.2. [DOI] [PubMed] [Google Scholar]
- 12.Minniti G, Muni R, Lanzetta G, Marchetti P, Enrici RM. Chemotherapy for glioblastoma: current treatment and future perspectives for cytotoxic and targeted agents. Anticancer Res. 2009;29:5171–5184. [PubMed] [Google Scholar]
- 13.Murphy AC, Weyhenmeyer B, Noonan J, Kilbride SM, Schimansky S, Loh KP, Kogel D, Letai AG, Prehn JH, Murphy BM. Modulation of Mcl-1 sensitizes glioblastoma to TRAIL-induced apoptosis. Apoptosis. 2014;19:629–642. doi: 10.1007/s10495-013-0935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei JS, Liu CC, Hsu YN, Lin LL, Wang SC, Chung JG, Bau DT, Lin SS. Amentoflavone induces cell-cycle arrest and apoptosis in MCF-7 human breast cancer cells via mitochondria-dependent pathway. In Vivo. 2012;26:963–970. [PubMed] [Google Scholar]
- 15.Puliyappadamba VT, Hatanpaa KJ, Chakraborty S, Habib AA. The role of NF-ĸB in the pathogenesis of glioma. Mol Cell Oncol. 2014;1:e963478. doi: 10.4161/23723548.2014.963478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, Kaina B. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 17.Safa AR. c-FLIP, a master anti-apoptotic regulator. Exp Oncol. 2012;34:176–184. [PMC free article] [PubMed] [Google Scholar]
- 18.Saggioro FP, Neder L, Stavale JN, Paixao-Becker AN, Malheiros SM, Soares FA, Pittella JE, Matias CC, Colli BO, Carlotti CG Jr., Franco M. Fas, FasL and cleaved caspases 8 and 3 in glioblastomas: a tissue microarray-based study. Pathol Res Pract. 2014;210:267–273. doi: 10.1016/j.prp.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Shin DH, Bae YC, Kim-Han JS, Lee JH, Choi IY, Son KH, Kang SS, Kim WK, Han BH. Polyphenol amentoflavone affords neuroprotection against neonatal hypoxic-ischemic brain damage via multiple mechanisms. J Neurochem. 2006;96:561–572. doi: 10.1111/j.1471-4159.2005.03582.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsai JJ, Pan PJ, Hsu FT. Regorafenib induces extrinsic and intrinsic apoptosis through inhibition of ERK/NF-kappaB activation in hepatocellular carcinoma cells. Oncology Rep. 2017;37:1036–1044. doi: 10.3892/or.2016.5328. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Wang H, Zhang W, Huang HJ, Liao WS, Fuller GN. Analysis of the activation status of AKT, NFkappaB and STAT3 in human diffuse gliomas. Lab Invest. 2004;84:941–951. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- 22.Wang WH, Chiang IT, Ding K, Chung JG, Lin WJ, Lin SS, Hwang JJ. Curcumin-induced apoptosis in human hepatocellular carcinoma J5 cells: critical role of ca(+2)-dependent pathway. Evid Based Complement Alternat Med. 2012;2012:512907. doi: 10.1155/2012/512907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Jia L, Jin X, Liu Q, Cao W, Gao X, Yang M, Sun B. NF-kappaB inhibitor reverses temozolomide resistance in human glioma TR/U251 cells. Oncol Lett. 2015;9:2586–2590. doi: 10.3892/ol.2015.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Wang Y, Guo H, Guo M. Synergistic anticancer effects of icariin and temozolomide in glioblastoma. Cell Biochem Biophys. 2015;71:1379–1385. doi: 10.1007/s12013-014-0360-3. [DOI] [PubMed] [Google Scholar]