Abstract
Background/aim: Geriatric oncology practice should be based on dedicated studies and real-world experience. Therefore, we evaluated survival outcomes after palliative thoracic radiotherapy in octogenarian patients with lung cancer and analyzed prognostic factors. Patients and Methods: We carried out a retrospective analysis of 51 patients with a median age of 83 years. Three different fractionation regimens were compared: two fractions of 8.5 Gy, 10 fractions of 3 Gy, and higher doses than 30 Gy (maximum biologically equivalent dose in 2-Gy fractions (EQD2) was always lower than 50 Gy). No concomitant chemotherapy was prescribed. Patients with incomplete radiotherapy (16%) were included, in line with the intention-to-treat principle, i.e. based on prescribed rather than accumulated dose. Results: Median survival was 3.4 months. We observed a relatively high proportion of patients who received radiotherapy in the last 30 days of life (24%). Nevertheless, approximately 10% of patients were alive 3-5 years after treatment. Prognosis was similar for those with stage III and IV disease. Multivariate analysis identified four significant prognostic factors for shorter survival: reduced performance status, serum C-reactive protein (CRP) ≥30 mg/l, leukocytosis, and prescribed radiation dose ≤30 Gy (EQD2=33 Gy). The three different radiotherapy regimens resulted in median survival of 2.4, 2.6 and 11.8 months, respectively. Conclusion: Survival outcomes were highly variable. Given that survival after 10 fractions of 3 Gy was indistinguishable from that after two fractions of 8.5 Gy, we suggest that the latter regimen should be considered for patients with poor prognosis. Patients with favorable prognostic factors should be treated with higher radiation doses, e.g. 15 fractions of 3 Gy.
Keywords: Palliation, radiotherapy, geriatrics, prognostication, lung cancer
Octogenarians with lung cancer are not a homogeneous patient population (1-4). While many patients present with a long history of smoking and considerable pulmonary and cardiovascular comorbidity, often compromising activities of daily living, others are physically active and well functioning. If diagnosed early, curative treatment is often feasible (5,6). Besides surgery, different highly efficacious radiotherapy approaches are available (7-9). However, many patients are referred to radiation oncologists for palliation of thoracic symptoms in the context of stage III or IV disease of small cell or non-small cell histology (SCLC, NSCLC). The reasons why curative approaches were not recommended in such cases include compromised organ function, frailty, disease extent and sometimes patient preference (10). This heterogeneity implies that individually-tailored concepts for palliative thoracic radiotherapy might be pursued, aiming, for example, at palliation of pain or hemoptysis, or prolonged survival as a result of tumor-growth arrest.
Given that most developed countries are facing ageing populations and increasing numbers of newly diagnosed patients with cancer (11), dedicated studies addressing the unique challenges associated with geriatric oncology are urgently needed. Treatment decisions should be based on comprehensive assessments of organ function, comorbidity and the patient’s ability to function independently rather than on their biological age (12-17). Studies focusing on palliative radiotherapy in octogenarians with lung cancer are scarce. Important questions include i) Are these patients at increased risk of dropout from long-course regimens, and ii) Do they survive long enough to experience the benefits from better local tumor control? We hypothesized that short survival might be common and, therefore, short-course radiotherapy should be preferred. To shed light on these questions, a retrospective audit of our Institution’s patterns of care and survival outcomes was performed.
Patients and Methods
We included 51 unselected, consecutive patients with lung cancer who received palliative thoracic radiotherapy without concomitant systemic treatment at a single academic teaching hospital during the time period June 2007-2016. Minimum age was 80 years (maximum 90 years, median 83 years). Typical fractionation regimes were two fractions of 8.5 Gy (day 1 and 8), 10 fractions of 3 Gy or 15 fractions of 2.8 Gy prescribed to the International Commission on Radiation Units < Measurements reference point. However, other fractionations were also prescribed for some patients. Stereotactic radiotherapy was not included in the present study. Typically, a 3-5 field 3-D conformal beam arrangement was used, without inclusion of uninvolved lymph node regions. The treating physician who was responsible for dose/fractionation also recorded each patient’s medical history and Eastern Cooperative Oncology Group performance status (ECOG PS) at pre-treatment consultation. On the same day, blood tests were analyzed. Comorbidity was retrospectively scored by use of the Charlson comorbidity index, a validated and widely used tool (18). All medical records, treatment details and information on date of death or last contact were available in the hospital’s Electronic Patient Record system. At the time of analysis, 45 patients had died and six were still alive.The median follow-up for all living patients was 5 months, range=2-12 months. Survival time was measured from start of radiotherapy. IBM SPSS Statistics 23 (IBM Corp., Armonk, NY, USA) was employed for all statistical analyses. Actuarial survival curves were generated by Kaplan–Meier method and compared by log-rank test. The prognostic impact of all baseline variables included in Table I and the Results section was analyzed (intention-to-treat principle for radiation dose, i.e. number of prescribed rather than completed fractions). For multivariate analysis of survival, Cox regression analysis was used (backward stepwise method). Associations between different variables of interest were assessed with the chi-square test (when appropriate, Fisher exact probability test). A p-value of 0.05 or less was considered statistically significant. Two-tailed tests were performed.
Table I. Baseline characteristics before palliative thoracic radiotherapy.
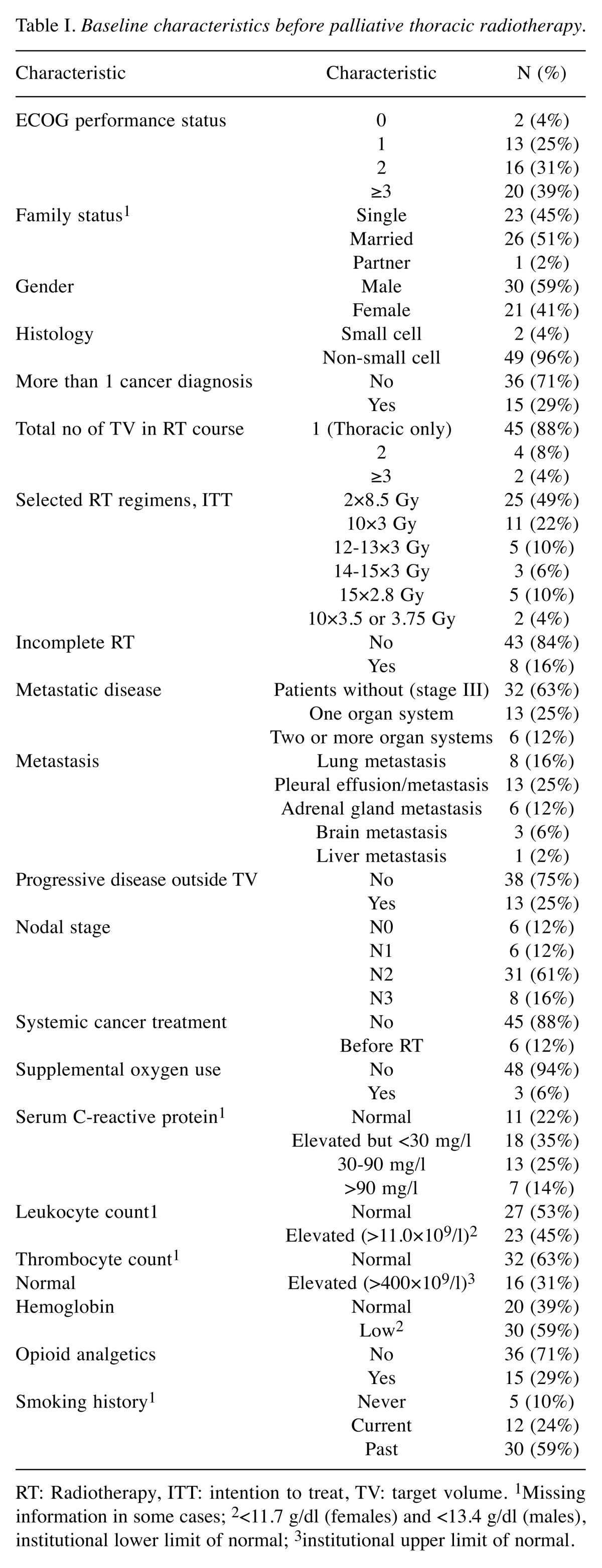
RT: Radiotherapy, ITT: intention to treat, TV: target volume. 1Missing information in some cases; 2<11.7 g/dl (females) and <13.4 g/dl (males),institutional lower limit of normal; 3institutional upper limit of normal.
Results
Forty-nine patients (96%) were treated for NSCLC. The median time from initial radiology diagnosis was 2 months (range=0-74 months). Thirty-five patients (69%) received up-front radiotherapy within 3 months of diagnosis. The others were treated with different other approaches first. Nineteen patients (36%) had stage IV disease, with median time from diagnosis of metastases of 1 month (range=0-25 months). Progression of metastases was recorded in 13 patients (25%). The median Charlson comorbidity index was 2 (range=0-7, presently treated lung cancer excluded). A diagnosis of diabetes mellitus was present in 20%, myocardial infarction in 24% and chronic obstructive pulmonary disease in 25%. Further baseline characteristics are shown in Table I.
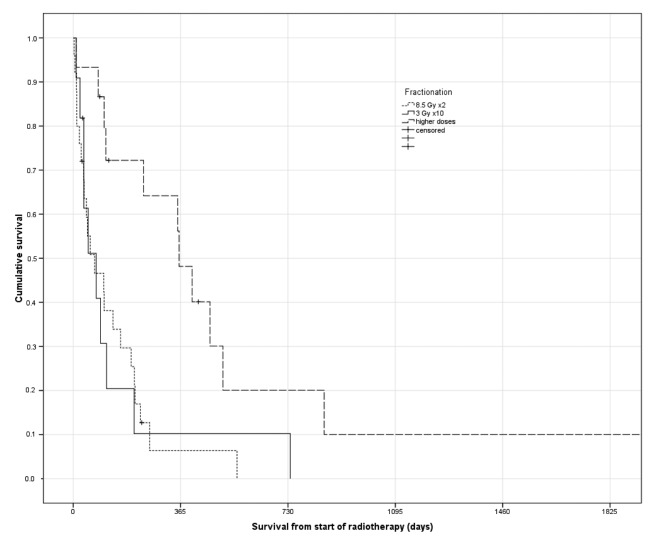
Eight patients (16%) were unable to complete the prescribed radiotherapy regimen. Four of these were assigned to 10 fractions, three to two fractions and one to 14 fractions. Median actuarial survival was 3.4 months and 20% of the patients were alive at 1 year. Twelve patients (24%) received radiotherapy shortly before they died, i.e. in the final 30 days of life. In terms of overall survival, no advantage was seen for 30 Gy over 17 Gy. However, a significantly better outcome was observed after higher doses (Figure 1). Median overall survival according to radiotherapy group was 2.4, 2.6 and 11.8 months (p=0.004), respectively. In the group treated with >30 Gy, 33% had stage IV disease, not significantly different from the groups treated with lower doses (39% for 17 and 30 Gy combined). However, ECOG PS was significantly different (0 or 1 in 53% of patients who received >30 Gy as compared to 19% when lower doses were prescribed, p=0.02).
Figure 1. Actuarial overall survival according to palliative thoracic radiotherapy (Kaplan–Meier estimates, log-rank test, p=0.004). Median survival of 2.4, 2.6 and 11.8 months, respectively.
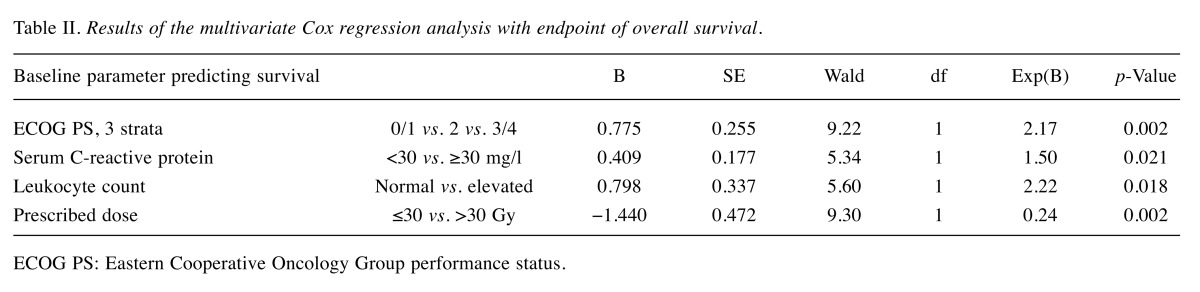
By univariate log-rank test, a total of eight parameters were significantly associated with survival: regimen (p=0.004), ECOG PS (p=0.00001), N stage (p=0.03), pleural effusion/metastasis (p=0.001), progressive distant metastasis (p=0.02), use of opioid analgesics (p=0.007), serum C-reactive protein (p=0.008) and leukocyte count (p=0.001). These were entered into a multivariate Cox regression analysis. Despite correlations between fractionation and ECOG PS, both remained associated with survival (Table II). Serum C-reactive protein and leukocyte count also contributed independent information.
Table II. Results of the multivariate Cox regression analysis with endpoint of overall survival.
ECOG PS: Eastern Cooperative Oncology Group performance status.
For 14 patients with ECOG PS 0-2, normal leukocyte count and serum C-reactive protein <30 mg/l (i.e. all three favorable prognostic factors), radiotherapy with 17 Gy or 30 Gy resulted in a median survival of 6.8 months (n=6), whereas higher doses resulted in survival of 15.2 months (n=8), p=0.056. All patients alive after more than 24 months had received total doses above 30 Gy.
Discussion
This retrospective analysis suggests that octogenarians with lung cancer who received palliative thoracic radiotherapy had limited median survival and a relatively high risk associated with receiving radiotherapy in the last 30 days of life. Nevertheless, approximately 10% of the patients were alive 3-5 years after treatment. Therapeutic nihilism is therefore not recommended. The role of radiotherapy with 30 Gy in a 10-fraction regimen appears questionable because two fractions of 8.5 Gy resulted in comparable survival and is more convenient to patients. Previous analyses, which were not limited to particular age groups, but often to NSCLC, suggested that symptom palliation could be achieved with low doses of radiation, e.g. 17 Gy in two fractions of 8.5 Gy (19), and that efficacy was not improved with higher doses (20,21). Most studies reported no impact of EQD2 or fractionation regimen on median survival. However, there was uncertainty about 1- and 2-year survival rates, which appeared better when higher radiation doses were prescribed, at least in patients with better baseline prognostic features. Recently, Janssen et al. reported that regimens with EQD2 of 47-52 Gy resulted in 2-year survival rates of 20%, whereas lower doses (31-46 Gy) resulted in approximately 15% (estimated from the published Kaplan–Meier graphs) (22). A Dutch randomized study with 303 patients with NSCLC found significantly improved survival after 10 fractions of 3 Gy compared to two fractions of 8 Gy (23). One-year survival was 20 versus 11%. The survival difference was driven by patients with performance status 0-1.
Van Oorschot et al. reported on 120 patients with NSCLC treated with 13-15 fractions of 3 Gy (24). The median survival of patients overall was 5.8 months. Those with non-metastatic disease survived significantly longer than patients with metastatic disease (median=11.7 months vs. 4.7 months, respectively). In the multivariate analysis, good general condition, non-metastatic disease, and a stable or improved general condition at the end of radiotherapy were significant. Schröder et al. compared two standard regimens used at their institution for palliation of SCLC and NSCLC: five fractions of 5 Gy (EQD2=31 Gy, 126 patients) and 20 fractions of 2.5 Gy (EQD2=52 Gy, 81 patients) (25). No significant survival difference was observed (median 4.8 vs. 5.3 months, respectively).
A study from the Karolinska Hospital, Sweden, included 452 patients with NSCLC aged 80 years or more (4). About a third each was diagnosed with stages I-II, III and IV. Best supportive care was given to 209 patients (46%); potentially curative therapy was administered to fewer than 20% (8% surgery, 11% stereotactic radiation therapy); and chemotherapy was given to 51 patients (11%). Median overall survival was 3.8 months in patients receiving best supportive care and 11.9 months in patients given any therapy. Data for palliative thoracic radiotherapy were not specified. Lee et al. surveyed radiation oncologists regarding their recommendations for treatment (chemoradiation, radiation alone, chemotherapy alone, or no therapy) for hypothetical patients with stage IIIB NSCLC who differed by age (55 vs. 80 years) and comorbid illness (none, moderate, or severe chronic obstructive pulmonary disease) (26). There was substantial variability in recommendations for an 80-year-old with moderate or severe chronic obstructive pulmonary disease. These data reflect the paucity of prospective studies for octogenarians, resulting in uncertainty about the optimum treatment approach.
Our policy was to discuss all patients in our multidisciplinary Lung Tumor Board and prescribe palliative thoracic radiotherapy when this was the consensus. Other options such as surgery, chemoradiation (27) or high-dose radiotherapy (7,8) were available and selected for appropriate patients. The actual study population was relatively inhomogeneous, even if most patients had NSCLC, stage III disease, and received upfront radiotherapy without preceding systemic treatment. In addition, the number of patients was limited. Radiation dose and fractionation were not assigned randomly, but rather according to physicians’ choice and taking into consideration different baseline parameters. The impact of radiation dose on survival was larger than expected and persisted in multivariate analysis. Nevertheless, residual bias cannot be excluded. Cachexia, differences in size of the gross tumor volume, number of involved nodal levels and difficulty in adhering to lung dose constraints might have been reasons to prescribe lower doses of radiation. In addition, development of distant metastases is a competing cause of death in those with stage III disease. Mutation status [epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) etc.] might also contribute to confounding results because increased survival can be caused by administration of targeted systemic therapy during follow-up. It is therefore necessary to embark on prospective controlled studies in octogenarians. These should also include toxicity and quality-of-life endpoints. Further studies are also required to firmly establish the prognostic impact of serum C-reactive protein and leukocytosis. These parameters outperformed N and M stage in our analysis.
Palliative radiotherapy is a well-established, widely used means of providing symptom improvement and, in selected patients with less advanced disease, increased overall survival (28,29). This treatment is often associated with improved quality of life and functional independence. However, incorrect expectations about the remaining lifespan might result in prescription of overly aggressive treatment near the end of life (30). Koshy et al. queried the US National Cancer Database from 2004 to 2012 for patients with stage IV lung cancer who received palliative chest radiation therapy (31). They reported that approximately half of all patients with metastatic lung cancer received a higher number of radiation fractions than recommended in guidelines. If the patient and the radiation oncologist mutually agree that symptom palliation, e.g. of hemoptysis, should be attempted even where the remaining lifespan is limited to a couple of weeks, a 10-fraction regimen would not be prescribed in our clinical practice. The benefits of palliative care should always be discussed in patients with limited lifespan.
Conclusion
For many patients, palliative radiotherapy is not expected to prolong survival. Therefore, care should be taken in assigning the right fractionation regimen in order to avoid lengthy treatment courses when survival is limited. The inconvenience and acute toxicity associated with higher radiation doses should not prevent clinicians from considering regimens with EQD2 >33 Gy in patients with favorable prognostic features who might benefit from tumor-growth arrest in terms of survival beyond 2 years. The role of regimens with intermediate EQD2, such as 30 Gy in 10 fractions of 3 Gy, is less clear.
Conflicts of Interest
The Authors declare no conflicts of interest in regard to this study.
Statement of Ethics
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 (in its most recently amended version).
References
- 1.Blanco R, Maestu I, de la Torre MG, Cassinello A, Nuñez I. A review of the management of elderly patients with non-small-cell lung cancer. Ann Oncol. 2015;26:451–463. doi: 10.1093/annonc/mdu268. [DOI] [PubMed] [Google Scholar]
- 2.Baimatova I, Smith C, Beckert L, Singh H. Treatment of octogenarians with lung cancer: A single-centre audit of treatments and outcomes. N Z Med J. 2015;128:29–34. [PubMed] [Google Scholar]
- 3.Chen KY, Chen JH, Shih JY, Yang CH, Yu CJ, Yang PC. Octogenarians with advanced non-small cell lung cancer: treatment modalities, survival, and prognostic factors. J Thorac Oncol. 2010;5:82–89. doi: 10.1097/JTO.0b013e3181c09b28. [DOI] [PubMed] [Google Scholar]
- 4.Koyi H, Hillerdal G, Kölbeck KG, Brodin D, Liv P, Brandén E. Non-small cell lung cancer (NSCLC) in octogenarians in clinical practice. Anticancer Res. 2016;36:5397–5402. doi: 10.21873/anticanres.11115. [DOI] [PubMed] [Google Scholar]
- 5.Zachariah B, Balducci L, Venkattaramanabalaji GV, Casey L, Greenberg HM, DelRegato JA. Radiotherapy for cancer patients aged 80 and older: a study of effectiveness and side effects. Int J Radiat Oncol Biol Phys. 1997;39:1125–1129. doi: 10.1016/s0360-3016(97)00552-x. [DOI] [PubMed] [Google Scholar]
- 6.Laurent M, Paillaud E, Tournigand C, Caillet P, Le Thuaut A, Lagrange JL, Beauchet O, Vincent H, Carvahlo-Verlinde M, Culine S, Bastuji-Garin S, Canouï-Poitrine F, ELCAPA Study Group Assessment of solid cancer treatment feasibility in older patients: a prospective cohort study. Oncologist. 2014;19:275–282. doi: 10.1634/theoncologist.2013-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda A, Sanuki N, Eriguchi T, Kaneko T, Morita S, Handa H, Aoki Y, Oku Y, Kunieda E. Stereotactic ablative body radiation therapy for octogenarians with non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;86:257–263. doi: 10.1016/j.ijrobp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Cannon NA, Iyengar P, Choy H, Timmerman R, Meyer J. Stereotactic ablative body radiation therapy for tumors in the lung in octogenarians: a retrospective single-institution study. BMC Cancer. 2014;14:971. doi: 10.1186/1471-2407-14-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karube M, Yamamoto N, Nakajima M, Yamashita H, Nakagawa K, Miyamoto T, Tsuji H, Fujisawa T, Kamada T. Single-fraction carbon-ion radiation therapy for patients 80 years of age and older with stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2016;95:542–548. doi: 10.1016/j.ijrobp.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues G, Sanatani M. Age and comorbidity considerations related to radiotherapy and chemotherapy administration. Semin Radiat Oncol. 2012;22:277–283. doi: 10.1016/j.semradonc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Borras JM, Lievens Y, Barton M, Corral J, Ferlay J, Bray F, Grau C. How many new cancer patients in Europe will require radiotherapy by 2025? An ESTRO-HERO analysis. Radiother Oncol. 2016;119:5–11. doi: 10.1016/j.radonc.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Kenis C, Bron D, Libert Y, Decoster L, Van Puyvelde K, Scalliet P, Cornette P, Pepersack T, Luce S, Langenaeken C, Rasschaert M, Allepaerts S, Van Rijswijk R, Milisen K, Flamaing J, Lobelle JP, Wildiers H. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24:1306–1312. doi: 10.1093/annonc/mds619. [DOI] [PubMed] [Google Scholar]
- 13.Spyropoulou D, Pallis AG, Leotsinidis M, Kardamakis D. Completion of radiotherapy is associated with the Vulnerable Elders Survey-13 score in elderly patients with cancer. J Geriatr Oncol. 2014;5:20–25. doi: 10.1016/j.jgo.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Nieder C, Angelo K, Haukland E, Pawinski A. Survival after palliative radiotherapy in geriatric cancer patients. Anticancer Res. 2014;34:6641–6645. [PubMed] [Google Scholar]
- 15.Rades D, Schild SE, Bajrovic A, Janssen S, Bartscht T. Personalized radiotherapeutic approaches for elderly patients with epidural cord compression from gastric cancer. In Vivo. 2016;30:69–72. [PubMed] [Google Scholar]
- 16.Käsmann L, Manig L, Janssen S, Rades D. Chemoradiation including paclitaxel for locally recurrent muscle-invasive bladder cancer in elderly patients. In Vivo. 2017;31:239–241. doi: 10.21873/invivo.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Felice F, Galdieri A, Abate G, Bulzonetti N, Musio D, Tombolini V. Definitive intensity-modulated radiation therapy in elderly patients with locally advanced oropharyngeal cancer. In Vivo. 2017;31:455–459. doi: 10.21873/invivo.11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Sundstrøm S, Bremnes R, Aasebø U, Aamdal S, Hatlevoll R, Brunsvig P, Johannessen DC, Klepp O, Fayers PM, Kaasa S. Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase III trial. J Clin Oncol. 2004;22:801–810. doi: 10.1200/JCO.2004.06.123. [DOI] [PubMed] [Google Scholar]
- 20.Fairchild A, Harris K, Barnes E, Wong R, Lutz S, Bezjak A, Cheung P, Chow E. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26:4001–4011. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 21.Stevens R, Macbeth F, Toy E, Coles B, Lester JF. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer. Cochrane Database Syst Rev. 2015;1:CD002143. doi: 10.1002/14651858.CD002143.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen S, Kaesmann L, Schild SE, Rades D. Impact of the radiation dose and completion of palliative radiotherapy on survival in patients treated for locally advanced lung cancer. Anticancer Res. 2016;36:1825–1828. [PubMed] [Google Scholar]
- 23.Kramer GW, Wanders SL, Nordijk EM, Vonk EJ, van Houwelingen HC, van den Hout WB, Geskus RB, Scholten M, Leer JW. Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small cell lung cancer. J Clin Oncol. 2005;23:2962–2970. doi: 10.1200/JCO.2005.01.685. [DOI] [PubMed] [Google Scholar]
- 24.Van Oorschot B, Assenbrunner B, Schuler M, Beckmann G, Flentje M. Survival and prognostic factors after moderately hypofractionated palliative thoracic radiotherapy for non-small cell lung cancer. Strahlenther Onkol. 2014;190:270–275. doi: 10.1007/s00066-013-0507-y. [DOI] [PubMed] [Google Scholar]
- 25.Schröder C, Ivo M, Buchali A. Does high-dose radiotherapy benefit palliative lung cancer patients. Strahlenther Onkol. 2013;189:771–776. doi: 10.1007/s00066-013-0360-z. [DOI] [PubMed] [Google Scholar]
- 26.Lee IH, Hayman JA, Landrum MB, Tepper J, Tao ML, Goodman KA, Keating NL. Treatment recommendations for locally advanced, non-small-cell lung cancer: the influence of physician and patient factors. Int J Radiat Oncol Biol Phys. 2009;74:1376–1384. doi: 10.1016/j.ijrobp.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieder C, Pawinski A, Andratschke NH. Combined radio- and chemotherapy for non-small cell lung cancer: systematic review of landmark studies based on acquired citations. Front Oncol. 2013;3:176. doi: 10.3389/fonc.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Oorschot B, Schuler M, Simon A, Schleicher U, Geinitz H. Patterns of care and course of symptoms in palliative radiotherapy: a multicenter pilot study analysis. Strahlenther Onkol. 2011;187:461–466. doi: 10.1007/s00066-011-2231-9. [DOI] [PubMed] [Google Scholar]
- 29.Nieder C, Mannsåker B, Dalhaug A, Pawinski A, Haukland E. Palliative radiotherapy in cancer patients with increased serum C-reactive protein level. In Vivo. 2016;30:581–586. [PubMed] [Google Scholar]
- 30.Berger B, Ankele H, Bamberg M, Zips D. Patients who die during palliative radiotherapy. Status survey. Strahlenther Onkol. 2014;190:217–220. doi: 10.1007/s00066-013-0471-6. [DOI] [PubMed] [Google Scholar]
- 31.Koshy M, Malik R, Mahmood U, Husain Z, Weichselbaum RR, Sher DJ. Prevalence and predictors of inappropriate delivery of palliative thoracic radiotherapy for metastatic lung cancer. J Natl Cancer Inst. 2015;107:djv278. doi: 10.1093/jnci/djv278. [DOI] [PMC free article] [PubMed] [Google Scholar]