Abstract
Natural history of prostate cancer (PCa) is extremely variable, as it ranges from indolent and slow growing tumors to highly aggressive histotypes. Genetic background and environmental factors co-operate to the genesis and clinical manifestation of the tumor and include among the others race, family, specific gene variants (i.e., BRCA1 and BRCA2 mutations), acute and chronic inflammation, infections, diet and drugs. In this scenario, remaining actual the clinical interest of bone scan (BS) in detecting skeletal metastases, an important role in diagnostic imaging may be also carried out by, positron emission tomography/computed tomography (PET/CT) and PET/magnetic resonance imaging (PET/MRI), which combine morphological information provided by CT and MRI with functional and metabolic data provided by PET acquisitions. With respect to PET radiotracers, being ancillary the usefulness of F-18 fluoro-deoxyglucose and not yet demonstrated the cost-effectiveness of F-18 Fluoride respect to BS, the main role is now played by choline derivatives, in particular by 11C-choline and 18F-fluorocholine. More recently, a greater interest for both diagnostic and therapeutic purposes has been associated with radiotracers directed to prostate-specific membrane antigen (PSMA), a transmembrane protein expressed on the cell surface, which showed high selective expression in PCa, metastatic lymph nodes and bone metastases. Several PSMA-targeted PET tracers have been developed many of which showing promising results for accurate diagnosis and staging of primary PCa and re-staging after biochemical recurrence, even in case of low prostate specific antigen values. In particular, the most widely used PSMA ligand for PET imaging is a 68Ga-labelled PSMA inhibitor, 68Ga-PSMA-HBED-CC (68Ga-PSMA-11). 99mTc-HYNIC-Glu-Urea-A for single photon emission computed tomography, and 177Lu-PSMA-617 for radioligand therapy has also been applied in humans, with interesting preliminary results related to a possible theranostic approach. A potential role of PSMA radioligands in radio-guided surgery has also been proposed.
Keywords: Choline, nuclear medicine, positron emission tomography/computed tomography, positron emission tomography/magnetic resonance imaging, prostate cancer, prostate-specific membrane antigen
Introduction
Prostate cancer (PCa) has always been described as a typical disease of the elderly, with a peak incidence at 80 years. In this paper, we review the pathophysiological basis of PCa as a substratum for a nuclear medicine digression on clinically used tracers, for relapse and secondary lesions detection. We will also discuss a possible role for nuclear medicine in primary cancer detection and/or at least as a better guide to biopsy, to end with future perspectives based on new radioligands, for both diagnosis and therapy.
Current Status
During the last few years, thanks to an earlier and more accurate diagnosis and due to the increase in medium lifespan, the incidence of PCa has grown to its highest levels, representing today the 11% of all malignancies and counting up to 2.6 million of new cases per year in Europe. Even though a complete comprehension of the causes of PCa is not possible yet, it is clear that several primary risk factors cooperate in its tumorigenesis: Aging above all, but also individual genetic predisposition, which promotes the interaction with environmental factors, such as inflammation and infections, diet and body mass index.[1]
Another key parameter to take in consideration, used for both diagnosis and follow-up, is represented by serum levels of prostate specific antigen (PSA), a glycoprotein produced by the prostatic gland, which increases in the plasma in case of tissue damage or of glandular enlargement, including benign prostatic hypertrophy, prostatitis, or PCa. At present, the only test that can fully confirm the diagnosis is still a biopsy, removing small tissue samples for microscopic examination to provide a grading score (Gleason score).[2]
Fortunately, whereas the lifetime risk of a PCa diagnosis is high, the mortality ratio is low; which means that most men diagnosed with PCa will not die of the disease. In this sense, PSA screening fostered the diagnosis of low-grade and low-volume cancers reducing the incidence of advanced disease and mortality but led in some cases to overdiagnosis and overtreatment. With some exceptions, PCa can be considered a slow growing tumor; hence, less invasive testing must be conducted before a biopsy, which has to be limited only to patients with high probability of cancer. In this context, although it cannot avoid biopsy that still is a mandatory step before surgery, diagnostic imaging plays a crucial role in the decisional algorithm of PCa, leading to changes in the management of patients.[3]
In this sense, even if it is more relevant in staging of patients already diagnosed with PCa and in the restaging of patients with high probability of relapse, diagnostic imaging must be employed also in the pretherapeutic phase. At this level, its role consists either in avoiding unneeded biopsies in patients in which cancer may be reliably excluded or guiding biopsies in patients highly suspicious for PCa with unclear lesions. It could also be useful for an under diaphragmatic evaluation in patients in which the detection of local invasion and/or lymph node enlargement may support the diagnosis of malignancy. In the pretherapeutic phase, transrectal ultrasound (TRUS) provides real-time visualization of the prostate, thus allowing the determination of gland volume and the distinction between peripheral zone and transition zone. Multiparametric magnetic resonance imaging (mpMRI) instead, is rightfully considered the most accurate, non-invasive, morphologic technique for PCa diagnosis.[4] T2 weighted (T2W) and diffusion weighted imaging (DWI) offer the best possibilities for mpMRI, with the former that grants high-resolution excellent anatomical detail, and the latter, which is based on Brownian motion of free water within tissues and identifies a restriction in water diffusion due to an increase in cellular density in malignancies when compared to normal tissue. Recent data proved that a combination of such parameters yield higher sensitivity, specificity and negative predictive value than T2W alone.[5] Either TRUS or magnetic resonance imaging (MRI) can help in defining the presence of a local extension of the malignant neoplasm, suggesting different therapeutic strategies. A pre-therapeutic staging has to include also bone scintigraphy (BS) that showed a high sensitivity in detecting bone metastases, although affected by a low specificity. Better results may be obtained performing BS with single photon emission computed tomography/computed tomography (SPECT/CT), significantly increasing diagnostic accuracy. Positron emission tomography/CT (PET/CT) with 18F sodium fluoride (18F-NaF) did not find a wide diffusion, because its cost-effectiveness has not yet been demonstrated, despite its greater sensitivity respect to BS with SPECT/CT. In this scenario, albeit the most important diagnostic techniques for PCa, TRUS, and MRI are however considered unable to detect early functional changes that happen at a molecular level and for which nuclear medicine examinations are highly sensitive. In fact, using targeted radionuclides, the early detection of such malignancies may be achieved although, most frequently, in the presence of an unsatisfactory specificity.
Risk Factors and Pathophysiology
PCa natural history is extremely variable, as it ranges from indolent and slow growing tumors to highly aggressive histotypes, frequently diagnosed based on clinical symptoms related to bone metastases. Genetic background has to be taken in consideration as suggested by PCa association with race, family, and specific gene variants (i.e., BRCA1 and BRCA2 mutations).[6] However, several environmental factors must cooperate to the genesis and clinical manifestation of the tumor, including among the others acute and chronic inflammation, infections, diet, and drugs. As concerning inflammation and infections, the main hypothesis links chronic processes to PCa, due to the enhanced production of inflammatory mediators by clinical prostatitis. In fact, Tumor Necrosis Alpha (TNF-α), reactive oxygen species, cyclooxygenase 2, and vascular endothelial growth factor, which are produced in case of inflammation by cell damage, promote, during the repairing process, the risk of mutation and malignant transformation. In addition, asymptomatic sexually transmitted infections from pathogens such as Trichomonas vaginalis and Mycoplasma species that may persist undetected in the urinary tract for longer periods may lead to the release of inflammatory mediators, cytokines and free radicals, which can contribute to malignant progression. An important promoting role has also been found for diet, with different protective and risk-increasing food associations. In particular, green tea, for its polyphenolic compounds, and soy products, rich in isoflavones, are reported to decrease risk of PCa by altering the expression of several genes, Estrogen 2 receptors, suppressing proliferation and down-regulating IL-8. On the other hand, obesity appears to increase the risk of PCa, through the release by adipose cells of inflammatory mediators, as well as the consumption of high levels of red meat, due to the presence of nitrites and nitrates. Finally, preclinical studies on animals suggested the protective role of aspirin and nonsteroidal anti-inflammatory drugs in PCa, which reduce the overall risk for advanced cancer of 17% and 19%, respectively.[6] In this context, a key role is played by the androgen receptor (AR), a transcription factor, which mediates the physiological effects of androgens such as testosterone and its metabolites, through direct binding of androgen-AR complex to specific DNA target sequences (androgen responsive elements) that produce inputs of cell survival and apoptosis regulation. In PCa, this function is aberrant and represents a possible therapeutic target, for example, androgen deprivation therapy is already used to sensitize PCa to radiation therapy. In addition, from a cellular point of view, the prostate accumulates zinc through active transportation mediated by ZIP1 protein and produces citrate, which is an important component of semen. This physiological process, however, requires high amounts of energy (ATP) thus being energy inefficient. PCa cells instead, do not have any zinc storage, most probably due to ZIP1 silencing; hence, they can channel all the extra energy in cellular growth, proliferation, and spreading.[7] Some preclinical research groups focused on the possibility to transport zinc into malignant prostate cells as it inhibits NF-κB pathways, suppresses proliferation and induces apoptosis in abnormal cells but the results have been conflicting and inconclusive. More recently, another antigen has been studied, prostate specific membrane antigen, which stimulates PCa development by increasing cell folate uptake and has considerable overexpression on most PCa cells, thus being a promising target molecule for diagnostic imaging and therapy.[8]
Clinical Scenario: Diagnostic Imaging and Nuclear Medicine
In 2016, the American Association of Urology produced the updated guidelines on PCa in which stated how aggressive screening policies increased the early detection rate and reduced the mortality. Nevertheless, there is still the concrete risk of overdiagnosis and overtreatment, therefore, the decision of an aggressive screening policy remains one of the most controversial topics in urological literature (still no level 1 evidence).[9] The diagnostic algorithm for PCa early detection starts as usual with clinical diagnosis, based on Digital Rectal Examination (DRE) and PSA serum levels, which include various forms, free PSA, PSA density (PSAd), PSA velocity and doubling time (PSAdt) and free/total PSA ratio; however, definite diagnosis is always linked to histopathological evaluation in prostate biopsy specimens. In this scenario, the role of diagnostic imaging is carried out by TRUS, mainly used to guide biopsies, being standard US affected by a lower diagnostic accuracy and mpMRI that showed an excellent sensitivity, in the presence of a low specificity. Nonetheless, a useful clinical information may be achieved with mpMRI mainly in advanced lesions (Gleason score > 7), through the association of T2W imaging with DWI and/or H1-spectroscopy.[5] However, thanks to the employment of new imaging modalities such as PET/CT and PET/MRI, which combine morphological information provided by CT and MRI with functional and metabolic data provided by PET acquisitions, an improvement in PCa detection and staging was possible.[10,11] In particular, since MRI is the most accurate morphologic technique that provides high-resolution and excellent anatomical detail in the evaluation of pelvic region, its combination with PET systems can be considered the best solution, even compared to PET/CT.[12] Moreover, as reported above, the implemental role of functional techniques may permit to PET/MRI the achievement of further information useful at diagnostic and/or prognostic level.
Although at present radionuclide procedures are affected by a low diagnostic accuracy in diagnosing primary PCa, radiolabeled agents can trace almost any pathophysiological pathway, thus being able to characterize the disease in an early stage and/or allowing a prognostic stratification. Among the used radiotracers, 18F-fluoro-deoxyglucose (18F-FDG), a biological analogue of glucose, occupies a pivotal role in a great number of neoplasms, due to the increased concentration of glucose and glucose transporters in malignant tumor cells where there is an enhanced anaerobic glycolysis (Warburg effect).[13] Nonetheless, 18F-FDG results in PCa are not as good as in other tumors because of the low metabolic activity usually present in PCa cells and due to an unfavorable lesion/background ratio determined by the high activity in the bladder [Figure 1]. Therefore, 18F-FDG PET is only limited in the restaging of selected PCa patients with high-grade hormone-resistant disease and poorly differentiated lesions, due to the increase of FDG uptake with increasing malignancy.[14]
Figure 1.
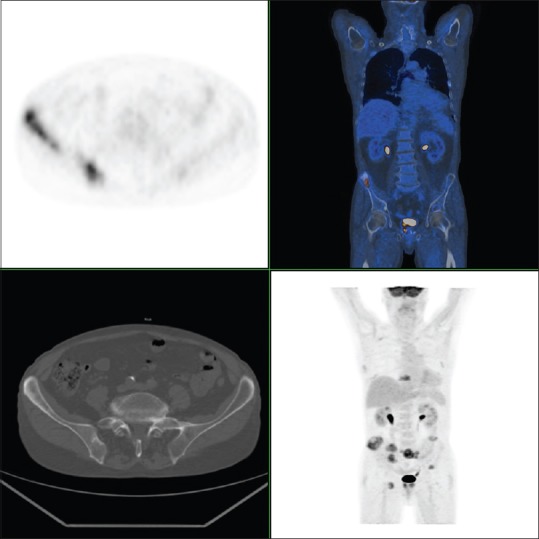
Fluoro-deoxyglucose - patient with untreated prostate cancer. Fluoro-deoxyglucose - positron emission tomography reveals prostate uptake and multiple bone locations (Courtesy of Prof. Stefano Fanti, Nuclear Medicine Unit, University of Bologna)
At present, either in staging or in restaging to detect skeletal metastases, is still consolidated the use of bone scan (BS) with 99mTc methyldiphosphonate (MDP) or other radiolabelled phosphonates, which takes advantage of increased osteoblastic activity seen in metastatic skeletal lesions, allowing their early detection. Unfortunately, a high uptake may be also observed in many nonmalignant skeletal alterations such as trauma and degenerative joint disease among the others, leading to false positive results.[15] A significant improvement may be achieved either with SPECT or even more with SPECT/CT, which allows a differential diagnosis also based on CT information. Despite a further improvement in sensitivity, the alternative use of PET/CT with 18F-NaF is not yet considered the method of choice due to its low cost-effectiveness and higher risk of false positive results, determined by the greater spatial resolution that highlights a larger number of concentrating lesions. Furthermore, neither BS and 18F-NaF PET can detect local relapse or to define lymph node involvement.[16]
For these reasons, different radiotracers for PCa imaging have been developed and extensively evaluated over the last few years. In particular, not having found a wide spreading of radiotracers labeled with γ-emitters, the clinical-care role is now carried out by PET/CT with choline derivatives, because of choline presence as a phospholipid in prostate cell membranes, whose turnover is elevated in case of malignancy. The most diffuse radiotracers are 11 C-choline, only available in PET centers owning their onsite cyclotron, and 18F-fluorocholine (18F-FCH).[17,18,19] The former takes advantage of a lower urinary excretion, which favors the analysis of the prostate bed, but is negatively affected by a short half-life (HL = 20 min). In fact, 11 C-choline needs faster procedures that have to be performed in the 1st min after intravenous (IV) administration.[20] Therefore, while local relapse may be more easily diagnosed respect to radio-fluorinated compounds, because of the favorable tumor/background ratio related to the absence of urinary activity, the procedure may be affected by false negative results in bone metastases, which do not usually show a significant lesion/background ratio few minutes after injection. Furthermore, the evaluation of local relapse has to be very careful and also based on morphostructural information, to discriminate possible false positive results in high flow benign inflammatory lesions. Conversely, 18F-FCH presents a higher urinary excretion, which makes more difficult the diagnosis of local relapse, but on the other hand allows a more comfortable procedure with the possibility of delayed scans, improving the diagnostic efficacy for lymph node and skeletal metastases.[21] It has, however, to be pointed out that a careful evaluation is ever needed when using each of the two radiotracers, being possible the presence of false positive results also in the evaluation of distant metastases.
As consequence of different Half Life, while when using 11C-choline, the procedure is more frequently based on a WB scan (from the upper thighs to the base of the skull), from 3 to 5 min after the IV injection. 18F-FCH instead, allows delayed acquisitions up to 2 h postinjection and more, being anyway preferable a dynamic study of pelvic region better to evaluate the early pathological uptake in prostate or prostatic bed, before urinary activity by entering distal ureters and bladder, compromises image interpretation [Figure 2].
Figure 2.
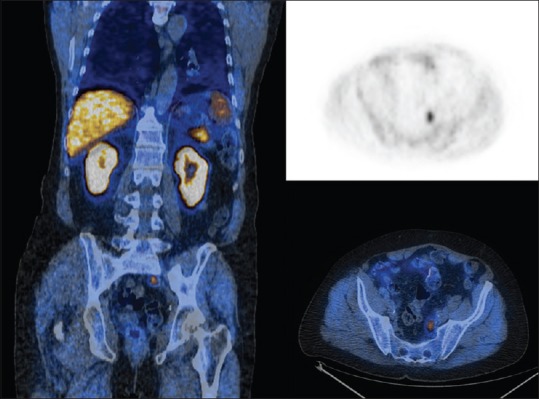
11C-choline - A 72-year-old patient, previously treated and increasing prostate specific antigen. 11C-positron emission tomography demonstrates disease relapse (SUVmax = 6.2) (courtesy of Prof. Stefano Fanti, Nuclear Medicine Unit, University of Bologna)
In a recent literature revision, Evangelista et al. stated the wide diffusion of 18F-FCH PET/CT in different countries (n = 1502/1932; 78%), stressing at the same time a great variability in acquisition methods, including PET protocols and patient preparation.[22] The same group proposed then a specific protocol that summarizes all precedent experiences. Patient preparation should start with a low-choline-food diet the week before the scan, with at least 4 h fasting before the examination and a water intake of 1.5–2 L, to significantly reduce bowel uptake. Acquisition protocol should include dynamic imaging (for 8 min at least from tracer administration) or very early static imaging (maximum 2 min after the injection) to avoid interference from physiological bladder uptake followed by a delayed WB scan for distant organs involvement, in particular, bone metastases.
New Directions: Prostate-Specific Membrane Antigen Ligands
Although radiocholine derivatives represent the current radionuclide diagnostic method of choice for PCa restaging and in some individual patients for staging, they showed in several studies different drawbacks that encouraged the research and testing of new compounds. In fact, choline-based radioligands are not tumor-specific tracers and showed limited diagnostic value in case of primary diagnosis, being also discussed their standard role in staging, with the main reference to an accurate pre-therapeutic assessment of nodal involvement, which can define different strategies.[23,24,25,26] Prostate-specific membrane antigen (PSMA) is a trans-membrane protein physiologically expressed on the cell surface of healthy prostate and other tissues such as salivary glands and kidneys that manifested about a thousand-fold increase in PCa.[27] From a pathophysiological point of view, PSMA functions as a folate hydrolase, thus being involved in PCa growth by increasing folate levels to support survival and proliferation.[8] In addition, an increased PSMA expression has been found in the peripheral stromal tissue of solid tumors, suggesting a possible involvement also in the neoangiogenesis process. Thanks to its selective overexpression either in PCa lesions, lymph nodes and bone metastases, it can be used as a target for both diagnostic and therapeutic purposes, particularly in those cases where choline derivatives showed low sensitivity and specificity for PCa detection. For example, in patients with low PSA values (lower than 1 ng/ml), in case of biochemical recurrence of PCa or with high Gleason scores where PSMA expression is usually higher, thus being also used to reduce the number of investigations (i.e., 18F-FDG PET/CT that showed a low sensitivity anyway).[28] Moreover, PSMA expression proved to be linked directly to tumor aggressiveness, metastatic and recurrent disease, thus giving also a prognostic indication.[29] The first specific PSMA-targeting probe was 111In-capromab pendetide (Prostascint®), a 111 In-labeled anti-PSMA antibody, whose application was quite limited due to its binding to an intracellular domain of PSMA. This characteristic implies that the uptake is possible only after internalization or in cells with disrupted membranes, with a resulting high non-specific uptake and poor T/B ratio.[17,30] Since then, several PSMA-targeted PET tracers have been developed from different study groups, all of which showing promising results for accurate staging of primary PCa and re-staging after biochemical recurrence, even in case of low PSA values. In particular, given that such radiotracers have been implemented in clinical routine protocols only in few centers worldwide and that few data are available yet, in Europe the most widely used PSMA ligands are a theranostic agent, 68Ga-labelled PSMAI and T (Imaging and Therapy) and a 68Ga-labelled PSMA inhibitor Glu-NH-CO-NH-Lys (Ahx)-HBED-CC(68Ga-PSMA-HBED-CC or 68Ga-PSMA-11).[17,31] PSMA-11 presents several advantages over Prostascint® as it is a urea-based inhibitor with very high affinity for PSMA binding motif; 68Ga labeling is a readily available and cost-effective production method and HBED-CC conjugate provides reduced nonspecific binding and considerably higher specific internalization.[32] Besides, 68Ga-PSMA-11 showed good kinetic properties, with fast blood and organ clearances, low liver accumulation, and high specific uptake in PSMA tumors. In a recent meta-analysis, von Eyben and Kairemo evaluated eighteen articles with 2213 patients, in which PET/CT was used for both staging and re-staging. The standard acquisition protocol used in all studies considered a dose generally in the range of 130–170 MBq, with an uptake time of 5 min in articles with 11C-choline PET/CT and 29 ± 24 min in articles with 18F-FCH PET/CT, and with imaging interpretation also based on SUVmax criteria. The detection rate for staging was 70%–80%, whereas in the restaging scenario PSA was positively associated with the detection rate: 50% in patients with PSA levels of 0.2–0.49 ng/ml, 53% for PSA of 0.5–0.99 ng/ml. It has to be evidenced that these values consider both local relapse and distant metastases.[33] In this direction, in recent studies 68Ga-PSMA-11 PET/CT outperformed 99mTc-MDP BS for bone involvement assessment, showing also a higher lymph node metastases detection rate compared to morphological imaging, which proved to be also affected by a higher rate of false positive results.[34] These data confirmed 68Ga-PSMA-11 high potential and its clinical usefulness in the detection of small recurrent PCa lesions in patients with low PSA values, in which choline derivatives, though widely used, demonstrated poor sensitivity.
More recently, another approach has been proved utilizing a modified PSMA ligand, designed with a DOTA chelator, referred to as PSMA-617, labeled with 68Ga.[35] Compared to PSMA-11, the former also permits stable bindings with therapeutic radioisotopes, such as 177Lu, thus being perfect for a theranostic approach[36,37] [Figure 3].
Figure 3.
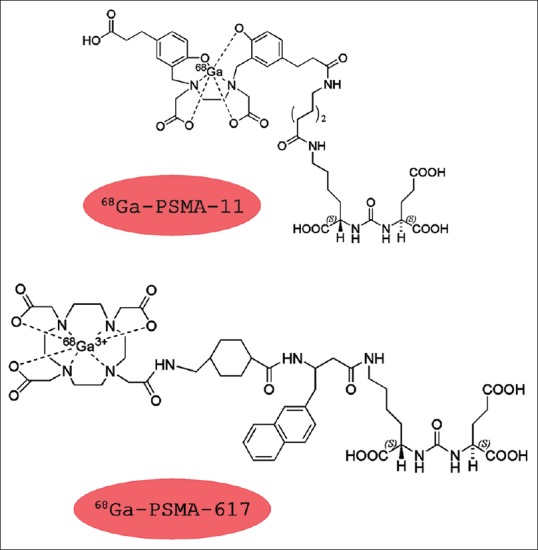
Different structures of the different prostate-specific membrane antigen agents
Rahbar et al. retrospectively evaluated tumor response, adverse effects, and survival rates in 28 patients with metastatic castration-resistant PCa undergoing radioligand therapy with 177Lu-PSMA-617 and compared the median overall survival with that of a recent historical cohort treated with best supportive care before the availability of 177Lu-PSMA-617. Their results showed optimally tolerated therapies, with a PSA decline in 75% of patients and a decline of 50% or greater in 50% of patients, with an increased overall survival, after two cycles of therapy.[38] A German group instead, evaluated dosimetry, safety and efficacy of 177Lu-PSMA-617 radioligand therapy in 15 patients with metastatic castration-resistant PCa using RECIST1.1 criteria to define treatment response; patient reported outcomes in terms of pain, quality of life and changes in PSA values as secondary endpoints. Their experience showed that radioligand therapy with 177Lu-PSMA-617 is effective and well tolerated, with a disease control rate of 67%; however, further studies, randomized, controlled and prospective, will be necessary to confirm these promising results.[39] A different approach has been proved at Paul Scherrer Institut, where Umbricht et al. proposed in a preclinical investigation on PCa cell lines, 44 Sc-PSMA-617 in tandem with 177Lu-PSMA-617 for radiotheranostics in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. Scandium-44 decays by positron emission (β+ branching of 94.3%) to stable 44Ca and can be produced with high radionuclidic purity (>99%) and at high activities (>2 GBq) via nuclear reaction 44Ca(p, n) 44 Sc in small cyclotrons already available in PET centers worldwide. Moreover, 44 Sc compared to 68Ga has almost a fourfold longer half-life (3.97 h versus 68 min), which enables the delivery to PET centers without cyclotron of 44Sc-based radiopharmaceuticals. In this study, the authors demonstrated the almost identical distribution profile of 44Sc-PSMA-617 and 177Lu-PSMA-617 for the investigated period of 6 h compared to 68Ga-PSMA-11. In particular, the main advantage of this new tracer lays in the chemical analogies between 44Sc and 177Lu and in similar ligand pharmacokinetics that allow the exact prediction of tissue distribution of the β-emitter, based on 44Sc-PSMA-617 PET imaging results.[40] Another factor to take in consideration is the possibility of delayed acquisitions that could enable the detection of small pathological lesions thanks to a greater T/B ratio. The latest feature is also typical of 64Cu labelled ligands,[41] because of 64Cu longer half-life that allows the utilization for both diagnostic and therapeutic purposes.[42]
Another PSMA-based tracer is 18F-DCFBC (PSMA target), developed at Johns Hopkins University by Rowe et al. who preliminarily tested it in different studies and proved its diagnostic potential.[43] Compared to conventional imaging modalities, 18F-DCFBC PET/CT detected an overall larger number of lesions (592 positive with 63 equivocal versus 520 positive with 61 equivocal), in both hormone-naïve and castration-resistant PCa patients.[44]
In parallel with diagnostic and therapeutic applications, PSMA ligands could also be used as a radio-guided surgery (RGS) tool for PCa lesion identification.[45,46,47] Surgical treatment based on radical prostatectomy still represents the gold standard technique for patients with PCa; however, in several cases, residual disease and/or micrometastases cannot be properly identified and removed during surgery, thus leading to potential recurrence of the disease. In this sense, as already proven by RGS efficacy in patients with breast cancer or cutaneous malignancies, PSMA-based tracers could provide real-time information to the surgeon regarding resection margins and extent of the disease. In a feasibility study, Maurer et al. preoperatively studied five patients with 68Ga-PSMA-11 PET/CT and subsequently injected them with 111In-PSMA investigation and therapy agent (111 In-PSMA I&T) 24 h before surgery. The intraoperative detection of metastatic lymph nodes was possible via a γ-probe with acoustic and visual feedback. In these patients, all PSMA-positive lesions detected in vivo corresponded to preoperative 68Ga-PSMA-11 PET/CT scan and were confirmed by ex vivo measurements and histopathology analysis. Moreover, intraoperative probing revealed two subcentimetric metastatic lymph nodes not seen on 68Ga-PSMA-11 PET/CT.[48,49] Although promising, the role of PSMA-radio-guided surgery is still not clear and greater clinical records and long-term follow-up data are needed. Unfortunately, PET/CT is not always available as conventional scintigraphy systems, thus representing a possible hindrance for PSMA imaging. In this sense, a Chinese study group evaluated the possible role of a PSMA-based SPECT imaging for those health-care structures that do not have access to PET systems.[50] The Authors analyzed fifty patients who received a histopathological diagnosis of PCa with a biochemical recurrence that underwent PSMA SPECT/CT (99mTc-HYNIC-Glu-Urea-A), pelvic MRI and BS within a 30-day period. PSMA SPECT/CT showed a better diagnostic efficiency for bone and lymph node metastases (50.0% and 42.0%) compared to BS (34.0% and 0.0%) and MRI (24.0% and 20.0%), respectively and provided also a higher detection rate at serum PSA levels of ≤1 ng/ml. Therefore, PSMA-SPECT/CT changed the therapeutic approach in 31 patients (62% of cases) leading to a possible enhancement of their clinical outcome.[50] Nonetheless, more studies are needed to confirm this promising SPECT tracer for detection of locally recurrent PCa and/or metastatic disease [Figure 4].
Figure 4.
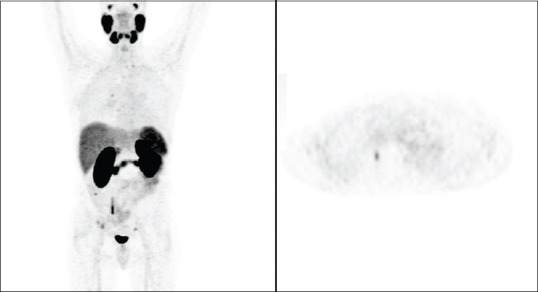
68GA-prostate-specific membrane antigen - A 49-year-old patient, previously treated. Prostate-specific membrane antigen positron emission tomography shows uptake at the level of the right acetabulum (SUVmax = 3.5), compatible with secondary localization (Courtesy of Prof. Stefano Fanti, Nuclear Medicine Unit, University of Bologna)
Future Perspectives: The Bombesin/Gastrin Releasing Peptide
The receptor of the bombesin/gastrin releasing peptide (GRPR) also represents a promising field of investigation with both diagnostic and therapeutic applications via β+ and β− emitters, respectively. In fact, ex vivo studies demonstrated GRPR overexpression in several human tumors, including in 63%–100% of human PCa, mainly those with neuroendocrine differentiation, making it a promising target for both imaging and therapy. Unfortunately, the majority of data available on the topic is preclinical, with limited studies published on men. In particular, Minmimoto et al. evaluated seven patients with biochemically recurrent PCa, who underwent both 68Ga-PSMA-11 PET/CT and 68Ga-RM2 PET/MRI scans, with the latter that is labeled through a DOTA chelator to 68Ga and acts as a synthetic bombesin receptor antagonist, targeting GRPR. The aim of their study was to directly compare the two tracers and their biodistribution; in particular, 68Ga-PSMA-11 showed as already known high accumulation in small intestine, kidneys, and bladder, whereas 68Ga-RM2 showed high accumulation in the pancreas and bladder. Due to this different pattern, 68Ga-RM2 may be more useful for the detection of abdominal and pelvic foci, since bowel uptake and/or clearance may mask small lesions. Wieser et al. instead studied GRPR antagonist 68Ga-RM2 in direct comparison with 18F-fluoroethylcholine (FECH) in 16 patients with biochemical recurrent PCa and in particular in those patients where 18F-FECH PET/CT was either negative or inconclusive. They had 62.5% (10 out of 16) positive scans thus concluding that albeit 68Ga-RM2-PET/CT was helpful to localize PCa recurrence in the majority of the cases, further investigation is necessary to define the role of this promising tracer.
Conclusion
The decisional algorithm of PCa takes in consideration a multidisciplinary approach for each step, from primary diagnosis up to staging, re-staging and therapy. In this context, nuclear medicine already plays a pivotal role in defining disease activity, characterizing the tumor from a functional point of view in patients with relapse and increased level of PSA.[51] Choline derivatives, and in particular 18F-FCH, are currently the most used tracers; however, their diagnostic value lowers in case of biochemical recurrence of PCa with low levels of PSA, where it proved to be not as satisfying. In this sense, PSMA ligands represent the most innovative compounds for PCa diagnosis and therapy, including lymph node and bone metastases assessment, even in patients with low PSA levels and high Gleason scores. The versatility of these radiopharmaceuticals lays in the possible use for diagnostic and therapeutic purposes, targeted radiation delivery or radio-guided surgery, depending on the employed radioisotope. At present, the most promising tracer for SPECT imaging of PCa seems to be 99mTc-MIP-1404, whereas 68Ga-labeled HBED-CC-PSMA is the tracer of choice for PET diagnostic imaging. However, further studies are needed to directly compare all the aforementioned agents under the same conditions and in a greater number of patients, in order to clarify either which agent is superior and in which conditions their use is preferable. Numerous studies with PSMA-ligands at different phases are currently ongoing and we hope that they will define and standardize the approach to PCa patients in any clinical scenario.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are gratefully thankful to Prof. Stefano Fanti and Dr. Andrea Farolfi (Nuclear Medicine Unit, S. Orsola-Malpighi University Hospital, University of Bologna) for their contribution.
References
- 1.Attard G, Parker C, Eeles RA, Schröder F, Tomlins SA, Tannock I, et al. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 2.Fandella A, Scattoni V, Galosi A, Pepe P, Fiorentino M, Gaudiano C, et al. Italian prostate biopsies group: 2016 updated guidelines insights. Anticancer Res. 2017;37:413–24. doi: 10.21873/anticanres.11333. [DOI] [PubMed] [Google Scholar]
- 3.Hövels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin Radiol. 2008;63:387–95. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Maurer MH, Härmä KH, Thoeny H. Diffusion-weighted genitourinary imaging. Radiol Clin North Am. 2017;55:393–411. doi: 10.1016/j.rcl.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Shaish H, Taneja SS, Rosenkrantz AB. Prostate MR imaging: An update. Radiol Clin North Am. 2017;55:303–20. doi: 10.1016/j.rcl.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 6.DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361:955–64. doi: 10.1016/S0140-6736(03)12779-1. [DOI] [PubMed] [Google Scholar]
- 7.Karanika S, Karantanos T, Li L, Corn PG, Thompson TC. DNA damage response and prostate cancer: Defects, regulation and therapeutic implications. Oncogene. 2015;34:2815–22. doi: 10.1038/onc.2014.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5. [PubMed] [Google Scholar]
- 9.NCCN Guidelines: Prostate Cancer. Fort Washington, Pennsylvania: National Comprehensive Cancer Network; 2015. [Google Scholar]
- 10.Mansi L, Ciarmiello A, Cuccurullo V. PET/MRI and the revolution of the third eye. Eur J Nucl Med Mol Imaging. 2012;39:1519–24. doi: 10.1007/s00259-012-2185-x. [DOI] [PubMed] [Google Scholar]
- 11.Cho SY, Szabo Z. Molecular imaging of urogenital diseases. Semin Nucl Med. 2014;44:93–109. doi: 10.1053/j.semnuclmed.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindenberg L, Ahlman M, Turkbey B, Mena E, Choyke P. Advancement of MR and PET/MR in prostate cancer. Semin Nucl Med. 2016;46:536–43. doi: 10.1053/j.semnuclmed.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Høilund-Carlsen PF, Poulsen MH, Petersen H, Hess S, Lund L. FDG in urologic malignancies. PET Clin. 2014;9:457–68. doi: 10.1016/j.cpet.2014.07.003. vi. [DOI] [PubMed] [Google Scholar]
- 14.Jadvar H. Is there use for FDG-PET in prostate cancer? Semin Nucl Med. 2016;46:502–6. doi: 10.1053/j.semnuclmed.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuccurullo V, Cascini G, Rossi A, Tamburrini O, Rotondo A, Mansi L. Pathophysiological premises to radiotracers for bone metastases. Q J Nucl Med Mol Imaging. 2011;55:353–73. [PubMed] [Google Scholar]
- 16.Cuccurullo V, Cascini GL, Tamburrini O, Rotondo A, Mansi L. Bone metastases radiopharmaceuticals: An overview. Curr Radiopharm. 2013;6:41–7. doi: 10.2174/1874471011306010007. [DOI] [PubMed] [Google Scholar]
- 17.Mease RC. Radionuclide based imaging of prostate cancer. Curr Top Med Chem. 2010;10:1600–16. doi: 10.2174/156802610793176774. [DOI] [PubMed] [Google Scholar]
- 18.FDA approves 11C-choline for PET in prostate cancer. J Nucl Med. 2012;53:11N. [PubMed] [Google Scholar]
- 19.Beheshti M, Haim S, Zakavi R, Steinmair M, Waldenberger P, Kunit T, et al. Impact of 18F-choline PET/CT in prostate cancer patients with biochemical recurrence: Influence of androgen deprivation therapy and correlation with PSA kinetics. J Nucl Med. 2013;54:833–40. doi: 10.2967/jnumed.112.110148. [DOI] [PubMed] [Google Scholar]
- 20.Cuccurullo V, Di Stasio GD, Evangelista L, Castoria G, Mansi L. Biochemical and pathophysiological premises to positron emission tomography with choline radiotracers. J Cell Physiol. 2017;232:270–5. doi: 10.1002/jcp.25478. [DOI] [PubMed] [Google Scholar]
- 21.Calabria F, Gallo G, Schillaci O, Cascini GL. Bio-distribution, imaging protocols and diagnostic accuracy of PET with tracers of lipogenesis in imaging prostate cancer: A comparison between 11C-choline, 18FFluoroethylcholine and 18F-methylcholine. Curr Pharm Des. 2015;21:4738–47. doi: 10.2174/1381612821666150818110422. [DOI] [PubMed] [Google Scholar]
- 22.Evangelista L, Cervino AR, Guttilla A, Zattoni F, Cuccurullo V, Mansi L. 18 F-fluoromethylcholine or 18F-fluoroethylcholine pet for prostate cancer imaging: Which is better? A literature revision. Nucl Med Biol. 2015;42:340–8. doi: 10.1016/j.nucmedbio.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Chondrogiannis S, Marzola MC, Ferretti A, Grassetto G, Maffione AM, Rampin L, et al. Is the detection rate of 18F-choline PET/CT influenced by androgen-deprivation therapy? Eur J Nucl Med Mol Imaging. 2014;41:1293–300. doi: 10.1007/s00259-014-2720-z. [DOI] [PubMed] [Google Scholar]
- 24.Chondrogiannis S, Marzola MC, Grassetto G, Maffione AM, Rampin L, Veronese E, et al. New acquisition protocol of 18F-choline PET/CT in prostate cancer patients: Review of the literature about methodology and proposal of standardization. Biomed Res Int. 2014;2014:215650. doi: 10.1155/2014/215650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mertens K, Slaets D, Lambert B, Acou M, De Vos F, Goethals I. PET with (18) F-labelled choline-based tracers for tumour imaging: A review of the literature. Eur J Nucl Med Mol Imaging. 2010;37:2188–93. doi: 10.1007/s00259-010-1496-z. [DOI] [PubMed] [Google Scholar]
- 26.Mansi L, Cuccurullo V, Evangelista L. Is radiocholine PET/CT already clinically useful in patients with prostate cancer? J Nucl Med. 2014;55:1401–3. doi: 10.2967/jnumed.114.142679. [DOI] [PubMed] [Google Scholar]
- 27.Haffner MC, Laimer J, Chaux A, Schäfer G, Obrist P, Brunner A, et al. High expression of prostate-specific membrane antigen in the tumor-associated neo-vasculature is associated with worse prognosis in squamous cell carcinoma of the oral cavity. Mod Pathol. 2012;25:1079–85. doi: 10.1038/modpathol.2012.66. [DOI] [PubMed] [Google Scholar]
- 28.Chakraborty PS, Kumar R, Tripathi M, Das CJ, Bal C. Detection of brain metastasis with 68Ga-labeled PSMA ligand PET/CT: A novel radiotracer for imaging of prostate carcinoma. Clin Nucl Med. 2015;40:328–9. doi: 10.1097/RLU.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 29.Santoni M, Scarpelli M, Mazzucchelli R, Lopez-Beltran A, Cheng L, Cascinu S, et al. Targeting prostate-specific membrane antigen for personalized therapies in prostate cancer: Morphologic and molecular backgrounds and future promises. J Biol Regul Homeost Agents. 2014;28:555–63. [PubMed] [Google Scholar]
- 30.Manyak MJ. Indium-111 capromab pendetide in the management of recurrent prostate cancer. Expert Rev Anticancer Ther. 2008;8:175–81. doi: 10.1586/14737140.8.2.175. [DOI] [PubMed] [Google Scholar]
- 31.Kitson SL, Cuccurullo V, Moody TS, Mansi L. Radionuclide antibody-conjugates, a targeted therapy towards cancer. Curr Radiopharm. 2013;6:57–71. doi: 10.2174/1874471011306020001. [DOI] [PubMed] [Google Scholar]
- 32.Giesel FL, Sterzing F, Schlemmer HP, Holland-Letz T, Mier W, Rius M, et al. Intra-individual comparison of (68)Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:1400–6. doi: 10.1007/s00259-016-3346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Eyben FE, Kairemo K. Acquisition with (11)C-choline and (18)F-fluorocholine PET/CT for patients with biochemical recurrence of prostate cancer: A systematic review and meta-analysis. Ann Nucl Med. 2016;30:385–92. doi: 10.1007/s12149-016-1078-7. [DOI] [PubMed] [Google Scholar]
- 34.Zang S, Shao G, Cui C, Li TN, Huang Y, Yao X, et al. 68Ga-PSMA-11 PET/CT for prostate cancer staging and risk stratification in Chinese patients. Oncotarget. 2017;8:12247–58. doi: 10.18632/oncotarget.14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Leeuwen PJ, Stricker P, Hruby G, Kneebone A, Ting F, Thompson B, et al. (68) Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016;117:732–9. doi: 10.1111/bju.13397. [DOI] [PubMed] [Google Scholar]
- 36.Benešová M, Schäfer M, Bauder-Wüst U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56:914–20. doi: 10.2967/jnumed.114.147413. [DOI] [PubMed] [Google Scholar]
- 37.Ahmadzadehfar H, Rahbar K, Kürpig S, Bögemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with 177Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: A two-centre study. EJNMMI Res. 2015;5:1. doi: 10.1186/s13550-015-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: A multicenter retrospective analysis. J Nucl Med. 2016;57:1334–8. doi: 10.2967/jnumed.116.173757. [DOI] [PubMed] [Google Scholar]
- 39.Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. Lutetium-177 PSMA radioligand therapy of metastatic castration-resistant prostate cancer: Safety and efficacy. J Nucl Med. 2016;57:1006–13. doi: 10.2967/jnumed.115.168443. [DOI] [PubMed] [Google Scholar]
- 40.Umbricht CA, Benešová M, Schmid RM, Türler A, Schibli R, van der Meulen NP, et al. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617-preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017;7:9. doi: 10.1186/s13550-017-0257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grubmüller B, Baum RP, Capasso E, Singh A, Ahmadi Y, Knoll P, et al. 64Cu-PSMA-617 PET/CT imaging of prostate adenocarcinoma: First in-human studies. Cancer Biother Radiopharm. 2016;31:277–86. doi: 10.1089/cbr.2015.1964. [DOI] [PubMed] [Google Scholar]
- 42.Calabria F, Gangemi V, Gullà D, Schillaci O, Cascini GL. 64Cu-PSMA uptake in meningioma: A potential pitfall of a promising radiotracer. Rev Esp Med Nucl Imagen Mol. 2017;36:335–6. doi: 10.1016/j.remn.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Rowe SP, Macura KJ, Ciarallo A, Mena E, Blackford A, Nadal R, et al. Comparison of prostate-specific membrane antigen-based 18F-DCFBC PET/CT to conventional imaging modalities for detection of hormone-naïve and castration-resistant metastatic prostate cancer. J Nucl Med. 2016;57:46–53. doi: 10.2967/jnumed.115.163782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowe SP, Gorin MA, Allaf ME, Pienta KJ, Tran PT, Pomper MG, et al. PET imaging of prostate-specific membrane antigen in prostate cancer: Current state of the art and future challenges. Prostate Cancer Prostatic Dis. 2016;19:223–30. doi: 10.1038/pcan.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demirkol MO, Acar Ö, Uçar B, Ramazanoglu SR, Saglican Y, Esen T. Prostate-specific membrane antigen-based imaging in prostate cancer: Impact on clinical decision making process. Prostate. 2015;75:748–57. doi: 10.1002/pros.22956. [DOI] [PubMed] [Google Scholar]
- 46.Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with Lu-177 labeled PSMA-617. J Nucl Med. 2016;57:1170–6. doi: 10.2967/jnumed.115.171397. [DOI] [PubMed] [Google Scholar]
- 47.Robu S, Schottelius M, Eiber M, Maurer T, Gschwend J, Schwaiger M, et al. Preclinical Evaluation and First Patient Application of 99mTc-PSMA-I&S for SPECT Imaging and Radioguided Surgery in Prostate Cancer. J Nucl Med. 2017;58:235–242. doi: 10.2967/jnumed.116.178939. [DOI] [PubMed] [Google Scholar]
- 48.Maurer T, Weirich G, Schottelius M, Weineisen M, Frisch B, Okur A, et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur Urol. 2015;68:530–4. doi: 10.1016/j.eururo.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 49.Maurer T, Schwamborn K, Schottelius M, Wester HJ, Schwaiger M, Gschwend JE, et al. PSMA theranostics using PET and subsequent radioguided surgery in recurrent prostate cancer. Clin Genitourin Cancer. 2016;14:e549–52. doi: 10.1016/j.clgc.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 50.Su HC, Zhu Y, Ling GW, Hu SL, Xu XP, Dai B, et al. Evaluation of 99mTc-labeled PSMA-SPECT/CT imaging in prostate cancer patients who have undergone biochemical relapse. Asian J Androl. 2017;19:267–71. doi: 10.4103/1008-682X.192638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu CY, Desai B, Ji L, Groshen S, Jadvar H. Comparative performance of PET tracers in biochemical recurrence of prostate cancer: A critical analysis of literature. Am J Nucl Med Mol Imaging. 2014;4:580–601. [PMC free article] [PubMed] [Google Scholar]


