Abstract
A cDNA encoding a chitinase-related receptor-like kinase, designated CHRK1, was isolated from tobacco (Nicotiana tabacum). The C-terminal kinase domain (KD) of CHRK1 contained all of the conserved amino acids of serine/threonine protein kinases. The putative extracellular domain was closely related to the class V chitinase of tobacco and to microbial chitinases. CHRK1 mRNA accumulation was strongly stimulated by infection with fungal pathogen and tobacco mosaic virus. Amino acid-sequence analysis revealed that the chitinase-like domain of CHRK1 lacked the essential glutamic acid residue required for chitinase activity. The recombinant chitinase-like domain did not show any catalytic activity for either oligomeric or polymeric chitin substrates. The recombinant KD of CHRK1 exhibited autophosphorylation, but the mutant KD with a mutation in the essential ATP-binding site did not, suggesting that CHRK1 encoded a functional kinase. CHRK1 was detected in membrane fractions of tobacco BY2 cells. Furthermore, CHRK1-GFP fusion protein was localized in plasma membranes when it was expressed in animal cells. This is the first report of a new type of receptor-like kinase containing a chitinase-like sequence in the putative extracellular domain.
Plant receptor-like kinases (RLKs) play a fundamental role in various cellular processes, including hormone signaling (Ecker, 1995; Li and Chory, 1997), self-incompatibility (Nasrallah et al., 1994), regulation of plant development (Becraft et al., 1996; Lee et al., 1996, 1997; Clark et al., 1997; Jinn et al., 2000), and plant-pathogen interactions (Martin et al., 1993; Song et al., 1995). Plant RLKs show variations in their structure, especially in the extracellular domain, which probably enable them to selectively respond to diverse extracellular signals (Clark, 1996; Lease et al., 1998).
Various biochemical studies (Dixon et al., 1994; Suzuki and Shinshi, 1995) and recent cloning of resistance genes (Martin et al., 1993; Song et al., 1995), such as Pto (encoding a Ser/Thr kinase) and Xa21 (encoding an RLK), indicate the central role of protein phosphorylation in pathogen signaling. Resistance genes are thought to encode receptors that interact with race-specific elicitors as ligands. When the signal is perceived, resistance gene products activate a wide array of defense responses, which result in highly effective disease resistance. Non-race-specific elicitors, such as oligosaccharides, peptides, and glycoproteins released from fungal or plant cell walls, also induce various biochemical responses that slow pathogen growth but often are not as effective as resistance gene-mediated responses in blocking disease (Bent, 1996). However, non-race-specific elicitors induce defense reactions against a broad spectrum of pathogens, in contrast to resistance gene-mediated responses. Although the molecular basis of signal transduction via non-race-specific elicitors is poorly understood, various studies have suggested that a specific receptor is involved in perceiving and transducing the signal (Benhamou, 1996).
High-affinity binding sites for non-race-specific elicitors have been identified in membrane preparations of several plant species. Soybean root cells contained a high affinity binding protein for hepta-β-glucoside (Cheong and Hahn, 1991), and recently Umemoto et al. (1997) isolated a β-glucan-elicitor-binding protein from soybean root cells. This protein showed homology to three proteins from yeast, the functions of which are unknown. Chitin oligosaccharides also induce various defense responses, including the oxidative burst, phosphorylation of specific proteins, phytoalexin biosynthesis, and transcriptional activation of defense genes (Benhamou, 1996). High-affinity binding sites for the chitin elicitor were found in both rice and tomato suspension-cultured cells (Shibuya et al., 1993; Baureithel et al., 1994), and a 75-kD protein that binds to the N-acetylchitooligosaccharide elicitor has been identified in rice using affinity labeling (Ito et al., 1997). Molecular characterization of the protein should be performed to understand the significance of its binding to the chitin elicitor and its biological function.
In this study we have isolated a cDNA encoding a novel RLK containing a chitinase-related sequence in its putative extracellular domain. Accumulation of chitinase-related RLK1 (CHRK1) mRNA was strongly stimulated by fungal pathogen and tobacco mosaic virus (TMV) infection. The CHRK1 kinase domain (KD) exhibited kinase activity, whereas the chitinase-related domain did not show any detectable chitinase activity. CHRK1 appears to be localized in membranes in plant cells. A possible function of this protein as a receptor for the chitin oligosaccharide signal is discussed based on these findings.
RESULTS
Isolation of a cDNA Clone Encoding a Chitinase-Related RLK
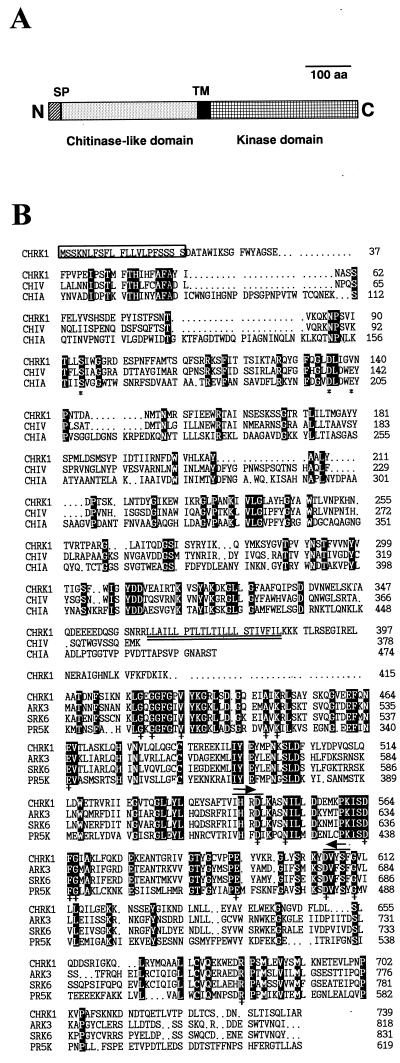
In an attempt to identify kinases that play a role in anther/pollen development, reverse transcriptase (RT)-PCR was performed with two degenerate kinase primers using total RNA from tobacco (Nicotiana tabacum) anthers. It resulted in amplified DNA fragments of about 210 bp, which putatively encode kinases. PCR products were cloned and sequenced. One of the clones that contained a sequence homologous to many plant RLKs was used as a probe to screen a tobacco flower bud cDNA library. From 7.5 × 105 phages screened, two independent positive clones were obtained, and both encoded the same protein. The longest cDNA was 2,943 bp in length and encoded a protein of 739 amino acids with structural features of an RLK (Fig. 1A). Its predicted Mr was 81,643. The N-terminal end of the protein contained a signal peptide of 21 amino acids, which showed conserved structural features of signal peptides characterized in yeast, animal, and plant systems (von Heijne, 1983). The putative extracellular domain was homologous to plant and microbial chitinases. A central transmembrane domain consisted of 23 hydrophobic amino acids (double underlined in Fig. 1B) followed by three positively charged residues. The sequence of the transmembrane region resembled the Leu zipper motif, which is involved in protein dimerization in many transcription factors (Busch and Sassone-Corsi, 1990). The cytoplasmic domain showed significant sequence similarity to other plant RLKs. The clone was designated CHRK1.
Figure 1.
Structure and amino acid sequence comparison of CHRK1. A, Schematic representation of the CHRK1 protein. The signal peptide (SP), the transmembrane region (TM), the putative extracellular domain, and the KD are indicated. B, Deduced amino acid sequence of CHRK1 and alignment with related sequences. The deduced amino acid sequence of the CD of CHRK1 was aligned with the sequences from class V chitinase (CHIV) from tobacco (Melchers et al., 1994) and chitinase A1 (CHIA) from B. circulans (Watanabe et al., 1990). The predicted KD was aligned with the KD of the plant RLKs, SRK6 of Brassica (Stein et al., 1991), and ARK3 (Dwyer et al., 1994) and PR5K (Wang et al., 1996) of Arabidopsis. The number on the right indicates the amino acid residues. Gaps, which were introduced to maximize alignment, are indicated by dots. Residues conserved among all sequences compared here are highlighted in reverse contrast letters. Asterisks indicate the three conserved residues that are important for chitinase activity. Crosses (+) indicate residues that are conserved in all Ser/Thr-type kinases. Asterisks and crosses were written below the relevant amino acids. The putative signal peptide is boxed. The transmembrane region is indicated with double underlines. The amino acid residues corresponding to the degenerate oligonucleotides for PCR amplification are marked with overlines and arrows.
Amino acid sequence comparison of CHRK1 with several plant and bacterial chitinases and the KDs of representative plant RLKs is shown in Figure 1B. The sequence alignment was performed using the CLUSTAL W multiple sequence alignment program (version 1.7; Thompson et al., 1994). The putative extracellular domain (361 amino acids) of CHRK1 shows homology to class V tobacco chitinases (41% amino acid sequence identity; Melchers et al., 1994), and to chitinases from Bacillus circulans WL-12 (23% identity; Watanabe et al., 1990) and Serratia marcescens (19% identity; Harpster and Dunsmuir, 1989). However, the domain is not closely related to other classes of plant chitinases. The KD (355 amino acids) of CHRK1 contains all of 11 conserved subdomains and 15 invariant amino acid residues of eukaryotic Ser/Thr protein kinases (Fig. 1B). This domain is closely related to plant RLKs, including ARK3 from Arabidopsis (50% identity; Dwyer et al., 1994), SRK6 from Brassica oleracea (47% identity; Stein et al., 1991), and PR5K from Arabidopsis (36% identity; Wang et al., 1996).
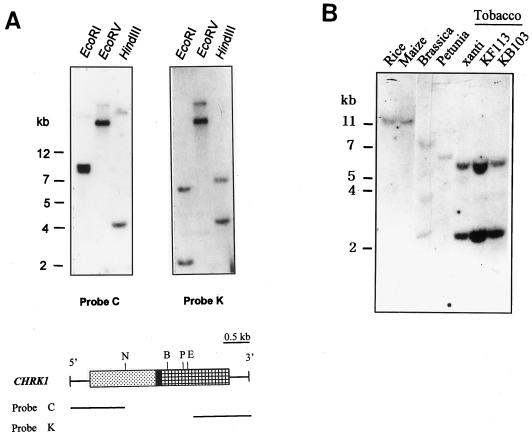
Genomic Southern Blot
Genomic Southern-blot analysis was carried out with two probes corresponding to the chitinase-like domain (CD) and the KD (Fig. 2A). Genomic DNA was digested with EcoRI, EcoRV, and HindIII. Two to three hybridizing bands were detected with both probes. In EcoRV digestion, the same band pattern was observed with both the chitinase probe and the kinase probe, suggesting that CHRK1 gene contained both domains. The difference in the hybridization patterns between the two probes in EcoRI and HindIII digestion may be due to the presence of an intron and the presence of an EcoRI site in the cDNA. Considering that tobacco is amphidiploid between Nicotiana tomentosiformis and Nicotiana sylvestris, these results suggest that CHRK1 gene is most likely present as a single copy in the tobacco genome. The chitinase probe did not cross-hybridize with the class V chitinase gene, which showed a different hybridization pattern (Melchers et al., 1994).
Figure 2.
DNA gel-blot analysis of CHRK1 gene. A, Genomic DNA gel- blot analysis. DNA gel blots were hybridized with the chitinase (C) or the kinase (K) probe. The DNA size markers are indicated in kb. Coding regions of CHRK1 cDNA are boxed. E, EcoRI; B, BglII; P, PstI; N, NdeI. B, Genomic Southern-blot analysis of CHRK1-related sequences in other plant species. Each lane represents 10 μg of genomic DNA fragmented with EcoRI. The blot was hybridized with the kinase probe. The DNA size markers are indicated in kb.
To determine whether homologs of the CHRK1 gene are present in other plant species, DNA gel-blot analysis was carried out with genomic DNAs from corn, rice, petunia, and cauliflower. Three cultivars of tobacco were also examined. Under stringent hybridization conditions, the kinase probe detected hybridizing bands in all the species examined, as shown in Figure 2B. This finding indicates that homologs of the CHRK1 gene exist in other species as well.
Expression Patterns of CHRK1
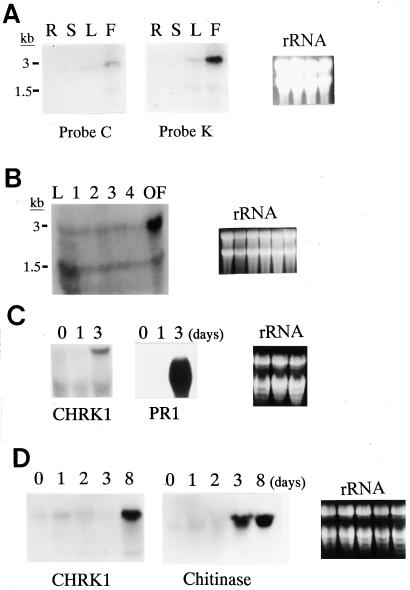
Tissue-specific expression of CHRK1 mRNA was examined by RNA gel-blot analysis using the chitinase and kinase probes (Fig. 3A). Under high stringency, both probes hybridized to 3- and 1.5-kb transcripts, which were present in flowers and leaves. In stems and roots, the transcripts were almost undetectable. The 3-kb transcript was consistent with the size of the isolated cDNA. The 1.5-kb transcript may have been produced by alternative splicing, because both the chitinase and kinase probes hybridized to the 1.5 kb-transcript, indicating that the transcript contained at least a part of both domains. During flower development, CHRK1 mRNA was most highly expressed at the open flower stage, but lower levels were detected in stages 1 to 4 (Fig. 3B).
Figure 3.
Expression of CHRK1 mRNA. A, Tissue-specific expression. Each lane represents 50 μg of total RNA from roots (R), stems (S), leaves (L), or flowers (F). The amount of ethidium bromide-stained rRNA was shown to verify equal loading of RNA in each lane. B, Expression of CHRK1 mRNA during flower development. Fifty micrograms of total RNA of flowers from stage 1 to open flower stage, and from leaves (L) is represented in each lane. The five developmental stages are defined by bud size: <1 cm, stage 1; 1 to 2 cm, stage 2; 2 to 3 cm, stage 3; 3 to 4 cm, stage 4; open flower, stage OF. The K probe was used. C, Expression of CHRK1 mRNA in response to TMV infection. Fifty micrograms of total RNA was used in each lane. Lane 1 contains RNA from uninfected leaves; lane 2 contains RNA from leaves 1 d after infection; lane 3 contains RNA from leaves 3 d after infection. Duplicate membranes were hybridized with PR-1a probe as a control. The size of the PR1 transcripts is approximately 0.9 kb. The K probe was used. D, Expression of CHRK1 mRNA in response to fungal pathogen infection. Young tobacco plants were inoculated through roots with P. parasitica, which causes black shank disease in tobacco. Total RNA was prepared from leaves collected at 0, 1, 2, 3, and 8 d after infection. Fifty micrograms of total RNA was used in each lane. The K probe was used. Duplicate membranes were hybridized with chitinase probe as a control. The size of the chitinase transcripts is approximately 1.2 kb.
Infection by TMV resulted in accumulation of higher levels of CHRK1 transcripts in leaves at 3 d after inoculation, but not at 1 d after inoculation (Fig. 3C, left). Mock-inoculation did not result in induction at either time (results not shown). Strong induction of the 0.9-kb PR-1 transcripts was observed in leaves at 3 d after inoculation (Fig. 3C, right). To investigate CHRK1 gene expression upon fungal pathogen infection, young tobacco plants were infected through roots with Phytophthora parasitica, which causes black shank disease in tobacco. The CHRK1 mRNA level increased in leaves at 8 d after infection when disease symptoms appeared in lower parts of stems and bottom leaves of the infected plants, but not at 1, 2, or 3 d after infection, when no visible symptoms were observed in the plants (Fig. 3D, left). Fungus infection also stimulated the accumulation of the 1.2-kb chitinase transcripts, but at 3 d after infection. Mock-inoculation did not result in induction at any time points (results not shown). In contrast, treatments of tobacco BY2 cells with chitosan, N-acetylchitooligosaccharides (N-acetylchitotriose and N-acetylchitotetraose), fungal elicitors, benzo[1,2,3]thiadiazole-7-carbothioic acid-S-methyl ester (BTH), or methyl jasmonate did not change the level of CHRK1 mRNA, whereas various transcripts for PR proteins accumulated to higher levels (results not shown).
Autophosphorylation of the KD of CHRK1
The CDs and KDs were expressed in Escherichia coli as fusion proteins with thioredoxin for biochemical analyses. Two cDNAs encoding the CD (residues 30–359) and the KD (residues 390–739) were subcloned into the pET32a vector, and the expression of the recombinant proteins was induced by isopropylthio-β-galactoside. The thioredoxin-KD and thioredoxin-CD fusion proteins were purified using nickel resin following the manufacturer's protocol. The protein profiles after purification are shown in Figure 4.
Figure 4.
Expression of the CD and the KD of CHRK1 in E. coli. The CD and KD of CHRK1 were expressed in E. coli as fusion proteins with thioredoxin using the pET32a vector. The protein profiles after purification were analyzed by SDS-PAGE. Arrowheads indicate the electrophoretic positions of thioredoxin (Trx), thioredoxin-KD, and thioredoxin-CD proteins. The sizes of molecular mass markers are also indicated.
To determine whether CHRK1 encodes an active protein kinase, the fusion protein containing the KD was digested with enterokinase to remove the thioredoxin moiety. The resulting KD was assayed for autophosphorylation (Fig. 5). A single 35-kD band was detected by autoradiography. When the KD was omitted from the reaction, no labeled products were produced (results not shown). To determine whether autophosphorylation requires kinase activity, the mutant form of the KD, which carries a mutation in the essential ATP-binding site (Lys-449 to Asn), was analyzed by the same assay. The mutation drastically reduced radiolabeling of the 35-kD band, suggesting that autophosphorylation activity was dependent on the functional kinase (Fig. 5).
Figure 5.
Autophosphorylation of the recombinant KD of CHRK1. Top, Autoradiography; Bottom, Coomassie Blue-stained gel showing the relative amounts of the recombinant proteins. Wild-type (N) and mutant (M) forms of the KD are indicated. In the mutant kinase the essential ATP-binding site, Lys-449, was mutated to Asn.
Lack of Chitinase Activity of CHRK1
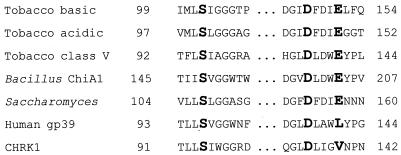
The putative extracellular domain of CHRK1 has homology to the class V chitinase of tobacco and bacterial chitinases, indicating that the domain may possess chitinase activity. In chitinase A1 from B. circulans WL-12, three amino acid residues, Ser, Asp, and Glu (marked with asterisks in Fig. 1B), which are highly conserved in plant and bacterial chitinases, were shown to be important for chitinase activity (Watanabe et al., 1993). The mutation in the Glu residue completely abolished chitinase activity of chitinase A1. Figure 6 shows a comparison of amino acid sequences from the catalytic center of representative chitinases. Among these, functional chitinases can be seen to contain the Glu residue, whereas human cartilage gp-39 protein (HC gp-39; Hakala et al., 1993), which is homologous to microbial chitinases but does not possess chitinase activity, shows the residue replaced by Leu. CHRK1 contains the Val residue (Val-139) in the position of the essential Glu. The presence of the Val residue was verified by sequencing of a PCR-amplified genomic DNA containing the CHRK1 gene.
Figure 6.
Sequence comparison of the conserved residues of chitinases with those of the CHRK1 CD. Three residues conserved in plant and microbial chitinases identified by Watanabe et al. (1993) are shown in bold. The sources of other chitinase sequences are basic and acidic class III chitinases from tobacco (Lawyton et al., 1992); class V chitinase from tobacco (Melchers et al., 1994); chitinase A1 from Bacillus subtilis (Watanabe et al., 1990); chitinase from Saccharomyces cerevisiae (Kuranda and Robbins, 1991); HC gp-39 (Hakala et al., 1993); and CHRK1.
To determine if CHRK1 exhibits catalytic activity, a chitinase assay was performed with the recombinant CD either as a fusion protein or as a separate moiety. S. marcescens chitinase was used as a control (Table I). Using 4-methylumbelliferyl-N,N′,N"-triacetylchitotriose [4-MU-(GlcNAc)3], 4-methylumbelliferyl-N,N′,N",N‴-tetraacetylchitotetraose [4-MU-(GlcNAc)4], chitin azure, and regenerated chitin as substrates, the recombinant CD in either form failed to exhibit either endo- or exo-chitinase activity (Table I).
Table I.
Chitinase assay of the recombinant chitinase-like domain of CHRK1
| Substrates | Control Chitinasea | CHRK1 |
|---|---|---|
| 4-MU-(GlcNAc)3 | >1,000 | <10 |
| 4-MU-(GlcNAc)4 | >1,000 | <10 |
| Chitin azureb | 0.26 | <0.005 |
| Regenerated chitin | 0.18 | <0.002 |
The recombinant CD, both as a fusion protein with thioredoxin and as a separate moiety, was assayed. Only data obtained with the fusion protein are presented, since the protein in both forms produced similar results. Measuring units for chitinase assays using 4-MU-(GlcNAc)3 and 4-MU-(GlcNAc)4 are fluorescence units. Measuring units for chitinase assays using chitin azure and regenerated chitin are optical density units.
As a control, S. marcescens chitinase was used.
Remazol Brilliant Violet conjugated to carboxymethyl-chitin.
Immunodetection of CHRK1 in the Membrane
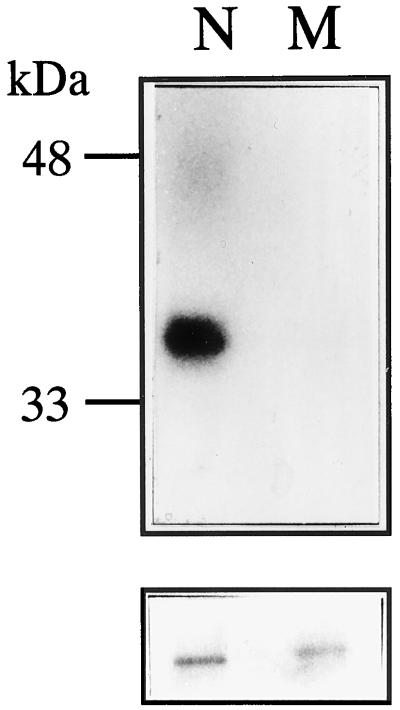
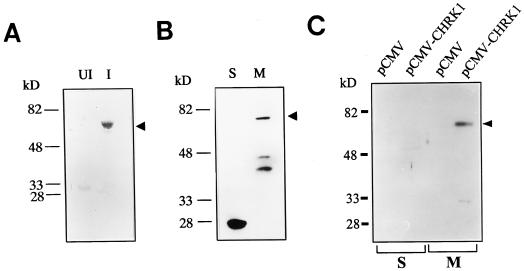
Polyclonal rabbit antiserum was raised using the recombinant CD. The antiserum specifically recognized the recombinant CD from E. coli total proteins only after induction (Fig. 7A). To detect CHRK1 in plant cells, immunoblotting of soluble proteins and membrane proteins of tobacco BY2 cells was performed using the antiserum (Fig. 7B). The antiserum detected a 75-kD protein and two other proteins of lower molecular mass in the membrane fraction. A single 28-kD protein was detected in the soluble fraction. The 75-kD protein in the membrane fraction likely represents CHRK1, whereas the other two proteins may represent the proteolytic fragments from CHRK1 or chitinases associated with the membrane (Fig. 7B). Since the predicted molecular mass of CHRK1 is approximately 80 kD, the apparent size discrepancy may be due to the structural conformation of CHRK1 in the membrane or post-translational modification. The 28-kD protein detected in the soluble fraction may be a cytosolic chitinase that has similar epitopes, since the antiserum was polyclonal and was raised against the whole CD. However, the cDNA probe corresponding to the CD did not cross-hybridize with other genes in genomic Southern-blot analysis (Fig. 2A), suggesting that the gene encoding the 28-kD protein is not closely related to CHRK1 in nucleotide sequences.
Figure 7.
Immunodetection of CHRK1 protein. A, Immunoblotting of the recombinant CD with anti-CHRK1 antiserum. Ten micrograms of protein extracts from uninduced (UI) and induced (I) E. coli cells that carry the pET32a-CD plasmid was subjected to immunoblotting. The recombinant CHRK1 is marked with the arrowhead. B, Immunodetection of CHRK1 proteins in membranes of tobacco BY2 cells. Thirty micrograms of soluble (S) and membrane (M) proteins from BY2 cells was subjected to immunoblotting. The CHRK1 protein is marked with the arrowhead. Molecular mass markers are indicated in kD. C, Immunodetection of CHRK1 protein expressed in animal cells. CHRK1 protein was transiently expressed in human 293T cells under the control of CMV promoter. Thirty micrograms of S and M proteins isolated from transfected cells with pCMV or pCMV-CHRK1 was subjected to immunoblotting. The expressed CHRK1 protein is marked with the arrowhead. Molecular mass markers are indicated in kD.
To gain evidence that the 75-kD protein detected in the membrane fraction of tobacco cells is CHRK1, we attempted to transiently express the CHRK1 cDNA in animal cells and to immunodetect the protein using the CHRK1 polyclonal antiserum (Fig. 7C). The recombinant plasmid containing the whole CHRK1 cDNA fused to the cytomegalovirus (CMV) promoter (pCMV-CHRK1), or the pCMV-Tag1 vector alone (pCMV) was transfected into human 293T cells, and soluble and membrane fractions were isolated from the cells. No protein band was detected by the antiserum from pCMV-transfected cells in either fraction, but a major protein band in size of approximately 75 kD was detected from pCMV-CHRK1-transfected cells only in the membrane fraction. These results indicate that the CHRK1 protein expressed in animal cells is targeted to the membranes and that the 75-kD protein detected in tobacco membranes is CHRK1.
Membrane Localization of the CHRK1-GFP Protein Expressed in Human Cells
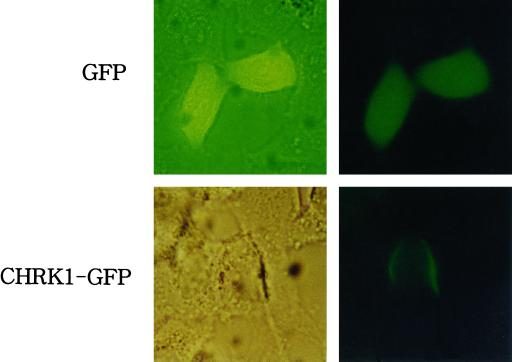
Membrane localization of CHRK1 was further examined by expressing a fusion protein between CHRK1 and a green fluorescent protein (CHRK1-GFP) in animal cells under the control of CMV promoter (Fig. 8). DNA constructs encoding CHRK1-GFP fusion protein or GFP alone were transfected into HeLa cells. After incubation for 72 h, expression of the introduced genes was examined under a fluorescent microscope. The CHRK1-GFP protein was clearly localized in the plasma membrane, whereas GFP was localized in the cytosol (Fig. 8). This result provides further evidence of membrane localization of CHRK1.
Figure 8.
Membrane localization of the CHRK1-GFP fusion protein expressed in animal cells. The CHRK1-GFP and GFP alone were transiently expressed in HeLa cells. The left and the right panel represent light microscopic image and fluorescent microscopic image of individual cells, respectively. A fluorescence filter set 09 (Zeiss, Jena, Germany) was used to observe the fluorescence signal.
DISCUSSION
Recent molecular genetic evidence has revealed that RLKs are involved in diverse processes of plant physiology (Braun and Walker, 1996; Clark, 1996; Lease et al., 1998). Based on the characteristics of the extracellular domain, plant RLKs have been divided into groups (Braun and Walker, 1996), but many new forms have recently been identified, suggesting their interaction with various ligands (He et al., 1996, 1999; Herve et al., 1996). CHRK1 encodes a previously unreported kind of RLK, which contains a chitinase-related sequence in its putative extracellular domain. The CHRK1 KD possesses functional kinase activity. In tobacco cells, CHRK1 is localized in the membrane. Furthermore, CHRK1-GFP fusion protein is clearly localized in the plasma membrane when the protein was expressed in human cells. The unique structure of CHRK1 and its membrane localization raises a possibility that this protein may be a cell surface receptor for transduction of the chitin oligosaccharide signal.
Although the putative extracellular domain of CHRK1 shows significant amino acid sequence homology to chitinases, we found that it does not possess chitinase activity. Watanabe et al. (1993) showed that two residues, Glu-204 and Asp-200, are critical for the catalytic activity of the chitinase A1 of B. circulans WL-12, proposing that the two residues are directly involved in the catalytic events of the enzyme. Most notably, the Glu residue was absolutely required for the activity. CHRK1, which lacks the essential Glu residue, does not possess any detectable chitinase activity.
Despite its sequence homology to chitinases, HC gp-39 does not possess any glycosidic activity against chitin substrates, and this protein also lacks the essential Glu residue (Hakala et al., 1993). According to Renkema et al. (1998), human chitotriosidase, which is highly homologous to HC gp-39 but possesses chitinase activity, has a similar amino acid sequence in its catalytic center, the major exception being that it contains the essential Glu residue. When the Glu residue of chitotriosidase was mutated to Leu, a loss of hydrolytic activity resulted. However, the capacity to tightly bind to chitin, like HC gp-39, was created. Based on the result, it was hypothesized that the slightly modified catalytic center of HC gp-39 is responsible for its lectin properties (Renkema et al., 1998). Similarly, the change from Glu to Val in CHRK1 may enable the protein to bind chitin molecules with higher affinity but not to hydrolyze them.
CHRK1 mRNA accumulation is significantly stimulated by fungal pathogen and TMV infection (Fig. 3, C and D), suggesting that CHRK1 may be involved in pathogen signaling. In many cases, the expression of genes encoding components of a given signaling pathway is up-regulated by the corresponding stimulus, as exemplified by CTR1 and Nr in ethylene signaling (Kieber et al., 1993; Wilkinson et al., 1995), NIMI in pathogen signaling (Ryals et al., 1997), and CIP1 in light signaling (Yamamoto et al., 1998). In plant cells the CHRK1 protein appears to be localized in the membrane (Fig. 7B). When fungal pathogens invade plants, the chitinases that are present in the extracellular space of plant cells may hydrolyze the fungal cell wall. The resulting chitin oligosaccharides may bind to the CD of CHRK1, which activates the KD of the protein. In this respect, it is interesting that tobacco class V chitinase, to which CHRK1 shows the highest homology, more readily hydrolyzes chitin oligomers than chitin polymers (Brunner et al., 1998), indicating that CHRK1 may have evolved to form a structure more suitable for binding to chitin oligomers. After chitin binding, the activated CHRK1 may transduce the elicitor signal by phosphorylating its substrates, and as a result of signal transduction, various cellular defense reactions may ensue. Despite this intriguing possibility, the in vivo functions of CHRK1 remain to be demonstrated. Molecular genetic approaches, such as antisense RNA techniques and dominant negative mutants, would be direct approaches to determine functions of CHRK1. In addition, identifying interacting signaling components may also provide insights into cellular functions of this kinase.
MATERIALS AND METHODS
Plant Material
Tobacco (Nicotiana tabacum cv Xanthi) plants were cultivated in a greenhouse under a regime of 16 h of light and 8 h of dark.
RT-PCR and cDNA Library Screening
Using RNA from tobacco anthers, RT-PCR was performed with degenerate oligonucleotides corresponding to amino acid residues IHRDL (sense primer) and DVWSF (antisense primer). Amplified DNA fragments of about 210 bp, which putatively encode kinases, were generated. PCR products were cloned and sequenced. One of the clones, which contained a sequence homologous to plant RLKs, was used as a probe to screen a λZAPII flower bud cDNA library of tobacco. The probe was 32P-labeled by Random Primed DNA labeling kit (Boehringer Mannheim, Mannheim, Germany). After phage lifting, the membranes (Hybond-N, Amersham, Buckinghamshire, UK) were hybridized overnight at 60°C in 6× SSC, 5× Denhardt's, 0.5% (w/v) SDS, and 100 μg mL−1 denatured, fragmented salmon sperm DNA. After hybridization, the membranes were washed for 15 min at room temperature in 1× SSC and 1% (w/v) SDS and then for 1 h at 60°C in 0.1× SSC and 0.1% (w/v) SDS. Two independent clones were obtained from 7.5 × 105 phages screened, and the positive phage clones were converted to phagemid following the manufacturer's instructions (Stratagene, La Jolla, CA). By sequencing, both of the clones were found to encode the same kinase. The longest cDNA clone was selected for further study. The nucleotide and amino acid sequence analysis was carried out using the PCGENE program (Intelligenetics, University of Geneva).
DNA Gel-Blot Analysis
The genomic DNA isolated from tobacco leaves was digested with EcoRI, EcoRV, and HindIII, electrophoresed on a 0.8% (w/v) agarose gel in the presence of ethidium bromide (0.1 μg mL−1), and blotted onto Hybond-N Nylon membrane (Amersham). The cDNA fragments corresponding to the KD and the CD of CHRK1 were labeled with [α-32P]dCTP using Random Primed DNA labeling kit (Boehringer Mannheim) and used as domain-specific probes. Prehybridization and hybridization was carried out in 6× SSC, 5× Denhardt's solution, and 0.5% (w/v) SDS at 60°C, overnight. The membranes were washed twice in 2× SSC and 1% (w/v) SDS at room temperature and then washed in 0.1× SSC and 0.1% (w/v) SDS at 60°C for 30 min.
RNA Gel-Blot Analysis
Total RNA was prepared by using Trizol reagent (Gibco/BRL, Cleveland) following the manufacturers' instructions. Approximately 50 μg of total RNA was electrophoresed on an agarose gel containing 5.1% (v/v) formaldehyde and blotted onto Hybond-N Nylon membrane (Amersham). Prehybridization, hybridization, and washing conditions were as described in DNA gel-blot analysis.
The expression of CHRK1 mRNA under conditions that induce PR proteins was examined. Young tobacco leaves were infected with TMV and collected at 1 or 3 d after inoculation. Young tobacco plants were infected through roots with Phytophthora parasitica, which causes black shank disease in tobacco. Total RNA was prepared from leaves collected at 0, 1, 2, 3, and 8 d after infection. As a control, leaves were also mock-inoculated. BY2 cells were separately treated with 100 μg mL−1 chitosan at 2, 6, and 24 h; 1 μm chitin oligosaccharides, N-acetylchitotriose and N-acetylchitotetraose, at 2 and 24 h; 30 μg mL−1 fungal elicitors at 2 and 24 h; 0.2 mm BTH at 1 and 2 d; and 0.1 mm methyl jasmonate at 6 and 24 h. Total RNA was prepared after the treatments and RNA gel-blot analyses were carried out as described above. As a probe, a 32P-labeled cDNA fragment corresponding to the KD of CHRK1 was used. At the same time, membranes were probed with other PR genes to test the efficiency of the treatments. For TMV infection, PR1a cDNA was used as a probe; for fungus infection, chitinase cDNA was used as a probe; for treatments of chitosan, chitin oligosaccharides, fungal elicitors, PAL cDNA were used as a probe; and for BTH and methyl jasmonate treatments, PR1a and PR2b cDNAs were used, respectively.
Expression of Domains of CHRK1 and Preparation of Polyclonal Antibody
PCR products corresponding to the CD (residues 30–359) and KD (residues 390–739) of CHRK1 were obtained using Pwo DNA polymerase (Boehringer Mannheim) and were subcloned into pET32a vector (Novagen, Madison, WI) using BamHI/HindIII sites. The two recombinant plasmids were designated pET32a-CD and pET32a-KD, respectively, corresponding to the CDs and KDs. After induction the thioredoxin-KD and thioredoxin-CD fusion proteins were purified using nickel resin following the manufacturer's instructions (Novagen).
For anti-CHRK1 antibody production, the purified thioredoxin-CD fusion protein was concentrated and dialyzed with Centriprep-10, according to the manufacturer's instructions (Amicon, Beverly, MA). Polyclonal rabbit antibody was prepared at Bio-Synthesis (Lewisville, TX). Before use, the antibody was purified using protein A-agarose beads and a sepharose column linked with Escherichia coli total proteins.
Autophosphorylation Assay
A point mutation from AAA (Lys) to AAC (Asn) was introduced into CHRK1 cDNA by recombinant PCR as described by Higuchi (1990). PCR reaction was carried out with primers MR (5′-ATTGCAATAAACCGGCTTTCAG-3′) and K2 (TCTCCCAATCTAACTGCAG) as a set, and MF (5′-GCTGAAAGCCGGTTTATTGCAA-3′) and K1 (GGATCCGAAGGGATCAG) as a set, using the CHRK1 KD cDNA as a template. After denaturation and annealing of the two PCR products, another PCR reaction was carried out with K1 and K2 primers. The resulting PCR product was digested with BamHI/PstI, and ligated with PstI/DraI fragment of CHRK1 cDNA. The ligated DNA fragment containing BamHI and DraI at the ends was cloned into pET32a vector using HindIII site that was made blunt ended by Klenow treatment and BamHI site.
The recombinant KD and its mutant form, both of which were fused to thioredoxin, were purified with nickel column. After dialysis in 50 mm Tris-HCl (pH 8.0), the protein was digested with enterokinase for 1 h at 37°C and passed through nickel column to remove the thioredoxin moiety. Unbound eluate containing the KD of CHRK1 was collected and used for the analysis. One microgram of the KD was incubated in a 20-μL phosphorylation buffer (25 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], pH 7.5, 1 mm dithiothreitol, 10 mm MgCl2, and 10 mm MnCl2) containing 10 μCi of [γ-32P]ATP (6,000 Ci/mmol) at 37°C for 1 h. The reactions were terminated by the addition of 5× Laemmli sample buffer and electrophoresed on a 10% (w/v) SDS-PAGE. The gel was blotted to nitrocellulose and exposed to x-ray film.
Chitinase Assay
The purified recombinant CD as a fusion protein with thioredoxin or as a separate moiety was used for chitinase assays. Chitinase activity using 4-MU-(GlcNAc)3 (Sigma, St. Louis) and 4-MU-(GlcNAc)4 (Sigma) as substrates was measured as described by Watanabe et al. (1993). One microgram of the purified recombinant protein in either form and 0.1 unit of a control chitinase from Serratia marcescens (Sigma) were incubated for 1 h at 37°C in a 0.1 m sodium phosphate buffer, pH 6.0, containing 5 mm 4-MU-(GlcNAc)3 or 4-MU-(GlcNAc)4. The reaction was terminated by the addition of 1 m Gly-NaOH buffer, pH 10.2. The amount of MU released from both substrates was measured spectrophotometrically with excitation at 360 nm and emission at 450 nm. For chitinase assay using chitin azure, 1 μg of the purified chitinase domain in either form and 0.1 unit of the control chitinase was diluted in 400 μL of 25 mm sodium phosphate, pH 7.0. The reaction was started by the addition of 200 μL of substrate (5 mg mL−1 chitin azure). After 1 h of incubation at 37°C, samples were cooled and spun for 10 min. The absorbance of 200 μL of supernatant solution was measured at 550 nm. Regenerated chitin was prepared according to Molano et al. (1977). One microgram of the purified thioredoxin-CD fusion protein and 5 units of the control chitinase were incubated with 3 g of regenerated chitin in 0.1 m phosphate buffer, pH 6.0, for 2 h at 37°C. The amount of released reducing sugar was measured using dinitrosalicylic acid as described by Chaplin (1986).
Immunoblotting
Immunoblotting was carried out as described by Bollag et al. (1996). The polyclonal rabbit antiserum raised against the CD (residues 30–359) of CHRK1 was used for the analysis. Nitrocellulose membrane containing 10 μg of the total proteins from uninduced and induced E. coli cells that carry pET32a-CD was prepared. The membrane was blocked with 1% (w/v) bovine serum albumin in Tris-buffered saline and 0.1% (w/v) Tween 20 (TBST), reacted with anti-CHRK1 antibody (1:2,000 dilution) in TBST, and washed with TBST. They were then reacted with alkaline phosphatase-conjugated goat anti-rabbit IgG secondary antibody (1:1,000 dilution; Sigma), and the signal was detected by nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate solution (Boehringer Mannheim).
For immunodetection of CHRK1 proteins in plant cells, soluble and microsomal membrane proteins from tobacco BY2 cells were prepared as previously described (Park et al., 1997). Protein concentration was measured by Bradford method (Bradford, 1976) using a protein assay kit (Bio-Rad, Hercules, CA) and bovine serum albumin as the standard. Thirty micrograms of soluble and membrane proteins was separated by SDS-PAGE, blotted to nitrocellulose, and incubated with the CHRK1 antibody (1:2,000 dilution). The proteins were then reacted with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:1,000 dilution; Amersham), and the signal was detected by ECL+Plus (Amersham).
For immunodetection of CHRK1 proteins expressed in the animal cells, the CHRK1 cDNA modified by PCR to contain BamHI and SalI sites at the 5′ and 3′ ends, respectively, was cloned into BamHI/SalI-digested pCMV-Tag1 vector (Stratagene) containing CMV promoter. The recombinant plasmid was designated pCMV-CHRK1. The pCMV-CHRK1 was transfected into human 293T cells as described by Spector et al. (1998). As a control, pCMV-Tag1 vector alone was also transfected into human 293T cells. After 72 h, soluble and microsomal membrane proteins were prepared as described by Spector et al. (1998). Thirty micrograms of the soluble and microsomal proteins from the transfected cells were separated by SDS-PAGE, blotted to nitrocellulose, and reacted with the CHRK1 antibody (1:1,000 dilution) and the secondary antibody as described above. The signal was detected using ECL+Plus (Amersham).
Membrane Localization of the CHRK1-GFP Fusion Protein
The pCMV-CHRK1 was digested with BamHI and SalI, and the resulting CHRK1 DNA fragment was isolated and cloned into BamHI/SalI-digested pEGFP-N1 vector (CLONTECH Laboratories, Palo Alto, CA) to generate a fusion protein between CHRK1 and GFP. To express the CHRK1-GFP fusion protein, the recombinant plasmid was transfected into HeLa cells as described by Spector et al. (1998). For expression of GFP as a control, pEGFP-N1 vector alone was also transfected into HeLa cells. After 72 h, individual cells were viewed under a fluorescent microscope with a fluorescence filter set 09 (Zeiss).
ACKNOWLEDGMENTS
The authors wish to thank Dr. Yong-Hwan Lee (Seoul National University) and Dr. Woon Hyung Yuh (Korea Ginseng and Tobacco Research Institute) for fungus infection, Dr. Gyn An (Pohang University of Science and Technology) for providing tobacco flower cDNA library, and Dr. Chee Harn (Nongwoo Seed Company) for helpful comments on the manuscript.
Footnotes
This work was supported by a grant from Korea Ministry of Agriculture and Forestry.
LITERATURE CITED
- Baureithel K, Felix G, Boller T. Specific, high affinity binding of chitin fragments to tomato cells and membranes: competitive inhibition of binding by derivatives of chitooligosaccharides and a Nod factor of Rhizobium. J Biol Chem. 1994;269:17931–17938. [PubMed] [Google Scholar]
- Becraft PW, Stinard PS, McCarty DR. CRINKLY4: a TNFR-like receptor kinase involved in maize epidermal differentiation. Science. 1996;273:1406–1409. doi: 10.1126/science.273.5280.1406. [DOI] [PubMed] [Google Scholar]
- Benhamou N. Elicitor-induced plant defense pathways. Trends Plant Sci. 1996;1:233–240. [Google Scholar]
- Bent AF. Plant disease resistance genes: function meets structure. Plant Cell. 1996;8:1757–1771. doi: 10.1105/tpc.8.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag DM, Rozycki MD, Edelstein SJ. Protein Methods. New York: Wiley-Liss; 1996. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braun DM, Walker JC. Plant membrane receptors: new pieces in the signaling puzzle. Trends Biochem Sci. 1996;21:70–73. [PubMed] [Google Scholar]
- Brunner F, Stintzi A, Fritig B, Legrand M. Substrate stobacco chitinases. Plant J. 1998;14:225–234. doi: 10.1046/j.1365-313x.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- Busch SJ, Sassone-Corsi P. Dimers, leucine zippers and DNA-binding domains. Trends Genet. 1990;6:36–40. doi: 10.1016/0168-9525(90)90071-d. [DOI] [PubMed] [Google Scholar]
- Chaplin MF. Monosaccharides. In: Chaplin MF, Kennedy JF, editors. Carbohydrate Analysis-Practical Approach. Oxford: IRL Press; 1986. p. 3. [Google Scholar]
- Cheong J-J, Hahn MG. A specific, high-affinity binding site for the hepta-beta-glucoside elicitor exists in soybean membranes. Plant Cell. 1991;3:137–147. doi: 10.1105/tpc.3.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE. Plant cell communication: the world outside the plasma membrane. Trends Plant Sci. 1996;1:406–407. [Google Scholar]
- Clark SE, Williams RW, Meyerowitz EM. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell. 1997;89:575–585. doi: 10.1016/s0092-8674(00)80239-1. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Harrison MJ, Lamb CJ. Early events in the activation of plant defense responses. Annu Rev Phytopathol. 1994;32:479–501. [Google Scholar]
- Dwyer KG, Kandasamy MK, Mahosky DI, Acciai J, Kudish BI, Miller JE, Nasrallah ME, Nasrallah JB. A superfamily of S locus-related sequences in Arabidopsis: diverse structures and expression patterns. Plant Cell. 1994;6:1829–1843. doi: 10.1105/tpc.6.12.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JR. The ethylene signal transduction pathway in plants. Science. 1995;268:667–675. doi: 10.1126/science.7732375. [DOI] [PubMed] [Google Scholar]
- Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803–25810. [PubMed] [Google Scholar]
- Harpster MH, Dunsmuir P. Nucleotide sequence of the chitinase B gene of Serratia marcescens QMB1466. Nucleic Acids Res. 1989;17:5395. doi: 10.1093/nar/17.13.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He ZH, Cheeseman I, He D, Kohorn BD. A cluster of five cell wall-associated receptor kinase genes, Wak1–5, are expressed in specific organs of Arabidopsis. Plant Mol Biol. 1999;39:1189–1196. doi: 10.1023/a:1006197318246. [DOI] [PubMed] [Google Scholar]
- He ZH, Fujiki M, Kohorn BD. A cell wall-associated receptor-like protein kinase. J Biol Chem. 1996;271:19789–19793. doi: 10.1074/jbc.271.33.19789. [DOI] [PubMed] [Google Scholar]
- Herve C, Dabos P, Galaud J-P, Rouge P, Lescure B. Characterization of an Arabidopsis thaliana gene that defines a new class of putative plant receptor kinases with an extracellular lectin-like domain. J Mol Biol. 1996;258:778–788. doi: 10.1006/jmbi.1996.0286. [DOI] [PubMed] [Google Scholar]
- Higuchi R. Recombinant PCR. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols. New York: Academic Press; 1990. pp. 177–183. [Google Scholar]
- Ito Y, Kaku H, Shibuya N. Identification of a high-affnity binding protein for N-acetylchitooligosaccharide elicitor in the plasma membrane of suspension-cultured rice cells by affinity labeling. Plant J. 1997;12:347–356. doi: 10.1046/j.1365-313x.1997.12020347.x. [DOI] [PubMed] [Google Scholar]
- Jinn TL, Stone JM, Walker JC. HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev. 2000;14:108–117. [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldman KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of the protein kinase. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Kuranda MJ, Robbins PW. Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J Biol Chem. 1991;266:19758–19767. [PubMed] [Google Scholar]
- Lawyton K, Payne G, Moyer M, Ryals J. Acidic and basic class III chitinase mRNA accumulation in response to TMV infection of tobacco. Plant Mol Biol. 1992;19:735–743. doi: 10.1007/BF00027070. [DOI] [PubMed] [Google Scholar]
- Lease K, Ingham E, Walker JC. Challenges in understanding RLK function. Curr Opin Plant Biol. 1998;1:388–392. doi: 10.1016/s1369-5266(98)80261-6. [DOI] [PubMed] [Google Scholar]
- Lee H-S, Chung Y-Y, Das C, Karunanandaa B, van Went JL, Mariani C, Kao T-H. Embryo sac development is affected in Petunia inflata plants transformed with an antisense gene encoding the extracellular domain of receptor kinase PRK1. Sex Plant Reprod. 1997;10:341–350. [Google Scholar]
- Lee H-S, Karunanandaa B, McCubbin A, Gilroy S, Kao T-H. PRK1, a receptor-like kinase of Petunia inflata, is essential for post-meiotic development of pollen. Plant J. 1996;9:613–624. [Google Scholar]
- Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Martin GB, Brommonschenkel SH, Chungwongse J, Frary A, Ganal MW, Spivey R, Wu T, Earle ED, Tanksley SD. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science. 1993;262:1432–1436. doi: 10.1126/science.7902614. [DOI] [PubMed] [Google Scholar]
- Melchers LS, Apotheker M, van der Knaap J, Ponstein AS, Sela-Buurlage MB, Bol JF, Cornelissen BJC, van den Elzen PJM, Linthorst HJM. A new class of tobacco chitinases homologous to bacterial exo-chitinases displays antifungal activity. Plant J. 1994;5:469–480. doi: 10.1046/j.1365-313x.1994.5040469.x. [DOI] [PubMed] [Google Scholar]
- Molano J, Duran A, Cabib E. A rapid and sensitive assay for chitinase using tritiated chitin. Anal Biochem. 1977;83:648–656. doi: 10.1016/0003-2697(77)90069-0. [DOI] [PubMed] [Google Scholar]
- Nasrallah JB, Stein JC, Kandasamy MK, Nasrallah ME. Signaling the arrest of pollen tube development in self-incompatible plants. Science. 1994;266:1505–1508. doi: 10.1126/science.266.5190.1505. [DOI] [PubMed] [Google Scholar]
- Park JM, Kang SG, Pih KT, Jang HJ, Piao HL, Yoon HW, Cho MJ, Hwang I-H. A dynamin-like protein, ADL1, is present in membranes as a high-molecular-mass complex in Arabidopsis thaliana. Plant Physiol. 1997;115:763–771. doi: 10.1104/pp.115.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkema GH, Boot RG, Au FL, Donker-Koopman WE, Strijland A, Muijsers AO, Hrebicek M, Aerts JM. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem. 1998;251:504–509. doi: 10.1046/j.1432-1327.1998.2510504.x. [DOI] [PubMed] [Google Scholar]
- Ryals J, Weymann K, Lawton K, Friedrich L, Ellis D, Steiner HY, Johnson J, Delaney TP, Jesse T, Vos P, Uknes S. The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor IκB. Plant Cell. 1997;9:425–439. doi: 10.1105/tpc.9.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya N, Kaku H, Kuchitsu K, Maliarik MJ. Identification of a novel high-affinity binding site for N-acetylchitooligosaccharide elicitor in the membrane fraction from suspension-cultured rice cells. FEBS Lett. 1993;329:75–78. doi: 10.1016/0014-5793(93)80197-3. [DOI] [PubMed] [Google Scholar]
- Song W-Y, Wang G-L, Chen L-L, Kim H-S, Pi L-Y, Holsten T, Gardner J, Wang B, Zhai W-X, Zhu L-H, Fauquet C, Ronald P. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science. 1995;270:1804–1806. doi: 10.1126/science.270.5243.1804. [DOI] [PubMed] [Google Scholar]
- Spector DL, Goldman RD, Leinwand LA. Cells: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1998. [Google Scholar]
- Stein JC, Howlett B, Boyes DC, Nasrallah ME, Nasrallah JB. Molecular cloning of a putative receptor kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc Natl Acad Sci USA. 1991;88:8816–8820. doi: 10.1073/pnas.88.19.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Shinshi H. Transient activation and tyrosine phosphorylation of a protein kinase in tobacco cells treated with a fungal elicitor. Plant Cell. 1995;7:639–647. doi: 10.1105/tpc.7.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DJ, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto N, Kakitani M, Iwamatsu A, Yoshikawa M, Yamaoka N, Ishida I. The structure and function of a soybean beta-glucan-elicitor-binding protein. Proc Natl Acad Sci USA. 1997;94:1029–1034. doi: 10.1073/pnas.94.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983;133:17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Zafian P, Choudhary M, Lawton M. The PR5K receptor protein kinase from Arabidopsis thaliana is structurally related to a family of plant defense proteins. Proc Natl Acad Sci USA. 1996;93:2598–2602. doi: 10.1073/pnas.93.6.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Kobori K, Miyashita K, Fujii T, Sakai H, Uchida M, Tanaka H. Identification of glutamic acid 204 and aspartic acid 200 in chitinase A1 of Bacillus circulans WL-12 as essential residues for chitinase activity. J Biol Chem. 1993;268:18567–18572. [PubMed] [Google Scholar]
- Watanabe T, Suzuki K, Oyanagi W, Ohnishi K, Tanaka H. Gene cloning of chitinase A1 from Bacillus circulans WL-12 revealed its evolutionary relationship to Serratia chitinase and to the type III homology units of fibronectin. J Biol Chem. 1990;265:15659–15665. [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ. An ethylene-inducible component of signal transduction encoded by never-ripe. Science. 1995;270:1807–1809. doi: 10.1126/science.270.5243.1807. [DOI] [PubMed] [Google Scholar]
- Yamamoto YY, Matsui M, Ang LH, Deng XW. Role of COP1 interacting protein in mediating light-regulated gene expression in Arabidopsis. Plant Cell. 1998;10:1083–1094. doi: 10.1105/tpc.10.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]