Abstract
Purpose:
To provide the clinical recommendations for the administration of intravitreal anti-vascular endothelial growth factor (VEGF) drugs especially bavacizumab for ocular vascular diseases including diabetic macular edema, neovascular age-related macular degeneration, myopic choroidal neovascularization, retinal vein occlusion and central serous chorioretinopathy.
Methods:
Twenty clinical questions were developed by the guideline technical committee. Relevant websites and databases were searched to find out the pertinent clinical practice guidelines to answer the questions. The technical committee provided possible answers (scenarios) according to the available evidences for each question. All scenarios along with their levels of evidence and the supported articles were sent to the experts for external review. If the experts did not agree on any of the scenarios for one particular clinical question, the technical committee reviewed all scenarios and their pertinent evidences and made the necessary decision. After that, the experts were asked to score them again. All confirmed scenarios were gathered as the final recommendations.
Results:
All the experts agreed on at least one of the scenarios. The technical committee extracted the agreed scenario for each clinical question as the final recommendation. Finally, 56 recommendations were developed for the procedure of intravitreal anti-VEGF injection and their applications in the management of ocular vascular diseases.
Conclusion:
The implementation of this guideline can standardize the management of the common ocular vascular diseases by intravitreal injection of anti-VEGF agents. It can lead to better policy-making and evidence-based clinical decision by ophthalmologists and optimal evidence based eye care for patients.
Keywords: Age-related Macular Degeneration, Anti-vascular Endothelial Growth Factor, Intravitreal Injection, Diabetic Macular Edema, Retinal Vein Occlusion
INTRODUCTION
Ocular vascular diseases are among the leading causes of visual impairment (VI) and blindness worldwide.[1] Diabetic retinopathy (DR), diabetic macular edema (DME), age-related macular degeneration (AMD), and retinal vein occlusion (RVO) are the most prevalent ocular vascular disorders. DR is an important cause of acquired vision loss among the world's working-age population.[2,3,4] It is estimated that the number of people with DR and sight-threatening DR will increase to 191 million and 56.3 million, respectively by 2030.[5] In Iran, a population-based study in Yazd province reported that DR accounted for 50% of blindness and 17% of VI.[6] AMD is also the main cause of severe VI among the elderly globally.[7,8] Due to the worldwide aging of the population, the global burden of AMD is expected to increase, affecting an estimated 196 million people in 2020 and 288 million in 2040.[9]
In recent years, the introduction of vascular endothelial growth factor (VEGF) inhibitors (also known as anti-VEGFs) has revolutionized the treatment of—and prognosis for—individuals with AMD, DME, and RVO.[10,11,12,13,14,15,16,17,18,19,20] Anti-VEGFs have an important role in treating common vision-threatening retinal diseases and multiple studies have demonstrated the efficacy of intravitreal anti-VEGF injection for management of these diseases.[21,22,23] Thirty percent of patients with neovascular AMD reported an increase in visual acuity, and 90% reported preserved visual acuity following intravitreal anti-VEGF injections.[13,24,25,26] The off-label anti-VEGF drug (bevacizumab; Avastin®, Genentech/Roche), and US “Food and Drug Administration” approved anti-VEGF agents including ranibizumab (Lucentis®;Genentech/Novartis), and aflibercept (Eylea; Regeneron/Bayer)—are used in Iran; however, the most common one is bevacizumab due to the lower cost and the insurance coverage. Although the safety and efficacy of these agents have been already reported, their rare complications can be devastating, leading to permanent visual loss.[21,22,23,27,28] On the other hand, anti-VEGF injections need to be repeated due to their short-term efficacy.[29]
National clinical practice guidelines (CPGs) are developed to optimize the efficacy of interventions and to provide equity in access to treatment for all inhabitants of a country. CPGs contain clinical recommendations developed on the basis of high-level available evidence that are adapted considering their efficacy, safety and cost of interventions, and the nation's requirements. CPGs can help physicians and patients make appropriate decisions. They can also guide policymakers to improve the quality of care and reduce the costs.[30,31]
To the best of our knowledge, previously published protocols have specifically focused on intravitreal injection techniques, and there has been no CPG focused on the indications of anti-VEGF agents for ocular vascular diseases. This CPG was developed under the supervision of the Office for Healthcare Standards, Deputy of Curative Affairs, Iran Ministry of Health and Medical Education to help choosing the appropriate anti-VEGF agent and an appropriate interval for each individual. Furthermore, the recommendations of this CPG will be useful for increasing the safety of the injections by providing specific recommendations. The recommendations in this CPG were also revised based on the customized criteria to help ophthalmologists make the best evidence-based clinical decisions specifically applicable to Iran.
METHODS
This CPG for intravitreal injection of anti-VEGF agents for the treatment of ocular vascular diseases was developed at the Knowledge Management Unit (KMU) at the Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The technical committee comprised the steering technical committee, eight retina specialists, three ophthalmologists, four Master of Science degree holders in optometry; and the head of the Office for Healthcare Standards and Deputy of Curative Affairs, Iran Ministry of Health and Medical Education.
Finding the Relevant Clinical Practice Guidelines
A large number of websites and databases such as Guidelines International Network, National Institute for Clinical Excellence, National Guidelines Clearinghouse, Scottish Intercollegiate Guidelines Network, New Zealand Guidelines Group, National Health and Medical Research Council, Cochrane, Bandolier, Canadian Agency for Drugs and Technologies in Health, Trip Database, PubMed, Google Scholar, SID, Medlib, and Magiran were searched to find the pertinent CPGs.
Screening the Extracted Clinical Practice Guidelines
Two protocols and CPGs, including “Guidelines for intravitreal injections procedure” (The Royal College of Ophthalmologists 2009) and “Intravitreal injection” (American Academy of Ophthalmology 2015) were extracted.[32,33] The researchers chose the reference guidelines using the Appraisal of Guidelines for Research and Evaluation (AGREE) tool.[34] These guidelines focused more on the intravitreal injection procedure.
Designing the Clinical Questions
Twenty clinical questions were designed by the technical committee. Two reference guidelines were reviewed to find the answers to the questions. The questions, along with the answers were extracted from the reference guidelines and were entered into Table 1.
Table 1.
Analysis of recommendations

Appraising and Summarizing Additional Evidence
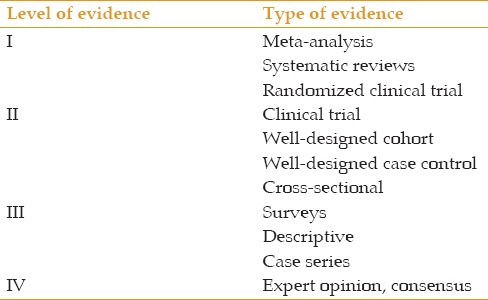
Relevant articles were excerpted from the above-mentioned databases to provide additional evidence to answer the clinical questions. These focused on indications of anti-VEGF agents for treating ocular vascular diseases. The details of this evidence were summarized and entered into Table 2. The level of evidence was determined based on the parameters described in Table 3.
Table 2.
Analysis of evidences

Table 3.
Level of evidence
Providing Scenarios
The technical committee developed all scientifically possible answers (scenarios) based on the available evidence for each question and entered them into Tables 4 and 5.
Table 4.
Clinical benefit of the recommendations[30]

Table 5.
Adaptability of the recommendations (external validity)[30]

External Review (Consensus)
All scenarios—along with Tables 1, 2, 4, and 5, and references—were sent to the retina specialists who were experts in this field. We requested them to review each question's different scenarios and specify the best one by score. The score of 1 represented the worst and the score of 9 represented the best choice, considering the clinical benefits and customizing criteria.
Analyzing the Scores and Providing Final Recommendations
The level of agreement for each scenario was determined according to the experts’ scores. The agreed-upon scenario for each clinical question was considered as the final answer. If the experts did not agree on any of the scenarios of one clinical question, the technical committee made the necessary corrections by reviewing the evidences again. After that, we asked experts to score them again.
All confirmed scenarios were gathered as the final recommendations that were provided in the results along with their evidence levels (ELs).
RESULTS
General Recommendations and Recommendations for Intravitreal Anti-VEGF Injection Procedure
-
Anti-VEGFs should be used cautiously in patients with a history of systemic vascular diseases such as stroke or myocardial infarction (MI) during the past three months. Appropriate consultations should be made before administration of anti-VEGF injections.[35,36,37,38,39,40]
EL: Consensus
-
Bilateral intravitreal injection is not recommended. However, it is not contraindicated and can be performed at the surgeon's discretion. Separate gloves, surgical preps, and vials with different batches should be used for each eye.[41]
EL: Consensus
-
The procedure can be conducted in the outpatient sterile operating room.
EL: Consensus
-
Individual sterile gloves should be used for each patient.[41]
EL: Consensus
Physicians should wear surgical masks when performing the injection. Physicians and patients should minimize speaking during the procedure.[41,42]
The patient's name, anti-VEGF agent type, and laterality should be checked immediately before intravitreal injection.[41]
It is recommended that topical anesthetics be used before prep and drapes to minimize patient discomfort.[33,41]
Eyelids and the lid margins should be sterilized with povidone-iodine (10%).[41]
-
The eyelids should be retracted from the intended injection site by a sterile spaculum and the needle should not have any touch with the lid margins.[41]
EL: Consensus
Diluted povidine-iodine (5%) should be applied to the conjunctival injection site for at least 30 seconds before injection.[41]
-
It is recommended that a 29 or 30 -gauge needle be used to perform anti-VEGF intravitreal injections.[41]
EL: Consensus
-
It is recommended that intravitreal injection be performed between the horizontal and vertical rectus muscles at the pars plana 3 and 4mm posterior to the limbus in pseudophakic and aphakic eyes, prospectively. However, the quadrant selection can be chosen using patient-specific considerations and preference of the physician. In the majority of settings, a simple perpendicular injection approach is preferred.[41]
EL: Consensus
-
It is not necessary to prescribe topical antibiotics immediately and/or for a few days after intravitreal injection. A growing body of evidence discourages the post-injection antibiotics.[43,44,45,46,47,48]
EL: Consensus
It is recommended that intravitreal bevacizumab, aflibercept and ranibizumab be injected at a dosage of 1.25 mg/0.05 ml, 2 mg/0.05 ml and 0.5 mg/0.05 ml, respectively in patients with ocular vascular diseases.[20,49]
-
An information brochure about the signs and symptoms of post-injection complications and emergency contact details should be presented to patients after injection. Patients should be aware of the necessity of urgent visit in case of ocular pain and visual impairment. Therefore, a routine first day post-injection visit is not necessary.
EL: Consensus
-
In patients at risk for optic nerve damage due to the rise in intraocular pressure (IOP) after intravitreal injection, topical anti-glaucoma drugs or anterior chamber paracentesis should be administered.[50]
EL: Consensus
-
One of the following strategies can be used for injecting intravitreal anti-VEGF agents based on the clinician's priority:[51,52,53,54,55,56,57,58]
- Three consecutive monthly injections, followed by as-needed injections (PRN)
- Three consecutive monthly injections, followed by treatment intervals that will be sequentially lengthened by 2 weeks. However, the interval should not exceed 3 months (treat and extend)
-
One injection at first followed by PRN injectionsEL: Consensus
-
Although the rhegmatogenous retinal detachment (RRD) following intravitreal anti-VEGF injections is rare (incidence = 0.013%), the risk of RRD should be considered, especially among myopic patients, who should be monitored after each injection.[45,59,60,61]
EL: II
Recommendations for Management of Retinal Vascular Diseases
Diabetic macular edema
General recommendations
-
All anti-VEGF agents, including intravitreal ranibizumab (IVR), intravitreal bevacizumab (IVB), and intravitreal aflibercept are effective in inducing visual improvement and central macular thickness (CMT) reduction.[21,62,63]
EL: I
-
Evidence showed that the 2-year visual outcomes of IVB, IVR and aflibercept are the same in patients with baseline visual acuity of 20/40 or better. Among patients with baseline visual acuity of 20/50 or worse, intravitreal injection of aflibercept resulted in the best visual outcomes compared with bevacizumab and ranibizumab at 1-year follow-up. However, the superiority of aflibercept disappeared at year 2.[21]
EL: I
-
According to the literature, short- and long-term safety and efficacy of the IVB injection has been proved in DME patients.[64]
EL: I
-
Intravitreal injection of bevacizumab is recommended as the first line treatment in patients with diabetes due to its effectiveness in reducing the VEGF level in the ocular media.[65,66,67]
EL: I
Indications
-
5.
Periodic injection of IVB is recommended for patients with naive DME.[67,68,69,70,71,72,73,74,75,76,77,78,79,80,81]
EL: I
-
6.
In patients with chronic DME, 5-6 monthly injections of IVB are recommended.[82]
EL: Consensus
-
7.
IVB injection is recommended in patients with diffuse DME.[71,80,83,84,85]
EL: II
-
8.
Three loading doses of IVB is recommended in patients with refractory DME.[86,87]
EL: I
-
9.
Patients with proliferative diabetic retinopathy (PDR) who have been scheduled for vitrectomy may receive intravitreal anti-VEGF injection within one week before surgery to reduce intraoperative and early postoperative hemorrhage.[88]
EL: I
-
10.
Intravitreal injection of anti-VEGF drugs may increase the risk of tractional retinal detachment in patients with extensive fibrovascular tissue. Therefore, it is recommended to perform the intravitreal injection 3-5 days before surgery in this subset of patients.[89,90,91,92,93,94,95,96]
EL: Consensus
-
11.
Either panretinal photocoagulation (PRP) or the combination of IVB and PRP is recommended in patients with high risk PDR.[97,98,99,100,101]
EL: I
Comorbidities
Neovascular age-related macular degeneration
General Recommendations
-
Considering the effectiveness, safety, and rare and transient complications of IVB and other anti-VEGF drugs injections, it is recommended these drugs be used to treat patients with neovascular AMD.[62,105]
EL: I
-
It is recommended that patients be given sufficient information regarding the need for repeated, frequent intravitreal anti-VEGF injections for the treatment of neovascular AMD.[106]
EL: III
-
Multiple intravitreal anti-VEGF injections do not reduce retinal nerve fiber layer thickness. Therefore, it is recommended that intravitreal anti-VEGF injections be repeated as needed.[107]
EL: III
-
In unilateral anti-VEGF injections, it is recommended that physicians consider the condition of the fellow eye.[108,109]
EL: I
-
5. It is also recommended that IVB injection be used to treat patients with active neovascular AMD coexisting with retinal pigment epithelium (RPE) tear to improve their visual acuity.[110]
EL: III
Risk Factors
-
6.
It is recommended that risk factors for poor visual acuity outcome—such as older age, larger choroidal neovascularization (CNV), and elevated pigment epithelial detachment (PED)—be considered before treating patients with neovascular AMD and inform them about the possibility of less favorable visual outcomes.[45,59,60,61]
EL: II
Complications
-
7.
In patients with neovascular AMD who are undergoing anti-VEGF treatment, there is a risk of scar formation especially in the cases of classic CNV, increased central retinal thickness, and the presence of excessive subfoveal fluids or deposits.[111]
EL: II
-
8.
To stabilize the visual and anatomic (CMT) outcomes in patients with persistent neovascular AMD (unresponsive to IVB), it is recommended that IVR or aflibercept injections be used. The presence of intraretinal fluid has an adverse effect on visual acuity improvement. However, residual subretinal fluid does not impede visual improvement and may even improve the visual acuity prognosis.[58,111,112]
EL: I
Polypoidal choroidal vasculopathy
Myopic choroidal neovascularization
General Recommendations
-
It is recommended that intravitreal anti-VEGF drugs be used in patients with myopic CNV to improve the vision and to reduce CMT.[115,116]
EL: I
-
In patients with myopic CNV, it is recommended that IVB be injected first, and photodynamic therapy (PDT) should then be performed in cases resistant to the treatment.[115,116,117]
EL: I
Risk Factors
-
3.
Vision improvement following anti-VEGF intravitreal injections was higher in patients with myopic CNV who were aged less than 50 years. Therefore, it is recommended that funduscopy be performed in young patients with high degrees of myopia to ensure early detection and timely treatment of CNV.[118]
EL: III
-
4.
Older patients with high degrees of myopia and subfoveal CNV, and/or those with higher levels of myopia, and/or those with primary extensive CNV, hemorrhage, and choroidal thickness reduction are at risk of CNV recurrence after IVB treatment. Therefore, it is recommended that they undergo periodic examinations at appropriate intervals.[119,120]
EL: III
Other types of Choroidal Neovascularization
-
5.
Due to the effectiveness and safety of intravitreal anti-VEGF injection in pediatric patients with CNV, it is recommended that these drugs be used for pediatric's CNV.[121]
EL: III
-
6.
It is recommended that IVB injections be used for patients with idiopathic subfoveal CNV or cases with previous inflammation.[115,116,122,123,124]
EL: I
Branch retinal vein occlusion
General Recommendations
-
1.
IVB is more effective in terms of visual acuity improvement and CMT reduction compared with the other treatment modalities (IVT, laser) for treating branch retinal vein occlusion (BRVO). Therefore, IVB injection is recommended for patients with macular edema secondary to BRVO.[125,126,127,128,129,130]
EL: I
-
Since IVB or IVR injection has long-term effects on visual acuity improvement in patients with perfused BRVO, it is recommended that these agents be injected in patients with macular edema secondary to perfused BRVO.[131]
EL: I
-
Better therapeutic effects can be achieved in patients with macular edema due to BRVO after early treatment (within two weeks after the diagnosis). Therefore, it is recommended that IVB be injected in these patients early after diagnosis.[132]
EL: III
Procedure
Risk Factors
-
5.
Lower baseline visual acuity, older age, longer duration, and non-perfused BRVO are risk factors for visual improvement after IVB injection. Hence, it is recommended that these risk factors be considered and that patients be informed before injections.[139]
EL: III
-
6.
IVB in BRVO patients with vitreomacular adhesion (VMA) leads to better visual and anatomical outcomes (more CMT reduction). Therefore, VMA is not considered as a risk factor in this regard.[140]
EL: III
Central retinal vein occlusion
General Recommendations
-
Anti-VEGF intravitreal injection is an effective and safe treatment for macular edema secondary to central retinal vein occlusion (CRVO) for up to 2 years; delayed treatment would lead to poor visual outcome.[132]
EL: III
-
Despite acceptable short-term visual outcomes after intravitreal steroid injection and intraocular steroid implants in patients with CRVO, possible side effects include cataract formation and increased IOP. Therefore, anti-VEGF agents are the preferred treatment in these cases.[141,142,143,144,145,146,147,148]
EL: I
Procedure
Risk Factors
-
4.
In patients with CRVO who have disruption of the external limiting membrane (ELM), IVB or IVR injection is associated with reduced visual acuity improvement. Therefore, it is recommended that the integrity of the ELM be evaluated before treatment to determine the prognosis for visual outcomes.[149]
EL: III
Central serous chorioretinopathy
-
The use of anti-VEGF intravitreal injections for treating eyes with central serous chorioretinopathy (CSC) remains controversial. Therefore, recommendations regarding intravitreal anti-VEGF injections for the treatment of CSC are summarized as follows:[150,151,152,153,154]
- It is recommended that patients with acute CSC only be followed up
- In patients with chronic CSC, half-dose photodynamic therapy is recommended
EL: I
DISCUSSION
Anti-VEGF agents have changed the treatment pattern for ocular vascular diseases.[1] The importance of timely treatment of the diseases previously described through the use of VEGF inhibitors for preventing vision loss[3] and the lack of CPGs for defining proper indication of these agents encouraged us to develop such CPG.[3] Development of CPGs for intravitreal injection of anti-VEGF agents in ocular vascular diseases was undertaken at KMU, Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran, in collaboration with the Ministry of Health and Medical Education.
This guideline includes 56 recommendations for the indication and management of DME, neovascular AMD, myopic CNV, RVO, and CSC using anti-VEGF agents.
Providing multiple scenarios for each clinical question was the strength of our research. In this approach, we presented different treatment modalities based on available evidence and asked experts to choose the best treatment strategy considering clinical and individual criteria. Therefore, we could evaluate their agreement on answers to each clinical question more accurately.
Experts agreed on at least one of the scenarios for each clinical question. Thirteen questions had more than one agreed-upon scenarios. These questions, along with their scenarios, were reviewed again by the technical committee to select the best scenario as the final recommendation.
The role of anti-VEGF agents in treatment of CSC has remained controversial and there is limited high-level evidence for managing this condition. Although a number of scenarios were developed in this regard, most of them were refused by experts in the external review process. Future multicenter research is needed to determine the effect of anti-VEGF agents in patients with CSC.
In conclusion, the CPG for intravitreal injection of anti-VEGFs for ocular vascular diseases was developed using existing high-level evidence to improve the equity in access to the best available evidence-based treatments for all society members. At the national level, this work complies with the strategic objective of the Ministry of Health and Medical Education. This objective includes developing, adapting, and implementing the CPGs and extending healthcare services.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Brand CS. Management of retinal vascular diseases: A patient-centric approach. Eye (Lond) 2012;26(Suppl 2):S1–S16. doi: 10.1038/eye.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105:998–1003. doi: 10.1016/S0161-6420(98)96025-0. [DOI] [PubMed] [Google Scholar]
- 3.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalm Epidemiol. 2007;14:179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 4.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 5.International Diabetes Federation. Diabetes atlas. 6th ed. [Last accessed on 2017 Mar 20]. Available from: https://www.idf.org/e-library/epidemiology./diabetes-atlas/19-atlas-6th-edition.html .
- 6.Katibeh M, Pakravan M, Yaseri M, Pakbin M, Soleimanizad R. Prevalence and causes of visual impairment and blindness in central Iran; The Yazd eye study. J Ophthalm Vis Res. 2015;10:279–85. doi: 10.4103/2008-322X.170362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291:1900–1901. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 8.Congdon N, O'Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 9.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 10.Yancopoulos GD. Clinical application of therapies targeting VEGF. Cell. 2010;143:13–16. doi: 10.1016/j.cell.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K; SEVEN-UP Study Group. Seven-year outcomes in ranibizumab -treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 12.Peden MC, Suñer IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receiving fixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology. 2015;122:803–808. doi: 10.1016/j.ophtha.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 14.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 15.Krüger Falk M, Kemp H, Sørensen TL. Four-year treatment results of neovascular age-related macular degeneration with ranibizumab and causes for discontinuation of treatment. Am J Ophthalmol. 2013;155:89–95. doi: 10.1016/j.ajo.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Subhi Y, Henningsen GØ, Larsen CT, Sørensen MS, Sørensen TL. Foveal morphology affects self-perceived visual function and treatment response in neovascular age-related macular degeneration: A cohort study. PLoS One. 2014;9:e91227. doi: 10.1371/journal.pone.0091227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, et al. Intravitreal Aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–2254. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, et al. Ranibizumab for macular edema following central retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124–1133. doi: 10.1016/j.ophtha.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Tufail A, Narendran N, Patel PJ, Sivaprasad S, Amoaku W, Browning AC, et al. Ranibizumab in myopic choroidal neovascularization: The 12-month results from the REPAIR study. Ophthalmology. 2013;120:1944–1945. doi: 10.1016/j.ophtha.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Sarwar S, Clearfield E, Soliman MK, Vaz-Carneiro A. Aflibercept for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2016;2:CD011346. doi: 10.1002/14651858.CD011346.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: Two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–1359. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ba J, Peng RS, Xu D, Li YH, Shi H, Wang Q, et al. Intravitreal anti-VEGF injections for treating wet age-related macular degeneration: A systematic review and meta-analysis. Drug Des Devel Ther. 2015;9:5397–5405. doi: 10.2147/DDDT.S86269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam FC, Chia SN, Lee RM. Macular grid laser photocoagulation for branch retinal vein occlusion. Cochrane Database Syst Rev. 2015;5:CD008732. doi: 10.1002/14651858.CD008732.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116:57–65. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF Trap-Eye) in wet age related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Brynskov T, Kemp H, Sørensen TL. No cases of endophthalmitis after 20,293 intravitreal injections in an operating room setting. Retina. 2014;34:951–957. doi: 10.1097/IAE.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 28.Entezari M, Karimi S, Ahmadieh H, Mahmoudi AH, Parhizgar H, Yaseri M. A large outbreak of fulminant bacterial endophthalmitis after intravitreal injection of counterfeit bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2016;254:1851–1856. doi: 10.1007/s00417-016-3426-7. [DOI] [PubMed] [Google Scholar]
- 29.Shin HJ, Kim SN, Chung H, Kim TE, Kim HC. Intravitreal anti-vascular endothelial growth factor therapy and retinal nerve fiber layer loss in eyes with age-related macular degeneration: A Meta-Analysis. Invest Ophthalmol Vis Sci. 2016;57:1798–1806. doi: 10.1167/iovs.15-18404. [DOI] [PubMed] [Google Scholar]
- 30.Clinical Practice Guidelines Office for Healthcare Standards, Deputy of Curative Affairs, Ministry of Health and Medical Education, Tehran, Iran. 2015 [Google Scholar]
- 31.Jacobson PD. Transforming clinical practice guidelines into legislative mandates: Proceed with abundant caution. JAMA. 2008;299:208–210. doi: 10.1001/jama.2007.12. [DOI] [PubMed] [Google Scholar]
- 32.The Royal College of Ophthalmologists. Guidelines for intravitreal injections procedure. 2009. [Last accessed on 2016 Jan 10]. Available from: https://www.rcophth.ac.uk/wp-content/uploads/2015/01/2009-SCI-012_Guidelines_for_Intravitreal_Injections_Procedure_1.pdf .
- 33.Intravitreal injection-2015. American Academy of Ophthalmology. [Last accessed on 15 Jan 2016]. Available from: https://www.aao.org/clinical-statement/intravitreal-injections-statement .
- 34.Appraisal of Guidelines for Research and Evaluation. [Last accessed on 2016 Jan 10]. Available from: http://www.agreetrust.org/
- 35.Shikari H, Silva PS, Sun JK. Complications of intravitreal injections in patients with diabetes. Semin Ophthalmol. 2014;29:276–289. doi: 10.3109/08820538.2014.962167. [DOI] [PubMed] [Google Scholar]
- 36.Berg K, Pedersen TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122:146–152. doi: 10.1016/j.ophtha.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 37.Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: One-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258–1267. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- 40.Thulliez M, Angoulvant D, Le Lez ML, Jonville-Bera AP, Pisella PJ, Gueyffier F, et al. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: Systematic review and meta-analysis. JAMA Ophthalmol. 2014;132:1317–1326. doi: 10.1001/jamaophthalmol.2014.2333. [DOI] [PubMed] [Google Scholar]
- 41.ICO Guidelines for Diabetic Eye Care (Updated 2017) International Council of Ophthalmology. 2017. [Last accessed on 2017 Jan 01]. Available from: http://www.icoph.org/downloads/ICOGuidelinesforDiabeticEyeCare.pdf .
- 42.McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31:654–61. doi: 10.1097/IAE.0b013e31820a67e4. [DOI] [PubMed] [Google Scholar]
- 43.Meredith TA, McCannel CA, Barr C, Doft BH, Peskin E, Maguire M, et al. Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Postinjection endophthalmitis in the comparison of age-related macular degeneration treatments trials (CATT) Ophthalmology. 2015;122:817–821. doi: 10.1016/j.ophtha.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casparis H, Wolfensberger TJ, Becker M, Eich G, Graf N, Ambresin A, et al. Incidence of presumed endophthalmitis after intravitreal injection performed in the operating room: A retrospective multicenter study. Retina. 2014;34:12–17. doi: 10.1097/IAE.0b013e31829f74b0. [DOI] [PubMed] [Google Scholar]
- 45.Goldberg RA, Flynn HW Jr, Miller D, Gonzalez S. An outbreak of streptococcus endophthalmitis after intravitreal injection of bevacizumab. Am J Ophthalmol. 2012;153:204–208e1. doi: 10.1016/j.ajo.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart JM, Srivastava SK, Fung AE, Mahmoud TH, Telander DG, Hariprasad SM, et al. Bacterial contamination of needles used for intravitreal injections: A prospective, multicenter study. Ocul Immunol Inflamm. 2011;19:32–38. doi: 10.3109/09273948.2010.520405. [DOI] [PubMed] [Google Scholar]
- 47.Inoue M, Kobayakawa S, Sotozono C, Komori H, Tanaka K, Suda Y, et al. Evaluation of the incidence of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor. Ophthalmologica. 2011;226:145–150. doi: 10.1159/000329863. [DOI] [PubMed] [Google Scholar]
- 48.Bhavsar AR, Stockdale CR, Ferris FL, Brucker AJ, Bressler NM, Glassman AR. Diabetic Retinopathy Clinical Research Network. Update on risk of endophthalmitis after intravitreal drug injections and potential impact of elimination of topical antibiotics. Arch Ophthalmol. 2012;130:809–810. doi: 10.1001/archophthalmol.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arevalo JF, Sanchez JG, Wu L, Maia M, Alezzandrini AA, Brito M, et al. Primary intravitreal bevacizumab for diffuse diabetic macular edema: The Pan-American Collaborative Retina Study Group at 24 months. Ophthalmology. 2009;116:1488–1497. doi: 10.1016/j.ophtha.2009.03.016. 1497e1. [DOI] [PubMed] [Google Scholar]
- 50.Katayama BY, Bonini-Filho MA, Messias AM, Paula JS, Martin LF, Costa R, et al. Comparison of acetazolamide, brimonidine, and anterior chamber paracentesis for ocular hypertension control after initial intravitreal bevacizumab injection: A randomized clinical trial. J Glaucoma. 2014;23:461–463. doi: 10.1097/IJG.0b013e3182948476. [DOI] [PubMed] [Google Scholar]
- 51.Gemenetzi M, Patel PJ. A Systematic Review of the Treat and Extend Treatment Regimen with Anti-VEGF Agents for Neovascular Age-Related Macular Degeneration. Ophthalmol Ther. 2017;6:79–92. doi: 10.1007/s40123-017-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wecker T, Ehlken C, Bühler A, Lange C, Agostini H, Böhringer D, et al. Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. Br J Ophthalmol. 2017;101:353–359. doi: 10.1136/bjophthalmol-2016-308668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L, et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology. 2015;122:2514–2522. doi: 10.1016/j.ophtha.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Li X, Hu Y, Sun X, Zhang J, Zhang M. Neovascular Age-Related Macular Degeneration Treatment Trial Using Bevacizumab (NATTB). Bevacizumab for neovascular age-related macular degeneration in China. Ophthalmology. 2012;119:2087–2093. doi: 10.1016/j.ophtha.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 55.Lushchyk T, Amarakoon S, Martinez-Ciriano JP. Bevacizumab in age-related macular degeneration: A randomized controlled trial on the effect of injections every 4 weeks, 6 weeks and 8 weeks. Bevacizumab in age-related macular degeneration: A randomized controlled trial on the effect of injections every 4 weeks, 6 weeks and 8 weeks. Acta Ophthalmol. 2013;91:e456–461. doi: 10.1111/aos.12119. [DOI] [PubMed] [Google Scholar]
- 56.Peden MC, Suñer IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receiving fixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology. 2015;122:803–808. doi: 10.1016/j.ophtha.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 57.Barikian A, Mahfoud Z, Abdulaal M, Safar A, Bashshur ZF. Induction with intravitreal bevacizumab every two weeks in the management of neovascular age-related macular degeneration. Am J Ophthalmol. 2015;159:131–137. doi: 10.1016/j.ajo.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Arnold JJ, Campain A, Barthelmes D, Simpson JM, Guymer RH, Hunyor AP, et al. Fight Retinal Blindness Study Group. Two-year outcomes of “treat and extend” intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology. 2015;122:1212–1219. doi: 10.1016/j.ophtha.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Ying GS, Huang J, Maguire MG, Jaffe GJ, Grunwald JE, Toth C, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120:122–129. doi: 10.1016/j.ophtha.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maguire MG, Daniel E, Shah AR, Grunwald JE, Hagstrom SA, Avery RL, et al. Incidence of choroidal neovascularization in the fellow eye in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120:2035–2041. doi: 10.1016/j.ophtha.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyer CH, Michels S, Rodrigues EB, Hager A, Mennel S, Schmidt JC, et al. Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta Ophthalmol. 2011;89:70–75. doi: 10.1111/j.1755-3768.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- 62.Fasih U, Shaikh N, Rahman A, Sultan S, Fehmi MS, Shaikh A. A one-year follow-up study of ocular and systemic complications of intravitreal injection of bevacizumab (Avastin) J Pak Med Assoc. 2013;63:707–710. [PubMed] [Google Scholar]
- 63.Stefanini FR, Badaró E, Falabella P, Koss M, Farah ME, Maia M. Anti-VEGF for the management of diabetic macular edema. J Immunol Res. 2014;2014:632307. doi: 10.1155/2014/632307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ho AC, Scott IU, Kim SJ, Brown GC, Brown MM, Ip MS, et al. Anti-vascular endothelial growth factor pharmacotherapy for diabetic macular edema: A report by the American Academy of Ophthalmology. Ophthalmology. 2012;119:2179–2188. doi: 10.1016/j.ophtha.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 65.Cancarini A, Costagliola C, Dell'omo R, Romano M, Morescalchi F, Agnifili L, et al. Effect of intravitreal bevacizumab on serum, aqueous, and vitreous humor levels of erythropoietin in patients with proliferative diabetic retinopathy. Minerva Endocrinol. 2014;39:305–311. [PubMed] [Google Scholar]
- 66.Sohn EH, He S, Kim LA, Javaheri M, Spee C, Dustin L, et al. Angiofibrotic response to vascular endothelial growth factor inhibition in diabetic retinal detachment: Report no. 1. Arch Ophthalmol. 2012;130:1127–1134. doi: 10.1001/archophthalmol.2012.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Funk M, Schmidinger G, Maar N, Bolz M, Benesch T, Zlabinger GJ, et al. Angiogenic and inflammatory markers in the intraocular fluid of eyes with diabetic macular edema and influence of therapy with bevacizumab. Retina. 2010;30:1412–1419. doi: 10.1097/IAE.0b013e3181e095c0. [DOI] [PubMed] [Google Scholar]
- 68.Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, et al. Diabetic Retinopathy Clinical Research Network. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:1860–1867. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 70.Solaiman KA, Diab MM, Abo-Elenin M. Intravitreal bevacizumab and/or macular photocoagulation as a primary treatment for diffuse diabetic macular edema. Retina. 2010;30:1638–1645. doi: 10.1097/IAE.0b013e3181e1ed07. [DOI] [PubMed] [Google Scholar]
- 71.Arevalo JF, Lasave AF, Wu L, Diaz-Llopis M, Gallego-Pinazo R, Alezzandrini AA, et al. Intravitreal bevacizumab plus grid laser photocoagulation or intravitreal bevacizumab or grid laser photocoagulation for diffuse diabetic macular edema: Results of the Pan-american Collaborative Retina Study Group at 24 months. Retina. 2013;33:403–413. doi: 10.1097/IAE.0b013e3182695b83. [DOI] [PubMed] [Google Scholar]
- 72.Soheilian M, Garfami KH, Ramezani A, Yaseri M, Peyman GA. Two-year results of a randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus laser in diabetic macular edema. Retina. 2012;32:314–321. doi: 10.1097/IAE.0b013e31822f55de. [DOI] [PubMed] [Google Scholar]
- 73.Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: Report 3. Arch Ophthalmol. 2012;130:972–979. doi: 10.1001/archophthalmol.2012.393. [DOI] [PubMed] [Google Scholar]
- 74.Zechmeister-Koss I, Huic M. Vascular endothelial growth factor inhibitors (anti-VEGF) in the management of diabetic macular oedema: A systematic review. Br J Ophthalmol. 2012;96:167–178. doi: 10.1136/bjophthalmol-2011-300674. [DOI] [PubMed] [Google Scholar]
- 75.Yilmaz T, Cordero-Coma M, Gallagher MJ, Teasley LA. Systematic review of intravitreal bevacizumab injection for treatment of primary diabetic macular oedema. Acta Ophthalmol. 2011;89:709–717. doi: 10.1111/j.1755-3768.2010.01918.x. [DOI] [PubMed] [Google Scholar]
- 76.Baker CW, Jiang Y, Stone T. Recent advancements in diabetic retinopathy treatment from the Diabetic Retinopathy Clinical Research Network. Curr Opin Ophthalmol. 2016;27:210–216. doi: 10.1097/ICU.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang YS, Li X, Wang HY, Zhang ZF, Li MH, Su XN. Intravitreal bevacizumab combined with/without triamcinolone acetonide in single injection for treatment of diabetic macular edema. Chin Med J (Engl) 2011;124:352–358. [PubMed] [Google Scholar]
- 78.Liu X, Zhou X, Wang Z, Li T, Jiang B. Intravitreal bevacizumab with or without triamcinolone acetonide for diabetic macular edema: A meta-analysis of randomized controlled trials. Chin Med J (Engl) 2014;127:3471–3476. [PubMed] [Google Scholar]
- 79.Kriechbaum K, Prager S, Mylonas G, Scholda C, Rainer G, Funk M, et al. Intravitreal bevacizumab (Avastin) versus triamcinolone (Volon A) for treatment of diabetic macular edema: One-year results. Eye (Lond) 2014;28:9–15. doi: 10.1038/eye.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Penha FM, Maia M, Cardillo JA, Arevalo JF, Wu L, Rodriguez FJ, et al. Comparison of a single intravitreal injection of bevacizumab versus triamcinolone acetonide as primary treatment for diffuse diabetic macular oedema. Acta Ophthalmol. 2012;90:e160–161. doi: 10.1111/j.1755-3768.2010.02098.x. [DOI] [PubMed] [Google Scholar]
- 81.Lim JW, Lee HK, Shin MC. Comparison of intravitreal bevacizumab alone or combined with triamcinolone versus triamcinolone in diabetic macular edema: A randomized clinical trial. Ophthalmologica. 2012;227:100–106. doi: 10.1159/000331935. [DOI] [PubMed] [Google Scholar]
- 82.Erol N, Gursoy H, Kimyon S, Topbas S, Colak E. Vision, retinal thickness, and foveal avascular zone size after intravitreal bevacizumab for diabetic macular edema. Adv Ther. 2012;29:359–369. doi: 10.1007/s12325-012-0009-9. [DOI] [PubMed] [Google Scholar]
- 83.Haritoglou C, Kook D, Neubauer A, Wolf A, Priglinger S, Strauss R, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26:999–1005. doi: 10.1097/01.iae.0000247165.38655.bf. [DOI] [PubMed] [Google Scholar]
- 84.Arevalo JF, Sanchez JG, Wu L, Maia M, Alezzandrini AA, Brito M Pan-American Collaborative Retina Study Group. Primary intravitreal bevacizumab for diffuse diabetic macular edema: the Pan-American Collaborative Retina Study Group at 24 months. Ophthalmology. 2009;116:1488–1497. doi: 10.1016/j.ophtha.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 85.Koss MJ, Naser H, Sener A, Ackermann H, Al-Sarireh F, Singh P, et al. Combination therapy in diabetic macular oedema and retinal vein occlusion-past and present. Acta Ophthalmol. 2012;90:580–589. doi: 10.1111/j.1755-3768.2010.01962.x. [DOI] [PubMed] [Google Scholar]
- 86.Ahmadieh H, Ramezani A, Shoeibi N, Bijanzadeh B, Tabatabaei A, Azarmina M, et al. Intravitreal bevacizumab with or without triamcinolone for refractory diabetic macular edema; a placebo- controlled, randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2008;246:483–489. doi: 10.1007/s00417-007-0688-0. [DOI] [PubMed] [Google Scholar]
- 87.Shoeibi N, Ahmadieh H, Entezari M, Yaseri M. Intravitreal Bevacizumab with or without Triamcinolone for Refractory Diabetic Macular Edema: Long-term Results of a Clinical Trial. J Ophthalmic Vis Res. 2013;8:99–106. [PMC free article] [PubMed] [Google Scholar]
- 88.Ahmadieh H, Shoeibi N, Entezari M, Monshizadeh R. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: A randomized clinical trial. Ophthalmology. 2009;116:1943–1948. doi: 10.1016/j.ophtha.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Zhao LQ, Zhu H, Zhao PQ, Hu YQ. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. Br J Ophthalmol. 2011;95:1216–1222. doi: 10.1136/bjo.2010.189514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rizzo S, Genovesi-Ebert F, Di Bartolo E, Vento A, Miniaci S, Williams G. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR) Graefes Arch Clin Exp Ophthalmol. 2008;246:837–842. doi: 10.1007/s00417-008-0774-y. [DOI] [PubMed] [Google Scholar]
- 91.Yeoh J, Williams C, Allen P, Buttery R, Chiu D, Clark B, et al. Avastin as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: A prospective case series. Clin Experiment Ophthalmol. 2008;36:449–454. [PubMed] [Google Scholar]
- 92.Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology. 2009;116:927–938. doi: 10.1016/j.ophtha.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 93.Zhang ZH, Liu HY, Hernandez-Da Mota SE, Romano MR, Falavarjani KG, Ahmadieh H, et al. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: A meta-analysis of randomized controlled trials. Am J Ophthalmol. 2013;156:106–115e2. doi: 10.1016/j.ajo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 94.Moradian S, Ahmadieh H, Malihi M, Soheilian M, Dehghan MH, Azarmina M. Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246:1699–1705. doi: 10.1007/s00417-008-0914-4. [DOI] [PubMed] [Google Scholar]
- 95.Hernández-Da Mota SE, Nuñez-Solorio SM. Experience with intravitreal bevacizumab as a preoperative adjunct in 23-G vitrectomy for advanced proliferative diabetic retinopathy. Eur J Ophthalmol. 2010;20:1047–1052. doi: 10.1177/112067211002000604. [DOI] [PubMed] [Google Scholar]
- 96.Preti RC, Vasquez Ramirez LM, Ribeiro Monteiro ML, Pelayes DE, Takahashi WY. Structural and functional assessment of macula in patients with high-risk proliferative diabetic retinopathy submitted to panretinal photocoagulation and associated intravitreal bevacizumab injections: A comparative, randomised, controlled trial. Ophthalmologica. 2013;230:1–8. doi: 10.1159/000348605. [DOI] [PubMed] [Google Scholar]
- 97.Preti RC, Ramirez LM, Monteiro ML, Carra MK, Pelayes DE, Takahashi WY. Contrast sensitivity evaluation in high risk proliferative diabetic retinopathy treated with panretinal photocoagulation associated or not with intravitreal bevacizumab injections: A randomised clinical trial. Br J Ophthalmol. 2013;97:885–889. doi: 10.1136/bjophthalmol-2012-302675. [DOI] [PubMed] [Google Scholar]
- 98.Yang CS, Hung KC, Huang YM, Hsu WM. Intravitreal bevacizumab (Avastin) and panretinal photocoagulation in the treatment of high-risk proliferative diabetic retinopathy. J Ocul Pharmacol Ther. 2013;29:550–555. doi: 10.1089/jop.2012.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahmad M, Jan S. Comparison between panretinal photocoagulation and panretinal photocoagulation plus intravitreal bevacizumab in proliferative diabetic retinopathy. J Ayub Med Coll Abbottabad. 2012;24:10–13. [PubMed] [Google Scholar]
- 100.Forte R, Cennamo G, Breve MA, Vecchio EC, de Crecchio G. Functional and anatomic response of the retina and the choroid to intravitreal bevacizumab for macular edema. J Ocul Pharmacol Ther. 2012;28:69–75. doi: 10.1089/jop.2010.0181. [DOI] [PubMed] [Google Scholar]
- 101.Gallego-Pinazo R, Dolz-Marco R, Berrocal M, Wu L3, Maia M4, Serrano M, et al. Outcomes of cataract surgery in diabetic patients: Results of the Pan American Collaborative Retina Study Group. Arq Bras Oftalmol. 2014;77:355–359. doi: 10.5935/0004-2749.20140089. [DOI] [PubMed] [Google Scholar]
- 102.Fard MA, Yazdanei Abyane A, Malihi M. Prophylactic intravitreal bevacizumab for diabetic macular edema (thickening) after cataract surgery: Prospective randomized study. Eur J Ophthalmol. 2011;21:276–281. doi: 10.5301/EJO.2010.1405. [DOI] [PubMed] [Google Scholar]
- 103.Castillo J, Aleman I, Rush SW, Rush RB. Preoperative Bevacizumab Administration in Proliferative Diabetic Retinopathy Patients Undergoing Vitrectomy: A Randomized and Controlled Trial Comparing Interval Variation. Am J Ophthalmol. 2017;183:1–10. doi: 10.1016/j.ajo.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 104.Salehi A, Beni AN, Razmjoo H, Beni ZN. Phacoemulcification with intravitreal bevacizumab injection in patients with cataract and coexisting diabetic retinopathy: Prospective randomized study. J Ocul Pharmacol Ther. 2012;28:212–218. doi: 10.1089/jop.2011.0069. [DOI] [PubMed] [Google Scholar]
- 105.Van der Reis MI, La Heij EC, De Jong-Hesse Y, Ringens PJ, Hendrikse F, Schouten JS. A systematic review of the adverse events of intravitreal anti-vascular endothelial growth factor injections. Retina. 2011;31:1449–1469. doi: 10.1097/IAE.0b013e3182278ab4. [DOI] [PubMed] [Google Scholar]
- 106.Lad EM, Hammill BG, Qualls LG, Wang F, Cousins SW, Curtis LH. Anti-VEGF treatment patterns for neovascular age-related macular degeneration among medicare beneficiaries. Am J Ophthalmol. 2014;158:537–543. doi: 10.1016/j.ajo.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 107.Shin HJ, Shin KC, Chung H, Kim HC. Change of retinal nerve fiber layer thickness in various retinal diseases treated with multiple intravitreal antivascular endothelial growth factor. Invest Ophthalmol Vis Sci. 2014;55:2403–2411. doi: 10.1167/iovs.13-13769. [DOI] [PubMed] [Google Scholar]
- 108.Zehetner C, Kirchmair R, Huber S, Kralinger MT, Kieselbach GF. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br J Ophthalmol. 2013;97:454–459. doi: 10.1136/bjophthalmol-2012-302451. [DOI] [PubMed] [Google Scholar]
- 109.Matsuyama K, Ogata N, Matsuoka M, Wada M, Nishimura T, Takahashi K. Effects of intravitreally injected bevacizumab on vascular endothelial growth factor in fellow eyes. J Ocul Pharmacol Ther. 2011;27:379–383. doi: 10.1089/jop.2010.0194. [DOI] [PubMed] [Google Scholar]
- 110.Coco RM, Sanabria MR, Hernandez AG, Fernández Muñoz M. Retinal pigment epithelium tears in age-related macular degeneration treated with antiangiogenic drugs: A controlled study with long follow-up. Ophthalmologica. 2012;228:78–83. doi: 10.1159/000338730. [DOI] [PubMed] [Google Scholar]
- 111.Daniel E, Toth CA, Grunwald JE, Jaffe GJ, Martin DF, Fine SL, et al. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:656–666. doi: 10.1016/j.ophtha.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Singh RP, Srivastava S, Ehlers JP, Bedi R, Schachat AP, Kaiser PK. A single-arm, investigator-initiated study of the efficacy, safety and tolerability of intravitreal aflibercept injection in subjects with exudative age-related macular degeneration, previously treated with ranibizumab or bevacizumab: 6-month interim analysis. Br J Ophthalmol. 2014;98(Suppl 1):i22–27. doi: 10.1136/bjophthalmol-2013-304798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang W, He M, Zhang X. Combined intravitreal anti-VEGF and photodynamic therapy versus photodynamic monotherapy for polypoidal choroidal vasculopathy: A systematic review and meta-analysis of comparative studies. PLoS One. 2014;9:e110667. doi: 10.1371/journal.pone.0110667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yong M, Zhou M, Deng G. Photodynamic therapy versus anti-vascular endothelial growth factor agents for polypoidal choroidal vasculopathy: A meta-analysis. BMC Ophthalmol. 2015;15:82. doi: 10.1186/s12886-015-0064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang E, Chen Y. Intravitreal anti-vascular endothelial growth factor for choroidal neovascularization secondary to pathologic myopia: Systematic review and meta-analysis. Retina. 2013;33:1375–1392. doi: 10.1097/IAE.0b013e31827d260a. [DOI] [PubMed] [Google Scholar]
- 116.Iacono P, Parodi MB, Papayannis A, Kontadakis S, Sheth S, Cascavilla ML, et al. Intravitreal ranibizumab versus bevacizumab for treatment of myopic choroidal neovascularization. Retina. 2012;32:1539–1546. doi: 10.1097/IAE.0b013e31826956b7. [DOI] [PubMed] [Google Scholar]
- 117.Ng DS, Kwok AK, Chan CW. Anti-vascular endothelial growth factor for myopic choroidal neovascularization. Clin Experiment Ophthalmol. 2012;40:e98–e110. doi: 10.1111/j.1442-9071.2011.02684.x. [DOI] [PubMed] [Google Scholar]
- 118.Ruiz-Moreno JM, Montero JA, Arias L, Araiz J, Gomez-Ulla F, Silva R, et al. Twelve-month outcome after one intravitreal injection of bevacizumab to treat myopic choroidal neovascularization. Retina. 2010;30:1609–1615. doi: 10.1097/IAE.0b013e3181e22659. [DOI] [PubMed] [Google Scholar]
- 119.Yang HS, Kim JG, Kim JT, Joe SG. Prognostic factors of eyes with naïve subfoveal myopic choroidal neovascularization after intravitreal bevacizumab. Am J Ophthalmol. 2013;156:1201–1210e2. doi: 10.1016/j.ajo.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 120.Ruiz-Moreno JM, Arias L, Montero JA, Carneiro A, Silva R. Intravitreal anti-VEGF therapy for choroidal neovascularisation secondary to pathological myopia: 4-year outcome. Br J Ophthalmol. 2013;97:1447–1450. doi: 10.1136/bjophthalmol-2012-302973. [DOI] [PubMed] [Google Scholar]
- 121.Kozak I, Mansour A, Diaz RI, Calzada JI, Pichi F, Cruz-Villegas V, et al. Outcomes of treatment of pediatric choroidal neovascularization with intravitreal antiangiogenic agents: The results of the KKESH International Collaborative Retina Study Group. Retina. 2014;34:2044–2052. doi: 10.1097/IAE.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 122.Zhang H, Liu ZL, Sun P, Gu F. Intravitreal bevacizumab for treatment of subfoveal idiopathic choroidal neovascularization: Results of a 1-year prospective trial. Am J Ophthalmol. 2012;153:300–306e1. doi: 10.1016/j.ajo.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 123.Cha DM, Kim TW, Heo JW, Woo SJ, Park KH, Yu HG, et al. Comparison of 1-year therapeutic effect of ranibizumab andbevacizumab for myopic choroidal neovascularization: A retrospective, multicenter, comparative study. BMC Ophthalmol. 2014;14:69. doi: 10.1186/1471-2415-14-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Iacono P, Parodi MB, Papayannis A, Kontadakis S, Sheth S, Cascavilla ML, et al. Intravitreal ranibizumab versus bevacizumab for treatment of myopic choroidal neovascularization. Retina. 2012;32:1539–1546. doi: 10.1097/IAE.0b013e31826956b7. [DOI] [PubMed] [Google Scholar]
- 125.Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K, et al. Two-year outcomes of intravitreal bevacizumab therapy for macular oedema secondary to branch retinal vein occlusion. Br J Ophthalmol. 2014;98:195–199. doi: 10.1136/bjophthalmol-2013-303121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yilmaz T, Cordero-Coma M. Use of bevacizumab for macular edema secondary to branch retinal vein occlusion: A systematic review. Graefes Arch Clin Exp Ophthalmol. 2012;250:787–793. doi: 10.1007/s00417-012-2016-6. [DOI] [PubMed] [Google Scholar]
- 127.Ramezani A, Esfandiari H, Entezari M, Moradian S, Soheilian M, Dehsarvi B, et al. Three intravitreal bevacizumab versus two intravitreal triamcinolone injections in recent-onset branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2012;250:1149–1160. doi: 10.1007/s00417-012-1941-8. [DOI] [PubMed] [Google Scholar]
- 128.Higashiyama T, Sawada O, Kakinoki M, Sawada T, Kawamura H, Ohji M. Prospective comparisons of intravitreal injections of triamcinolone acetonide and bevacizumab for macular oedema due to branch retinal vein occlusion. Acta Ophthalmol. 2013;91:318–324. doi: 10.1111/j.1755-3768.2011.02298.x. [DOI] [PubMed] [Google Scholar]
- 129.Ho M, Liu DT, Lam DS, Jonas JB. Retinal vein occlusions, from basics to the latest treatment. Retina. 2016;36:432–448. doi: 10.1097/IAE.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 130.Oellers P, Grewal DS, Fekrat S. Role of aflibercept for macular edema following branch retinal vein occlusion: Comparison of clinical trials. Clin Ophthalmol. 2016;10:411–418. doi: 10.2147/OPTH.S98853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mitry D, Bunce C, Charteris D. Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion. Cochrane Database Syst Rev. 2013;1:CD009510. doi: 10.1002/14651858.CD009510.pub2. [DOI] [PubMed] [Google Scholar]
- 132.Yoon YH1, Kim HK, Yoon HS, Kang SW, Kim JG, Park KH, et al. Improved visual outcome with early treatment in macular edema secondary to retinal vein occlusions: 6-month results of a Korean RVO study. Jpn J Ophthalmol. 2014;58:146–154. doi: 10.1007/s10384-014-0305-9. [DOI] [PubMed] [Google Scholar]
- 133.Zhou S, Gao J, Xu X. Antivascular endothelial growth factors in the treatment of macular oedema secondary to central retinal vein occlusion: A meta-analysis. Clin Experiment Ophthalmol. 2014;42:637–649. doi: 10.1111/ceo.12286. [DOI] [PubMed] [Google Scholar]
- 134.Epstein DL, Algvere PV, von Wendt G, Seregard S, Kvanta A. Benefit from bevacizumab for macular edema in central retinal vein occlusion: Twelve-month results of a prospective, randomized study. Ophthalmology. 2012;119:2587–2591. doi: 10.1016/j.ophtha.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 135.Epstein DL, Algvere PV, von Wendt G, Seregard S, Kvanta A. Bevacizumab for macular edema in central retinal vein occlusion: A prospective, randomized, double-masked clinical study. Ophthalmology. 2012;119:1184–1189. doi: 10.1016/j.ophtha.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 136.Zhang H, Liu ZL, Sun P, Gu F. Intravitreal bevacizumab for treatment of macular edema secondary to central retinal vein occlusion: Eighteen-month results of a prospective trial. Ocul Pharmacol Ther. 2011;27:615–621. doi: 10.1089/jop.2011.0050. [DOI] [PubMed] [Google Scholar]
- 137.Algvere PV, Epstein D, von Wendt G, Seregard S, Kvanta A. Intravitreal bevacizumab in central retinal vein occlusion: 18-month results of a prospective clinical trial. Eur J Ophthalmol. 2011;21:789–795. doi: 10.5301/EJO.2011.6522. [DOI] [PubMed] [Google Scholar]
- 138.Lazić R, Boras I, Vlašić M, Gabrić N, Tomić Z. Anti-VEGF in treatment of central retinal vein occlusion. Coll Antropol. 2010;34(Suppl 2):69–72. [PubMed] [Google Scholar]
- 139.Jaissle GB1, Szurman P, Feltgen N, Spitzer B, Pielen A, Rehak M, et al. Predictive factors for functional improvement after intravitreal bevacizumab therapy for macular edema due to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2011;249:183–192. doi: 10.1007/s00417-010-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Terao R, Yuda K, Kure K, Inoue T, Ohtsu H, Yanagi Y. Effect of vitreomacular adhesion on antivascular endothelial growth factor therapy for macular edema secondary to branch retinal vein occlusion. Jpn J Ophthalmol. 2014;58:139–145. doi: 10.1007/s10384-013-0302-4. [DOI] [PubMed] [Google Scholar]
- 141.Yeh S, Kim SJ, Ho AC, Schoenberger SD, Bakri SJ, Ehlers JP, et al. Therapies for macular edema associated with central retinal vein occlusion: A report by the American Academy of Ophthalmology. Ophthalmology. 2015;122:769–778. doi: 10.1016/j.ophtha.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 142.Gado AS, Macky TA. Dexamethasone intravitreous implant versus bevacizumab for central retinal vein occlusion-related macular oedema: A prospective randomized comparison. Clin Experiment Ophthalmol. 2014;42:650–655. doi: 10.1111/ceo.12311. [DOI] [PubMed] [Google Scholar]
- 143.Ramezani A, Esfandiari H, Entezari M, Moradian S, Soheilian M, Dehsarvi B, et al. Three intravitreal bevacizumab versus two intravitreal triamcinolone injections in recent onset central retinal vein occlusion. Acta Ophthalmol. 2014;92:e530–539. doi: 10.1111/aos.12317. [DOI] [PubMed] [Google Scholar]
- 144.Abraldes MJ, Zapata MA, Gómez-Ulla F, García-Arumí J. From scientific evidence to clinical practice: Treatment regimens for macular edema secondary to retinal vein occlusion. Arch Soc Esp Oftalmol. 2012;87(Suppl 1):54–62. doi: 10.1016/s0365-6691(12)70052-7. [DOI] [PubMed] [Google Scholar]
- 145.Jin ZY, Zhu D, Tao Y, Wong IY, Jonas JB. Meta-analysis of the effect of intravitreal bevacizumab versus intravitreal triamcinolone acetonide in central retinal vein occlusion. J Ocul Pharmacol Ther. 2013;29:826–831. doi: 10.1089/jop.2013.0061. [DOI] [PubMed] [Google Scholar]
- 146.Thapa R, Poudyal G. Short term results of intra-vitreal bevacizumab for the treatment of macular edema secondary to retinal vein occlusion. Nepal J Ophthalmol. 2013;5:63–68. doi: 10.3126/nepjoph.v5i1.7824. [DOI] [PubMed] [Google Scholar]
- 147.Demir M, Dirim B, Acar Z, Sendul Y, Oba E. Comparison of the effects of intravitreal bevacizumab and triamcinolone acetonide in the treatment of macular edema secondary to central retinal vein occlusion. Indian J Ophthalmol. 2014;62:279–283. doi: 10.4103/0301-4738.105769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ding X, Li J, Hu X, Yu S, Pan J, Tang S. Prospective study of intravitreal triamcinolone acetonide versus bevacizumab for macular edema secondary to central retinal vein occlusion. Retina. 2011;31:838–845. doi: 10.1097/IAE.0b013e3181f4420d. [DOI] [PubMed] [Google Scholar]
- 149.Wolf-Schnurrbusch UE, Ghanem R, Rothenbuehler SP, Enzmann V, Framme C, Wolf S. Predictors of short-term visual outcome after anti-VEGF therapy of macular edema due to central retinal vein occlusion. Invest Ophthalmol Vis Sci. 2011;52:3334–3337. doi: 10.1167/iovs.10-6097. [DOI] [PubMed] [Google Scholar]
- 150.Salehi M, Wenick AS, Law HA, Evans JR, Gehlbach P. Interventions for central serous chorioretinopathy: A network meta-analysis. Cochrane Database Syst Rev. 2015;12:CD011841. doi: 10.1002/14651858.CD011841.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Beger I, Koss MJ, Koch F. Treatment of central serous chorioretinopathy: MicroPulse photocoagulation versus bevacizumab. Ophthalmologe. 2012;109:1224–1232. doi: 10.1007/s00347-012-2688-7. [DOI] [PubMed] [Google Scholar]
- 152.Jamil AZ, Rahman FU, Iqbal K, Ansari HM, Iqbal W, Mirza KA. Intravitreal bevacizumab in central serous chorioretinopathy. J Coll Physicians Surg Pak. 2012;22:363–366. [PubMed] [Google Scholar]
- 153.Chung YR, Seo EJ, Lew HM, Lee KH. Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: Meta-analysis and review. Eye (Lond) 2013;27:1339–1346. doi: 10.1038/eye.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lim SJ, Roh MI, Kwon OW. Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina. 2010;30:100–106. doi: 10.1097/IAE.0b013e3181bcf0b4. [DOI] [PubMed] [Google Scholar]


