Abstract
Infections of the orbit and periorbita are relatively frequent. Identifying unusual organisms is crucial because they can cause severe local and systemic morbidity, despite their rarity. Opportunistic infections of the orbit should be considered mainly in debilitated or immunocompromised patients. The key to successful management includes a high index of suspicion, prompt diagnosis, and addressing the underlying systemic disease. This review summarizes unusual infectious processes of the orbit, including mycobacterial, fungal, and parasitic infections, as well as their pathophysiology, symptoms, signs, and treatment.
Keywords: Fungal Orbital Infections, Unusual Orbital Infections, Parasitic Orbital Infections
MYCOBACTERIUM
Mycobacterium tuberculosis usually causes pulmonary disease, but adnexal involvement can rarely occur, usually by lymphatic or haematogenous spread, or by direct extension from the paranasal sinuses. Disease patterns in the orbit are generally divided into periostitis, soft tissue tuberculoma (with or without bony involvement), orbital spread from the paranasal sinuses, or TB dacryoadenitis.
Rose et al recently reported a cohort of 8 patients presenting to a London specialist orbital clinic with periocular or orbital tuberculosis,[1] although no ‘typical’ presentation was observed, most patients reported ocular discomfort on eye movement, and none had intraocular disease. Such non-specific features often lead to diagnostic delays, and the clinician should be lead by clinical suspicion, and not a positive culture result.
Where extrapulmonary TB is strongly suspected, patients should be investigated with a chest X-ray and triple anti-tuberculous therapy (ATT) commenced. A positive culture for M. Tuberculosis is the diagnostic ‘gold standard', but where this is negative, supportive tests such as tuberculin skin tests, PCR-based analysis and Interferon-Y release assays (IGRA) should be considered. Although surgical intervention can alleviate symptoms and prevent visual loss, a complete course of ATT is paramount to control the disease.
Finally, atypical mycobacteria and Mycobacterium hominis infections of the orbit can also occur, but are rare.[2,3,4,5,6] These organisms can involve any periorbital or orbital tissue (including the periosteum and bony walls), can extend into the adjacent sinuses or intracranial cavity,[3,7] and can also occur in the absence of pulmonary disease.[4,8] Clinical features include a “cold” orbital abscess, a discharging cutaneous sinus, or, rarely, optic neuropathy complicating sphenoidal osteomyelitis.[9]
FUNGAL INFECTIONS
Although rare, opportunistic fungal infections of the orbit should be considered in debilitated or immunocompromised patients. Such organisms include the Phycomycetes (Mucor and Rhizopus spp.), Ascomycetes (Aspergillus spp.), Blastomyces,[10] Sporothrix spp.,[11,12] and Phaeohyphomyces (Bipolarina spp.),[13] the most common of which are Mucor and Aspergillus spp. Ubiquitous in the environment, these organisms reach the orbit by direct extension from the sinuses and, unless the underlying risk factor(s) are addressed, the prognosis for sight and even life may be dire. In recent years, a new strain of Mucor infections (Apophysomyces elegans) has been reported in healthy immunocompetent patients with grave sequelae or death as a consequence. This strain of Mucor has a high mortality rate in immunocompromised patients, with 100% mortality reported in rhino-orbital-cerebral disease. This species of Mucor is found in warm climates and is generally transmitted through a traumatic break in the skin or by an insect bite.
Orbital Fungal Infection: Phycomycetes
Overview
Mucoris, a genera of the order Mucorales, and a subset of the class Phycomycetes, are ubiquitous spores that are pathogenic among debilitated patients [Table 1]. Their pathogenicity is due to their ability to invade blood vessels, leading to endothelial damage, ischemia, and necrosis. Half of all patients with Mucoris infections develop rhino-orbital-cerebral disease, presenting with facial pain, sinusitis, pharyngitis, or nasal discharge, and the characteristic necrotic nasal eschar may even be absent. Management includes treatment with lipid formulations of amphotericin B and thorough debridement of necrotic tissue, although mortality rates remain in the region of 30%. Finally, iron chelation with deferasirox may play a critical role in the future, although clinical experience remains limited.[14]
Table 1.
Predisposing factors for Mucoris infection

Pathophysiology
With increasing numbers of immunosuppressed patients, rhino-orbital-cerebral fungal infections are a growing cause of morbidity and mortality,[15,16] although infection rarely occurs in immunocompetent patients.[17] rhino-orbital-cerebral invasion begins in the nasal passages and spreads to the maxillary and ethmoid sinuses, before involving the orbit.[18,19,20] Intracranial involvement occurs as a result of extension via the orbital roof and apex through the cribriform plate.[18,21]
Understanding this pathogen and its mechanism of invasion is essential in managing the disease. Being angioinvasive, the fungus dissects the internal vascular lamina from the media, causing extensive endothelial damage, with thrombosis, ischemia, and infarction, thus limiting the efficacy of systemic medical treatment and allowing the fungus to invade the surrounding tissues. In addition, these fungi have the facultative ability to grow in both aerobic and anaerobic conditions.[19,21] Tissue ischemia and infarction result in lactic acidosis, which reduces phagocytosis, thus promoting fungal invasion outside the vessels.
Clinical Presentation and Diagnosis
The systemic and orbital features of rhino-orbital mucormycosis infection are shown in Tables 2 and 3, respectively. Although characteristic of the disease, necrotic black nasal or oral eschar resulting from vascular thrombosis and tissue infarction is a late finding and is occasionally absent [Figures 1 and 2].[18,19,21]
Table 2.
Systemic features of rhino-orbital phycomycete infection

Table 3.
Orbital symptoms and signs in rhino-orbital phycomycete infection

Figure 1.
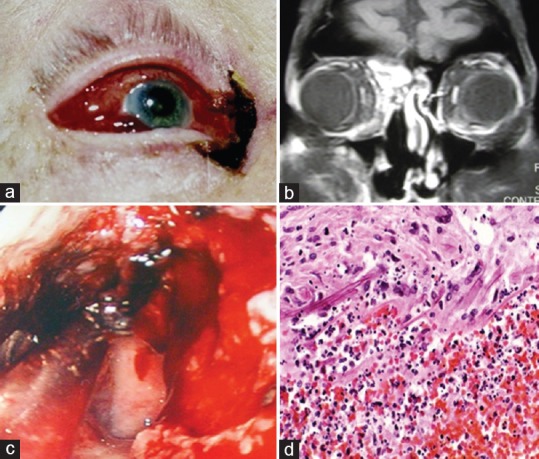
Rhino-orbital-cerebral mucor. A 65-year-old man presented with necrosis at the medial canthus and a fistula communicating with the ethmoid sinus and nasal cavity (a). Magnetic resonance imaging identified involvement of the ethmoid sinus (b), and nasal endoscopic views revealed (c). Histology confirmed the presence of angiothrombosis and broad nonseptate fungal hyphae (d) Courtesy of Prof. Dinesh Selva, FRANZCO, Department of Ophthalmology and Visual Sciences, University of Adelaide, Australia.
Figure 2.
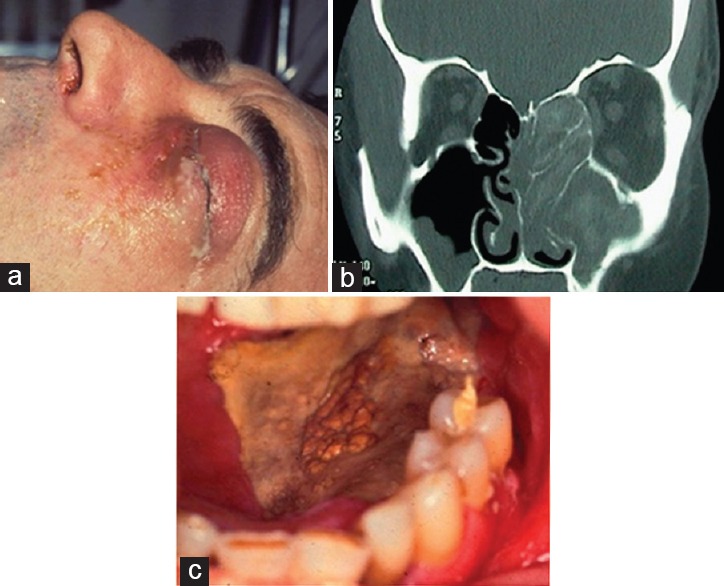
Rhino-orbital-cerebral mucor. A 58-year-old man presented with lid swelling, purulent discharge, and proptosis (a). Computed tomography identified disease in the nose and maxillary and ethmoid sinuses, with erosion into the orbit (b). Necrotic eschar was noted on the roof of the mouth (c). Courtesy of Mr Abbad Toma, FRCS (Ed), FRCS (ORL), Department of Otolaryngology, Head and Neck Surgery, St George's Hospital Medical School, University of London, UK.
Investigations
Blood tests occasionally identify a raised erythrocyte sedimentation rate or white blood cell count, but blood cultures are typically non-contributory.[21,22] Histological specimens show broad hyposeptate hyphae that branch irregularly and, when folded, give a characteristic twisted-ribbon appearance [Figure 1].[23] Relevant stains include Gomori's methenamine silver stain, potassium hydroxide preparation, and hematoxylin and eosin stain, and cultures are most likely to be achieved on Sabouraud's agar without inhibitors.[21,24]
The extent of disease is determined with computed tomography (CT) or magnetic resonance imaging (MRI). CT may show mucosal thickening, bony destruction, and venous filling defects suggestive of thrombosis [Figure 2],[25] and MRI of the sinuses typically identifies mucosal thickening or even obliteration of the sinus cavity with a hyperintense signal on T2-weighted images [Figure 2].[26]
Management of Mucormycosis
The keys to successful management include a high index of suspicion, prompt diagnosis, and addressing the underlying systemic disease. Prompt diagnosis can be achieved through early nasal endoscopic examination with biopsy of any areas with suspected mucosa. In addition, urgent biopsy of orbital tissue should be performed to determine the necessity of debridement or orbital exenteration. Early wide debridement of devitalized tissue and adjunctive antifungal therapy are essential and lifesaving.[27] Medical treatment includes liposomal amphotericin B (a fungistatic agent active against most yeasts and molds) at a dose of 3–10 mg/kg/d, with recent experience suggesting a role for posaconazole, a new triazole antifungal agent with a wide in vitro spectrum of activity that can be used for patients with elevated creatinine secondary to amphotericin B.[28,29,30,31,32] Repeated and extensive tissue debridement may be necessary; however, despite limited mucormycosis having been managed successfullywithout exenteration,[19,33,34,35] the indications for this destructive procedure remain uncertain because of the rarity of the disease.[36] Orbital exenteration is justified for more posterior disease, but may not always be indicated for more anterior involvement.[37] Recently, it was proposed that serial debridement should be performed in addition to endoscopic follow-up evaluation each week.[38] Augmentation of systemic antifungal therapy and surgical debridement, including hyperbaric oxygen and local amphotericin B packing in the area of disease involvement, are reported to exert a major beneficial clinical effect, although the literature contains no prospective studies supporting this impression. It is logical and likely that these will become part of the recommended therapy in the treatment of this disease. Future therapies are likely to include granulocyte-macrophage colony-stimulating factor therapy, and iron chelation.[14,34,39,40,41]
Prognosis
Historically, the prognosis of rhino-orbital phycomycete infection has been very poor, although, with the introduction of amphotericin B, overall survival rates have risen from 10% to approximately 70%. These figures reflect both the aggressive nature of Mucor and Rhizopus, and the frequent delay in diagnosing a debilitated patient who may be simultaneously receiving treatment for other life-threatening diseases.[18,21,22,24] Hematologic malignancy, advanced age, and extension to the cranium or orbit are among the negative prognostic factors. Early diagnosis and awareness of this disease among diabetic and immunocompromised patients improves the prognosis. The infection should be suspected in immunocompromised patients with facial discomfort, pain, paresthesia, facial swelling, cellulitis, fever, and new-onset progressive sinusitis over a period of hours or days. Immediate management with biopsy, antifungal therapy, and prompt surgical debridement should improve the prognosis and potential for survival.[38]
Aspergillus
Overview
Aspergillus is a saprophytic, ubiquitous fungus that causes both invasive and non-invasive sinus infections.[39,40] The non-invasive form occurs in immunocompetent patients, presenting with allergic sinusitis or sinonasal fungal ball,[41,42,43,44] whereas the invasive form occurs in immunocompromised patients. The latter is associated with arteritis and secondary thrombosis (because Aspergillus can invade vessel walls), as well as aneurysm formation, with mortality rates of approximately 28% despite intensive treatment.[45,46]
Pathophysiology
Although Aspergillus typically occurs in compromised patients, it also occurs in healthy patients.[47] In debilitated patients, the invasive disease often extends posteriorly through the orbit into the cavernous sinus and brain. Frequently, coexistent hematological diseases are present, such as leukemia, lymphoma, or HIV infection[48,49,50]; however, invasive aspergillosis very rarely occurs in healthy patients.[51] By contrast, although the non-invasive form causes chronic sinusitis, or isolated aspergillomas, orbital involvement is infrequent.[52] Aspergillus requires specific conditions to proliferate and invade, including a previous mucosal lesion caused by a previous infection or manipulation, mucosal hypertrophy, and chronic bacterial sinusitis.[53]
Clinical Presentation and Diagnosis
Aspergillus infections frequently occur in warm, humid climates (with the exception of Mucor and Rhizopus occurring equally in both warm and cold climates), and tend to be relatively indolent, generally progressing over many weeks, months, or even years. Nevertheless, it can lead to blindness and death. Isolated reports have highlighted the diagnostic dilemma that arises with such a slow process, with one case of steroidresponsive optic neuropathy, and another describing Aspergillus infection of the orbit following dental infection, both ultimately resulting in death.[54,55]
Colonization of the sinuses occurs from the nose, with invasion continuing both through the orbital apex and into the brain, and into the infratemporal fossa via the inferior orbital fissure. However, when isolated sphenoid sinus disease is present, optic neuritis can lead to visual loss without direct orbital involvement.[56] When the orbit is involved, gradually progressive proptosis occurs, with visual loss resulting from chronic fibrosing granulomatous disease of the sinuses.[57] An accurate and timely diagnosis requires a high index of clinical suspicion, and histology demonstrating a typical branching septate hyphae (often at 45° angles) on Gomori methenamine silver and periodic acid–Schiff stains. Cultures on Sabouraud's agar without inhibitors remain the gold standard for diagnosis.
Imaging with CT identifies a hyperdense mass expanding or destroying the paranasal sinuses, and extending into the orbit or cranium [Figure 3]. On MRI, Aspergillus lesions are hypointense on T2-weighted images, and isointense on T1-weighted images.
Figure 3.
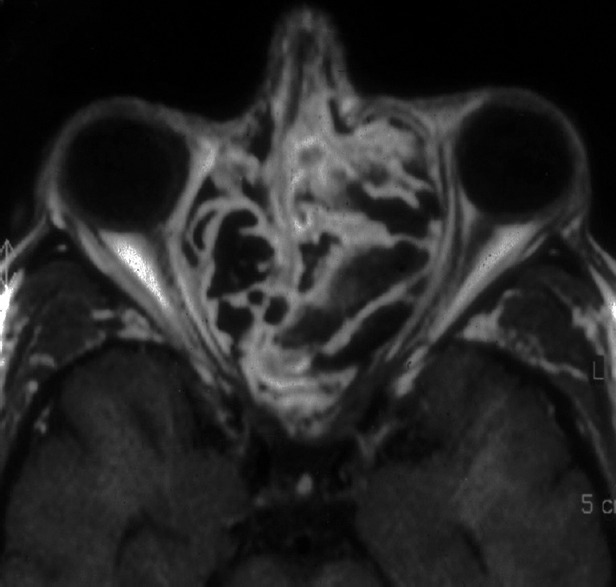
Aspergillus: Allergic non-invasive fungal sinusitis. Magnetic resonance imaging revealed signal voids in the expanded ethmoid sinuses Courtesy of Prof. Valerie J Lund, MS, FRCS, FRCS (Ed), Royal National Throat, Nose and Ear Hospital, London, UK.
Orbital Aspergillosis: Management and Prognosis
Aspergillosis has a comparatively favorable prognosis in immunocompetent patients if treated appropriately and in a timely fashion.[58] Management of orbital aspergillosis involves extensive debridement of the infected tissues, extending to the adjacent sinuses and skull base area, with intensive intravenous liposomal amphotericin B followed by flucytosine or itraconazole.[59,60] Recently, oral voriconazole, which is also highly active against Aspergillus spp., has gained favor in the management of invasive aspergillosis.[61,62,63]
Invasive aspergillosis has a very poor prognosis, being fatal in approximately one-third of all cases, including immunocompetent patients, with mortality rates for intracerebral aspergillosis in debilitated patients lying between 66% and 100%.[43,64]
Parasitic Disease
Parasitic orbital diseases, including Echinococcus, microfilaria, and cysticercosis infections, are rare in Europe and the United States, but commonly encountered in South America, Africa, and the Middle East.
Echinococcus Orbital Infection
Hydatid disease, caused by the parasite Echinococcus granulosus, occurs in agricultural regions of the Mediterranean, Middle East, South America, New Zealand, Australia, and Southeast Asia.[65,66] Echinococcosus parasites live in the intestines of dogs and shed their eggs within dog feces. These are then ingested by an intermediate host (such as a cow or sheep), and the eggs hatch in the animal's gut.[67] Hydatid cysts disseminate through the bloodstream to a wide variety of tissues and, when ingested by the definitive host (humans), progress to the final larval stage. Hepatic and pulmonary deposits are the most common clinical manifestations, and orbital hydatid disease occurs in fewer than 1% of cases,[67,68,69] although in endemic areas it may account for up to a quarter of all cystic orbital lesions.[67,70,71]
The clinical presentation of orbital hydatic disease is that of an gradually progressive, painless solitary intraconal lesion,[67] with proptosis and motility restriction, in patients under 20 years of age.[67,72] One study from Iran reported 28 cases, with the age at presentation ranging from 5 to 54 years (mean, 15 years), and with visual acuities ranging from 6/6 to no light perception.[65,72] Although serological testing is unreliable because the parasite can remain encysted,[67,73] ultrasonography identifies a well-circumscribed cystic structure, and CT imaging show a cyst with aqueous density and a contrast-enhancing rim. During surgery, excised ovoid lesions are found to be surrounded by an adventitial fibrous membrane in approximately half of all cases [Figure 4].[67,72]
Figure 4.
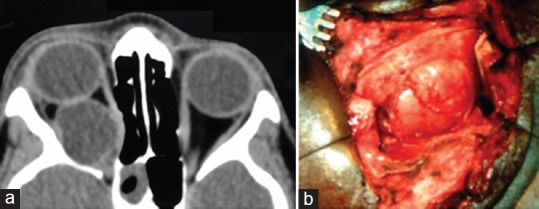
Computed tomography identifying a hydatid cyst in the intraconal space (a*). Surgical exposure of an exocyst (containing an endocyst) within the intraconal space (b) *Courtesy of Mr Yassir Abu-Raya, FRCS, Moorfields Eye Hospital, London, UK.
Orbital hydatid disease has traditionally been treated with surgical excision. However, since the introduction of albendazole in 1983, some authors have advocated for extended medical therapy alone, whereas others have favored its use it in conjunction with surgery.[73,74,75,76,77]
Microfilaria Infection
Dirofilaria is a parasitic worm that infests domestic and wild animals, but, rarely, can be transmitted to humans by an insect vector (Culex and Aedes mosquitoes). Ophthalmic involvement includes subconjunctival, intraocular, or periorbital infestation, and can also involve the extraocular muscles, leading to pain, chemosis, and proptosis.[78,79] The treatment is surgical excision.
Cysticercosis of the Orbit
Cysticercosis, caused by the parasite Taenia solium, is a potentially fatal infection, and is endemic to Latin America, sub-Saharan Africa, and parts of Asia. In human cysticercosis, T. solium eggs in raw pork hatch in the stomach and release larvae that disseminate through the bloodstream to all organs in the body, including the periorbita and eye.[80,81,82]
The most common sites in the eye for cysticercosis is vitreous and subretinal spaces, followed by orbital and adnexal tissues, among which the extraocular muscles are the most common site of cysticercosis.[83] Management includes surgical removal of the cyst and medical therapy with cestocides (albendazole or praziquantel), alongside steroids used to reduce the inflammatory response around the dying cysticerci.[84,85]
Ophthalmomyiasis
Myiasis is caused by infestation of human tissue by botfly larvae, typically the sheep botfly Oestrus ovis and the human botfly Diptera hominis. Specifically, it has been reported in the context of ulcerating periorbital cutaneous neoplasia.[86,87] The eggs are deposited on the skin by a vector, such as a mosquito, where the larvae hatch before penetrating the skin. Ophthalmomyiasis occurs in 5% of all cases, with larval invasion of the periorbita, conjunctiva, or cornea, resulting in major tissue destruction.[88,89] Symptoms include pain, itching, and a sensation of tissue movement because of the mobile larvae. The treatment is surgical excision, but, because of the numerous small spicules on the surface of the organism, this is not easily achieved [Figure 5]. Soaking in hydrogen peroxide or Dakin's solution is also advocated and has been found to be helpful. Severe, ulcerative orbital myiasis may require exenteration. However, in internal myiasis, larvae can be destroyed using laser photocoagulation or removed through pars plana vitrectomy.[90]
Figure 5.
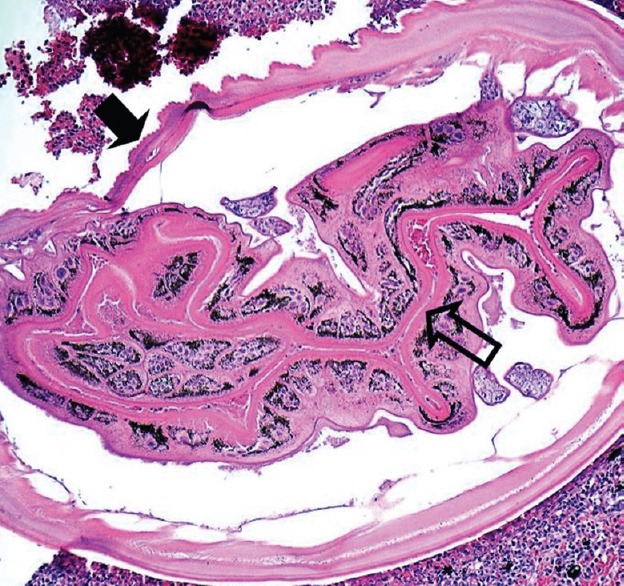
Ocular myiasis: Histological specimen exhibiting a larval cuticle with surface bosselations (closed arrow) and convoluted gut tube lined with pigmented columnar cells (open arrow) Courtesy of Dr HS Mudhar, PhD, MRC (Path), Royal Hallamshire Hospital, Sheffield, UK.
SUMMARY
Although rare, fungal and parasitic organisms can be fatal. Therefore, clinicians should be vigilant and aware of the clinical signs, etiology, and management. Unusual infections of the orbit should be considered mainly in debilitated or immunocompromised patients. Management should be adapted to the organism, but generally it includes systemic antibiotics, debridement, and occasionally more extensive surgery. Taking meticulous history from the patient, and awareness of the disease improve the prognosis and increase the survival rate.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Salam T, Uddin JM, Collin JR, Verity DH, Beaconsfield M, Rose GE. Periocular tuberculous disease: Experience from a UK eye hospital. Br J Ophthalmol. 2015;99:582–5. doi: 10.1136/bjophthalmol-2013-304516. [DOI] [PubMed] [Google Scholar]
- 2.Levine RA. Infection of the orbit by an atypical Mycobacterium. Arch Ophthalmol. 1969;82:608–610. doi: 10.1001/archopht.1969.00990020604007. [DOI] [PubMed] [Google Scholar]
- 3.Sen DK. Tuberculosis of the orbita and lacrimal gland: A clinical study of 14 cases. J Pediatr Ophthalmol Strabismus. 1980;17:232–238. doi: 10.3928/0191-3913-19800701-09. [DOI] [PubMed] [Google Scholar]
- 4.Khali M, Lindley S, Matouk E. Tuberculosis of the orbit. Ophthalmology. 1985;92:1624–1627. doi: 10.1016/s0161-6420(85)33818-6. [DOI] [PubMed] [Google Scholar]
- 5.Oakhill A, Shah KJ, Thompson AG, Stokes MJ, Mann JR. Orbital tuberculosis in childhood. Br J Ophthalmol. 1982;66:396–397. doi: 10.1136/bjo.66.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sardana K, Koranne RV, Langan U, Sharma RC, Bhatnagar SK. Ocular scrofuloderma with unilateral proptosis. J Dermatol. 2002;29:232–234. doi: 10.1111/j.1346-8138.2002.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal D, Suri A, Mahapatra AK. Orbital tuberculosis with abscess. J Neuroophthalmol. 2002;22:208–210. doi: 10.1097/00041327-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 8.van Assen S, Lutterman JA. Tuberculous dacryoadenitis: A rare manifestation of tuberculosis. Neth J Med. 2002;60:327–329. [PubMed] [Google Scholar]
- 9.Das JC, Singh K, Sharma P, Singla R. Tuberculous osteomyelitis and optic neuritis. Ophthalmic Surg Lasers Imaging. 2003;34:409–412. [PubMed] [Google Scholar]
- 10.Vida L, Moel SA. Systemic North American blastomycosis with orbital involvement. Am J Ophthalmol. 1974;77:240–242. doi: 10.1016/0002-9394(74)90680-1. [DOI] [PubMed] [Google Scholar]
- 11.Jakobiec FA, Jones IS. Orbital inflammations. In: Clinical Ophthalmology. Duane TD, editor. Philadelphia: JB Lippincott; 1988. [Google Scholar]
- 12.Streeten BW, Rabuzzi DD, Jones DB. Sporotrichosis of the orbital margin. Am J Ophthalmol. 1974;77:750–755. doi: 10.1016/0002-9394(74)90544-3. [DOI] [PubMed] [Google Scholar]
- 13.Maskin SL, Fetchick RJ, Leone CR Jr, Sharkey PK, Rinaldi MG. Bipolaris hawaiiensis caused phaeohyphomycotic orbitopathy. Ophthalmology. 1989;96:175–179. doi: 10.1016/s0161-6420(89)32917-4. [DOI] [PubMed] [Google Scholar]
- 14.McNab AA, McKelvie P. Iron overload is a risk factor for zygomycosis. Arch Ophthalmol. 1997;115:919–921. doi: 10.1001/archopht.1997.01100160089018. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Diaz JB, Palau L, Pankey G. Resolution of rhinocerebral zygomycosis associated with adjuvant administration of granulocyte-macrophage colony-stimulating factor. Clin Infect Dis. 2001;32:e166–e170. doi: 10.1086/320767. [DOI] [PubMed] [Google Scholar]
- 16.Georgopoulou S, Kounougeri E, Katsenos C, Rizos M, Michalopoulos A. Rhinocerebral Mucormycosis in a patient with cirrhosis and chronic renal failure. Hepatogastroenterology. 2003;50:843–845. [PubMed] [Google Scholar]
- 17.Fairley C, Sullivan TJ, Bartley P, Allworth T, Lewandowski R. Survival after rhino-orbital-cerebral mucormycosis in an immunocompetent patient. Ophthalmology. 2000;107:555–558. doi: 10.1016/s0161-6420(99)00142-6. [DOI] [PubMed] [Google Scholar]
- 18.Schwartze GM, Kilgo GR, Ford CS. Internal ophthalmoplegia resulting from acute orbital phycomycosis. J Clin Neuroophthalmol. 1984;4:105–108. [PubMed] [Google Scholar]
- 19.Kohn R, Hepler R. Management of limited rhino orbital mucormycosis without exenteration. Ophthalmology. 1985;92:1440–1444. doi: 10.1016/s0161-6420(85)33844-7. [DOI] [PubMed] [Google Scholar]
- 20.Hosseini SM, Borghei P. Rhinocerebral mucormycosis: Pathways of spread. Eur Arch Otorhinolaryngol. 2005;262:932–938. doi: 10.1007/s00405-005-0919-0. [DOI] [PubMed] [Google Scholar]
- 21.Bray WH, Giangiacomo J, Ide CH. Orbital apex syndrome. Surv Ophthalmol. 1987;32:136–140. doi: 10.1016/0039-6257(87)90106-8. [DOI] [PubMed] [Google Scholar]
- 22.Ferry AP, Abedi S. Diagnosis and management of rhino orbito cerebral mucormycosis (phycomycosis) Ophthalmology. 1983;90:1096–1104. doi: 10.1016/s0161-6420(83)80052-9. [DOI] [PubMed] [Google Scholar]
- 23.Virmani R, Connor DH, McAllister HA. Cardiac mucormycosis: A case report of five patients and review of 14 previously reported cases. Am J Clin Pathol. 1982;78:42–47. doi: 10.1093/ajcp/78.1.42. [DOI] [PubMed] [Google Scholar]
- 24.Lazzaro EC, Sloan B. Mucormycosis: Case presentation and discussion. Ann Ophthalmol. 1982;14:660–662. [PubMed] [Google Scholar]
- 25.Gamba JL, Woodruff WW, Djang WT, Yeates AE. Craniofacial mucormycosis: Assessment with CT. Radiology. 1986;160:207–212. doi: 10.1148/radiology.160.1.3715034. [DOI] [PubMed] [Google Scholar]
- 26.Press GA, Weindling SM, Hesselink JR, Ochi JW, Harris JP. Rhinocerebral mucormycosis: MR manifestations. J Comput Assist Tomogr. 1988;12:744–749. doi: 10.1097/00004728-198809010-00005. [DOI] [PubMed] [Google Scholar]
- 27.Petrikkos G, Skiada A, Sambatakou H, Toskas A, Vaiopoulos G, Giannopoulou M, et al. Mucormycosis: Ten-year experience at a tertiary-care center in Greece. Eur J Clin Microbiol Infect Dis. 2003;22:753–756. doi: 10.1007/s10096-003-1035-y. [DOI] [PubMed] [Google Scholar]
- 28.Sorensen J, Becker M, Porto L, Lambrecht E, Schuster T, Beske F, et al. Rhinocerebral zygomycosis in a young girl undergoing allogeneic stem cell transplantation for severe aplastic anaemia. Mycoses. 2006;49(Suppl 1):31–36. doi: 10.1111/j.1439-0507.2006.01300.x. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg RN, Mullane K, van Burik JA, Raad I, Abzug MJ, Anstead G, et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother. 2006;50:126–133. doi: 10.1128/AAC.50.1.126-133.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmons JH, Zeitler PS, Fenton LZ, Abzug MJ, Fiallo-Scharer RV, Klingensmith GJ. Rhinocerebral mucormycosis complicated by internal carotid artery thrombosis in a pediatric patient with type 1 diabetes mellitus: A case report and review of the literature. Pediatr Diabetes. 2005;6:234–238. doi: 10.1111/j.1399-543X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 31.Sun QN, Fothergill AW, McCarthy DI, Rinaldi MG, Graybill JR. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B and fluconazole against 37 clinical isolates of Zygomycetes. Antimicrob Agents Chemother. 2002;46:1581–1582. doi: 10.1128/AAC.46.5.1581-1582.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: A retrospective summary of 91 cases. Clin Infect Dis. 2006;42:e61–65. doi: 10.1086/500212. [DOI] [PubMed] [Google Scholar]
- 33.Pelton RW, Peterson EA, Patel BCK, Davis K. Successful treatment of rhino-orbital mucormycosis without exenteration: The use of multiple treatment modalities. Ophthal Plast Reconstr Surg. 2001;17:62. doi: 10.1097/00002341-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Nithyanandam S, Jacob MS, Battu RR, Thomas RK, Correa MA, D'Souza O. Rhino-Orbito-Cerebral Mucormycosis. A Retrospective Analysis of Clinical Features and Treatment Outcomes. Indian J Ophthalmol. 2003;51:231–236. [PubMed] [Google Scholar]
- 35.Songu M, Unlu HH, Gunhan K, Ilker SS, Nese N. Orbital exenteration: A dilemma in mucormycosis presented with orbital apex syndrome. Am J Rhinol. 2008;22:98–103. doi: 10.2500/ajr.2008.22.3121. [DOI] [PubMed] [Google Scholar]
- 36.Hargrove R, Wesley RE, Klippenstein KA, Fleming JC, Haik BG. Indications for Orbital Exenteration in Mucormycosis. Ophthal Plast Reconstr Surg. 2006;22:286–291. doi: 10.1097/01.iop.0000225418.50441.ee. [DOI] [PubMed] [Google Scholar]
- 37.Dhiwakar M, Thakar A, Bahadur S. Invasive sino-orbital aspergillosis: Surgical decisions and dilemmas. J Laryngol Otol. 2003;117:280–285. doi: 10.1258/00222150360600887. [DOI] [PubMed] [Google Scholar]
- 38.Bakhshaee M, Bojdi A, Allahyari A, Majidi MR, Tavakol S, Najafzadeh MJ, et al. Acute invasive fungal rhinosinusitis: Our experience with 18 cases. Eur Arch Otorhinolaryngol. 2016;273:4281–4287. doi: 10.1007/s00405-016-4109-z. [DOI] [PubMed] [Google Scholar]
- 39.Seif SR, Choo PH, Carter SR. Role of local amphotericin B therapy for sino-orbital fungal infections. Ophthal Plast Reconstr Surg. 1999;15:28–32. doi: 10.1097/00002341-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Abzug MJ, Walsh TJ. Interferon-gamma and colony-stimulating factors as adjuvant therapy for refractory fungal infections in children. Pediatr Infect Dis J. 2004;23:769–773. doi: 10.1097/01.inf.0000134314.65398.bf. [DOI] [PubMed] [Google Scholar]
- 41.Luna JD, Ponssa XS, Rodriguez SD, Luna NC, Juarez CP. Intraconal amphotericin B for the treatment of rhino-orbital mucormycosis. Ophthal Surg Lasers. 1996;27:706–708. [PubMed] [Google Scholar]
- 42.Kargi S, Kargi AE, Akduman D, Hanioglu S. Invasive fungal sinusitis. Plast Recon Surg. 2004;113:1067. doi: 10.1097/01.prs.0000107647.11339.c7. [DOI] [PubMed] [Google Scholar]
- 43.Chang WJ, Tse DT, Bressler KL, Casiano RR, Rosa RH, Johnson TE. Diagnosis and management of allergic fungal sinusitis with orbital involvement. Ophthal Plast Reconstr Surg. 2000;16:72–74. doi: 10.1097/00002341-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 44.Michaels L, Lloyd G, Phelps P. Origin and spread of allergic fungal disease of the nose and paranasal sinuses. Clin Otolaryngol Allied Sci. 2000;25:518–525. doi: 10.1046/j.1365-2273.2000.00401.x. [DOI] [PubMed] [Google Scholar]
- 45.Yumoto E, Kitani S, Okomura H, Yanagihara N. Sino orbital aspergillosis associated with total ophthalmoplegia. Laryngoscope. 1985;95:190–192. doi: 10.1288/00005537-198502000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Siddiqui A, Shah A, Bashir S. Craniocerebral aspergillosis of sinonasal origin in immunocompetent patients: Clinical spectrum and outcome in 25 Cases. Neurosurgery. 2004;55:602–613. doi: 10.1227/01.neu.0000134597.94269.48. [DOI] [PubMed] [Google Scholar]
- 47.Dortzbach R, Segrest DR. Orbital aspergillosis. Ophthalmic Surg. 1983;14:240–244. [PubMed] [Google Scholar]
- 48.Gulen H, Erbay A, Gulen F, Kazanci E, Vergin C, Demir E, et al. Sinopulmonary aspergillosis in children with hematological malignancy. Minerva Pediatr. 2006;58:319–324. [PubMed] [Google Scholar]
- 49.Levin LA, Avery R, Shore J, Woog JJ, Baker AS. The spectrum of orbital aspergillosis: A clinicopathological review. Surv Ophthalmol. 1996;41:142–154. doi: 10.1016/s0039-6257(96)80004-x. [DOI] [PubMed] [Google Scholar]
- 50.Johnson TE, Casiano RR, Kronish JW, Tse DT, Meldrum M, Chang W. Sino-orbital aspergillosis in acquired immunodeficiency syndrome. Arch Ophthalmol. 1999;117:57–64. doi: 10.1001/archopht.117.1.57. [DOI] [PubMed] [Google Scholar]
- 51.Sivak-Callcott JA, Livesley N, Nugent RA, Rasmussen SL, Saeed P, Rootman J. Localised invasive sino-orbital aspergillosis: Characteristic features. Br J Ophthalmol. 2004;88:681–687. doi: 10.1136/bjo.2003.021725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green WR, Font RL, Zimmerman LE. Aspergillosis of the orbit. Arch Ophthalmol. 1969;82:302–313. doi: 10.1001/archopht.1969.00990020304002. [DOI] [PubMed] [Google Scholar]
- 53.Stammberger H. Endoscopic surgery for mycotic and chronic recurring sinusitis. Ann Otol Rhinol Laryngol Suppl. 1985;119:1–11. doi: 10.1177/00034894850940s501. [DOI] [PubMed] [Google Scholar]
- 54.Spoor TC, Hartel WC, Harding S, Kocher G. Aspergillosis presenting as a corticosteroid responsive optic neuropathy. J Clin Neuroophthalmol. 1982;2:103–107. [PubMed] [Google Scholar]
- 55.Case 38–1982. Case records of the Massachusetts General Hospital: A 66 year old diabetic woman with sinusitis and cranial nerve abnormalities. N Engl J Med. 1982;307:806–814. doi: 10.1056/NEJM198209233071308. [DOI] [PubMed] [Google Scholar]
- 56.Nielsen EW, Weisman RA, Savino PJ, Schatz NJ. Aspergillosis of the sphenoid sinus presenting as orbital pseudotumor. Otolaryngol Head Neck Surg. 1983;91:699–703. doi: 10.1177/019459988309100620. [DOI] [PubMed] [Google Scholar]
- 57.Mauriello JA, Jr, Yepez N, Mostafavi R, Barofsky J, Kapila R, Baredes S, et al. Invasive rhinosino-orbital aspergillosis with precipitous visual loss. Can J Ophthalmol. 1995;30:124–130. [PubMed] [Google Scholar]
- 58.Green WR, Font RL, Zimmerman LE. Asperillosis of the orbit. Report of ten cases and review of the literature. Arch Ophthalmol. 1969;82:302–313. doi: 10.1001/archopht.1969.00990020304002. [DOI] [PubMed] [Google Scholar]
- 59.Steinbach WJ, Stevens DA, Denning DA. Combination and sequential antifungal therapy for invasive aspergillosis: Review of published in vitro and in vivo interactions and 6281 clinical cases from 1966 to 2001. Clin Infect Dis. 2003;37(Suppl 3):S188–S224. doi: 10.1086/376524. [DOI] [PubMed] [Google Scholar]
- 60.Walsh TJ, Goodman JL, Pappas P, Bekersky I, Buell DN, Roden M, et al. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: Maximum tolerated dose study. Antimicrob Agents Chemother. 2001;45:3487–3496. doi: 10.1128/AAC.45.12.3487-3496.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 62.O'Doherty M, Hannan M, Fulcher T. Voriconazole in the treatment of fungal osteomyelitis of the orbit in the immunocompromised host. Orbit. 2005;24:285–289. doi: 10.1080/01676830500187696. [DOI] [PubMed] [Google Scholar]
- 63.Parsonage MJ, Stafford ND, Lillie P, Moss PJ, Barlow G, Thaker H. Oral voriconazole for invasive fungal skull base infection. J Laryngol Otol. 2010;124:1010–1013. doi: 10.1017/S0022215109992507. [DOI] [PubMed] [Google Scholar]
- 64.Denning DW. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 65.Akhan O, Dincer A, Gokoz A, Sayek I, Havlioglu S, Abbasoglu O, et al. Percutaneous treatment of abdominal hydatid cysts with hypertonic saline and alcohol: An experimental study in sheep. Invest Radiol. 1993;28:121–127. doi: 10.1097/00004424-199302000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Basset D, Girou C, Nozais IP, D'Hermies F, Hoang C, Gordon R, et al. Neotropical echinococcosis in Suriname: Echinococcus oligarthrus in the orbit and Echinococcus vogeli in the abdomen. Am J Trop Med Hyg. 1998;59:787–790. doi: 10.4269/ajtmh.1998.59.787. [DOI] [PubMed] [Google Scholar]
- 67.Morales AG, Croxatto JO, Crovetto L, Ebner R. Hydatid cysts of the orbit: A review of 35 cases. Ophthalmology. 1988;95:1027–1032. doi: 10.1016/s0161-6420(88)33067-8. [DOI] [PubMed] [Google Scholar]
- 68.Xiao A, Xueyi C. Hydatid cysts of the orbit in Xinjiang: A review of 18 cases. Orbit. 1999;18:151–155. doi: 10.1076/orbi.18.3.151.2705. [DOI] [PubMed] [Google Scholar]
- 69.Abbassioun K, Amirjamshidi A. Diagnosis and Management of Hydatid Cyst of the Central Nervous System: Part 2: Hydatid Cysts of the Skull, Orbit, and Spine. Neurosurg Quart. 2001;11:10–16. [Google Scholar]
- 70.Belmekki M, El Bakkali M, Abdellah H, Benchrifa F, Berraho A. Epidemiologie des processus orbitaires chez l'enfant. A propos de 54 cas. J Fr Ophthalmol. 1999;22:394–398. [PubMed] [Google Scholar]
- 71.Talib H. Orbital hydatid disease in Iraq. Br J Surg. 1972;59:391–394. doi: 10.1002/bjs.1800590517. [DOI] [PubMed] [Google Scholar]
- 72.Kars Z, Kansu T, Ozcan OE, Erbengi A. Orbital echinococcosis: Report of two cases studied by computerized tomography. J Clin Neuroophthalmol. 1982;2:197–199. [PubMed] [Google Scholar]
- 73.Senyuz OF, Yesildag E, Celayir S. Albendazole therapy in the treatment of hydatid liver disease. Surg Today. 2001;31:487–491. doi: 10.1007/s005950170106. [DOI] [PubMed] [Google Scholar]
- 74.Sihota R, Sharma T. Albendazole therapy for a recurrent orbital hydatid cyst. Indian J Ophthalmol. 2000;48:142–143. [PubMed] [Google Scholar]
- 75.Horton RJ. Albendazole in treatment of human cystic echinococcosis: 12 years of experience. Acta Trop. 1997;64:79–93. doi: 10.1016/s0001-706x(96)00640-7. [DOI] [PubMed] [Google Scholar]
- 76.Ciurea AV, Giuseppe G, Machinis TG, Coman TC, Fountas KN. Orbital hydatid cyst in childhood: A report of two cases. South Med J. 2006;99:620–624. doi: 10.1097/01.smj.0000217492.03019.16. [DOI] [PubMed] [Google Scholar]
- 77.Stringfellow G, Francis I, Coroneo MT, Walker J. Orbital dirofilariasis. Clin Exp Ophthalmol. 2002;30:378–380. doi: 10.1046/j.1442-9071.2002.00562.x. [DOI] [PubMed] [Google Scholar]
- 78.Angunawela RI, Ataullah S, Whitehead KJ, Sullivan TJ, Rosser P. Dirofilarial infection of the orbit. Orbit. 2003;22:41–46, 2003. doi: 10.1076/orbi.22.1.41.14008. [DOI] [PubMed] [Google Scholar]
- 79.Strianese D, Martini A, Molfino G, Falabella L, Tranfa F. Orbital dirofilariasis. Eur J Ophthalmol. 1998;8:258–262. doi: 10.1177/112067219800800410. [DOI] [PubMed] [Google Scholar]
- 80.Pushker N, Bajaj MS, Balasubramanya R. Disseminated cysticercosis involving orbit, brain and subcutaneous tissue. J Infect. 2005;51:245–248. doi: 10.1016/j.jinf.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 81.Gulliani BP, Dadeya S, Malik KPS, Jain DC. Bilateral Cysticercosis of the Optic Nerve. J Neuro-Ophthalmol. 2001;21:217–218. doi: 10.1097/00041327-200109000-00013. [DOI] [PubMed] [Google Scholar]
- 82.Walrath JD, Lalin SC, Leib ML. Cysticercosis isolated to the orbit. Ophthal Plast Reconstr Surg. 2003;19:243–244. doi: 10.1097/01.iop.0000064991.06048.dd. [DOI] [PubMed] [Google Scholar]
- 83.Damani M, Mehta V, Baile R, Nakwa B. Orbital cysticercosis: A case report. Saudi J Ophthalmol. 2012;26:457–458. doi: 10.1016/j.sjopt.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jones TC. Cestodes (tapeworm) Principles and Practice of Infectious Diseases. In: Mandell GL, Douglas RG, Bennett J, editors. 3rd ed. New York: Churchill Livingstone; 1990. p. 2154. [Google Scholar]
- 85.Sotelo J, Escobedo F, Rodriguez Carbajal J, Torres B, Rubio-Donnadieu F. Therapy of parenchymal brain cysticercosis with praziquantel. N Engl J Med. 1984;310:1001–1007. doi: 10.1056/NEJM198404193101601. [DOI] [PubMed] [Google Scholar]
- 86.Yeung JC, Chung CF, Lai JS. Orbital myiasis complicating squamous cell carcinoma of eyelid. Hong Kong Med J. 2010;16:63–65. [PubMed] [Google Scholar]
- 87.Abalo-Lojo JM, López-Valladares MJ, Llovo J, Garcia A, Gonzalez F. Palpebro-orbital myiasis in a patient with basal cell carcinoma. Br J Ophthalmol. 2009;19:683–685. doi: 10.1177/112067210901900426. [DOI] [PubMed] [Google Scholar]
- 88.Wilhelmus KR. Myiasis palpebrarum. Am J Ophthalmol. 1986;101:496–498. doi: 10.1016/0002-9394(86)90661-6. [DOI] [PubMed] [Google Scholar]
- 89.Devoto MH, Zaffaroni MC. Orbital myiasis in a patient with a chronically exposed hydroxyapatite implant. Ophthal Plast Reconstr Surg. 2004;20:395–396. doi: 10.1097/01.iop.0000139526.01850.d1. [DOI] [PubMed] [Google Scholar]
- 90.Ranjan R, Jain A. External ophthalmomyaisis. Oman J Ophthalmol. 2014;7:160–161. doi: 10.4103/0974-620X.142607. [DOI] [PMC free article] [PubMed] [Google Scholar]


