Abstract
Purpose:
To compare mean posterior corneal power and astigmatism in normal versus keratoconus affected eyes and determine the optimal cut-off points to maximize sensitivity and specificity in discriminating keratoconus from normal corneas.
Methods:
A total of 204 normal eyes and 142 keratoconus affected eyes were enrolled in this prospective comparative study. Mean posterior corneal power and astigmatism were measured using a dual Scheimpflug camera. Correlation coefficients were calculated to assess the relationship between the magnitudes of keratometric and posterior corneal astigmatism in the study groups. Receiver operating characteristic curves were used to compare the sensitivity and specificity of the measured parameters and to identify the optimal cut-off points for discriminating keratoconus from normal corneas.
Results:
The mean posterior corneal power was −6.29 ± 0.20 D in the normal group and −7.77 ± 0.87 D in the keratoconus group (P < 0.001). The mean magnitudes of the posterior corneal astigmatisms were −0.32 ± 0.15 D and −0.94 ± 0.39 D in the normal and keratoconus groups, respectively (P < 0.001). Significant correlations were found between the magnitudes of keratometric and posterior corneal astigmatism in the normal (r=−0.76, P < 0.001) and keratoconus (r=−0.72, P < 0.001) groups. The mean posterior corneal power and astigmatism were highly reliable characteristics that distinguished keratoconus from normal corneas (area under the curve, 0.99 and 0.95, respectively). The optimal cut-off points of mean posterior corneal power and astigmatism were −6.70 D and −0.54 D, respectively.
Conclusion:
Mean posterior corneal power and astigmatism measured using a Galilei analyzer camera might have potential in diagnosing keratoconus. The cut-off points provided can be used for keratoconus screening.
Keywords: Keratoconus, Posterior Corneal Astigmatism, Posterior Corneal Power
INTRODUCTION
Keratoconus is a non-inflammatory and progressive corneal disease with unknown etiology. It leads to thinning and bulging of the cornea and, consequently, irregular astigmatism and myopia. Detection of keratoconus among refractive surgery patients is crucial because the prevalence of keratoconus is higher in patients with such eyes than in the general population, and operating on an undetected keratoconic cornea is the major cause of post-refractive surgery ectasia.[1,2,3] The diagnosis of clinical keratoconus is based on biomicroscopic findings along with additional paraclinical tests, such as pachymetry, keratometry, and corneal topography.[4] Placido disk-based videokeratography and measurement of central corneal thickness are widely used methods in the diagnosis of keratoconus.[5,6,7] Placido disk-based corneal topography examines only the central 7-8 mm diameter of the anterior corneal surface, and is unable to evaluate the elevation of the posterior corneal surface, which is considered to be a significant feature, particularly in early stage keratoconus detection.[8,9,10]
Recently, the development of new technologies, such as slit-scanning devices, Scheimpflug devices, and optical coherence tomography has made the quantitative measurement of the posterior corneal curvature in a clinical setting possible. The Galilei dual Scheimpflug system (Ziemer Ophthalmic System AG, Zurich, Switzerland) is a non-invasive diagnostic instrument designed for analysis of anterior eye segment characteristics.[11,12] It combines Placido imaging, which provides curvature data, and Scheimpflug imaging, which is optimal for precise elevation measurements. This technology enables the quantitative measurement of the posterior corneal curvature from which the mean posterior corneal power and astigmatism can be determined. Studies conducted on the posterior corneal surface in eyes with keratoconus have all focused on the radius of the posterior best-fit sphere and posterior maximum elevation.[13,14,15] Recent studies have been conducted on the magnitude and the axis orientation of posterior corneal astigmatism for keratoconus with respect to each clinical stage of the disease.[16] Kamiya et al[16] reported that the mean magnitude of posterior astigmatism was approximately 1.0 D with a range of 0.0 to 2.90 D. They found no statistically significant increase in anterior, posterior, or total corneal astigmatism with the progressive stages of keratoconus.[16] Shajari et al[17] reported that the posterior corneal astigmatism was 0.69 ± 0.40 D in eyes across all keratoconus stages. They found a significant increase of posterior corneal astigmatism with disease progression and a significant correlation between anterior and posterior corneal astigmatism.[17] Savini et al[18] reported that posterior corneal astigmatism produces large, variable magnitudes and is correlated to total corneal astigmatism in eyes with keratoconus. Naderan et al[19] reported a trend for increasing anterior against-the-rule (ATR) and posterior with-the-rule (WTR) astigmatism as well as decreasing oblique astigmatism on both corneal surfaces by increasing the keratoconus severity. They found a cut-off value of 0.4 D for posterior corneal astigmatism with 89.5% sensitivity and 85.0% specificity for discriminating keratoconus from normal corneas.[19] The purpose of the present study was to measure and compare the magnitudes of mean posterior corneal power and astigmatism between normal and keratoconus corneas by using a dual Scheimpflug analyzer. Additionally, an attempt was made to identify optimal cut-off points to maximize sensitivity and specificity in discriminating keratoconus from normal corneas.
METHODS
In this prospective comparative study, the right eyes of 204 normal (90 male subjects) and 142 eyes of 98 patients with keratoconus (56 male patients) were enrolled. The study was approved by the Institutional Review Board of the Ophthalmic Research Center, which is affiliated with Shahid Beheshti University of Medical Sciences, Tehran, Iran, and followed the tenets of the Declaration of Helsinki. Informed consent, signed by the participants, was obtained after the purpose of the study was thoroughly explained.
In the normal group, the only ocular problem was refractive error (sphere between −13.0 and +0.75 D and refractive astigmatism lower than 6.5 D), and a history or diagnosis of ocular pathology such as dry eye, keratoconus, glaucoma, retinal disease, or any previous ocular surgery led to subject exclusion. The keratoconus group consisted of patients with clinical keratoconus. Eyes with previous acute corneal hydrops, stromal scar, other corneal and ocular diseases, or a history of any ocular surgery were excluded from the keratoconus group. Contact lens users were asked to discontinue the use of lenses for at least 2 weeks or 1 month before the preoperative examination for soft and gas permeable contact lenses, respectively.
A complete ocular examination, including slit-lamp biomicroscopy, manifest refraction, uncorrected and best-spectacle corrected visual acuity (UCVA and BSCVA, respectively) measurement using a Snellen acuity chart, keratometry, intraocular pressure measurement, and dilated funduscopy was performed. The diagnosis of keratoconus was based on clinical slit-lamp findings (stromal thinning, conical protrusion, Fleischer ring, and Vogt striae) and associated characteristics of Placido disk-based (TMS-2, Tomey, Nagoya, Japan) topographic patterns. Keratoconic eyes were divided into the following three groups per mean keratometry (K) readings: mild (K < 48.0 D), moderate (48.0 D ≤ K ≤ 52.0 D), and severe (K > 52.0 D).
For measurements with the Galilei dual Scheimpflug analyzer (software version 5.2; Ziemer Ophthalmic System AG), the participants were seated with their chin on a chinrest and forehead against the forehead strap while focusing on the target. The alignment of the scan center with the corneal apex was checked using an initial Scheimpflug image formed on the monitor together with a guideline. The measurements were checked under a quality-specification window. High quality dual Scheimpflug analyzer scans (indicated by a green check mark displayed on the map) were included in the study. If the comments were marked yellow or red (i.e., not acceptable), the examination was repeated. The dual Scheimpflug–Placido measurements included simulated mean keratometry and keratometric astigmatism, mean posterior corneal power and astigmatism, and mean total corneal power and astigmatism. All measurements were made across the 1.0–4.0 mm central zone of the cornea.
Simulated keratometric astigmatism is defined as the difference in simulated keratometry between the flattest and steepest meridians, calculated using the standard keratometric index (1.3375) and the radius of anterior corneal curvature. Posterior corneal astigmatism is defined as the difference in keratometry between the flattest and steepest meridians, calculated using the refractive index of the cornea (1.376) and the aqueous humor (1.336) and radius of posterior corneal curvature. Mean simulated keratometry and posterior corneal power were the average of the powers in the flat and steep meridians. Total corneal power and astigmatism were calculated using ray tracing through the anterior and posterior corneal surfaces according to Snell's law. Simulated keratometric and total corneal astigmatism was classified as WTR when the steep meridian was within 60–120° and as ATR when the steep meridian was within 0–30° or 150–180°. The remaining astigmatisms were classified as oblique astigmatism. Because the dioptric power of the posterior corneal surface was negative, posterior corneal astigmatism was classified as WTR when the steep meridian was within 0–30° or 150–180° and as ATR when the steep meridian was within 60–120°. The remaining astigmatisms were classified as oblique astigmatism.
Statistical Analysis
Considering an α error of 5.0%, study power of 80%, difference in posterior corneal astigmatism between the two study groups (d) of 0.2 D, and standard deviation of difference in posterior corneal astigmatism of 0.3 D, the sample size was calculated to be 135 patients in each group. Data were analyzed using Statistical Package for the Social Sciences version 21 (IBM Corp., Armonk, NY, USA). Values indicating means and standard deviations, ranges, frequencies, and percentages were used to express the data. The normal distribution of continuous variables was verified using a Kolmogorov–Smirnov test and Q-Q plot. The chi-square test was used for comparing percentages between the groups. Generalized estimating equation models were used to compare the study groups to prevent the confounding effects of potential correlations between measurements from both corneas of the same patient. The Pearson or partial correlation coefficient was calculated in the normal and keratoconus groups, respectively, to assess the relationship between the magnitudes of keratometric and posterior corneal astigmatism. The latter coefficient was also used to determine the correlation between the two eyes of each patient in the keratoconus group. To distinguish clinical keratoconus from normal eyes, receiver operating characteristic (ROC) curves were calculated and quantified using the area under the curve (AUC) for the mean corneal power and astigmatism of each component. The AUC describes the predictive accuracy of the indices; an area of 1 implies that the test perfectly discriminates between the study groups. Optimal cut-off points to distinguish keratoconus from normal corneas were calculated for the measured parameters. The positive likelihood ratio [sensitivity/(1-specificity)] and negative likelihood ratio [(1-sensitivity)/specificity] for these cut-off points were determined. A P value of less than 0.05 was considered statistically significant and all P values were two sided.
RESULTS
Table 1 presents and compares demographic and refractive data between the two study groups. In the keratoconus group, mild keratoconus was observed in 41 eyes (28.9%), whereas 46 eyes (32.4%) and 55 eyes (38.7%) had moderate and severe keratoconus, respectively. Table 2 lists and compares the simulated keratometric, posterior, and mean total corneal power and astigmatism between the study groups. Comparisons of the magnitudes of keratometric, posterior, and mean total corneal power and astigmatism among the different keratoconus severity subgroups are presented in Table 3. The table shows that the mean corneal power and astigmatism of the different components were significantly higher in the keratoconus group than in the normal group and increased with the disease stage.
Table 1.
Comparisons of demographic and refractive data between normal eyes and eyes with keratoconus.
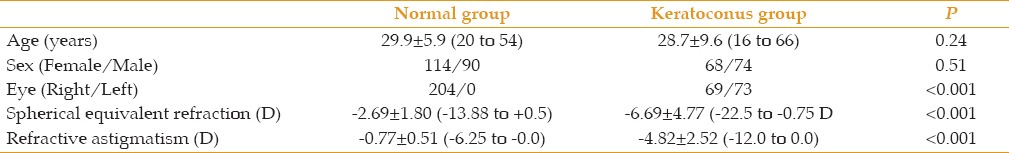
Table 2.
Comparisons of keratometric, posterior and total corneal mean power and astigmatism (mean±standard deviation; range) between normal eyes and eyes with keratoconus.
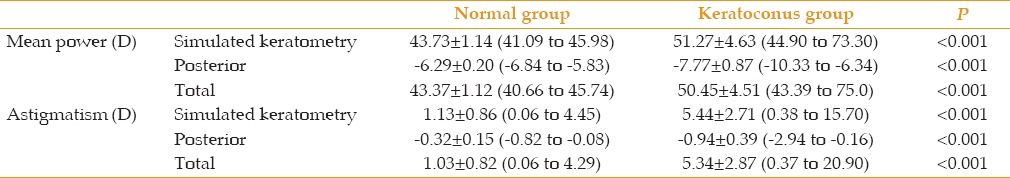
Table 3.
Comparisons of keratometric, posterior and total corneal mean power and astigmatism (mean±standard deviation; range) among different subgroups of keratoconus severity.

Significant correlations were found between the magnitudes of keratometric and posterior corneal astigmatism in the normal (r=−0.76, P < 0.001) and keratoconus (r=−0.72, P < 0.001) groups. Posterior corneal astigmatism could be predicted from keratometric astigmatism in the normal and keratoconus groups by using the following linear regression equations: posterior corneal astigmatism = −0.171 – 0.129 × keratometric astigmatism and posterior corneal astigmatism = −0.363 – 0.105 × keratometric astigmatism, respectively. In the subgroup analysis, the association between keratometric and posterior corneal astigmatism was weak in the mild keratoconus subgroup (r = −0.38, P = 0.02). However, this association was strong in the moderate (r = −0.87, P < 0.001) and severe (r = −0.86, P < 0.001) keratoconus subgroups.
In the normal group, WTR, ATR, and oblique keratometric astigmatism were found in 156 eyes (76.5%), 20 eyes (9.8%), and 28 eyes (13.7%), respectively. Corresponding figures in the keratoconus group were 103 (72.0%), 5 (3.5%), and 35 (24.5%), respectively (P = 0.01). WTR, ATR, and oblique astigmatism of the posterior corneal surface were found in 2 eyes (1.0%), 198 eyes (97.0%), and 4 eyes (2.0%) in the normal group and in 4 eyes (2.8%), 114 eyes (79.7%), and 25 eyes (17.5%) in the keratoconus group, respectively (P < 0.001). These values indicate that more eyes showed oblique astigmatism of both the anterior (P = 0.02) and posterior (P < 0.001) corneal surfaces in the keratoconus group than in the normal group. The subgroup analysis revealed that the axis orientation of the keratometric and posterior corneal surfaces remained unchanged regardless of the disease stage (P = 0.81 and 0.92, respectively) [Table 4].
Table 4.
Comparison of axis orientations of keratometric and posterior corneal astigmatism among different subgroups of keratoconus severity

Figure 1 compares the results of the ROC curve analyses, and Table 5 presents the AUC of keratometric, posterior, and mean total corneal power and astigmatism for the keratoconus group versus the normal group. The AUC was close to 1.0 for all measurements, indicating that they can effectively differentiate keratoconus from normal corneas. The posterior corneal steep meridian had the strongest power to distinguish keratoconus from normal corneas. Table 6 lists the optimal cut-off points and their sensitivity, specificity, and positive and negative likelihood ratios for keratometric, posterior, and mean total corneal power and astigmatism to distinguish keratoconus from normal eyes. As demonstrated, the cut-off points of all parameters except for the flat meridian of total corneal power had high sensitivity and specificity for identifying keratoconus.
Figure 1.
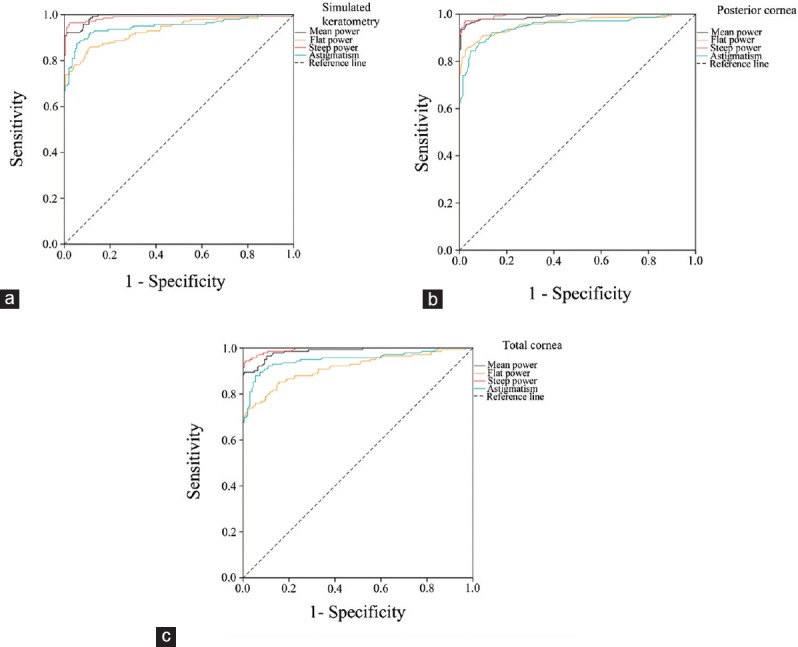
Receiver operating characteristic graphs showing the sensitivity and specificity of the mean corneal power and astigmatism values to distinguish eyes with keratoconus from controls. (a) Simulated keratometric measurements. (b) Posterior corneal surface measurements. (c) Total corneal measurements.
Table 5.
Area under the receiver operating characteristics curve (AUC) of keratometric, posterior, total corneal mean power and astigmatism for keratoconus versus normal corneas

Table 6.
Cut-off points of keratometric, posterior and total corneal mean power and astigmatism and their sensitivity, specificity, and positive and negative likelihood ratios (LR) to distinguish eyes with keratoconus from controls

DISCUSSION
The current study reported the characteristics of the posterior corneal surface measured with a dual Scheimpflug topographer and compared the results of eyes with keratoconus and those with normal corneas. Previous studies have shown that the Galilei Scheimpflug analyzer has excellent repeatability for corneal curvature measurements.[20,21] Our results indicated that the magnitude of mean posterior corneal power and astigmatism was significantly higher in keratoconus corneas than in normal corneas. A recent study evaluated the posterior corneal astigmatism in keratoconic corneas by using Pentacam.[16] It reported that the mean magnitude of posterior astigmatism was approximately 1.0 D with a range of 0.0–2.90 D, which corresponds to the range of values reported in the present study. Kamiya et al[16] found no statistically significant increase in anterior, posterior, or total corneal astigmatism with the progress of keratoconus. Their finding contrasts with our results, which indicated that the magnitude of the mean posterior corneal power and astigmatism significantly increased with disease progression. This difference can be explained by the fact that Kamiya et al[16] used a different classification of keratoconus severity that accounted for astigmatism, corneal power, corneal transparency, and corneal thickness. It is unclear which is the more reliable method for classification of keratoconus severity.
We found a significantly higher prevalence of oblique astigmatism of both the anterior and posterior corneal surfaces in the keratoconus group than in the normal group. However, in most keratoconic eyes, the axis orientations of the keratometric and posterior corneal astigmatism were WTR and ATR, respectively, and did not change as the disease severity increased. These data suggest that posterior corneal astigmatism provides a compensatory effect for the astigmatism of the anterior corneal surface in keratoconus eyes irrespective of the stage of the disease.
Kamiya et al[16] reported a significant correlation between the magnitudes of anterior and posterior corneal astigmatism in keratoconus. Coupled with these findings, the results of the current study show that the magnitude of posterior corneal astigmatism is significantly related to the magnitude of keratometric astigmatism at the different stages of keratoconus. This association is weak in mild keratoconus but strong in moderate and severe keratoconus. This observation can be attributed to the fact that manifestations of keratoconus occur at the posterior corneal surface in early stages of the disease, when the anterior surface demonstrates subtle topographic changes.[8,9,10] With the progression of the disease, however, an increase in the anterior corneal curvature occurs in parallel with the posterior corneal curvature, which results in a linear relationship between the two surfaces at a constant ratio. The significant correlation between the keratometric and posterior corneal astigmatism is of clinical importance; this correlation allows the estimation of total corneal astigmatism from the keratometric value when the posterior corneal measurement is unavailable.[22] Toric intraocular lenses have been developed for the correction of astigmatic errors in normal and keratoconic eyes with cataracts.[23,24,25,26] The magnitude of corneal astigmatism power determined using anterior corneal measurements should be adjusted based on the posterior corneal astigmatism to improve the accuracy of predicted corneal astigmatism correction achieved by these lenses, particularly in keratoconus eyes in which the role of the posterior cornea is magnified.[27] Devices that measure the posterior corneal surface are not available in all eye clinics; the intercept and slope parameters reported in the current study can be used in such situations to predict posterior corneal astigmatism.
The ability of the mean power and astigmatism of the posterior corneal surface to discriminate keratoconus from normal eyes was investigated using the area under the ROC curves. The results indicated that mean posterior corneal power and astigmatism are strong enough to distinguish eyes with keratoconus from normal eyes and the measurements of the posterior corneal steep meridian were the coefficients with the highest discriminative ability. This is congruent with previous studies that have demonstrated that manifestations of keratoconus occur at the posterior corneal surface at early stages of the disease.[8,9,10] Furthermore, the current study attempted to define the cut-off points for posterior corneal surface measurements to differentiate keratoconus and normal eyes. The values provided in this study can be used in clinical settings, particularly for the screening of keratoconus in refractive surgery candidates. For example, we found that posterior corneal astigmatism >0.54 D indicates keratoconus with a sensitivity and specificity of 88% and 92%, respectively. Naderan et al[19] reported a cut-off value of 0.4 D for posterior corneal astigmatism with 89.5% sensitivity and 85.0% specificity for discriminating keratoconus from normal corneas. Posterior corneal astigmatism >0.50 D has been reported in 9% and 12.5% of normal corneas.[28,29] In the current study, this rate was 11.3% in the normal group and 88.7% in the keratoconus group.
There were two limitations in this study. First, two eyes of the same patient were evaluated in some cases in the keratoconus group. We attempted to exclude the confounding effects of potential correlations between measurements from both corneas of the same patient by using generalized estimating equation models and partial correlation coefficients. Second, this study included patients with clinical keratoconus and excluded patients with subclinical forms. A method of distinguishing keratoconus from normal eyes is more valuable when it can also identify subclinical forms with acceptable sensitivity and specificity. Additionally, the threshold of cut-off points may vary if subclinical forms of keratoconus are considered.
CONCLUSION
In conclusion, since posterior corneal imaging became possible, it has received increasing clinical interest, particularly for the accurate correction of astigmatic errors using toric intraocular lenses. Posterior corneal measurements using a dual Scheimpflug camera seem to have potential in diagnosing keratoconus patients. Additional studies are advisable to confirm the usefulness of the power of posterior corneal surface measurements for the identification of keratoconus and subclinical keratoconus.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Wilson SE, Klyce SD. Screening for corneal topographic abnormalities before refractive surgery. Ophthalmology. 1994;101:147–152. doi: 10.1016/s0161-6420(94)31372-8. [DOI] [PubMed] [Google Scholar]
- 2.Varssano D, Kaiserman I, Hazarbassanov R. Topographic patterns in refractive surgery candidates. Cornea. 2004;23:602–607. doi: 10.1097/01.ico.0000121699.74077.f0. [DOI] [PubMed] [Google Scholar]
- 3.Seiler T, Quurke AW. Iatrogenic keratectasia after LASIK in a case of forme fruste keratoconus. J Cataract Refract Surg. 1998;24:1007–1009. doi: 10.1016/s0886-3350(98)80057-6. [DOI] [PubMed] [Google Scholar]
- 4.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 5.Rabinowitz YS. Videokeratographic indices to aid in screening for keratoconus. J Refract Surg. 1995;11:371–379. doi: 10.3928/1081-597X-19950901-14. [DOI] [PubMed] [Google Scholar]
- 6.Ambrósio R Jr, Klyce SD, Wilson SE. Corneal topographic and pachymetric screening of keratorefractive patients. J Refract Surg. 2003;19:24–29. doi: 10.3928/1081-597X-20030101-05. [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Hutchings H, Rouland JF, Guell J, Burillon C, Arné JL, et al. Videokeratographic anomalies in familial keratoconus. Ophthalmology. 2004;111:867–874. doi: 10.1016/j.ophtha.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 8.de Sanctis U, Loiacono C, Richiardi L, Turco D, Mutani B, Grignolo FM. Sensitivity and specificity of posterior corneal elevation measured by Pentacam in discriminating keratoconus/subclinical keratoconus. Ophthalmology. 2008;115:1534–1539. doi: 10.1016/j.ophtha.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Schlegel Z, Hoang-Xuan T, Gatinel D. Comparison of and correlation between anterior and posterior corneal elevation maps in normal eyes and keratoconus-suspect eyes. J Cataract Refract Surg. 2008;34:789–795. doi: 10.1016/j.jcrs.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 10.Sonmez B, Doan MP, Hamilton DR. Identification of scanning slit-beam topographic parameters important in distinguishing normal from keratoconic corneal morphologic features. Am J Ophthalmol. 2007;143:401–408. doi: 10.1016/j.ajo.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 11.Swartz T, Marten L, Wang M. Measuring the cornea: The latest developments in corneal topography. Curr Opin Ophthalmol. 2007;18:325–333. doi: 10.1097/ICU.0b013e3281ca7121. [DOI] [PubMed] [Google Scholar]
- 12.Konstantopoulos A, Hossain P, Anderson DF. Recent advances in ophthalmic anterior segment imaging: A new era for ophthalmic diagnosis? Br J Ophthalmol. 2007;91:551–557. doi: 10.1136/bjo.2006.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Sanctis U, Aragno V, Dalmasso P, Brusasco L, Grignolo F. Diagnosis of subclinical keratoconus using posterior elevation measured with 2 different methods. Cornea. 2013;32:911–915. doi: 10.1097/ICO.0b013e3182854774. [DOI] [PubMed] [Google Scholar]
- 14.Ishii R, Kamiya K, Igarashi A, Shimizu K, Utsumi Y, Kumanomido T. Correlation of corneal elevation with severity of keratoconus by means of anterior and posterior topographic analysis. Cornea. 2012;31:253–258. doi: 10.1097/ICO.0B013E31823D1EE0. [DOI] [PubMed] [Google Scholar]
- 15.Jafarinasab MR, Feizi S, Karimian F, Hasanpour H. Evaluation of corneal elevation in eyes with subclinical keratoconus and keratoconus using Galilei double Scheimpflug analyzer. Eur J Ophthalmol. 2013;23:377–384. doi: 10.5301/ejo.5000226. [DOI] [PubMed] [Google Scholar]
- 16.Kamiya K, Shimizu K, Igarashi A, Miyake T. Assessment of anterior, posterior, and total central corneal astigmatism in eyes with keratoconus. Am J Ophthalmol. 2015;160:851–857. doi: 10.1016/j.ajo.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Shajari M, Friderich S, Pour Sadeghian M, Schmack I, Kohnen T. Characteristics of Corneal Astigmatism of Anterior and Posterior Surface in a Normal Control Group and Patients With Keratoconus. Cornea. 2017;36:457–462. doi: 10.1097/ICO.0000000000001143. [DOI] [PubMed] [Google Scholar]
- 18.Savini G, Næser K, Schiano-Lomoriello D, Mularoni A. Influence of Posterior Corneal Astigmatism on Total Corneal Astigmatism in Eyes With Keratoconus. Cornea. 2016;35:1427–1433. doi: 10.1097/ICO.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 19.Naderan M, Rajabi MT, Zarrinbakhsh P. Distribution of Anterior and Posterior Corneal Astigmatism in Eyes With Keratoconus. Am J Ophthalmol. 2016;167:79–87. doi: 10.1016/j.ajo.2016.03.051. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Shirayama M, Koch DD. Repeatability of corneal power and wavefront aberration measurements with a dual-Scheimpflug Placido corneal topographer. J Cataract Refract Surg. 2010;36:425–430. doi: 10.1016/j.jcrs.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 21.Savini G, Carbonelli M, Barboni P, Hoffer KJ. Repeatability of automatic measurements performed by a dual Scheimpflug analyzer in unoperated and post-refractive surgery eyes. J Cataract Refract Surg. 2011;37:302–309. doi: 10.1016/j.jcrs.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 22.Eom Y, Rhim JW, Kang SY, Kim SW, Song JS, Kim HM. Toric intraocular lens calculations using ratio of anterior to posterior corneal cylinder power. Am J Ophthalmol. 2015;160:717–724. doi: 10.1016/j.ajo.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Visser N, Bauer NJ, Nuijts RM. Toric intraocular lenses: Historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J Cataract Refract Surg. 2013;39:624–637. doi: 10.1016/j.jcrs.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Sauder G, Jonas JB. Treatment of keratoconus by toric foldable intraocular lenses. Eur J Ophthalmol. 2003;13:577–579. doi: 10.1177/112067210301300612. [DOI] [PubMed] [Google Scholar]
- 25.Nanavaty MA, Lake DB, Daya SM. Outcomes of pseudophakic toric intraocular lens implantation in keratoconic eyes with cataract. J Refract Surg. 2012;28:884–889. doi: 10.3928/1081597X-20121106-02. [DOI] [PubMed] [Google Scholar]
- 26.Alió JL, Peña-García P, Abdulla Guliyeva F, Soria FA, Zein G, Abu-Mustafa SK. MICS with toric intraocular lenses in keratoconus: Outcomes and predictability analysis of postoperative refraction. Br J Ophthalmol. 2014;98:365–370. doi: 10.1136/bjophthalmol-2013-303765. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Sy ME, Mai H, Yu F, Hamilton DR. Effect of posterior corneal astigmatism on refractive outcomes after toric intraocular lens implantation. J Cataract Refract Surg. 2015;41:84–89. doi: 10.1016/j.jcrs.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 28.Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012;38:2080–2087. doi: 10.1016/j.jcrs.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 29.Nemeth G, Berta A, Lipecz A, Hassan Z, Szalai E, Modis L., Jr Evaluation of posterior astigmatism measured with Scheimpflug imaging. Cornea. 2014;33:1214–1218. doi: 10.1097/ICO.0000000000000238. [DOI] [PubMed] [Google Scholar]


