Abstract
Talazoparib inhibits PARP catalytic activity, trapping PARP1 on damaged DNA and causing cell death in BRCA1/2-mutated cells. We evaluated talazoparib therapy in this two-part, phase I, first-in-human trial. Antitumor activity, MTD, pharmacokinetics, and pharmacodynamics of once-daily talazoparib were determined in an open-label, multicenter, dose-escalation study (NCT01286987). The MTD was 1.0 mg/day, with an elimination half-life of 50 hours. Treatment-related adverse events included fatigue (26/71 patients; 37%) and anemia (25/71 patients; 35%). Grade 3 to 4 adverse events included anemia (17/71 patients; 24%) and thrombocytopenia (13/71 patients; 18%). Sustained PARP inhibition was observed at doses ≥0.60 mg/day. At 1.0 mg/day, confirmed responses were observed in 7 of 14 (50%) and 5 of 12 (42%) patients with BRCA mutation– associated breast and ovarian cancers, respectively, and in patients with pancreatic and small cell lung cancer. Talazoparib demonstrated single-agent antitumor activity and was well tolerated in patients at the recommended dose of 1.0 mg/day.
INTRODUCTION
The most-studied PARP enzymes are PARP1 and PARP2, which play critical roles in DNA damage detection and repair (1, 2), including the repair of single-strand DNA breaks through the base excision repair pathway (3–5). It has been hypothesized that single-strand DNA breaks persist when PARP function is compromised, leading to the creation of double-strand DNA breaks during replication (6); these double-strand DNA breaks are usually repaired by homologous recombination repair (HRR), allowing replication to continue (6). However, loss of PARP activity becomes lethal when HRR is compromised. This phenomenon, known as synthetic lethality, is well established for deleterious mutations of BRCA1 and BRCA2 (7–9).
The PARP inhibitor olaparib was recently approved for the treatment of advanced ovarian cancer and remains the only approved agent. PARP inhibitors have also demonstrated antitumor activity against other tumor types with DNA repair deficiencies, including breast and prostate cancers (10–13). Talazoparib (also known as MDV3800 and BMN 673) is a novel, potent, and selective inhibitor of PARP1/2 that achieves antitumor cell responses and elicits DNA repair markers at notably lower concentrations than earlier-generation PARP1/2 inhibitors (14, 15). In addition to inhibiting PARP catalytic activity, talazoparib is currently the most potent PARP1/2 inhibitor in vitro at trapping PARP–DNA complexes at sites of single-strand DNA breaks (16). Preclinically, talazoparib has favorable metabolic stability, oral bioavailability, and pharmacokinetics (PK) that support its daily schedule in clinical trials (14).
We conducted a first-in-human, phase I dose-escalation (Part 1) trial of talazoparib in patients with advanced solid malignancies and an expansion cohort (Part 2) in patients with tumors predicted to be potentially sensitive to PARP inhibition. These included tumors harboring germline BRCA1/2 mutations; triple-negative breast cancers; high-grade serous and/or undifferentiated ovarian, fallopian tube, or peritoneal cancers; and castration-resistant prostate and pancreatic cancers. Patients with Ewing sarcoma or small cell lung cancer (SCLC) were also studied; the former was based on a 1,000-cell line screen demonstrating antitumor activity (17, 18), and the latter was based on SCLC platinum sensitivity, increased PARP1 expression, and sensitivity of SCLC cell lines and animal models to PARP inhibition (19, 20).
RESULTS
Between January 3, 2011, and August 21, 2014, 113 patients with advanced solid tumors were enrolled at a total of six centers: five in the United States and one in the United Kingdom. A total of 110 patients received talazoparib (Table 1). Thirty-nine patients participated in Part 1 and received talazoparib at nine dose levels ranging from 0.025 to 1.1 mg/day (Fig. 1). An additional 71 patients were treated with talazoparib 1.0 mg/day in Part 2. As of the date of database cutoff (March 31, 2015), 2 patients in Part 1 and 5 patients in Part 2 continue to be treated (Fig. 1).
Table 1.
Demographics and baseline clinical characteristics
| Demographic parameter | Dose escalation (Part 1; n = 39) | Dose expansion (Part 2; n = 71) | Overall (N = 110) |
|---|---|---|---|
| Median age, years (range) | 58.0 (19–81) | 57.0 (18–88) | 57.0 (18–88) |
|
| |||
| Male, n (%) | 6 (15.4) | 28 (39.4) | 34 (30.9) |
|
| |||
| ECOG performance status, n (%) | |||
| 0 | 23 (59.0) | 37 (52.1) | 60 (54.5) |
| 1 | 16 (41.0) | 34 (47.9) | 50 (45.5) |
|
| |||
| Tumor type, n (%) | |||
| Breast | 8 (20.5) | 12 (16.9) | 20 (18.2) |
| Ovarian/peritoneal | 23 (59.0) | 11 (15.5) | 34 (30.9) |
| Prostate | 1 (2.6) | 3 (4.2) | 4 (3.6) |
| Pancreatic | 3 (7.7) | 10 (14.1) | 13 (11.8) |
| Ewing sarcoma | 2 (5.1) | 12 (16.9) | 14 (12.7) |
| SCLC | 0 | 23 (32.4) | 23 (20.9) |
| Colorectal | 2 (5.1) | 0 | 2 (1.8) |
|
| |||
| Deleterious mutation, n (%) | |||
| gBRCA1 | 16 (41.0) | 13 (18.3) | 29 (26.4) |
| gBRCA2 | 7 (17.9) | 20 (28.2) | 27 (24.5) |
| gBRCA1/2 | 1 (2.6) | 2 (2.8) | 3 (2.7) |
|
| |||
| Median prior chemotherapy regimens, n (range) | 4.0 (1.0–13.0) | 2.0 (0.0–6.0) | 2.5 (0.0–13.0) |
|
| |||
| Median prior platinum regimens, n (range) | 2.0 (0.0–4.0) | 1.0 (0.0–4.0) | 1.0 (0.0–4.0) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; gBRCA, germline BRCA mutated.
Figure 1.
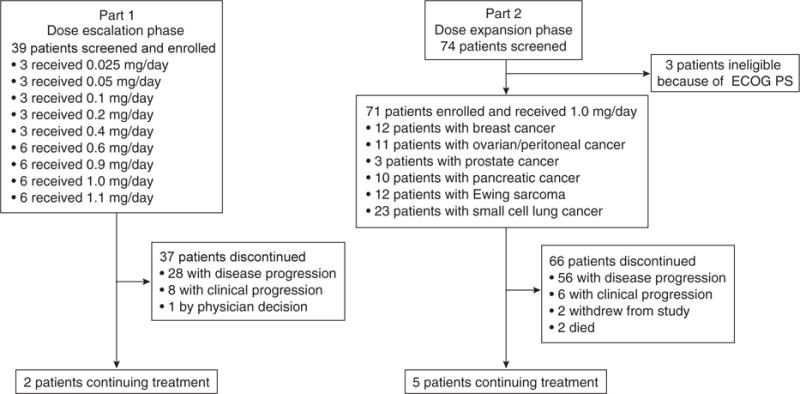
Patient enrollment and disposition. Abbreviation: ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Safety
The number of patients per dose level, observed dose-limiting toxicities (DLT), dose reductions, and median time on study are provided in Table 2. Dose-limiting thrombocytopenia in cycle 1 occurred in 1 of 6 patients at 0.9 mg/day and 2 of 6 patients assessable for DLT at 1.1 mg/day. The patient treated at 0.9 mg/day experienced grade 3 thrombocytopenia with grade 3 anemia. Of the 2 patients treated at 1.1 mg/day, both experienced grade 3 thrombocytopenia; for 1 of these patients, it became grade 4 thrombocytopenia. All DLTs resolved after temporary interruption of study drug; no hemorrhage was noted. Because 2 patients experienced a DLT at the 1.1 mg/day dose level, an interim dose of 1.0 mg/day was investigated. No DLTs were observed at this dose level in a group of 6 assessable patients. This dose was therefore determined to be the MTD and the recommended dose for Part 2.
Table 2.
Part 1 dose escalation schema, DLTs, dose reductions, and common AEs (>15%) or grade 3 to 4 AEs (>4%) assessed by investigator as related at the recommended dose
| Dose level | Patients (n = 39) | DLTs in first cycle | Dose reductions (any cycle) | Number of treatment days | |
|---|---|---|---|---|---|
| Number | Description | Number | Median (range) | ||
| 0.025 mg | 3 | 0 | — | 2 | 35 (35-98) |
| 0.05 mg | 3 | 0 | — | 2 | 99 (34-205) |
| 0.10 mg | 3 | 0 | — | 2 | 119 (65-253) |
| 0.20 mg | 3 | 0 | — | 2 | 281 (35-427) |
| 0.40 mg | 3 | 0 | — | 1 | 226 (97-268) |
| 0.60 mg | 6 | 0 | — | 4 | 185 (58-289) |
| 0.90 mg | 6 | 1 | Grade 3 TCP | 5 | 261 (30-1114) |
| 1.00 mg | 6 | 0 | — | 5 | 214 (84-960) |
| 1.10 mg | 6a | 2 | Grade 3-4 TCP | 4 | 60 (14-196) |
| Adverse event | All grade (n = 71) | Grade 3-4 (n = 71) |
|---|---|---|
| Any treatment-emergent AE, n (%) | 55 (77) | 32 (45) |
|
| ||
| Blood and lymphatic system disorders,n (%) | 40 (56) | 30 (42) |
| Anemia | 25 (35) | 16 (23) |
| TCP | 15 (21) | 13 (18) |
| Neutropenia | 11 (15) | 7 (10) |
|
| ||
| Gastrointestinal disorders, n (%) | 27 (38) | — |
| Nausea | 23 (32) | — |
|
| ||
| General disorders and administration site conditions, n (%) | 27 (38) | 2 (3) |
| Fatigue | 26 (37) | 2 (3) |
|
| ||
| Skin and subcutaneous tissue disorders, n (%) | 19 (27) | — |
| Alopecia | 14 (20) | — |
Abbreviation: TCP, thrombocytopenia.
One patient discontinued from the trial on study day 21 for progressive disease, having received only 8 days of continuous dosing.
In Part 2, 71 patients received talazoparib at 1.0 mg/day via continuous daily dosing. The median relative dose intensity was high at 97.2%, and the dose was well tolerated. Table 2 presents the most common toxicities at this dose related to the study drug, including fatigue (37%), anemia (35%), nausea (32%), thrombocytopenia (21%), alopecia (20%), and neutropenia (15%). Grade 3 or 4 adverse events (AE) assessed by investigator as related were reported in 32 (45%) patients, with the most frequent being anemia (23%), thrombocytopenia (18%), and neutropenia (10%).
Of the 77 patients receiving the 1.0 mg/day dose, 26 patients (34%) reported at least one dose reduction, the majority of whom (20 patients) had reductions due to an AE such as anemia, thrombocytopenia, and neutropenia. Although transient dose holidays were needed as a result of these AEs, no patients permanently withdrew from treatment because of them in either Part 1 or Part 2 of the trial.
There were eight deaths associated with an AE during the study, none of which were considered to be related to study treatment. Two of the deaths occurred in patients with breast cancer enrolled in Part 1 at the entry dose of 1.1 mg/day talazoparib (both related to disease progression). Six of the deaths occurred in patients in Part 2 at the 1.0 mg/day dose of talazoparib (2 patients with pancreatic cancer, both from disease progression; 2 patients with Ewing sarcoma, 1 from dyspnea and the other from respiratory failure; and 2 patients with SCLC, 1 from hypoxia secondary to lung metastases and the other from lung infection).
Pharmacokinetics
Mean talazoparib plasma concentration–time profiles following single and multiple doses of talazoparib are provided in Fig. 2A–D. Talazoparib PK parameters resulting from the analysis of the plasma concentration–time profiles are provided in Table 3.
Figure 2.
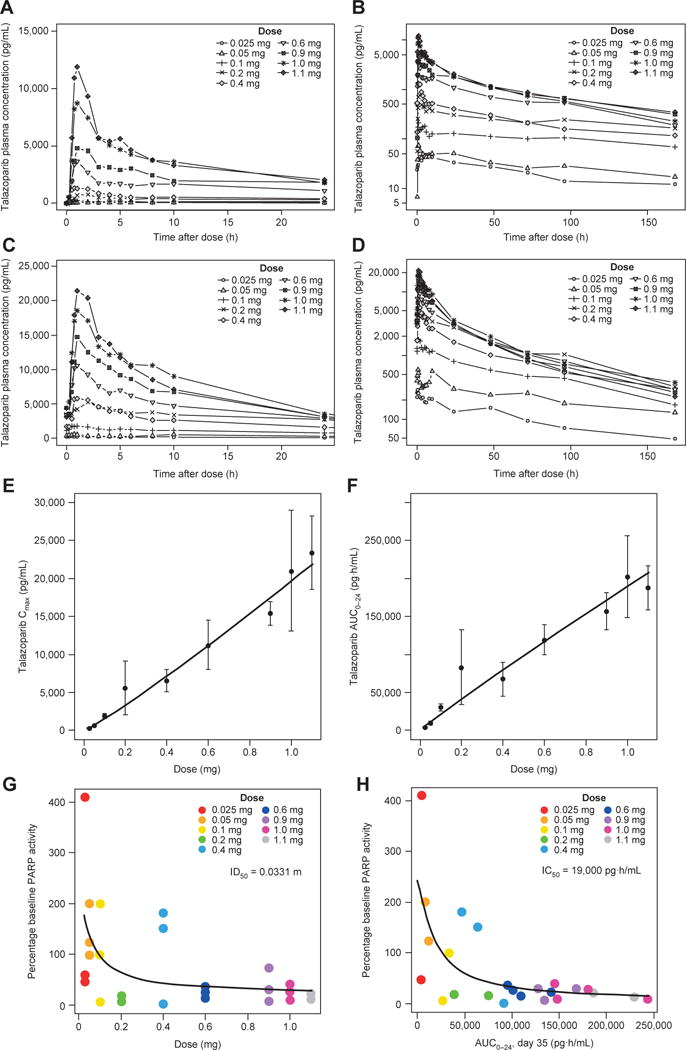
PK and PD features of talazoparib. A–D, Mean concentration– time profiles of talazoparib. Linear mean talazoparib plasma concentration–time profiles over the initial 24 hours after dose and log-linear mean talazoparib plasma concentration–time profiles over the complete sampling interval following (A and B) single doses of talazoparib and (C and D) multiple daily doses of talazoparib. E–H, Dose proportionality of talazoparib PK and dose–response and exposure–response relationships between talazoparib and PBMC PARP activity. E, Plasma Cmax following multiple daily doses ranging from 0.025 to 1.1 mg. F, AUC0–24 following multiple daily doses ranging from 0.025 to 1.1 mg. Filled circles represent the mean value at each dose level, and error bars represent the standard deviations. Solid line represents the power model fit through the data. G, Dose–response relationship between talazoparib and PBMC PARP activity. H, Exposure–response relationship between talazoparib and PBMC PARP activity. Percentage baseline PBMC PARP activity defined as the mean of the predose PARP activity assessments during the multiple dosing assessment phase (i.e., predose assessments on days 15, 22, and 35 of cycle 1). Abbreviations: AUC0–24, AUC from 0 to 24 h; IC50, half maximal inhibitory concentration; ID50, inhibitory dose 50%; PD, pharmacodynamic.
Table 3.
PK parameters and PARP inhibition following single and multiple daily dosing
| PK parameter | Single talazoparib dose, mg | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.025 (n = 3) | 0.05 (n = 3) | 0.1 (n = 3) | 0.2 (n = 3) | 0.4 (n = 3) | 0.6 (n = 6) | 0.9 (n = 6) | 1.0 (n = 5) | 1.1 (n = 7) | |
| Tmax, median (min, max), h | 7.92 (1.95, 9.95) | 1.00 (0.80, 1.02) | 1.02 (1.00, 3.98) | 1.03 (1.00, 2.32) | 2.03 (0.75, 2.95) | 0.835 (0.75, 1.95) | 2.00 (1.02, 9.98) | 1.03 (0.73, 2.07) | 1.00 (0.73, 2.05) |
| Cmax, mean (SD), pg/mL | 60.0 (15.9) | 79.7 (7.50) | 214 (50.9) | 788 (369) | 1,830 (699) | 4,100 (1,400) | 6,100 (3,060) | 10,600 (4,220) | 13,200 (3,220) |
| AUC0–24, mean (SD), pg·h/mL | 952 (386) | 1,160 (166) | 3,160 (1,270) | 9,130 (3,540) | 13,500 (5,200) | 37,900 (12,900) | 58,200 (24,300) | 85,100 (29,100) | 91,600 (31,800) |
| AUC0–t, mean (SD), pg·h/mL | 3,600 (1,360) | 5,340 (1,960) | 16,600 (5,320) | 39,300 (11,700) | 43,700 (15,000) | 97,900 (30,000) | 160,000 (66,100) | 182,000 (62,400) | 201,000 (93,400) |
| AUC0-∞, mean (SD), pg·h/mL | 5,330 (1,840) | 8,320 (1,960) | 37,600 (6,620) | 92,700 (48,500) | 60,100 (15,900) | 120,000 (26,000) | 188,000 (85,700) | 200,000 (64,000) | 235,000 (111,000) |
| t1/2, mean (SD), h | 100 (11.9) | 129 (42.6) | 229 (158) | 212 (126) | 102 (27.2) | 58.6 (17.3) | 60.4 (10.9) | 52.9 (13.4) | 71.0 (20.6) |
| CL/F, mean (SD), L/h | 5.17 (2.10) | 6.27 (1.66) | 2.72 (0.532) | 2.61 (1.35) | 6.95 (1.71) | 5.19 (0.99) | 5.49 (2.08) | 5.39 (1.59) | 5.32 (1.64) |
| Vz/F, mean (SD), L | 756 (351) | 1,240 (742) | 839 (487) | 678 (217) | 1,050 (431) | 441 (143) | 468 (169) | 415 (170) | 549 (232) |
| Multiple daily talazoparib dosing, mg/day | |||||||||
| 0.025 (n = 3) | 0.05 (n = 2) | 0.1 (n = 2) | 0.2 (n = 3) | 0.4 (n = 3) | 0.6 (n = 6) | 0.9 (n = 5) | 1.0 (n = 6) | 1.1 (n = 4) | |
| Tmax, median (min, max), h | 1.02 (0.58, 3.98) | 5.43 (0.77, 10.1) | 0.76 (0.75, 0.82) | 1.97 (1.00, 3.02) | 0.98 (0.75, 2.00) | 1.04 (0.73, 5.98) | 1.02 (0.97, 2.07) | 1.02 (0.75, 2.00) | 1.48 (0.98, 2.00) |
| Cmax, mean (SD), pg/mL | 300 (78.8) | 615 (74.2) | 1,880 (332) | 5,620 (3,530) | 6,560 (1,500) | 11,300 (3,230) | 15,400 (1,540) | 21,000 (7,990) | 23,400 (4,810) |
| AUC0-24, mean (SD), pg·h/mL | 3,960 (759) | 9,770 (2,440) | 30,000 (4,490) | 83,100 (49,300) | 67,300 (22,600) | 119,000 (19,900) | 157,000 (24,500) | 202,000 (54,000) | 188,000 (29,200) |
| t1/2, mean (SD), h | 107 (84.2) | 132 (12.3) | 98.2 (4.83) | 50.9 (19.1) | 90.7 (32.7) | 63.7 (12.7) | 71.0 (14.5) | 50.0 (16.6) | 52.8 (23.2) |
| CLss/F, mean (SD), L/h | 6.43 (1.23) | 5.28 (1.32) | 3.37 (0.502) | 3.12 (1.91) | 6.40 (2.07) | 5.15 (0.897) | 5.86 (0.951) | 5.24 (1.39) | 5.96 (0.837) |
| Vz/F, mean (SD), L | 1,070 (971) | 1,020 (345) | 475 (47.8) | 264 (249) | 818 (326) | 477 (136) | 604 (169) | 373 (144) | 472 (254) |
| Cmin, mean (SD), pg/mL | 169 (58.0) | 299 (133) | 1,020 (107) | 2,880 (1,710) | 2,230 (957) | 3,470 (1,050) | 3,180 (802) | 3,720 (1,590) | 2,910 (803) |
| PARP activity, % baseline | |||||||||
| 0.025 (n = 3) | 0.05 (n = 3) | 0.1 (n = 3) | 0.2 (n = 3) | 0.4 (n = 3) | 0.6 (n = 4) | 0.9 (n = 4) | 1.0 (n = 4) | 1.1 (n = 2) | |
| PARP activity, mean (SD) | 172 (206) | 141 (52.5) | 102 (98.0) | 14.7 (5.04) | 111 (96.5) | 24.7 (8.19) | 34.7 (27.4) | 21.1 (14.9) | 16.3 (5.63) |
Abbreviations: AUC0–24, AUC from 0 to 24 h; AUC0–∞, AUC from time 0 extrapolated to infinity; AUC0–t, AUC from time 0 to last quantifiable concentration; CLss/F, CL/F at steady state; SD, standard deviation; Tmax, time to Cmax.
Talazoparib demonstrated rapid absorption, with maximum plasma concentration (Cmax) generally reached within 2 hours after all evaluated doses and following both single and multiple daily dosing. Steady-state plasma concentrations were reached by 2 weeks of daily dosing across all doses evaluated. Talazoparib was well distributed into tissue compartments, with apparent volume of distribution (Vz/F) estimates well in excess of the volume of the systemic circulatory space. Plasma elimination followed biphasic kinetics with a long terminal half-life (t1/2). Linear elimination across dose levels was apparent following both single and multiple daily dosing as evidenced by parallel terminal phases of the log-linear profiles and similar apparent oral clearance (CL/F) estimates across dose levels. At the MTD dose of 1.0 mg/day, t1/2 was approximately 2 days, and mean accumulation ratio was 2.4-fold at steady state.
Plasma concentrations, Cmax, and area under the plasma concentration–time curve (AUC) estimates increased approximately with doses ranging from 0.025 to 1.1 mg following multiple daily dosing as shown in Fig. 2E–H. Estimates [95% confidence interval (CI)] of the dose proportionality parameter, β, for Cmax and AUC from 0 to 24 hours (AUC0–24) following multiple daily doses of talazoparib were 1.11 (1.01–1.20) and 0.95 (0.84–1.05), respectively.
Results for urinary elimination of the parent compound suggest linear urinary elimination kinetics after daily talazoparib dosing between the 0.025 and 1.1 mg dose levels. Following single doses in Part 1, mean values for the amount of the analyte excreted in urine from 0 to 24 hours (Ae0–24) and the fraction of urine excretion from 0 to 24 hours (Fe0–24) generally increased with dose, and average renal clearance from time 0 to 24 hours postdose (ARC0–24) values were similar across dose levels. Following multiple daily doses in Part 1, Ae0–24 increased with increasing dose, whereas mean Fe0–24 and ARC0–24 values were generally similar across the 0.025 and 1.1 mg/day dose levels.
Pharmacodynamics
The mean percentage baseline peripheral blood mononuclear cell (PBMC) PARP activities with multiple-dose talazoparib by dose level are provided in Table 3 (Supplementary Fig. S1). Overall, PBMC PARP activity decreased with talazoparib dose across the evaluated dose range.
The dose–response and concentration–response relationships between talazoparib and PBMC PARP activity are shown in Fig. 2E–H, and maximum inhibitory effect model parameter estimates are provided in Supplementary Table S1. In the exposure–response curve, an estimated half maximal inhibitory concentration of AUC0–24 was 19,000 pg · h/mL.
Efficacy
In 14 patients with breast cancer (all with deleterious BRCA1/2 mutations) treated with talazoparib at 1.0 mg/day, the objective response rate (ORR) was 50% and included one complete response (CR; Table 4). Five patients had stable disease (SD) lasting at least 24 weeks, resulting in a clinical benefit rate (CBR) of 86% for at least 24 weeks. Median progression-free survival (PFS) was 34.6 weeks (95% CI, 27.1–54.0; Table 4). For the total of 18 patients with breast cancer with deleterious BRCA1/2 mutations treated at any talazoparib dose level, the ORR and CBR were higher in patients whose tumors carried the BRCA2 mutation (ORR, 55%, 6/11 patients; CBR, 91%, 10/11 patients) compared with those who had the BRCA1 mutation (ORR, 38%, 3/8 patients; CBR, 50%, 4/8 patients; percentage change in target lesion size summarized in Fig. 3A). Of note, 1 patient had aberrations in both BRCA1 and BRCA2, although the BRCA2 aberration detected may not be deleterious (Y3098X). Interestingly, in the patients with BRCA-mutated breast cancer, higher antitumor activity was observed in patients with non–triple-negative breast cancer (n = 9) than in those with triple-negative disease [n = 9; CBR, 89% vs. 56% ≥24 weeks; median PFS, 38.3 weeks (95% CI, 2.6–67.4) vs. 20.4 weeks (95% CI, 3.1–36.1)]. Six of the 18 patients with BRCA-mutated breast cancer had received prior platinum therapy, of whom 2 had an objective response.
Table 4.
Clinical response rate (RECIST) by cancer type in patients treated with talazoparib 1.0 mg/day (recommended phase II dose)
| Response | Breasta (n = 14) | Ovarian/peritoneala (n = 12) | SCLC (n = 23) | Pancreatic (n = 10) | Ewing sarcoma (n = 13) |
|---|---|---|---|---|---|
| ORR,% | 50.0 | 41.7 | 8.7 | 20.0 | 0 |
| CR, n | 1 | 1 | 0 | 0 | 0 |
| PR, n | 6 | 4 | 2 | 2 | 0 |
| SD, n | 5 | 3 | 4 | 1 | 3 |
| CBR,%b | 85.7 | 66.7 | 26.1 | 30.0 | 23.1 |
| Median PFS, weeks | 34.6 | 36.4c | 11.1 | ND | ND |
Abbreviations: ND, not determined; SD, stable disease.
Patients had BRCA1/2 mutation.
Clinical benefit = CR + PR + SD ≥24 weeks for breast and ovarian cancers, and CR + PR + SD ≥16 weeks for SCLC, pancreatic cancer, and Ewing sarcoma.
Analysis on 14 patients, as 2 patients who did not have measurable disease at baseline were included in the PFS analysis but not in the response analysis.
Figure 3.
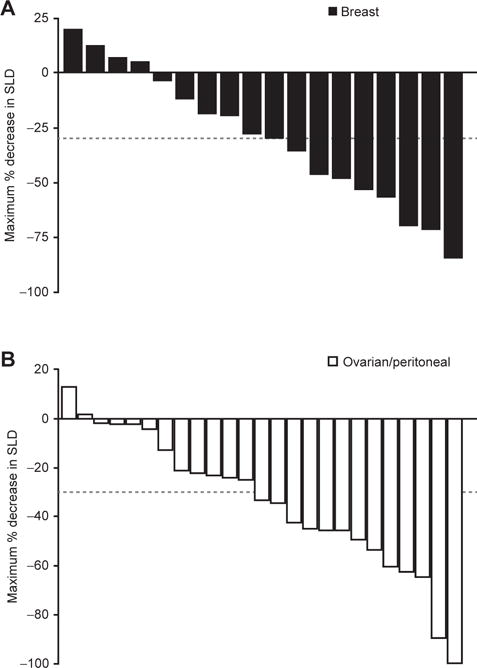
Percentage change in target lesion for patients undergoing treatment with talazoparib who have (A) gBRCA breast cancer and (B) gBRCA ovarian cancer. Positive values indicate tumor growth, negative values indicate tumor reduction, and the dashed line represents the definition of partial response from RECIST guidelines. Abbreviations: gBRCA, germline BRCA mutated; SLD, sum of longest diameter.
In 12 patients with ovarian cancer with deleterious germline BRCA1/2 mutations with measurable disease treated with talazoparib 1.0 mg/day, ORR and CBR lasting at least 24 weeks equaled 42% and 67%, respectively, with a median PFS of 36.4 weeks (Table 4). For all patients with BRCA-mutated ovarian cancer treated at any talazoparib dose level with measurable disease (n = 25), ORR and CBR lasting at least 24 weeks were 48% (including one CR) and 76%, respectively (percentage change in target lesion size is summarized in Fig. 3B). All 25 patients had received prior platinum-based chemotherapy; the ORR in platinum-sensitive patients was 55% (11/20 patients) compared with 20% (1/5 patients) in platinum-resistant patients.
All 23 patients with SCLC were enrolled in Part 2 and treated with 1.0 mg/day. Median number of prior regimens was 1, ranging from 0 to 2. Two patients had a partial response (PR; ORR, 9%, with duration of response 12.0 and 15.3 weeks, respectively), and a further 4 had SD lasting at least 16 weeks (CBR, 26% ≥16 weeks; Table 4). For the 2 patients with an objective response, both had had an objective response to the last prior platinum therapy, with a platinum-free interval of 6 months or less. Median PFS for this group was 11.1 weeks (95% CI, 4.3–13.0).
Of the 13 patients with pancreatic cancer from Part 1 and Part 2, 4 had clinical benefit (CBR, 31% ≥16 weeks): Two patients had a PR, 1 with BRCA2 mutation, the other with a PALB2 mutation (Table 4). For patients with Ewing sarcoma, no objective response was observed, and the CBR (SD ≥ 16 weeks) was 23%.
For the 7 patients currently receiving talazoparib on the study as of the data cutoff of March 31, 2015, 4 have ovarian cancer (continuing on study for 27.4, 28.1, 31.5, and 36.6 months), and 1 patient each has breast, pancreatic, or prostate cancer (24.2, 22.8, and 8.4 months, respectively). The starting dose for these patients ranged between 0.9 and 1.0 mg/day; current dose is between 0.5 and 1.0 mg/day.
DISCUSSION
Talazoparib is a potent oral PARP1/2 inhibitor that has equivalent catalytic activity to olaparib and rucaparib, but is superior in trapping PARP–DNA at the site of DNA damage by comparison (16). This first-in-human study demonstrated that talazoparib results in single-agent activity in patients harboring germline deleterious BRCA mutations or whose tumors harbor other mutations sensitive to PARP inhibition. The clinical activity observed with talazoparib suggests that targeting PARP1/2 may also be an effective strategy for those patients whose tumors harbor other genomic abnormalities involved in DNA repair mechanisms (13).
Talazoparib was well tolerated overall. The primary toxicity of talazoparib was hematologic, with transient and reversible cytopenias (thrombocytopenia, neutropenia, and anemia), primarily managed with drug interruption and/or dose reduction and otherwise routine medical intervention; transfusions were uncommon. All episodes of DLT involved brief thrombocytopenia without hemorrhage. Nonhematologic toxic effects were mild in severity and manageable. The relative dose intensity was high at 97.2%, and overall the dose was well tolerated. Furthermore, no patients permanently withdrew from talazoparib treatment because of toxicity, in either Part 1 or Part 2 of this study.
Talazoparib demonstrated favorable PK properties with good oral bioavailability, rapid absorption, and dose-proportional increases in total exposure (AUC) over a wide dose range (0.025–1.1 mg/day). Steady state was reached approximately 2 weeks after initiation of daily dosing. Linear urinary elimination kinetics were reported with daily dosing. At the recommended phase II dose of 1.0 mg/day, the t1/2 was approximately 2 days upon multiple dosing; trough talazoparib plasma concentrations were maintained above 10 nmol/L, suggesting that systemic concentrations of talazoparib are sufficient to inhibit PARP activity.
In pharmacodynamic (PD) testing, talazoparib demonstrated PARP inhibition in PBMCs over a relatively wide range of doses. For doses at and above 0.6 mg/day, PARP activity was consistently inhibited in all patients evaluated. PD results suggest that effective PARP inhibition could still be achieved at reduced dose levels.
Talazoparib demonstrated promising antitumor activity in patients with heavily pretreated breast and ovarian cancers associated with deleterious germline BRCA1/2 mutations. Single-agent activity in patients with advanced breast cancer (including patients with triple-negative disease) equaled 50% (ORR) and 86% (CBR). Similarly, in the 12 patients with BRCA-mutated ovarian cancer treated with 1.0 mg/day of talazoparib, ORR and CBR equaled 42% and 67%, respectively.
Of note, 1 responding patient with pancreatic cancer harbored a PALB2 mutation (21); as this mutation is known to recruit BRCA2 and RAD51 to DNA breaks, such findings support a trial in a broader population (those with additional DNA repair deficiencies as opposed to BRCA mutations only), potentially expanding applications for PARP inhibitor therapy.
In conclusion, the findings from this study demonstrate the effectiveness of single-agent talazoparib for the treatment of patients with and without germline BRCA1/2 mutations in ovarian, breast, small cell lung, and pancreatic cancers. Talazoparib has a tolerable safety profile in multiple patients seen over a treatment period exceeding 2 years. The PK properties of talazoparib support once-daily dosing. Data from this phase I trial support a role for talazoparib in the treatment of patients with advanced tumors (inherited and sporadic cancers with DNA repair deficiencies). Talazoparib is currently undergoing further clinical investigation against multiple tumor types, including a phase III trial in patients with metastatic breast cancer with a deleterious BRCA1/2 mutation.
METHODS
Study Design and Participants
We undertook a phase I study of talazoparib in patients with advanced solid tumors and either germline BRCA1/2 mutations or a strong preclinical rationale for use of a PARP inhibitor. Eligible patients were age 18 years or older and had histologically or cytologically documented unresectable, locally advanced, or metastatic solid tumors not suitable for established therapy or for which standard therapy had failed; Eastern Cooperative Oncology Group Performance Status of 0 or 1; and adequate hematologic and liver function.
Patients enrolled in Part 1 (dose escalation) had tumors known to harbor DNA repair deficiencies (Supplementary Methods); provision of documentation (genomic or immunohistochemistry) was not required. Enrollment in Part 2 was restricted to patients with selected tumors with confirmed BRCA1/2 germline pathogenic or deleterious mutations by BRACAnalysis (Myriad Genetics) or local laboratory evaluation (ovarian or peritoneal, breast, prostate, or pancreatic cancers), patients with DNA repair deficiency, or patients with SCLC or Ewing sarcoma (Supplementary Methods). Patient eligibility, including a full list of exclusion criteria, is provided in Supplementary Methods.
The study was conducted in accordance with the protocol, good clinical practice standards, and the Declaration of Helsinki and the International Conference on Harmonisation. The appropriate Institutional Review Board or ethics committee at each participating institution approved the protocol. All enrolled patients provided written informed consent before undergoing study-specific procedures.
Study Treatment
For Part 1, fasted patients received a single dose of talazoparib at the start of the study and then underwent PK and PD assessments 1 week later. Following assessments, patients received talazoparib once daily, continuously for 28 days, again followed by a 1-week break from treatment (defined as cycle 1) to assess PK and PD. Dosing was continuous thereafter without breaks except as needed for toxicity. A standard 3+3 design was used for dose escalation (22), with a starting talazoparib dose of 0.025 mg/day. Dose doubling occurred provided toxicities were Common Terminology Criteria for Adverse Events grade 1 or less during cycle 1; dose escalations were limited to 25% to 50% once grade 2 drug-related toxicities were observed (25% for grade 3 drug-related toxicity). For each cohort, the first patient was observed for 15 days prior to additional patient enrollment. To be eligible for DLT assessment, a patient must have received at least 24 of the planned 28 doses of talazoparib between days 8 and 35. A DLT was defined as any of the following events occurring during cycle 1: grade 4 neutropenia associated with grade 2 or greater infection or lasting at least 5 days; grade 4 thrombocytopenia (or grade 3 with either hemorrhage or dose interruption for ≥5 days); any AE of grade 3 or greater considered related to talazoparib, except a nonhematologic asymptomatic grade 3 laboratory AE, grade 3 nausea, vomiting, and/ or diarrhea medically managed to grade 2 or less within 24 hours, or grade 3 fatigue that improved to grade 2 or less in no more than 5 days (additional information provided in Supplementary Methods).
Enrollment in Part 2 proceeded once the MTD was determined. Patients received talazoparib at the MTD of 1.0 mg/day starting from cycle 1, day 1 (28-day cycles). Participation in the study could be discontinued at any time at the discretion of the investigator and in accordance with clinical judgment.
AEs were recorded from the time of first dose of talazoparib until 30 days after the last dose.
Study Procedures
At screening, patients underwent physical examination (with vital signs and performance status assessment). Safety laboratory tests (complete blood count with differential and platelets, chemistry) were obtained weekly; coagulation and urinalysis were obtained weekly (cycle 1) and at the beginning of each cycle thereafter. Hematology evaluations were conducted more frequently upon observation of grade 2 or greater neutropenia or thrombocytopenia. Further details of study procedures are given in Supplementary Methods.
Pharmacokinetic Analysis
Plasma and urine samples were assayed for talazoparib concentrations using a validated high-performance LC/MS-MS detection method. For plasma, the lower limit of quantitation (LLOQ) was 5.0 pg/mL; for urine, the LLOQ was 25.0 pg/mL. Talazoparib PK parameters (following single and multiple daily dosing) were obtained using standard noncompartmental analysis methods in Phoenix Win-Nonlin Version 6.4 (Certara L.P.). PK parameters estimated included Cmax; time to Cmax; AUC0–24, AUC from time 0 to time of last quantifiable concentration, and AUC from time 0 extrapolated to infinity; CL/F; Vz/F; and t1/2. The multiple-dose PK parameters also estimated included minimum plasma concentration and CL/F at steady state. Dose proportionality following single and multiple daily dosing of talazoparib was assessed using a power model approach (23).
Pharmacodynamic Analysis
See Supplementary Methods for details.
Statistical Analysis
The primary objective in Part 1 of this study was to determine the MTD and recommended dose of daily oral talazoparib; secondary objectives included safety, PK, and PD profiles. For Part 2, efficacy parameters in the selected tumor types were investigated per a prespecified analysis based on RECIST version 1.1 through investigator assessment of lesion measurements, including ORR (in patients with measurable disease) or disease-specific changes in tumor markers using standard definitions (24–26). The number and percentage of patients achieving a response were summarized with an exact 95% CI calculated using the Clopper– Pearson method. The PFS was summarized using the Kaplan–Meier method. The data cutoff was March 31, 2015. SAS Analytics Software (version 9.1; SAS Institute, Inc.) was used for data analyses.
Supplementary Material
SIGNIFICANCE.
In this clinical trial, we show that talazoparib has single-agent antitumor activity and a tolerable safety profile. At its recommended phase II dose of 1.0 mg/day, confirmed responses were observed in patients with BRCA mutation–associated breast and ovarian cancers and in patients with pancreatic and small cell lung cancer.
Acknowledgments
The authors would like to thank the study patients and the following persons from the sponsors for their contributions to data collection and analysis, assistance with statistical analysis, or critical review of the manuscript. From BioMarin: Andrew Dorr, MD, Gilles Gallant, PhD, Don Musson, PhD, Charles O’Neill, PhD, Evelyn W. Wang, PhD, Charlie Zhang, PhD, and Huiyu Zhou, PhD; from Medivation (acquired by Pfizer, Inc., in September 2016): Alison L. Hannah, MD. Copyediting and formatting support funded by Medivation (acquired by Pfizer, Inc., in September 2016) was provided by Edwin Thrower, PhD, and Shannon Davis of Ashfield Healthcare Communications.
Grant Support
Medivation, Inc., has assumed responsibility for talazoparib effective October 6, 2015, and was involved in the trial, data analysis, and interpretation; Medivation was acquired by Pfizer, Inc., in September 2016. BioMarin Pharmaceutical, Inc., was involved in the study design, data collection, analysis, and interpretation. All authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
J. de Bono acknowledges support from The Institute of Cancer Research/Royal Marsden Drug Development Unit through a Cancer Research UK Centre grant, an Experimental Cancer Medical Centre (ECMC) grant from Cancer Research UK and the Department of Health (Ref: C51/A7401), and NHS funding to the NIHR Biomedical Research Centre to the Royal Marsden.
J. de Bono reports receiving honoraria from the Speakers Bureaus of AstraZeneca, Genentech, GSK, Merck, and Pfizer, and is a consultant/ advisory board member for AstraZeneca, Genentech, GSK, Merck, and Pfizer. R.K. Ramanathan has received travel reimbursement from BioMarin. R. Chugh reports receiving commercial research grants from BioMarin and Medivation. S. Kaye is a consultant/advisory board member for AstraZeneca. J. Heymach is a consultant/advisory board member for BioMarin. N.J. Curtin reports receiving a commercial research grant from BioMarin and commercial research support from Pfizer; has ownership interest (including patents) in Newcastle University and Pfizer; and is a consultant/advisory board member for AbbVie and Tesaro. Z.A. Wainberg is a consultant/advisory board member for Medivation.
Footnotes
Authors’ Contributions
Conception and design: J. de Bono, L. Mina, J. Glaspy, S. Rafii, S. Kaye, J. Heymach, J.W. Henshaw, L.A. Byers
Development of methodology: J. de Bono, S. Rafii, S. Kaye, J.W. Henshaw, L.A. Byers
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): J. de Bono, R.K. Ramanathan, R. Chugh, J. Glaspy, S. Rafii, S. Kaye, J. Sachdev, D.C. Smith, A. Herriott, M. Patterson, N.J. Curtin, L.A. Byers, Z.A. Wainberg
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): J. de Bono, R. Chugh, S. Rafii, S. Kaye, J.W. Henshaw, A. Herriott, M. Patterson, L.A. Byers, Z.A. Wainberg
Writing, review, and/or revision of the manuscript: J. de Bono, R.K. Ramanathan, L. Mina, R. Chugh, J. Glaspy, S. Rafii, S. Kaye, J. Sachdev, J. Heymach, D.C. Smith, J.W. Henshaw, N.J. Curtin, L.A. Byers, Z.A. Wainberg
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): J. de Bono, S. Rafii, A. Herriott
Study supervision: J. de Bono, L. Mina, S. Rafii, J. Sachdev, N.J. Curtin, L.A. Byers, Z.A. Wainberg
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed by the other authors.
Note: Supplementary data for this article are available at Cancer Discovery Online (http://cancerdiscovery.aacrjournals.org/).
L.A. Byers and Z.A. Wainberg are co–senior authors of this article.
References
- 1.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satoh MS, Lindahl T. Role of poly(ADP-ribose) formation in DNA repair. Nature. 1992;356:356–58. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- 3.Ame JC, Rolli V, Schreiber V, Niedergang C, Apiou F, Decker P, et al. PARP-2, a novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J Biol Chem. 1999;274:17860–68. doi: 10.1074/jbc.274.25.17860. [DOI] [PubMed] [Google Scholar]
- 4.Shall S, de Murcia G. Poly(ADP-ribose) polymerase-1: What have we learned from the deficient mouse model? Mutat Res. 2000;460:1–15. doi: 10.1016/s0921-8777(00)00016-1. [DOI] [PubMed] [Google Scholar]
- 5.Dantzer F, Schreiber V, Niedergang C, Trucco C, Flatter E, De La Rubia G, et al. Involvement of poly(ADP-ribose) polymerase in base excision repair. Biochimie. 1999;81:69–75. doi: 10.1016/s0300-9084(99)80040-6. [DOI] [PubMed] [Google Scholar]
- 6.De Vos M, Schreiber V, Dantzer F. The diverse roles and clinical relevance of PARPs in DNA damage repair: Current state of the art. Biochem Pharmacol. 2012;84:137–46. doi: 10.1016/j.bcp.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 8.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 9.Lord CJ, Tutt AN, Ashworth A. Synthetic lethality and cancer therapy: Lessons learned from the development of PARP inhibitors. Annu Rev Med. 2015;66:455–70. doi: 10.1146/annurev-med-050913-022545. [DOI] [PubMed] [Google Scholar]
- 10.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 11.Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, et al. Poly(ADP)-ribose polymerase inhibition: Frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–9. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 12.Lee JM, Ledermann JA, Kohn EC. PARP Inhibitors for BRCA1/2 mutation-associated and BRCA-like malignancies. Ann Oncol. 2014;25:32–40. doi: 10.1093/annonc/mdt384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Y, Rehman FL, Feng Y, Boshuizen J, Bajrami I, Elliott R, et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res. 2013;19:5003–15. doi: 10.1158/1078-0432.CCR-13-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aoyagi-Scharber M, Gardberg AS, Yip BK, Wang B, Shen Y, Fitzpatrick PA. Structural basis for the inhibition of poly(ADP-ribose) polymerases 1 and 2 by BMN 673, a potent inhibitor derived from dihydropyridophthalazinone. Acta Crystallogr F Struct Biol Commun. 2014;70:1143–9. doi: 10.1107/S2053230X14015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murai J, Huang SY, Renaud A, Zhang Y, Ji J, Takeda S, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther. 2014;13:433–43. doi: 10.1158/1535-7163.MCT-13-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–5. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HJ, Yoon C, Schmidt B, Park DJ, Zhang AY, Erkizan HV, et al. Combining PARP-1 inhibition and radiation in Ewing sarcoma results in lethal DNA damage. Mol Cancer Ther. 2013;12:2591–600. doi: 10.1158/1535-7163.MCT-13-0338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Byers LA, Wang J, Nilsson MB, Fujimoto J, Saintigny P, Yordy J, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2:798–811. doi: 10.1158/2159-8290.CD-12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardnell RJ, Feng Y, Diao L, Fan YH, Masrorpour F, Wang J, et al. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer. Clin Cancer Res. 2013;19:6322–28. doi: 10.1158/1078-0432.CCR-13-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89:1138–47. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 23.Gough K, Hutchison M, Keene O, Byrom B, Ellis S, Lacey L, et al. Assessment of dose proportionality: Report from the statisticians in the pharmaceutical industry/pharmacokinetics UK joint working party. Drug Inf J. 1995;29:1039–48. [Google Scholar]
- 24.Rustin GJ, Quinn M, Thigpen T, du Bois A, Pujade-Lauraine E, Jakobsen A, et al. New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer) J Natl Cancer Inst. 2004;96:487–8. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- 25.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melnik MK, Webb CP, Richardson PJ, Luttenton CR, Campbell AD, Monroe TJ, et al. Phase II trial to evaluate gemcitabine and etoposide for locally advanced or metastatic pancreatic cancer. Mol Cancer Ther. 2010;9:2423–9. doi: 10.1158/1535-7163.MCT-09-0854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


