Abstract
Adhesion to wet and dynamic surfaces, including biological tissues, is important in many fields, but has proven extremely challenging. Existing adhesives are either cytotoxic, adhere weakly to tissues, or cannot be utilized in wet environments. We report a bio-inspired design for adhesives consisting of two layers: an adhesive surface and a dissipative matrix. The former adheres to the substrate by electrostatic interactions, covalent bonds, and physical interpenetration. The latter amplifies energy dissipation through hysteresis. The two layers synergistically lead to higher adhesion energy on wet surfaces than existing adhesives. Adhesion occurs within minutes, independent of blood exposure, and compatible with in vivo dynamic movements. This family of adhesives may be useful in many areas of application, including tissue adhesives, wound dressings and tissue repair.
Adhesives that can bond strongly to biological tissues would have broad applications ranging from tissue repair (1, 2), drug delivery (3, 4), wound dressings (5, 6), and biomedical devices (7, 8). However, existing tissue adhesives are far from ideal. Cyanoacrylate (Superglue) is the strongest class of tissue adhesive (9), but is cytotoxic, incompatible with wet surfaces as it solidifies immediately upon exposure to water, and forms rigid plastics that cannot accommodate dynamic movements of tissues (10). Nanoparticle (11) and mussel-inspired adhesives (12) adhere weakly to tissues, as their adhesion mainly relies on relatively weak physical interactions, typically leading to low adhesion energies of 1–10 Jm−2. Commercial adhesives, such as the fibrin glue TISSEEL (Baxter) (13) and polyethylene glycol-based adhesives (14) like COSEAL (Baxter) and DURASEAL (Confluent Surgical Inc.), can form covalent bonds with tissues. However, their matrix toughness and adhesion energy are on the order of 10 Jm−2 (15). Such brittle adhesives are vulnerable to debonding, because of cohesive failure in the adhesive matrix. For comparison, cartilage constitutes a matrix of high toughness (1000 Jm−2) and bonds to bones with adhesion energy of 800 Jm−2 (16).
Achieving high adhesion energy requires the synergy of two effects. First, the adhesive should form strong bonds with the substrate. Second, materials inside either the adhesive or the substrate (or both) should dissipate energy by hysteresis. Tissue adhesives must also show compatibility with fluids within the body, and compatibility with cells and tissues. Here we report a design of tough adhesive (TA) for biological applications to meet those requirements. The design is inspired by a defensive mucus secreted by slugs (Arion subfuscus) that strongly adheres to wet surfaces (17). This slug adhesive consists of a tough matrix with interpenetrating positively charged proteins (18). Our tough adhesive consists of two layers - an adhesive surface containing an interpenetrating positively charged polymer and a dissipative matrix (Fig. 1A). The adhesive surface can bond to the substrate through electrostatic interactions, covalent bonds and physical interpenetration, while the matrix dissipates energy through hysteresis under deformation.
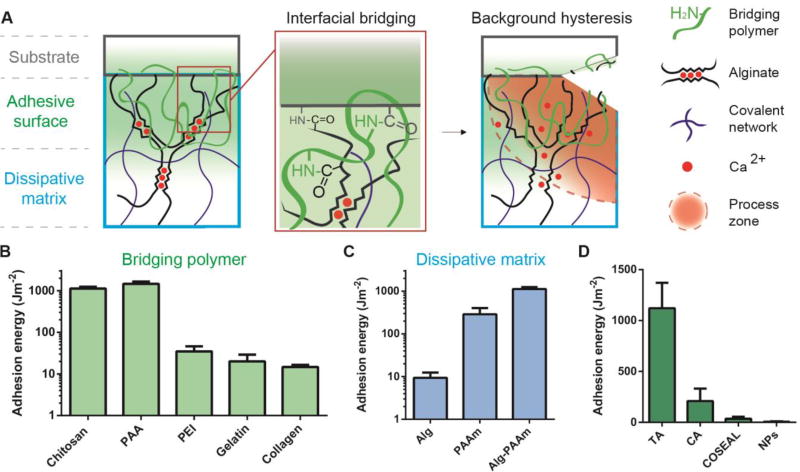
Fig. 1. Design of tough adhesive (TA).
(A) TA consists of a dissipative matrix (light blue square) made of a hydrogel containing both ionically (calcium) crosslinked and covalently crosslinked polymers (black and blue lines), and an adhesive surface that contains a bridging polymer with primary amines (green lines). The bridging polymer penetrates into TA and the substrate (light green region). When a crack approaches, a process zone (orange area) dissipates significant energy as ionic bonds between alginate chains and calcium ions break. (B) Adhesion energy on porcine skin was measured using different bridging polymers. (C) Adhesion energy varies with the hydrogel matrix. (D) Comparison between TA and other adhesives. Error bars show standard deviation; N=4.
The TA was designed based on two criteria: [1] the adhesive surface must wet negatively charged surfaces of tissues and cells, and form covalent bonds across the interface while being compliant to dynamic movements of tissues; [2] the dissipative matrix must be tough and capable of dissipating energy effectively when the interface is stressed. To satisfy the first criterion, we employed a bridging polymer that bears positively charged primary amine groups under physiological conditions. The primary amine found in the slug adhesive is believed to play a major role in its mechanics and adhesion (19). Such a polymer can be absorbed to the tissue surface via electrostatic attractions, and provide primary amine groups to bind covalently with carboxylic acid groups from the hydrogel matrix and the tissue surface (Fig. 1A). If the target surface is permeable, the bridging polymer can also penetrate into the target surface, forming physical entanglements, and then chemically anchor the adhesive. The second criterion is satisfied by using a hydrogel capable of dissipating energy as the dissipative matrix. Alginate-polyacrylamide hydrogels, as an example, effectively dissipate energy under deformation (20). We hypothesize that by combining the interfacial bridging and the background hysteresis, TA could form strong adhesion on wet surfaces.
Using these design principles, we fabricated a family of tough adhesives that can adhere to wet surfaces. We chose porcine skin as the first model tissue, as it closely resembles human skin and is robust, ensuring that ultimate adhesive failure occurs at the interface. To identify an appropriate bridging polymer, we tested five polymers including chitosan, polyallylamine (PAA), polyethylenimine (PEI), collagen and gelatin. The bridging polymer penetrated rapidly into the hydrogel matrix (Fig. S1), forming a positively charged surface (Fig. S2). Coupling reagents, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysulfosuccinimide (sulfo-NHS), were applied to facilitate covalent bond formation (21, 22). There also exist other coupling reagents or enzymes like transglutaminase for interfacial bridging (23). TA was then applied on the epidermis of porcine skin with compression, and the resulting adhesion was quantified by the adhesion energy (Fig. S3) (24). Among the tested polymers, polyallylamine and chitosan led to adhesion energy >1000 Jm−2 (Figs. 1B and S4), likely due to the high concentration of primary amines present on these polymers. In comparison, use of the coupling reagents or the bridging polymer alone led to 14 Jm−2 and 303 Jm−2, respectively (Fig. S5). Adhesion energy was sensitive to the concentration, but not the molecular weight of the bridging polymer (Fig. S6).
The importance of the synergy between interfacial bridging and background hysteresis was next examined. TA was compared with adhesives formed with either alginate (Alg) or polyacrylamide (PAAm) single-network hydrogels, as these do not dissipate energy as effectively as the Alg-PAAm hydrogels (20). The coupling reagents and chitosan were again applied for interfacial bridging. The alginate hydrogel led to weak adhesion, as it is vulnerable to rupture and lacks effective energy dissipating mechanisms to toughen the interface. The PAAm hydrogel led to higher adhesion, but not as high as the tough matrix of Alg-PAAm hydrogel, which enables TA to integrate high adhesion energy and high matrix toughness simultaneously (Figs. 1C and S4). This unique combination is unprecedented among existing tissue adhesives (Fig. 1D and Fig. S7), including cyanoacrylate (CA), COSEAL and a nanoparticle-based adhesive (NPs). Commercial adhesives are either formed with a brittle matrix like COSEAL, or lack strong interaction with tissues in case of adhesive bandages (24). This finding is also echoed in many studies on adhesion between hard materials and rubbers (25, 26), and adhesion between hydrogels and inorganic oxidized surfaces (27).
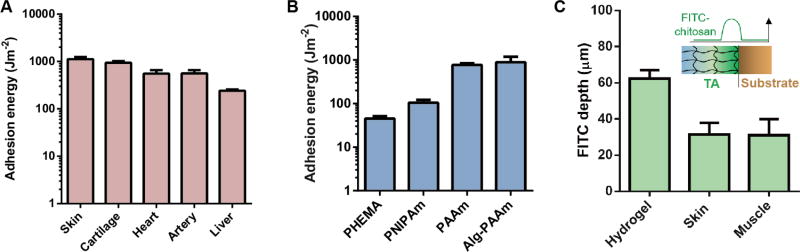
TA is applicable to a wide variety of wet surfaces, including tissues and hydrogels. It formed strong adhesion to porcine skin, cartilage, heart, artery and liver (Fig. 2A). Its adhesion energy on hydrogels is higher than that of nanoparticle-based adhesive (1–10 Jm−2) developed recently to glue hydrogels (Fig. 2B) (11). Unlike tissues, certain hydrogels like poly(hydroxyethyl methacrylate) (PHEMA) lack the functional groups (amine or carboxylic acid) utilized here to form covalent bonds at interfaces, but interestingly these hydrogels still adhere well to TA (Figs. S8 and S9). While the bridging polymer was found to interpenetrate into a variety of substrates, the penetration depth in a given time depended on the substrate permeability. As hydrogels are more permeable than tissues, the penetration depth of fluorescein isothiocyanate-labeled chitosan (FITC-chitosan) in hydrogels was larger than that found in skin or muscle (Figs. 2C and S10), and likely underlies the strong adhesion of TA to even chemically inert hydrogels.
Fig. 2. Adherence on diverse wet surfaces.
TA adheres to a variety of (A) tissue surfaces and (B) hydrogels, including poly(hydroxyethyl methacrylate) (PHEMA), poly(N’-isopropylacrylamide) (PNIPAm), polyacrylamide (PAAm) and alginate-polyacrylamide (Alg-PAAm) hydrogels. (C) Penetration depth of FITC-chitosan into PAAm hydrogels, skin and muscle. Error bars show standard deviation; N=4.
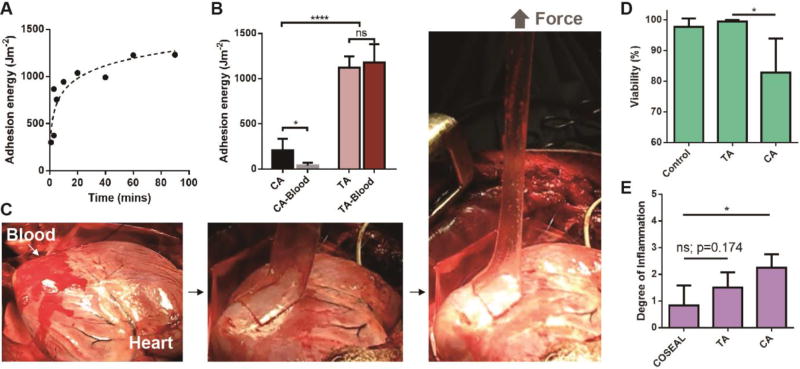
The capacity of TA as a tissue adhesive was next evaluated, and compared to the widely-used cyanoacrylate (CA). TA exhibited a rapid increase in adhesion energy to porcine skin over time (Fig. 3A). This rapid, but not immediate adhesion is likely to aid clinical translation and adoption of these tissue adhesives, as it allows the material to be applied in a facile manner. In contrast, cyanoacrylate solidifies upon contact with tissues, which makes handling and repositioning difficult (28). The formation of tissue adhesion is often complicated in vivo due to exposure to blood and dynamic movements. To simulate this in vitro, the porcine skin was first covered with blood before application of TA (Fig. S11 and Movie S2). The adhesion energy was found to be 1116 Jm−2, indicative of strong adhesion even with blood exposure. In contrast, the adhesion provided by cyanoacrylate deteriorates significantly upon exposure to blood (Figs. 3B and S12). TA was further tested on a beating porcine heart in vivo (Fig. 3C). Freshly drawn blood was spread on the heart surface at the site of application, followed by application of TA and peeling tests (Movie S3). A strong adhesion was formed on the dynamic curved surface with a peak strength of 83±31 kPa, which exceeds commercially available tissue adhesives (typically ~10 kPa) (29). TA was found to maintain strong adhesion (600 Jm−2) after being implanted into rats for 2 weeks (Fig. S13). TA also exhibited excellent biocompatibility. In an in vitro cell study, human dermal fibroblasts were able to maintain full viability after 24-hour culture in TA-conditioned medium, while the cells cultured in CA-conditioned medium were unable to spread and exhibited low viability (Figs. 3D and S14). The in vivo biocompatibility of TA was evaluated with subcutaneous implantation and myocardium attachment in rats (24). The histological assessment concluded TA led to lower degree of inflammatory reaction than CA, and was comparable to COSEAL (Figs. 3E and S15).
Fig. 3. Adhesion performance and biocompatibility.
(A) Adhesion kinetics of TA to porcine skin. (B) Comparison of TA to cyanoacrylate (CA) placed on porcine skin with and without exposure to blood. N=4–6. (C) In vivo test on a beating porcine heart with blood exposure. (D) In vitro cell compatibility was compared by quantifying the viability of human dermal fibroblasts. N=4. (E) In vivo biocompatibility was evaluated using subcutaneous implantation in rats. Degree of inflammation was determined by a pathologist (0=normal, 1=very mild, 2=mild, 3=moderate, 4=severe, 5=very severe). Error bars show standard deviation; N=4–6. P values were determined by the t-test; *, p≤0.05; ****, p≤0.0001; ns, not significant.
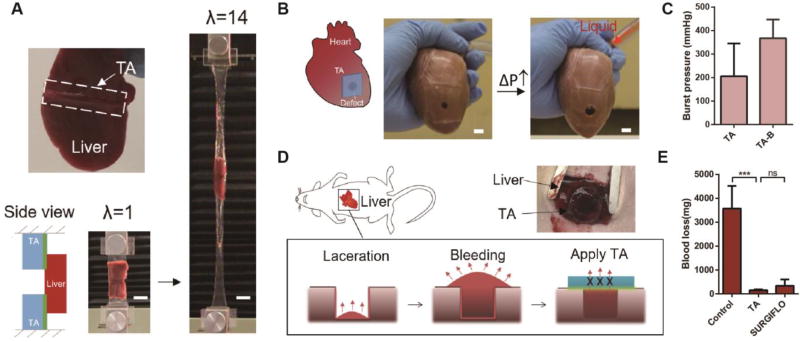
The design of TA can potentially enable many applications, including the gluing of tissues and attaching devices in vivo, tissue repair, and attaining hemostasis. Liver can be readily adhered to TA (Fig. 4A). Tensile testing demonstrated that TA remained highly stretchable and sustained 14 times its initial length before debonding from the liver. The combination of strong adhesion and large deformability is mission-critical when interfacing tissues and deformable devices, whereas existing adhesives hardly accommodate large deformation. For example, TA managed to anchor an actuator, recently developed to support heart function, onto myocardium surfaces (Fig. S16). It is also potentially useful as a skin wound dressing. TA adhered strongly to the epidermis of mice, and readily accommodated dynamic movements of this tissue on the living animal (Fig. S17 and Movie S4).
Fig. 4. Application enabled by TA.
(A) TA was used as tissue adhesives. TA adhered to the liver and sustained 14 times its initial length before debonding. Scale bar, 20mm. (B) TA served as heart sealants. The TA sealant prevented liquid (red) leakage as the porcine heart was inflated. Scale bar, 10mm. (C) Burst pressures of the TA sealant were measured without and with plastic backing (TA-B). (D) Use of TA as hemostatic dressing. A deep wound was created on liver in rats, and then sealed with TA to stop the blood flow (labelled with red arrows). (E) Blood loss with the treatment of TA, SURGIFLO hemostat and control without treatment. Error bars show standard deviation; sample size N=4. P values were determined by the t-test; ***, p≤0.001; ns, not significant.
TA can be used for tissue repair as either a preformed patch or injectable solution. TA was first tested as a sealant to close a large defect in a porcine heart (Fig. 4B). It was compliant and conformed closely to the geometry of the myocardium. While the heart was being inflated, the sealant expanded with the deformation, and no leakage was observed under strain up to 100%. A perfect seal was maintained after tens of thousands cycles of inflation-deflation (Fig. S18 and Movie S5). The measured burst pressures of the TA sealant with and without a plastic backing were 206 mmHg and 367 mmHg, respectively (Fig. 4C), which exceeds normal arterial blood pressure in humans (80–120 mmHg) and the performance of commercially available surgical sealants (24, 30). Notably, the TA sealant failed due to cohesive failure, indicative of strong adhesion interface (Fig. S18 and Movie S6). We also developed an injectable tough adhesive based on alginate-polyethylene glycol hydrogel (24). It can be injected via syringe into a defect site and form a tough matrix upon exposure to UV light (Fig. S19). As a proof-of-concept, the injectable TA was used to repair a cylindrical defect in explanted cartilage discs, resulting in recovery of the compressive properties (Fig. S20).
TA can be used as a hemostatic dressing due to its compatibility with blood exposure, as shown in a hepatic hemorrhage model. Heavy bleeding was created on the left lobe of the liver in rats using a circular laceration (24). Animals were treated immediately with the TA, a commercial hemostat SURGIFLO (Ethicon) as a positive control, or left untreated as a negative control (Fig. 4D). The blood loss was decreased significantly by the application of TA versus the negative control, and the performance was comparable to SURGIFLO (Fig. 4E). All animals survived for the experimental period of 2 weeks without secondary hemorrhage. However, substantial adhesions were found at the lesion site when untreated or treated with SURGIFLO; necrosis occurred in the livers of untreated animals (Fig. S21). Neither of these were found in the animals treated with the TA.
Taken together, we report design principles of biocompatible tough adhesives that combine chemical and physical processes at the interface and in the bulk of the adhesive to achieve high adhesion energy on various wet and dynamic surfaces. The mechanical performance and compatibility with cells and tissues allows these materials to meet key requirements for next-generation tissue adhesives.
Supplementary Material
Acknowledgments
This work was supported by NIH under award R01DE0130333. It was performed in part at the Center for Nanoscale Systems at Harvard University. A.D.C. acknowledges support from Marie Curie International Outgoing Fellowship funded by the European Commission (Agreement no. 629320). W.W. acknowledges support from Science Foundation Ireland under grant SFI/12/RC/2278. Q.Y. acknowledges a scholarship from Tsinghua University. Z.S. and J.J.V. acknowledge support from NSF under award CMMI-1404653. Z.S., J.J.V. and D.J.M. acknowledge support from the Harvard University MRSEC (DMR-1420570). Authors have a pending patent application on this technology.
Footnotes
Materials and Methods
References and Notes
- 1.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006;12:2171–2180. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 2.Sharma B, et al. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci. Transl. Med. 2013;5:167ra6. doi: 10.1126/scitranslmed.3004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prausnitz MR, Langer R. Transdermal drug delivery. Nat. Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016;1:16071. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghobril C, Charoen K, Rodriguez EK, Nazarian A, Grinstaff MW. A dendritic thioester hydrogel based on thiol–thioester exchange as a dissolvable sealant system for wound closure. Angew. Chem. Int. Ed. 2013;52:14070–14074. doi: 10.1002/anie.201308007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grinstaff MW. Designing hydrogel adhesives for corneal wound repair. Biomaterials. 2007;28:5205–5214. doi: 10.1016/j.biomaterials.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roche ET, et al. A bioinspired soft actuated material. Adv. Mater. 2014;26:1200–1206. doi: 10.1002/adma.201304018. [DOI] [PubMed] [Google Scholar]
- 8.Feiner R, et al. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 2016;15:679–685. doi: 10.1038/nmat4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vakalopoulos KA, et al. Mechanical strength and rheological properties of tissue adhesives with regard to colorectal anastomosis: an ex vivo study. Ann. Surg. 2015;261:323–331. doi: 10.1097/SLA.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 10.Vinters H, Galil K, Lundie M, Kaufmann J. The histotoxicity of cyanoacrylates. Neuroradiol. 1985;27:279–291. doi: 10.1007/BF00339559. [DOI] [PubMed] [Google Scholar]
- 11.Rose S, et al. Nanoparticle solutions as adhesives for gels and biological tissues. Nature. 2013;505:382–385. doi: 10.1038/nature12806. [DOI] [PubMed] [Google Scholar]
- 12.Barrett DG, Bushnell GG, Messersmith PB. Mechanically robust, negative-swelling, mussel-inspired tissue adhesives. Adv. Healthcare Mater. 2013;2:745–755. doi: 10.1002/adhm.201200316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sierra DH. Fibrin sealant adhesive systems: a review of their chemistry, material properties and clinical applications. J. Biomater. Appl. 1993;7:309–352. doi: 10.1177/088532829300700402. [DOI] [PubMed] [Google Scholar]
- 14.Wallace DG, et al. A tissue sealant based on reactive multifunctional polyethylene glycol. J. Biomed. Mater. Res. 2001;58:545–55. doi: 10.1002/jbm.1053. 5. [DOI] [PubMed] [Google Scholar]
- 15.Dastjerdi AK, Pagano M, Kaartinen M, McKee M, Barthelat F. Cohesive behavior of soft biological adhesives: experiments and modeling. Acta Biomater. 2012;8:3349–3359. doi: 10.1016/j.actbio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Moretti M, et al. Structural characterization and reliable biomechanical assessment of integrative cartilage repair. J. Biomech. 2005;38:1846–1854. doi: 10.1016/j.jbiomech.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Pawlicki J, et al. The effect of molluscan glue proteins on gel mechanics. J. Exp. Biol. 2004;207:1127–1135. doi: 10.1242/jeb.00859. [DOI] [PubMed] [Google Scholar]
- 18.Wilks AM, Rabice SR, Garbacz HS, Harro CC, Smith AM. Double-network gels and the toughness of terrestrial slug glue. J. Exp. Biol. 2015;218:3128–3137. doi: 10.1242/jeb.128991. [DOI] [PubMed] [Google Scholar]
- 19.Braun M, Menges M, Opoku F, Smith AM. The relative contribution of calcium, zinc and oxidation-based cross-links to the stiffness of Arion subfuscus glue. J. Exp. Biol. 2013;216:1475–1483. doi: 10.1242/jeb.077149. [DOI] [PubMed] [Google Scholar]
- 20.Sun JY, et al. Highly stretchable and tough hydrogels. Nature. 2012;489:133–136. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakajima N, Ikada Y. Mechanism of amide formation by carbodiimide for bioconjugation in aqueous media. Bioconjugate Chem. 1995;6:123–130. doi: 10.1021/bc00031a015. [DOI] [PubMed] [Google Scholar]
- 22.Gilles MA, Hudson AQ, Borders CL. Stability of water-soluble carbodiimides in aqueous solution. Anal. Biochem. 1990;184:244–248. doi: 10.1016/0003-2697(90)90675-y. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez JG, et al. Direct bonding of chitosan biomaterials to tissues using transglutaminase for surgical repair or device implantation. Tissue Eng. 2016;23:135–142. doi: 10.1089/ten.TEA.2016.0266. [DOI] [PubMed] [Google Scholar]
- 24.Materials and methods are available as supplementary materials on Science online.
- 25.Gent AN. Adhesion and strength of viscoelastic solids. Is there a relationship between adhesion and bulk properties? Langmuir. 1996;12:4492–4496. [Google Scholar]
- 26.Hutchinson JW, Suo Z. Mixed mode cracking in layered materials. Adv. Appl. Mech. 1992;29:191. [Google Scholar]
- 27.Yuk H, Zhang T, Lin S, Parada GA, Zhao X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016;15:190–196. doi: 10.1038/nmat4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefanov T, Ryan B, Ivanković A, Murphy N. Mechanical bulk properties and fracture toughness of composite-to-composite joints of an elastomer-toughened ethyl cyanoacrylate adhesive. Int. J. Adhes. Adhes. 2016;68:142–155. [Google Scholar]
- 29.Lang N, et al. A blood-resistant surgical glue for minimally invasive repair of vessels and heart defects. Sci. Transl. Med. 2014;6:218ra216. doi: 10.1126/scitranslmed.3006557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell PK, Bennett SL, Driscoll A, Sawhney AS. Evaluation of absorbable surgical sealant: In vitro testing. Confluent Surgical Inc; Waltham, MA: 2005. [Google Scholar]
- 31.Kong HJ, Kaigler D, Kim K, Mooney DJ. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules. 2004;5:1720–1727. doi: 10.1021/bm049879r. [DOI] [PubMed] [Google Scholar]
- 32.Nemir S, Hayenga HN, West JL. PEGDA hydrogels with patterned elasticity: novel tools for the study of cell response to substrate rigidity. Biotechnol. Bioeng. 2010;105:636–644. doi: 10.1002/bit.22574. [DOI] [PubMed] [Google Scholar]
- 33.Qaqish RB, Amiji MM. Synthesis of a fluorescent chitosan derivative and its application for the study of chitosan–mucin interactions. Carbohydr. Polym. 1999;38:99–107. [Google Scholar]
- 34.Li J, Illeperuma WRK, Suo Z, Vlassak JJ. Hybrid hydrogels with extremely high stiffness and toughness. ACS Macro Lett. 2014;3:520–523. doi: 10.1021/mz5002355. [DOI] [PubMed] [Google Scholar]
- 35.Rivlin RS, Thomas AG. Rupture of rubber. I. Characteristic energy for tearing. J. Polym. Sci. 1953;10:291–318. [Google Scholar]
- 36.Tang J, Li J, Vlassak JJ, Suo Z. Adhesion between highly stretchable materials. Soft Matter. 2016;12:1093–1099. doi: 10.1039/c5sm02305j. [DOI] [PubMed] [Google Scholar]
- 37.Roche ET, et al. Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials. 2014;35:6850–8. doi: 10.1016/j.biomaterials.2014.04.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roche ET, et al. Soft robotic sleeve supports heart function. Sci. Transl. Med. 2017;9:eaaf3925. doi: 10.1126/scitranslmed.aaf3925. [DOI] [PubMed] [Google Scholar]
- 39.Morgan CE, Prakash VS, Vercammen JM, Pritts T, Kibbe MR. Development and validation of 4 different rat models of uncontrolled hemorrhage. JAMA Surgery. 2015;150:316–324. doi: 10.1001/jamasurg.2014.1685. [DOI] [PubMed] [Google Scholar]
- 40.Gaharwar AK, et al. Shear-thinning nanocomposite hydrogels for the treatment of hemorrhage. ACS Nano. 2014;8:9833–9842. doi: 10.1021/nn503719n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Q, et al. Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3. Proc. Nat. Acad. Sci. 2007;104:3782–3786. doi: 10.1073/pnas.0607852104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guvendiren M, Messersmith PB, Shull KR. Self-assembly and adhesion of DOPA-modified methacrylic triblock hydrogels. Biomacromol. 2007;9:122–128. doi: 10.1021/bm700886b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn BK, et al. High-performance mussel-inspired adhesives of reduced complexity. Nat. Comm. 2015;6:8663. doi: 10.1038/ncomms9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kjaergard HK, et al. Development of a model for measurement of adhesion strength of fibrin sealant to human tissue. Eur. Surg. Res. 1999;31:491–6. doi: 10.1159/000008729. 6. [DOI] [PubMed] [Google Scholar]
- 45.Bundy K, Schlegel U, Rahn B, Geret V, Perren S. An improved peel test method for measurement of adhesion to biomaterials. J. Mater. Sci. Mater. Med. 2000;11:517–521. doi: 10.1023/a:1008965926086. [DOI] [PubMed] [Google Scholar]
- 46.Perrin BRM, et al. Surgical glues: are they really adhesive? Eur. J. Cardio-thoracic Surg. 2009;36:967–972. doi: 10.1016/j.ejcts.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Tirrell M, Korba GA, Pocius AV. Surface energy and adhesion studies on acrylic pressure sensitive adhesives. J. Adhes. 2001;76:307–334. [Google Scholar]
- 48.Pharr M, Sun J-Y, Suo Z. Rupture of a highly stretchable acrylic dielectric elastomer. J. Appl. Phys. 2012;111:104114. 10. [Google Scholar]
- 49.Newby BZ, Chaudhury MK. Effect of interfacial slippage on viscoelastic adhesion. Langmuir. 1997;13:1805–1809. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.