Abstract
In a broad sense, inflammation can be conveniently characterised by two phases: the first phase, which is a pro‐inflammatory, has evolved to clear infection and/or injured tissue; and the second phase concerns regeneration of normal tissue and restitution of normal physiology. Innate immune cell‐derived pro‐inflammatory cytokines and chemokines activate and recruit nonresident immune cells to the site of infection, thereby amplifying the inflammatory responses to clear infection or injury. This phase is followed by a cytokine milieu that promotes tissue regeneration. There is no absolute temporal distinction between these two phases, and cytokines may have dual pleiotropic effects depending on the timing of release, inflammatory microenvironment or concentrations. IL‐22 is a cytokine with reported pro‐ and anti‐inflammatory roles; in this review, we contend that this protein has primarily a function in restitution of normal tissue and physiology.
Keywords: hepatitis, inflammatory bowel disease, inflammatory diseases, innate lymphoid cells, interleukins, T helper 17 cells
Introduction
Inflammation is a normal physiological response to infection (nonsterile) and/or sterile injury.1 The inflammatory response can usefully be divided into two phases: the first phase of a systemic or local inflammatory reaction is to eliminate infections and/or remove damaged tissue; and the second phase is characterised by promotion of tissue repair and restitution of normal physiology once the danger has been cleared. Where there is infection, the adaptive immune system enables memory cells which recognise pathogens, and on a future encounter, will react with a faster and more specific elimination.1 On the one hand, the phases of the inflammatory response are not precisely compartmentalised, but on the other hand, there is a temporal sequence of cellular, molecular and physiological events.
The initial phase involves membrane receptors and soluble mediators from damaged cells and cells responding to infection, which signal initially to local resident phagocytic cells and other resident and circulating antigen‐presenting dendritic cells. If sufficiently activated, these cells attract more circulating immune cells that help eliminate damaged or infected cells. Once infection and damage have been cleared, other cellular and soluble mediators can initiate restitution to engender tissue repair.
In primary injury or infection, the inflammatory response to an acute insult is initiated by the activation of innate immune cells. These include resident macrophages (Kupffer cells), natural killer T cells (NKT), innate lymphoid cells (ILCs) and neutrophils. These cells express pattern recognition receptors (PRRs) that detect microbial components, pathogen‐associated molecular patterns (PAMPs, e.g. lipopolysaccharide) or host‐derived damage‐associated molecular patterns (DAMPs) released from injured cells (e.g. mitochondrial DNA).2, 3, 4, 5, 6 Following this detection, these cells activate a network of signalling pathways and cellular responses, including secretion of multiple cytokines, notably tumor necrosis factor (TNF‐α), interleukin‐1 beta (IL‐1β), interferon‐gamma (IFN‐y), IL‐17 and IL‐22 that further recruit, co‐ordinate and activate pro‐inflammatory and regulatory anti‐inflammatory cells.2, 3, 4, 5, 6
A major question in this field is what determines the evolution of inflammation towards the healing or restitution phase. The initial inflammatory response is associated with pro‐inflammatory cytokines (IL‐1β, IFN‐y, IL‐6, TNF‐α, IL‐17 and IL‐23), which activate and recruit immunoinflammatory cells to an injury. The latter healing phase is more associated with cytokines that promote healing (IL‐10, IL‐4, IL‐22) although the distinction between pro‐inflammatory and anti‐inflammatory effects is far from clear‐cut with many cytokines appearing to have dual roles (such as IL‐17 and IL‐22). In this review, we will argue that the dual roles may be more apparent than real and will depend on experimental and physiological context, in particular timing and the nature of the injurious insult.
This review focuses on IL‐22. The review will summarise current understanding of the cellular sources of this cytokine, the target cells and the effects of IL‐22 on inflammation in general with a focus on liver injury, and from a review of the literature, argue that the primary role of IL‐22 is in the second restitution phase of inflammation to restore normal tissue.
Interleukin‐22 (IL‐22)
IL‐22 was initially classified as a cytokine within the IL‐10 family, but more recently, it has been placed within the smaller IL‐20 subfamily, along with IL‐19, IL‐20, IL‐22, IL‐24 and IL‐26.7, 8 The human IL‐22 gene encodes a protein of 179 amino acid in length, which is secreted in a form of 146 amino acid polypeptide once a signal peptide of 33 amino acid is cleaved off.9, 10 IL‐22 has been investigated both in humans and mice. There is an approximately 80% sequence similarity between them.11, 12 The structure of IL‐22 consists of six alpha‐helices (referred to as helices A to F).9, 13
IL‐22 is currently being tested experimentally in murine animals as a therapeutic agent via administration with a number of delivery methods including adenovirus expressing IL‐22 and recombinant IL‐22 (rIL‐22). Our studies on wild‐type (WT) mice indicate that exogenous IL‐22 delivered via intraperitoneal injections of rIL‐22 has ~30 min half‐life. Exogenous IL‐22 may act locally or systemically, depending on the route of administration and the location of target tissue. In some murine models with metabolic disorders, treatments with a long half‐life IL‐22Fc protein (3 days) have been utilised.14, 15
Sources of IL‐22
It has been suggested that sources of IL‐22 or secreting cells in human disease may differ depending on the nature and severity of the disease.8 IL‐22 is initially secreted by innate lymphoid cells, part of innate immune response, during early onset of inflammation in response to a primary acute insult where disease has just commenced and at least where there is a first exposure to stimuli.7, 8, 16 The classic source of IL‐22, however, is T‐lymphocyte subsets as part of the adaptive immune response.
Innate lymphoid cells (ILCs)
Innate lymphoid cells (ILCs) are a recently recognised group of developmentally related innate immune cells.17 They share morphological features with T lymphocytes, but do not express recombinant antigen receptors and do not undergo clonal selection and expansion.18, 19 In addition to regulatory roles in the adaptive immune system mediated through crosstalk between ILCs and classical lymphocytes,20, 21, 22 ILCs express PRRs and respond to a number of other cytokines and chemokines related to inflammatory and infectious stimuli.18
ILCs contribute to co‐ordination of inflammatory responses through interactions with both haematopoietic cells, such as macrophages,23, 24, 25 and nonhaematopoietic stromal, endothelial and epithelial cells via secretion of signature inflammatory cytokines such as amphiregulin, IL‐4, IL‐13, IL‐17 and notably IL‐22.26, 27 The ILCs comprise three main groups: ILC1, ILC2 and ILC3, based on their transcription factor expression pattern and production of cytokines.28, 29 These groups mirror T‐lymphocyte subtypes T helper 1 (Th1), Th2 and Th17/Th22 that produce signature cytokines TNF‐α and IFN‐γ; IL‐4, IL‐5, IL‐9, IL‐10 and IL‐13; and IL‐17 and/or IL‐22, respectively.30
IL‐22 is mainly secreted by subsets of ILC3 consisting of three subtypes: lymphoid tissue inducer (LTi) and the other two subtypes that are classified as either positive or negative ILC3 for the natural cytotoxicity triggering receptor (NCR) and divided based on their expression of activating NK cell receptor NKp46 in mice and NKp44 in humans.31, 32, 33, 34, 35, 36, 37, 38 This group can produce IL‐17RB and/or IL‐22 and depend on the transcription factor RORγt for their development and function.34, 39, 40, 41, 42 A number of phenotyping studies have determined that NCR+ ILC3 expresses IL‐17Alow and IL‐22high, whereas NCR− ILC3 expresses IL‐17Ahigh and IL‐22low 36, 37, 38.
ILC3s are located in mucosal tissues, predominantly within the gastrointestinal tract, where they are associated with immunity against extracellular pathogens.31, 32, 33, 34, 35, 43 They respond to stimulation with the secretion of one or a combination of classic Th17 and Th22 cytokines,31, 44 depending on the stimulus. They are likely to be the main lymphocyte where there is primary infection prior to induction of immune memory. They appear to be local sentinels and wardens of tissue homeostasis, being present predominantly at mucosal sites and in nonlymphoid tissues as tissue‐resident cells that expand locally during acute inflammation.41, 45, 46
Other innate immune
Other innate immune cells such as macrophages, monocytes and mature or immature dendritic cells (DC) do not produce IL‐22,9, 47, 48 and IL‐22 is not produced by nonhaematopoietic tissue cells.9, 48, 49, 50
T helper lymphocytes (Th lymphocytes)
IL‐22 is previously known to be mainly expressed by Th17 cells along with IL‐17 secretion.51
Th17 cells and their secreted cytokines appear to drive inflammatory responses in extracellular infection and a number of disorders. Investigations have revealed that these RORγt transcription factor‐expressing cells can be induced by IL‐6 and TGF‐beta (TGF‐β) in vitro and IL‐23 in vivo to produce IL‐17A, IL‐21 and IL‐22.6, 52 IL‐22 plays a role in increasing the expression of IL‐23 receptor (IL‐23R) found on Th17 cells and consequently upregulates RORγt and activates signal transducer and activator of transcription 3 (STAT3), a transcription factor that is involved with IL‐22 functions.53
An important recent discovery is that of a Th22 subset. Th22 is probably the main source of IL‐22.54, 55 This newly identified Th22 population may have similarities with the Th17 subset but secretes only IL‐22, hence the name. It has been reported that the Th22 lymphocytes produce almost 50% of IL‐22 produced by all T lymphocytes.56 Th22 is found to express CCR4, CCR6 and CCR10 and requires transcription factor aryl hydrocarbon receptor (AHR) for the secretion of IL‐22.57, 58 The differentiation of Th22 appears to be largely dependent on the signalling of IL‐6 and TNF‐α.59, 60
Th1 and Th2 lymphocyte subsets do not secrete IL‐22. It has been previously described that Th1 can initiate the activation of STAT4, which can enable the differentiation into Th2 via stimulation of IL‐12.61 Th2 cells are characterised by production of anti‐inflammatory cytokines including IL‐4, IL‐5, IL‐10 and IL‐13. They are associated with modulation of allergic responses and clearance of parasitic pathogens. The Th2 effects in hepatic inflammation indicate an anti‐inflammatory function, which promotes tissue repairs, ameliorates inflammation and limits hepatocytes death and apoptosis,62 but on the one hand, there is no evidence of IL‐22 secretion by Th2 or Th1 for that matter in liver or elsewhere. On the other hand, there is evidence that another population of T cells, γδ T cells, secrete IL‐22 in mice infected with lymphocytic choriomeningitis virus (LCMV).60 Viral infections including LCMV induce the secretion of IL‐22 in spleen, thymus and liver.60
IL‐22 receptor (IL‐22R)
The IL‐22R is a type 2 cytokine receptor consisting of two subunits, IL‐22R1 and IL‐10R2.63, 64 IL‐22 has been shown to initially bind to IL‐22R1 and then to IL‐10R2.63, 65 This contrasts with the other members of IL‐10 family including IL‐19, IL‐20 and IL‐24 each of which relies on IL‐10R2 for activation that follows with the activation of STAT, JAK (Janus kinase) and Akt cascades.9 IL‐22R1 is known to be the responsible subunit for the binding and activation of STAT3 molecules through its cytoplasmic tail.10, 49, 66 Others have reported that the phosphorylation and recruitment of STAT3 are also dependent on IL‐22 and proper binding of IL‐22R.63 For example, lack or dysfunctional IL‐22 was found to result in the disruption and inactivation of STAT3 and downstream pathways in epithelial cells of murine intestine following chemically induced colitis and also in vivo in IL‐22 KO mice.63, 67, 68
The structure of mouse IL‐22R gene and secreted IL‐22 is closely similar and comparable to human component, indicating comparable physiological functions from one model to the other.64 As previously mentioned, IL‐22 is produced by ILC3 both in mouse as NKp46 ILC3 and humans as NCR ILC3. Moreover, the expression of IL‐22R is clinically and physiologically important in dictating the role of IL‐22 based on the site the receptor is found on either in human models or animal models. Th17 cells are found on human and mice secreting similar cytokines including IL‐17A, IL‐17F, IL‐21, IL‐22 and IL‐23, as well as similar chemokines including CCL20. Th17 cells express retinoic acid‐related orphan nuclear hormone receptor C (RORC) compared to the mice counterpart (RORyt). However, their expression of IL‐22R and secretion of IL‐22 seem to be similar clinically. For example, Th17 cells and its cytokines have been cast to drive inflammatory responses in patients with inflammatory bowel disease (IBD) and experimental mice models. GWAS (genome‐wide association study) has classified multiple IBD susceptibility genes involved with Th17 such as IL‐12 beta, IL‐23R, STAT3, CCR6 and JAK2.69 Overall expression of Th17 cells and the levels of IL‐17A, IL‐17F and IL‐23 were significantly high within the mucosa and lamina propria layer in patients with ulcerative colitis (UC) and Crohn's disease (CD) compared to healthy controls.70, 71, 72 Similarly, the administration of IL‐23 in mice has led to increase in the production of Th17 cells and consequently worsen colitis, whereas anti‐IL‐23 antibodies were able to improve colitis.73, 74 Similarly, Th22 cells are expressed in mouse and humans but only able to secrete IL‐22 only and do not produce IL‐17. Th22 cells have similar transcription factors to Th17 cells as they express RORC in human tissues and RORyt in mice.54
IL‐10R2 cell surface expression is required for the activation of class 2 cytokines, including IL‐10, IL‐22 and IFN‐L1. The antiviral activity of IFN‐L2 and IFN‐L3 is mediated through IFN‐LR1/IL‐10R2 dimer, which stimulates the activation of the JAK/STAT signalling pathway leading to the expression of IFN‐stimulated genes. Polymorphisms of IL‐10R2, one of two dimer receptor ligands of IL‐22, may play a role in modulating HCV disease outcome.75
While the biological effect of IL‐22 is believed to be mediated mainly via STAT3 signalling pathway through the binding of IL‐22R1 and IL‐10R2, high concentrations of IL‐22 induce the phosphorylation of other pathways and transcription factors including tyrosine kinases Jak1 and Tyk2. High concentrations of IL‐22 can also activate STAT1, STAT5, MAPKs (mitogen‐activated protein kinase), AKT (protein kinase B, PKB), NF‐κB (nuclear factor‐kappaB) and AP‐1 (activator protein‐1).76 This in turn induces other intracellular signalling cascades from MAP kinase pathways including JNK, p38 Kinase and Mek/Erk.13, 66
Location of IL‐22R
IL‐22R is mainly expressed by pancreatic islets, intestinal and respiratory epithelial cells and, to a lesser extent, by hepatocytes and keratinocytes.5, 8 Thus, IL‐22R is well positioned to allow T cells and ILCs, which secrete IL‐22 to regulate proliferation (and influence differentiation more generally) of epithelial cells, endothelial cells and fibroblasts.63
IL‐22R is not found on immune cells suggesting that IL‐22 does not regulate the function of these cells.49 IL‐22R is rather located at the outer‐body barriers and on parenchymal tissues such as those found on epithelial cells and tissues of respiratory and digestive systems, kidney, skin and liver.8 This is consistent with a role of IL‐22 and its receptor in proliferation, resolution and repair of injured tissues that the receptor is found on. Thus, IL‐22R is appropriately expressed in cell subsets in order to control the restitution phase of inflammation.63
Conflicting roles ascribed to IL‐22
It has been reported that IL‐22 and the IL‐22 receptor have dual roles being either protective or pathological in inflammation.60, 63, 67, 77, 78, 79, 80, 81, 82, 83, 84, 85
Anti‐inflammatory roles
In cerulein‐induced acute pancreatitis, IL‐22 appeared to be an anti‐inflammatory mediator due to its inhibition of inflammatory cell infiltrations mediated by the induction of Reg3 proteins in acinar cells.86 For example, on the one hand, injury was prevented via administration of recombinant IL‐22 (1ug/g) 2 hours prior the onset of the acute cerulean‐induced pancreatitis.87 (On the other hand, the injury of acute cerulein pancreatitis was not worse in IL‐22 KO mice;87 however, significant inflammatory cell infiltration was observed86). In cerulein‐induced chronic pancreatitis, IL‐22 administration with adenovirus ameliorated damage,86, 87 and IL‐22 deficiency in IL‐22 KO mice or blocking endogenous IL‐22 with monoclonal anti‐IL‐22 was associated with worse acinar damage.87
In a high‐fat diet (HFD) mouse model, IL‐22 played a role in prevention of oxidative and ER stress in pancreatic beta cells via STAT1 and STAT3.88 This effect was found to be due to both upregulation of antioxidant genes such as Gpx5 (glutathione peroxidase‐5), Prdx5 (peroxiredoxin‐5) and Sod2 (encoding superoxide dismutase‐2) and downregulation of oxidative stress‐inducing genes: Nos2 (nitric oxide synthase‐2 or iNos), Hsp90ab1 (mitochondrial heat‐shock protein) and Fth1 (ferritin heavy chain‐1). A similar mechanism was also found on intestinal epithelial cells, where a suppression of stress was associated with increased Muc 2 production and restoration of epithelial integrity.89
Interestingly in the viral LCMV‐infected mouse model, IL‐22 KO mice showed hypertrophy of immune organs – thymus and spleen – compared with atrophy following excessive induction of IL‐22 using an adenovirus‐mediated delivery system.60
GWAS analysis has identified polymorphisms in IL‐23R in IBD.90 A more recent study reported an intestinal protective role of IL‐23R signalling pathway via IL‐22. Mice deficient in IL‐23R in intestinal epithelial cells had impaired mucosal IL‐22 induction in response to IL‐23. Administration of αThy‐1 enhanced the colitis, which was alleviated by IL‐22 treatment, whereas Reg3β administration improved the DSS colitis in these mice by increasing the IL‐22 mucosal levels through recruitment of IL‐22‐producing neutrophils.91 Interestingly, a mutation in IL‐23R is associated with a reduced likelihood of Crohn's disease. While serum IL‐22 directly correlates with disease activity of Crohn's disease, the lowest serum level was observed in carriers of the minor allele of the p.Arg381Gln mutation, which represents the only coding IL23R SNP and the main CD‐protecting IL23R variant.92 These findings taken together are consistent with an anti‐inflammatory role for IL‐22.
Pro‐inflammatory roles
IL‐22 has been associated with the pathogenesis of a number of autoimmune and inflammatory diseases. A published study reported a correlation between the disease activity of patients with rheumatoid arthritis (RA) and the levels of IL‐22 measured in serum, possibly suggesting a pathogenic function.84, 93 A pathological function is supported by a significantly less severe form of arthritis in IL‐22 KO mice with collagen‐induced arthritis.94
Similarly, IL‐22 is highly expressed in skin lesions of patients with psoriasis, and IL‐22 levels found in serum were correlated with disease activity.95, 96 Furthermore, experimental psoriasis was significantly ameliorated in IL‐22 KO mice or following neutralisation of IL‐22 via anti‐IL‐22 in WT mice.97
There are other examples of IL‐22 resulting in adverse outcomes. For example, treatment with recombinant IL‐22 (rIL‐22) exacerbated airway inflammatory responses in mice with acute exposure to cigarette smoke;98 at 24 hours following rIL‐22 treatment, there was significantly more pathology in lungs of treated animals.98 This pathology was associated with induction of significant pro‐inflammatory cytokines and damaged epithelium of the lung. It is important to note that in this model, the rIL‐22 was administered half an hour before smoke exposure at a dose of 100 mg kg−1 per mouse.98
Another study has suggested a pro‐inflammatory function of IL‐22 in models of bleomycin‐induced airway inflammation,99 in both IL‐22 KO mice and by anti‐IL‐22 monoclonal antibody in wild‐type mice.99 The airway inflammation induced by bleomycin was lethal for WT mice.99 IL‐22 KO mice or neutralised IL‐22 WT mice were ameliorated against bleomycin‐induced disease,99 but interestingly, IL‐17α KO mice were completely protected against bleomycin, while on the other hand, the administration of IL‐22‐blocking agent in IL‐17α KO exacerbated airway inflammation following bleomycin induction, suggesting a local protection of IL‐22 in the absence of IL‐17α.99 This was supported by their in vitro studies claiming a protection of airway epithelial cells from bleomycin following administration of IL‐22, which was then reversed with co‐administration of IL‐17α.99
Reconciling anti‐inflammatory and pro‐inflammatory roles of IL‐22
Timing
Timing of an IL‐22 intervention appears critical as shown in the acute cerulein–pancreatitis mouse model.86 This is not so surprising because IL‐22 promotes proliferation of epithelial cells, which would be undesirable before infected or otherwise damaged cells in narrow pancreatic ducts were cleared by the inflammatory phase of inflammation. If clearance does not occur, then it is likely that the debris will block the narrow pancreatic ductules resulting in more pancreatitis. A similar explanation may explain the pro‐inflammatory effect of IL‐22 on lung of mice exposed acutely to smoke inhalation.98 The presence of large amounts of IL‐22 by promoting proliferation could counter clearance of damaged tissue debris in the inflammatory phase of the model, leading to obstructed small bronchioles.
The interrelationship with Th17
In the bleomycin mouse model, complete absence of IL‐22 was associated with protection,99 but this appeared to relate to the absence of IL‐17α. This is consistent with the requirement for IL‐22 in the evolution of Th17 pro‐inflammatory cells, for example by inducing IL‐23R. In the absence of Th17, IL‐22 is protective. Th17 was presumably absent in the IL‐17α KO. Similarly, they are also likely reduced once the first phase of inflammation is exhausted.
The paradoxical effect with proliferative disease pathologies
Psoriasis is a skin disorder associated with hyperproliferation of keratinocytes. It should therefore not be surprising that a cytokine which promotes proliferation of these cells could exacerbate the pathology or that an IL‐22 KO animal might be protective of psoriasis.
A similar consideration could apply to rheumatoid arthritis. IL‐22 could conceivably exacerbate arthritis as the disease evolves to irreversible joint deformity.
IL‐22 dose effects
In the mouse model of acute airway inflammation induced by smoke inhalation, the pro‐inflammatory response followed administration of 100 mg kg−1 of rIL‐22.98 However, this dose is 1000‐fold >0.1mg kg−1 used to induce a protective effect in the high‐fat diet murine model.88 It also contrasts with a murine model study of IL‐22's role in Pseudomonas aeruginosa lung damage,100 where neutralisation of IL‐22 resulted in increased neutrophil infiltration, and lung damage.100 The protection in this study followed treatment with 0.1 mg kg−1 of rIL‐22 via intratracheal administration 18 hours prior to induction of acute pneumonia with the bacterium.100 Similarly, a study of rIL‐22 treatment in an airway disease model triggered by inhalation of methacholine78 reported that IL‐22 was protective at the more physiological dose of 0.1–10 mg kg−1.78 Unlike the smoke inhalation study, the finding of rIL‐22 protection was supported by IL‐22 KO mice, which developed more inflammation following methacholine challenge.78
High‐dose rIL‐22 would activate many other pathways14, 15, 101 including pro‐inflammatory nonphysiological pathways.
Liver
In liver, the protective function of IL‐22 is mediated by activation of the STAT3 signal pathway and induction of several anti‐apoptotic proteins (such as B‐cell lymphoma 2 (Bcl‐2), B‐cell lymphoma‐extra‐large (Bcl‐xL) and myeloid cell leukaemia 1 (Mcl‐1)). (Mitogenic proteins (such as retinoblastoma‐like protein 2, cyclin D1 and cyclin‐dependent kinase 4 (CDK4)) enhance cellular proliferation during serum starvation, but not under normal growth conditions.76, 83) IL‐22 may act on liver stem/progenitor cells, which are important for regeneration following liver injury being able to generate hepatocytes and biliary epithelial cells.2, 8 Consistent with these findings, the total progenitor cell numbers in liver were positively correlated with increased levels of IL‐22 expression in hepatocytes in patients with hepatitis B virus (HBV) or hepatitis C virus (HCV).2
The anti‐oxidative effect of IL‐22 has been demonstrated in both acute and chronic murine models of liver injury, where there is increased expression of the antioxidants metallothionein 1 (MT1) and metallothionein 2 (MT2).76, 102 More recently, it has been demonstrated that IL‐22 can promote nuclear translocation of nuclear factor‐related factor (Nrf2) in an in vitro model of alcoholic liver fibrosis (ALF) using hepatic stellate cells to show reduced fibrosis. This effect is proposed to be regulated through induction of the antioxidant signalling cascades, resulting in elevated expression of its downstream target protein glutathione (GSH), and suppression of oxidative stress.103
The role of IL‐22 following sterile insults to liver
In a number of studies, following sterile insults, for example with alcohol or paracetamol (APAP), it appears that IL‐22 acts on both hepatic stellate cells (HSCs) and hepatocytes by promoting proliferation to ameliorate acute liver injury.104, 105, 106 These cells highly express local receptors for IL‐22.
APAP is a widely used over‐the‐counter antipyretic and analgesic medication.107 It has well‐known hepatotoxicity if an overdose is taken, causing direct liver injury via the accumulation of toxic metabolites, lowering glutathione in the liver and leading to hepatic damage.108, 109 This damage or necrosis develops within 3 to 48 hours, which makes it a hyperacute injury.110, 111 APAP‐induced hepatotoxicity is found to be mainly due to events occurring within the hepatocytes.112 The APAP injury is mediated by mitochondrial dysfunction, production of toxic metabolites and ER stress. The injury is in turn amplified by the innate immune system.109 Hepatic cell death activates Kupffer cells (hepatic macrophages). These cells produce IL‐12, IL‐18 and TNF‐α that may be responsible for the activation of NKT and NK cells.109, 113 This activation is believed to drive accumulation and recruitment of neutrophils and other immune cells including increased numbers of activated NKT and NK cells in the liver,113 well after the toxin has been catabolised and so increasing the severity of liver damage. Pretreatment with a single dose of recombinant IL‐22 in WT mice significantly reduced the levels of serum alanine aminotransferase (ALT) and histopathologic damage compared with nontreated animals following APAP acute toxicity. Others have shown that this protection of IL‐22 is dependent on STAT3.114, 115, 116
Injudicious alcohol causes a significant liver damage via acute or chronic consumption. Alcoholic liver disease or ALD is caused by prolonged and excessive use of alcohol resulting in alcoholic steatohepatitis and fibrosis where there is an intermittent exposure inducing recurrent episodes of injury and healing. This ultimately leads to cirrhosis.117 The initial hepatic injury is the result of oxidative and ER stress and mitochondrial damage induced by alcohol and its metabolites,118, 119, 120, 121, 122, 123 leading to the activation of chemokines that stimulate macrophages, IL‐8 and neutrophils.124, 125 Kupffer cells,117 T lymphocytes and infiltrating macrophages are also linked to ALD. Alcohol also alters gut permeability, which results in high levels of lipopolysaccharide (LPS) in both the hepatic portal vein and systemic circulations. Activation of toll‐like receptor 4 (TLR4) by LPS leads also to activation of a number of inflammatory cytokines.126, 127
IL‐22 has been shown to ameliorate steatosis and hepatic damage in acute ethanol feeding, chronic‐binge ethanol feeding and high‐fat diet‐induced fatty liver disease.128 This occurs with IL‐22 pretreatment in murine models of ALD.129, 130 Liver damage is also worse in IL‐22‐deficient (IL‐22 KO) mice.129, 130, 131 Conversely, in vivo and in vitro studies show liver regeneration or hepatocyte proliferation with overexpression of IL‐22.128, 132 Similarly, Ki et al.76 have shown a protection following IL‐22 administration in a murine model of chronic‐binge ethanol feeding. The protection in the chronic model was related to the upregulation of STAT3 pathway as the hepato‐protection was abolished following a genetic deletion of STAT3 in hepatocytes.
While IL‐22 appears to be associated with amelioration of liver injury in hepatic oxidative stress, alcohol‐induced liver injury or HFD models,76, 133, 134 HSCs were also associated with liver fibrogenesis and formation of scar tissue in response to chronic liver injury and expressed high levels of IL‐22R1.82 Specifically, IL‐22 administration induced HSCs and prevented their apoptosis in vivo and in vitro.82, 106 Furthermore, overexpression of IL‐22 via exogenous administration of expressed IL‐22 via adenovirus or via targeted gene (i.e. transgenic mice for IL‐22) resulted in amelioration of liver fibrosis apparently due to senescence of b‐galactosidase‐positive HSCs.82, 106 In contrast, a single dose of IL‐22 via the expression of adenovirus induced CXCL1 in the liver attracting circulating neutrophils to cause acute inflammation.77
IL‐22 is being investigated as a treatment for acute or chronic liver injury, either by use of IL‐22‐blocking agents or induction of IL‐22‐producing cells.67, 79, 102, 132, 135 Other approaches include developing small molecule agonists of the aryl hydrocarbon receptor to enable the activation of transcription factors that are responsible for the differentiation of ILC3 and to promote Th22 cells each of which can enhance the production of IL‐22 in the liver.8, 136 Another alternative is via increasing the presence of IL‐22‐producing cells via blocking agents such as chemokine ligand 20 (CCL20) or liver activation‐regulated chemokine (LARC) or via the use of neutralising antibodies that specifically target IL‐22 and inflammatory mediator cytokines such as IL‐22 or TNF‐α.8 However, these therapies that are at an early stage of investigation remain controversial because they are associated with high infection rates post‐treatment.
This section of the review focused on acute liver injury via APAP and both acute and chronic liver injuries due to ethanol. Other models of sterile livery injury,114 where IL‐22 appears anti‐inflammatory/protective, include carbon tetrachloride (CCL4)‐ and FAS ligand (FASL)‐induced hepatic injury,137 and T‐cell‐mediated hepatitis induced by concanavalin A, where there was prevention of the hepatitis in IL‐22 pretreated mice compared with mice injected with IL‐22‐neutralising antibodies.51, 129 In general, the timing of an IL‐22 therapeutic intervention does not appear to be pro‐inflammatory where the injury model involved a sterile insult. An exception was the acute infiltration of neutrophils with a single dose of 5 × 1010 particles of recombinant adenovirus that expressed IL‐22.77
The role of IL‐22 in liver following (nonsterile) infection
In contrast to sterile injuries, a number of studies have indicated that IL‐22 can promote hepatic inflammation in mice models following infection.5, 77 In these models, IL‐22 appears to exacerbate chronic liver inflammation and fibrosis.5 Some of the strongest evidence for a pro‐inflammatory role of IL‐22 is found in models of chronic HBV.5, 85, 133 In patients with chronic HBV, hepatic IL‐22 was highly upregulated and its expression was positively correlated with the severity of injury and inflammation.2, 5, 102, 129, 138, 139 Studies in humans found that Th17‐secreting cells drive the upregulation of IL‐22, and their levels were highly abundant in patients with HBV, associated with increased inflammation and fibrosis.133, 138, 140 In animal models with chronic HBV, IL‐22 is associated with the development of liver fibrosis and cirrhosis.
How to reconcile these pro‐inflammatory outcomes with the hypothesis that IL‐22 is involved in restitution after clearance of infection and damaged cells? The blockade of IL‐22 in the chronic HBV mouse model resulted in reduced Th17 recruitment, which was associated with increased progression of liver inflammation.5 While it is unclear whether other immune cells were affected by the blockade of IL‐22 in this study, reduced Th17 could have driven increased inflammation due to impaired clearance of infection. Also IL‐22 KO mice displayed a significant tissue damage not only in liver.129 These outcomes are consistent firstly with the hypothesis that healing responses are impaired in the absence of clearance of infection so leading to fibrosis and cirrhosis, and secondly that absent IL‐22 is associated with reduced restitution after injury.129
Others have suggested that the pathological effect of IL‐22 in chronic HBV liver injuries may be due to the activation of IL‐22BP, an inhibitor of IL‐22 preventing the binding with IL‐22R1 and IL‐10R1.133 This effect could be abrogated by blocking IL‐22BP to promote resolution of inflammation.133 It has been postulated that this activation may be the cause of a number of failed attempts to use IL‐22 injections as a treatment for hepatic inflammation. This receptor may also be activated following high dose of IL‐22 or sustained IL‐22 exposure leading to these adverse effects.
IL‐22 may also be protective against liver fibrosis in human patients with chronic HCV.135 This study reported an inverse correlation between the severity and progression of disease and IL‐22 concentration found in cultures from peripheral blood mononuclear cells (PBMC) leucocytes of those patients.135 It was concluded that on the one hand, IL‐22 levels were correlated inversely with hepatic fibrosis.135 On the other hand, others have reported that IL‐22 was positively associated with severity of liver fibrosis in HCV but administration of IL‐22 promoted proliferation and inhibited apoptosis of LX‐2 human hepatic stellate cells, a major driver for liver fibrosis and inflammation.141 Taking these studies together, an interpretation of the human literature, which fits our hypothesis that IL‐22 is protective, is that elevated levels of IL‐22 may be compensatory to ameliorate inflammation rather being a cause of inflammation.142
IL‐22 appeared to be protective against liver damage in a primary Plasmodium chabaudi malarial infection.79 The absence of IL‐22 in IL‐22 KO mice was associated with increased mortality and hepatic injury during the infection. In contrast, the rates of mortality and liver damage in IL‐17α KO mice were not affected in this model.79
Summary
In this review, we hypothesise that IL‐22 has a primary role in tissue restitution following clearance of infection and damaged cellular debris from any injury. Th22 lymphocytes typically appear later in inflammation. We contend that pro‐inflammatory effects in humans and in experimental models relate in part to underlying disease pathology (e.g. psoriasis), timing of an exogenous administration of IL‐22 (e.g. where infection is still active) and dose (pharmacological doses of IL‐22 conceivably activate not only STAT3 but also some classical inflammatory pathways) (Figure 1).
Figure 1.
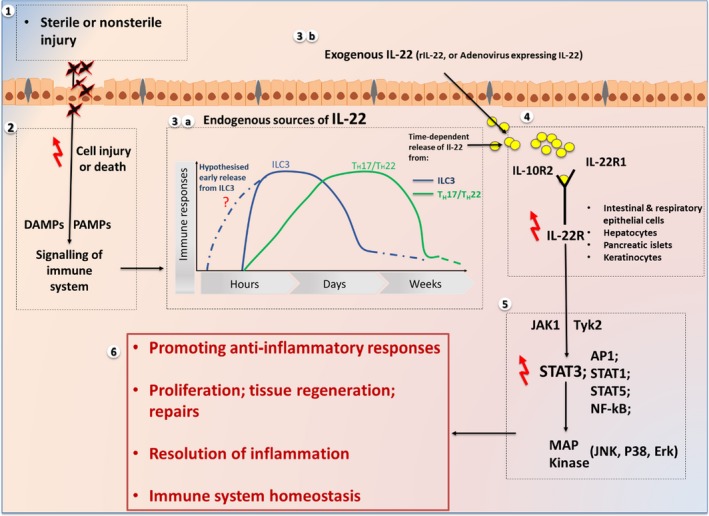
A diagram illustrating a summary of the pathways and roles of interleukin‐22 (IL‐22) following sterile and nonsterile injury. (1) The presence of sterile injury, for example by drugs, or nonsterile injury, for example by infections (2), will lead to cell injury and/or death activating damage‐associated molecular patterns (DAMP) and pathogen‐associated molecular patterns (PAMPs) that signal an immune response. (3)(a) Along with several chemokines and cytokines, endogenous IL‐22 is released from either group 3 innate lymphoid cells (ILC3) and/or Th17/Th22 hours after the induction of injury. It is also proposed that group 3 ILCs (ILC3) might secrete IL‐22 at an earlier stage when compared to TH17/TH22. (3)(B) Alternatively, in experimental‐ or clinical‐based studies, exogenous IL‐22 can be injected directly via recombinant IL‐22 (rIL‐22) or adenovirus expressing IL‐22. (4) Both endogenous and exogenous IL‐22 lead to the binding and activation of IL‐22 receptor (IL‐22R) via its two subunits, IL‐22R1 and IL‐10R2. IL‐22R is commonly found on the intestinal and respiratory epithelial cells, hepatocytes, pancreatic islets and keratinocytes. The initial binding of IL‐22 occurs with the IL‐22R1 subunit, unique to IL‐22 and unlike other cytokines from IL‐10 family, followed by the binding to IL‐10R2. (5) IL‐22 binding to IL‐22R mediates biological effects via phosphorylation of signalling pathways notably via signal transducer and activator of transcription‐3 (STAT3) and activating protein‐1 (AP1), STAT1, STAT5 and nuclear factor‐kappaB (NF‐kB). The signalling from these pathways stimulates the activation of several intracellular signalling processes such as mitogen‐activated protein kinase (MAPK) pathways including c‐Jun N‐terminal kinases (JNK), p38 and extracellular signal‐regulated kinases (Erk). (6) IL‐22 promotes anti‐inflammatory responses following injury by stimulating proliferation, regeneration and repair of injured tissue. IL‐22 thus plays an essential role in the resolution of injury and restoration of homeostasis in the immune system.
Potentially different from Th22, Th17 lymphocytes also infiltrate an injured tissue in the first inflammatory phase. The integrated response of Th17‐ and Th22‐derived cytokines may hold the fate of the inflammatory response. For example, IL‐22 by increasing the expression of IL‐23R on Th17 cells consequently upregulates RORγt and activates STAT3 during the first pro‐inflammatory phase of inflammation.53
The roles of Th‐derived IL‐22 versus ILC3‐derived IL‐22 remain largely unelucidated, but it is the same cytokine. Different effects may relate to time of release, with ILC3 playing a more significant role in the early inflammation.
Acknowledgments
Saleh Y Alabbas is sponsored for his PhD by the Saudi Arabian Cultural Mission (SACM) and the Saudi Ministry of Education. This work was supported by funding from the National Health and Medical Research Council (NHMRC) of Australia. We thank the IBD team, Dr Ran Wang and Katharine Irvine for their consistent support and encouragement.
Conflict of interest
We declare no conflict of interest.
References
- 1. Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov 2016; 15: 551–567. [DOI] [PubMed] [Google Scholar]
- 2. Feng D, Kong X, Weng H et al Interleukin‐22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology 2012a; 143: 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McHedlidze T, Waldner M, Zopf S et al Interleukin‐33‐dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 2013; 39: 357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sonnenberg GF, Monticelli LA, Alenghat T et al Innate lymphoid cells promote anatomical containment of lymphoid‐resident commensal bacteria. Science 2012; 336: 1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao J, Zhang Z, Luan Y et al Pathological functions of interleukin‐22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology 2014; 59: 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Molodecky NA, Soon IS, Rabi DM et al Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46–54. [DOI] [PubMed] [Google Scholar]
- 7. Basu R, O'Quinn DB, Silberger DJ et al Th22 cells are an important source of IL‐22 for host protection against enteropathogenic bacteria. Immunity 2012; 37: 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL‐22‐IL‐22R1 system. Nat Rev Drug Discov 2014; 13: 21–38. [DOI] [PubMed] [Google Scholar]
- 9. Sabat R, Witte E, Witte K et al IL‐22 and IL‐17: An Overview. IL‐17, IL‐22 and Their Producing Cells: role in Inflammation and Autoimmunity. Springer Basel: Basel, 2013, 11–35. [Google Scholar]
- 10. Xie MH, Aggarwal S, Ho WH et al Interleukin (IL)‐22, a novel human cytokine that signals through the interferon receptor‐related proteins CRF2‐4 and IL‐22R. J Biol Chem 2000; 275: 31335–31339. [DOI] [PubMed] [Google Scholar]
- 11. Wolk K, Sabat R. Interleukin‐22: a novel T‐ and NK‐cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev 2006; 17: 367–380. [DOI] [PubMed] [Google Scholar]
- 12. Dumoutier L, Van Roost E, Colau D et al Human interleukin‐10‐related T cell‐derived inducible factor: molecular cloning and functional characterization as an hepatocyte‐stimulating factor. Proc Natl Acad Sci USA 2000; 97: 10144–10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eyerich S, Eyerich K, Cavani A et al IL‐17 and IL‐22: siblings, not twins. Trends Immunol 2010; 31: 354–361. [DOI] [PubMed] [Google Scholar]
- 14. Park O, Ki SH, Xu M et al Biologically active, high levels of interleukin‐22 inhibit hepatic gluconeogenesis but do not affect obesity and its metabolic consequences. Cell Biosci 2015; 5: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang X, Ota N, Manzanillo P et al Interleukin‐22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 2014; 514: 237–241. [DOI] [PubMed] [Google Scholar]
- 16. Zheng Y, Valdez PA, Danilenko DM et al Interleukin‐22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 2008; 14: 282–289. [DOI] [PubMed] [Google Scholar]
- 17. Burkhard SH, Mair F, Nussbaum K et al T cell contamination in flow cytometry gating approaches for analysis of innate lymphoid cells. PLoS ONE 2014; 9: 94196–94200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cording S, Medvedovic J, Cherrier M et al Development and regulation of RORγt+ innate lymphoid cells. FEBS Lett 2014; 588: 4176–4181. [DOI] [PubMed] [Google Scholar]
- 19. Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol 2012; 30: 647–675. [DOI] [PubMed] [Google Scholar]
- 20. Halim Timotheus YF, Steer Catherine A, Mathä L et al Group 2 innate lymphoid cells are critical for the initiation of adaptive T Helper 2 Cell‐mediated allergic lung inflammation. Immunity 2014; 40: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Withers DR, Gaspal FM, Mackley EC et al Cutting edge: lymphoid tissue inducer cells maintain memory CD4 T cells within secondary lymphoid tissue. J Immunol 2012; 189: 2094–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gasteiger G, Rudensky AY. Opinion: Interactions of innate and adaptive lymphocytes. Nat Rev Immunol 2014; 14: 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Molofsky AB, Nussbaum JC, Liang H‐E et al Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 2013; 210: 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mortha A, Chudnovskiy A, Hashimoto D et al Microbiota‐dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014; 343: 1477–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Felix MW, Vedrana J, Sonja V et al NK cells link obesity‐induced adipose stress to inflammation and insulin resistance. Nat Immunol 2015; 16: 376–385. [DOI] [PubMed] [Google Scholar]
- 26. Monticelli LA, Sonnenberg GF, Abt MC et al Innate lymphoid cells promote lung‐tissue homeostasis after infection with influenza virus. Nat Immunol 2011; 12: 1045–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee M‐W, Odegaard Justin I, Mukundan L et al Activated Type 2 Innate Lymphoid Cells Regulate Beige Fat Biogenesis. Cell 2015; 160: 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spits H, Artis D, Colonna M et al Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol 2013; 13: 145–149. [DOI] [PubMed] [Google Scholar]
- 29. Kim CH, Hashimoto‐Hill S, Kim M. Migration and tissue tropism of innate lymphoid cells. Trends Immunol 2016; 37: 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. von Burg N, Turchinovich G, Finke D. Maintenance of Immune Homeostasis through ILC/T Cell Interactions. Front Immunol 2015; 6: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cella M, Fuchs A, Vermi W et al A human natural killer cell subset provides an innate source of IL‐22 for mucosal immunity. Nature 2009; 457: 722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luci C, Reynders A, Ivanov II et al Influence of the transcription factor RORgammat on the development of NKp46 + cell populations in gut and skin. Nat Immunol 2009; 10: 75–82. [DOI] [PubMed] [Google Scholar]
- 33. Sanos SL, Bui VL, Mortha A et al RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22‐producing NKp46 + cells. Nat Immunol 2009; 10: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Satoh‐Takayama N, Vosshenrich CAJ, Lesjean‐Pottier S et al Microbial flora drives interleukin 22 production in intestinal NKp46 + cells that provide innate mucosal immune defense. Immunity 2008; 29: 958–970. [DOI] [PubMed] [Google Scholar]
- 35. Takatori H, Kanno Y, Watford WT et al Lymphoid tissue inducer‐like cells are an innate source of IL‐17 and IL‐22. J Exp Med 2009; 206: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Forkel M, Mjösberg J. Dysregulation of group 3 innate lymphoid cells in the pathogenesis of inflammatory bowel disease. Curr Allergy Asthma Rep 2016; 16: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Flores‐Borja F, Irshad S, Gordon P et al Crosstalk between innate lymphoid cells and other immune cells in the tumor microenvironment. J Immunol Res 2016; 2016: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoorweg K, Peters C, Cornelissen F et al Functional differences between human NKp44‐ and NKp44 + RORC+ innate lymphoid cells. Front Immunol 2012; 3: 1664–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eberl G, Marmon S, Sunshine M‐J et al An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol 2004; 5: 64–73. [DOI] [PubMed] [Google Scholar]
- 40. Montaldo E, Juelke K, Romagnani C. Group 3 innate lymphoid cells (ILC3s): origin, differentiation, and plasticity in humans and mice. Eur J Immunol 2015; 45: 2171–2182. [DOI] [PubMed] [Google Scholar]
- 41. Gasteiger G, D'Osualdo A, Schubert DA et al Cellular innate immunity: an old game with new players. J Innate Immun 2016; 9: 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gronke K, Kofoed‐Nielsen M, Diefenbach A. Innate lymphoid cells, precursors and plasticity. Immunol Lett 2016; 179: 9–18. [DOI] [PubMed] [Google Scholar]
- 43. Sonnenberg GF, Monticelli LA, Elloso MM et al CD4 + lymphoid tissue‐inducer cells promote innate immunity in the gut. Immunity 2011; 34: 122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crellin NK, Trifari S, Kaplan CD et al Human NKp44 + IL‐22 + cells and LTi‐like cells constitute a stable RORC + lineage distinct from conventional natural killer cells. J Exp Med 2010; 207: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kazuyo M, Hiroki K, Masanobu T et al Interferon and IL‐27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol 2015; 17: 76–86. [DOI] [PubMed] [Google Scholar]
- 46. Gasteiger G, Fan X, Dikiy S et al Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 2015; 350: 981–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wolk K, Kunz S, Asadullah K et al Cutting edge: immune cells as sources and targets of the IL‐10 family members? J Immunol 2002; 168: 5397–5402. [DOI] [PubMed] [Google Scholar]
- 48. Wolk K, Witte K, Witte E et al Maturing dendritic cells are an important source of IL‐29 and IL‐20 that may cooperatively increase the innate immunity of keratinocytes. J Leukoc Biol 2008; 83: 1181–1193. [DOI] [PubMed] [Google Scholar]
- 49. Wolk K, Kunz S, Witte E et al IL‐22 increases the innate immunity of tissues. Immunity 2004; 21: 241–254. [DOI] [PubMed] [Google Scholar]
- 50. Kunz S, Wolk K, Witte E et al Interleukin (IL)‐19, IL‐20 and IL‐24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol 2006; 15: 991–1004. [DOI] [PubMed] [Google Scholar]
- 51. Lafdil F, Miller AM, Ki SH et al Th17 cells and their associated cytokines in liver diseases. Cell Mol Immunol 2010; 7: 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liang Z, Ivaylo II, Rosanne S et al IL‐6 programs TH‐17 cell differentiation by promoting sequential engagement of the IL‐21 and IL‐23 pathways. Nat Immunol 2007; 8: 967–974. [DOI] [PubMed] [Google Scholar]
- 53. Sarra M, Pallone F, Macdonald TT et al IL‐23/IL‐17 axis in IBD. Inflamm Bowel Dis 2010; 16: 1808–1813. [DOI] [PubMed] [Google Scholar]
- 54. Plank MW, Kaiko GE, Maltby S et al Th22 cells form a distinct Th lineage from Th17 cells in vitro with unique transcriptional properties and Tbet‐dependent Th1 plasticity. J Immunol 2017; 198: 2182–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eyerich S, Eyerich K, Pennino D et al Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 2009; 119: 3573–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Duhen T, Geiger R, Jarrossay D et al Production of interleukin 22 but not interleukin 17 by a subset of human skin‐homing memory T cells. Nat Immunol 2009; 10: 857–863. [DOI] [PubMed] [Google Scholar]
- 57. Trifari S, Kaplan CD, Tran EH et al Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)‐17, T(H)1 and T(H)2 cells. Nat Immunol 2009; 10: 864–871. [DOI] [PubMed] [Google Scholar]
- 58. Huang Y‐H, Cao Y‐F, Jiang Z‐Y et al Th22 cell accumulation is associated with colorectal cancer development. World J Gastroenterol 2015; 21: 4216–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fujita H, Nograles KE, Kikuchi T et al Human Langerhans cells induce distinct IL‐22‐producing CD4(+) T cells lacking IL‐17 production. Proc Natl Acad Sci USA 2009; 106: 21795–21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jia L, Wu C. The biology and functions of Th22 cells In: Sun B. ed. T Helper Cell Differentiation and Their Function. Springer, Netherlands: Dordrecht, 2014: 209–230. [DOI] [PubMed] [Google Scholar]
- 61. Gollob JA, Murphy EA, Mahajan S et al Altered interleukin‐12 responsiveness in Th1 and Th2 cells is associated with the differential activation of STAT5 and STAT1. Blood 1998; 91: 1341–1354. [PubMed] [Google Scholar]
- 62. López‐Navarrete G, Ramos‐Martínez E, Suárez‐Álvarez K et al Th2‐associated alternative kupffer cell activation promotes liver fibrosis without inducing local inflammation. Int J Biol Sci 2011; 7: 1273–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL‐22‐IL‐22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol 2010a; 107: 1–29. [DOI] [PubMed] [Google Scholar]
- 64. Tachiiri A, Imamura R, Wang Y et al Genomic structure and inducible expression of the IL‐22 receptor alpha chain in mice. Genes Immun 2003; 4: 153–159. [DOI] [PubMed] [Google Scholar]
- 65. Li J, Tomkinson KN, Tan XY et al Temporal associations between interleukin 22 and the extracellular domains of IL‐22R and IL‐10R2. Int Immunopharmacol 2004; 4: 693–708. [DOI] [PubMed] [Google Scholar]
- 66. Lejeune D, Dumoutier L, Constantinescu S et al Interleukin‐22 (IL‐22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL‐10. J Biol Chem 2002; 277: 33676–33682. [DOI] [PubMed] [Google Scholar]
- 67. Yang X, Zheng SG. Interleukin‐22: a likely target for treatment of autoimmune diseases. Autoimmun Rev 2014; 13: 615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pickert G, Neufert C, Leppkes M et al STAT3 links IL‐22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 2009; 206: 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Andre F, Dermot PBM, Jeffrey CB et al Genome‐wide meta‐analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet 2010; 42: 1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kobayashi T, Okamoto S, Hisamatsu T et al IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut 2008; 57: 1682–1689. [DOI] [PubMed] [Google Scholar]
- 71. Olsen T, Rismo R, Cui G et al TH1 and TH17 interactions in untreated inflamed mucosa of inflammatory bowel disease, and their potential to mediate the inflammation. Cytokine 2011; 56: 633–640. [DOI] [PubMed] [Google Scholar]
- 72. Rovedatti L, Di Sabatino A, Kudo T et al Differential regulation of interleukin‐17 and interferon‐gamma production in inflammatory bowel disease. Gut 2009; 41: 1629–1636. [DOI] [PubMed] [Google Scholar]
- 73. Yen D, Cheung J, Scheerens H et al IL‐23 is essential for T cell‐mediated colitis and promotes inflammation via IL‐17 and IL‐6. J Clin Invest 2006; 116: 1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Elson CO, Cong Y, Weaver CT et al Monoclonal Anti‐Interleukin 23 Reverses Active Colitis in a T Cell‐Mediated Model in Mice. Gastroenterology 2007; 132: 2359–2370. [DOI] [PubMed] [Google Scholar]
- 75. Hennig BJ, Frodsham AJ, Hellier S et al Influence of IL‐10RA and IL‐22 polymorphisms on outcome of hepatitis C virus infection. Liver Int 2007; 27: 1134–1143. [DOI] [PubMed] [Google Scholar]
- 76. Ki SH, Park O, Zheng M et al Interleukin‐22 treatment ameliorates alcoholic liver injury in a murine model of chronic‐binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 2010; 52: 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liang SC, Nickerson‐Nutter C, Pittman DD et al IL‐22 induces an acute‐phase response. J Immunol 2010; 185: 5531–5538. [DOI] [PubMed] [Google Scholar]
- 78. Taube C, Tertilt C, Gyülveszi G et al IL‐22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease. PLoS ONE 2011; 6: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mastelic B, do Rosario APF, Veldhoen M et al IL‐22 protects against liver pathology and lethality of an experimental blood‐stage malaria infection. Front Immunol 2012; 3: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hanash A, Dudakov J, Hua G et al IL‐22 protects intestinal stem cells from immune‐mediated tissue damage and regulates sensitivity to graft vs. host disease. J Immunol 2012; 188: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Matsumoto A, Kanai T, Mikami Y et al IL‐22‐Producing ROR[gamma]t‐Dependent Innate Lymphoid Cells Play a Novel Protective Role in Murine Acute Hepatitis. PLoS ONE 2013; 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kong X, Feng D, Wang H et al Interleukin‐22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 2012; 56: 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Radaeva S, Sun R, Hn P et al Interleukin 22 (IL‐22) plays a protective role in T cell‐mediated murine hepatitis: IL‐22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 2004a; 39: 1332–1342. [DOI] [PubMed] [Google Scholar]
- 84. Mühl H. Pro‐Inflammatory Signaling by IL‐10 and IL‐22: bad Habit Stirred Up by Interferons? Front Immunol 2013; 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wilson MS, Feng CG, Barber DL et al Redundant and pathogenic roles for IL‐22 in mycobacterial, protozoan, and helminth infections. J Immunol 2010; 184: 4378–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Huan C, Kim D, Ou P et al Mechanisms of interleukin‐22's beneficial effects in acute pancreatitis. World J Gastrointest Pathophysiol 2016; 7: 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Feng D, Park O, Radaeva S et al Interleukin‐22 ameliorates cerulein‐induced pancreatitis in mice by inhibiting the autophagic pathway. Int J Biol Sci 2012b; 8: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hasnain SZ, Borg DJ, Harcourt BE et al Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med 2014; 20: 1417–1426. [DOI] [PubMed] [Google Scholar]
- 89. Hasnain SZ, Dawson PA, Lourie R et al Immune‐driven alterations in mucin sulphation is an important mediator of Trichuris muris helminth expulsion. PLoS Pathog 2017; 13: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Duerr RH, Taylor KD, Brant SR et al A genome‐wide association study identifies IL23R as an inflammatory bowel disease gene. Science 2006; 314: 1461–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Aden K, Rehman A, Falk‐Paulsen M et al Epithelial IL‐23R signaling licenses protective IL‐22 responses in intestinal inflammation. Cell Rep 2016; 16: 2208–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schmechel S, Konrad A, Diegelmann J et al Linking genetic susceptibility to Crohn's disease with Th17 cell function: IL‐22 serum levels are increased in Crohn's disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis 2008; 14: 204–212. [DOI] [PubMed] [Google Scholar]
- 93. Leipe J, Schramm MA, Grunke M et al Interleukin 22 serum levels are associated with radiographic progression in rheumatoid arthritis. Ann Rheum Dis 2011; 70: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 94. Geboes L, Dumoutier L, Kelchtermans H et al Proinflammatory role of the Th17 cytokine interleukin‐22 in collagen‐induced arthritis in C57BL/6 mice. Arthritis Rheumatol 2009; 60: 390–395. [DOI] [PubMed] [Google Scholar]
- 95. Boniface K, Guignouard E, Pedretti N et al A role for T cell‐derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol 2007; 150: 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nakajima H, Nakajima K, Tarutani M et al Kinetics of circulating Th17 cytokines and adipokines in psoriasis patients. Arch Dermatol Res 2011; 303: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Van Belle AB, de Heusch M, Lemaire MM et al IL‐22 is required for imiquimod‐induced psoriasiform skin inflammation in mice. J Immunol 2012; 188: 462–469. [DOI] [PubMed] [Google Scholar]
- 98. J‐r L, W‐x Z, K‐w H et al Interleukin‐22 exacerbates airway inflammation induced by short‐term exposure to cigarette smoke in mice. Acta Pharmacol Sin 2014; 35: 1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sonnenberg GF, Nair MG, Kirn TJ et al Pathological versus protective functions of IL‐22 in airway inflammation are regulated by IL‐17A. J Exp Med 2010b; 207: 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Broquet A, Jacqueline C, Davieau M et al Interleukin‐22 level is negatively correlated with neutrophil recruitment in the lungs in a Pseudomonas aeruginosa pneumonia model. Sci Rep 2017; 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sabat R, Wolk K. Deciphering the role of interleukin‐22 in metabolic alterations. Cell Biosci 2015; 5: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Park O, Wang H, Weng H et al In vivo consequences of liver‐specific interleukin‐22 expression in mice: Implications for human liver disease progression. Hepatology 2011; 54: 252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ni Y‐H, Huo L‐J, Li T‐T. Antioxidant axis Nrf2‐keap1‐ARE in inhibition of alcoholic liver fibrosis by IL‐22. World J Gastroenterol 2017; 23: 2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liu M, Zhang C. The role of innate lymphoid cells in immune‐mediated liver diseases. Front Immunol 2017; 8: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cuff AO, Robertson FP, Stegmann KA et al Eomeshi NK cells in human liver are long‐lived and do not recirculate but can be replenished from the circulation. J Immunol 2016; 197: 4283–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Heymann F, Tacke F. Immunology in the liver–from homeostasis to disease. Nat Rev Gastroenterol Hepatol 2016; 13: 88–110. [DOI] [PubMed] [Google Scholar]
- 107. Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen‐induced liver necrosis. Handb Exp Pharmacol 2010; 196: 369–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Black M. Acetaminophen hepatotoxicity. Gastroenterology 1980; 78: 382–392. [PubMed] [Google Scholar]
- 109. Chun LJ, Tong MJ, Busuttil RW et al Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol 2009; 43: 342–349. [DOI] [PubMed] [Google Scholar]
- 110. Dargan PI, Jones AL. Acetaminophen poisoning: an update for the intensivist. Crit Care 2002; 6: 108–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Makin AJ, Williams R. Acetaminophen‐induced hepatotoxicity: predisposing factors and treatments. Adv Intern Med 1997; 42: 453–483. [PubMed] [Google Scholar]
- 112. Zhu X, Uetrecht J. A novel T H 17‐type cell is rapidly increased in the liver in response to acetaminophen‐induced liver injury: T H 17 cells and the innate immune response. J Immunotoxicol 2013; 10: 287–291. [DOI] [PubMed] [Google Scholar]
- 113. Liu Z‐X, Kaplowitz N. Role of innate immunity in acetaminophen‐induced hepatotoxicity. Expert Opin Drug Metab Toxicol 2006; 2: 493–503. [DOI] [PubMed] [Google Scholar]
- 114. Scheiermann P, Bachmann M, Goren I et al Application of interleukin‐22 mediates protection in experimental acetaminophen‐induced acute liver injury. Am J Pathol 2013; 182: 1107–1113. [DOI] [PubMed] [Google Scholar]
- 115. Muhl H. STAT3, a key parameter of cytokine‐driven tissue protection during sterile inflammation ‐ the case of experimental acetaminophen (Paracetamol)‐induced liver damage. Front Immunol 2016; 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Feng D, Wang Y, Wang H et al Acute and chronic effects of IL‐22 on acetaminophen‐induced liver injury. J Immunol 2014; 193: 2512–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Szabo G. Gut‐liver axis in alcoholic liver disease. Gastroenterology 2015; 148: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Cederbaum A, Lu Y, Wu D. Role of oxidative stress in alcohol‐induced liver injury. Arch Toxicol 2009; 83: 519–548. [DOI] [PubMed] [Google Scholar]
- 119. Dolganiuc A, Thomes PG, Ding W et al Autophagy in alcohol‐induced liver diseases. Alcohol Clin Exp Res 2012; 36: 1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fernandez‐Checa J, Kaplowitz N, Garcia‐Ruiz C et al Mitochondrial glutathione: importance and transport. Semin Liver Dis 1998; 18: 389–401. [DOI] [PubMed] [Google Scholar]
- 121. Leung T‐M, Nieto N. CYP2E1 and oxidant stress in alcoholic and non‐alcoholic fatty liver disease. J Hepatol 2013; 58: 395–398. [DOI] [PubMed] [Google Scholar]
- 122. Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004; 34: 9–19. [DOI] [PubMed] [Google Scholar]
- 123. Nassir F, Ibdah JA. Role of mitochondria in alcoholic liver disease. World J Gastroenterol 2014; 20: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mandrekar P, Ambade A, Lim A et al An essential role for monocyte chemoattractant protein‐1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 2011; 54: 2185–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Szabo G, Petrasek J, Bala S. Innate immunity and alcoholic liver disease. J Dig Dis 2012; 30: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Petrasek J, Mandrekar P, Szabo G. Toll‐like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol Res Pract 2010; 8: 1354–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Uesugi T, Froh M, Arteel GE et al Toll‐like receptor 4 is involved in the mechanism of early alcohol‐induced liver injury in mice. Hepatology 2001; 34: 101–108. [DOI] [PubMed] [Google Scholar]
- 128. C‐x P, Tang J, X‐y W et al Role of Interleukin‐22 in liver diseases. Inflamm Res 2014; 63: 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Carmo RF, Cavalcanti MSM, Moura P. Role of Interleukin‐22 in chronic liver injury. Cytokine 2016; 98: 107–114. [DOI] [PubMed] [Google Scholar]
- 130. Zenewicz LA, Yancopoulos GD, Valenzuela DM et al Interleukin‐22 but not interleukin‐17 provides protection to hepatocytes during acute liver inflammation. Immunity 2007; 27: 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dudakov JA, Hanash AM, van den Brink MRM. Interleukin‐ 22: immunobiology and Pathology. Annu Rev Immunol 2015; 33: 747–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Brand S, Dambacher J, Beigel F et al IL‐22‐mediated liver cell regeneration is abrogated by SOCS‐1/3 overexpression in vitro. Am J Physiol Gastrointest Liver Physiol 2007; 292: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 133. Khawar MB, Azam F, Sheikh N et al How Does Interleukin‐22 Mediate Liver Regeneration and Prevent Injury and Fibrosis? J Immunol Res 2016; 2016: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yang L, Zhang Y, Wang L et al Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin‐22. J Hepatol 2010; 53: 339–347. [DOI] [PubMed] [Google Scholar]
- 135. Sertorio M, Hou X, Carmo RF et al IL‐22 and IL‐22 binding protein (IL‐22BP) regulate fibrosis and cirrhosis in hepatitis C virus and schistosome infections. Hepatology 2015; 61: 1321–1331. [DOI] [PubMed] [Google Scholar]
- 136. Monteleone I, Rizzo A, Sarra M et al Aryl hydrocarbon receptor‐induced signals up‐regulate IL‐22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011; 141: 237–248. [DOI] [PubMed] [Google Scholar]
- 137. Pan H, Hong F, Radaeva S et al Hydrodynamic gene delivery of interleukin‐22 protects the mouse liver from concanavalin A‐, carbon tetrachloride‐, and Fas ligand‐induced injury via activation of STAT3. Cell Mol Immunol 2004; 1: 43–49. [PubMed] [Google Scholar]
- 138. Zhang Y, Cobleigh MA, Lian JQ et al A proinflammatory role for interleukin‐22 in the immune response to hepatitis B virus. Gastroenterology 2011; 141: 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Xiang X, Gui H, King NJ et al IL‐22 and non‐ELR‐CXC chemokine expression in chronic hepatitis B virus‐infected liver. Immunol Cell Biol 2012; 90: 611–619. [DOI] [PubMed] [Google Scholar]
- 140. Kronenberger B, Rudloff I, Bachmann M et al Interleukin‐22 predicts severity and death in advanced liver cirrhosis: a prospective cohort study. BMC Med 2012; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wu L‐Y, Liu S, Liu Y et al Up‐regulation of interleukin‐22 mediates liver fibrosis via activating hepatic stellate cells in patients with hepatitis C. Clin Immunol 2015; 158: 77–87. [DOI] [PubMed] [Google Scholar]
- 142. Wu L, Zhao J. Does IL‐22 protect against liver fibrosis in hepatitis C virus infection? Hepatology 2015; 62: 1919. [DOI] [PubMed] [Google Scholar]


