Abstract
Background
Hypercholesterolemia is a well established risk factor for coronary heart disease and is highly prevalent among human immunodeficiency virus (HIV)-positive persons. Antiretroviral therapy (ART) can both directly modify total cholesterol and have drug-drug interactions with statins. This makes investigating modifiable behavioral predictors of total cholesterol a pertinent task.
Methods
To explore the association between diet and physical activity with cross-sectionally measured total cholesterol, we administered a validated Food-Frequency-Questionnaire to participants of the Swiss HIV Cohort Study ≥45 years old. Linear mixed-effects models were applied to explore the associations between dietary patterns and physical activity with total cholesterol, after adjustment for clinical and demographic covariates.
Results
In total, 395 patients were included. Forty percent (158 of 395) had elevated total cholesterol (>5.2 mmol/L), and 41% (164 of 395) were not regularly physically active. In multivariable analysis, 2 factors were positively associated with total cholesterol; female sex (β = 0.562; 95% confidence interval [CI], 0.229–0.896) and the combined consumption of meat, refined/milled grains, carbonated beverages, and coffee (β = 0.243; 95% CI, 0.047–0.439). On the other hand, regular physical activity (β = −0.381; 95% CI, −0.626 to −0.136), lipid-lowering drugs (β = −0.443; 95% CI −0.691 to −0.196), ART containing tenofovir (β = −0.336; 95% CI −0.554 to −0.118), and black ethnicity (β = −0.967; 95% CI −1.524 to −0.410) exhibited a negative association.
Conclusions
We found independent associations between certain dietary patterns and physical activity with total cholesterol. Increasing physical activity might achieve cardiovascular and other health benefits in HIV-positive individuals. The clinical relevance of the identified dietary patterns requires further investigation in prospective cohort studies and randomized controlled trials.
Keywords: aging, diet, HIV, physical activity, total cholesterol
Although life expectancy in human immunodeficiency virus (HIV)-positive people on antiretroviral therapy (ART) has improved worldwide in recent years, it remains shorter in comparison with the general population [1].
Total cholesterol is a well established and low-cost marker of coronary heart disease (CHD), even across cultures [2]. A recent systematic review and meta-analysis showed that total cholesterol is a strong risk factor for CHD in the general population, with 1-mmol/L increase in total cholesterol associated with 20% higher risk of CHD in women and 24% in men [3].
Both HIV infection per se and its treatment may be associated with high total cholesterol [4]. Certain ART agents are associated with a range of metabolic abnormalities—including HIV lipodystrophy, dyslipidemia, diabetes mellitus, and insulin resistance [5]—and contribute to the elevated risk of cardiovascular disease (CVD) [6]. In addition, individuals who are HIV positive generally tend to have a higher rate of traditional risk factors for CVD such as smoking and hyperlipidemia [7].
Although statins can significantly reduce total cholesterol, their use might present a challenge for HIV-positive patients due to drug-drug interactions with ART [8] and in light of the main treatment goal to achieve viral suppression, which often requires change of regimen. Moreover, older patients who are HIV positive have an elevated risk of polypharmacy [9].
All this makes understanding the contribution of behavioral factors like dietary habits and physical activity a pertinent task which carries a possible prevention potential [10, 11], because both are modifiable risk factors. Although current guidelines encourage the implementation of lifestyle changes before statins initiation for CVD prevention [12], no empirical data about the association of diet and exercise with total cholesterol in aging HIV-positive individuals are available.
Therefore, the main aims of this study were as follows: (1) describe the predictors of total cholesterol in an aging HIV-positive population living in a resource-rich setting; (2) describe the major dietary patterns of this population; (3) and examine whether behavioral determinants such as dietary patterns and physical activity independently correlate with total cholesterol after adjustment for highly active ART, lipid-lowering drugs, and other confounders.
METHODS
Swiss Human Immunodeficiency Virus Cohort Study
The Swiss HIV Cohort Study (SHCS) is a large, ongoing, multicenter cohort study of HIV-positive individuals with a prospective recruitment since 1988 [13]. During the biannual outpatient visits, comprehensive clinical and behavioral data (including leisure physical activity) are collected. The patients described in this study are all enrolled in the SHCS nested project “Metabolism and Aging”, which includes participants ≥45 years of age undergoing dual energy x-ray absorptiometry scan, neurocognitive testing, and in the 2 centers of Zurich and Geneva additionally coronary computed tomography scan. For this project, Metabolic and Aging study participants of the Zurich, Berne, and Geneva centers were enrolled. Ethical approval of the SHCS and written informed consent from all participants were obtained upon cohort enrollment.
Dietary Assessment
Information on diet was obtained by a short and validated Food-Frequency Questionnaire (FFQ). This qualitative FFQ was originally used in the large INTERHEART study conducted in 52 countries [14] and was administered thereafter in several other large international studies [15, 16]. In this questionnaire, participants are asked, “In the last 12 months, how often did you consume foods from each of the following categories?” A list of 23 broad food categories is then presented, and the subject states the number of consumed items per month, week, or day. Because this FFQ was designed for use in international studies, it contains all of the main food groups, ie, dairy, meat, fish, fruits, and vegetables, and has been found to be applicable to different countries despite regional dietary differences [14]. Although we intentionally kept the original questionnaire intact to maintain its validity, we added a short supplementary part that gathered additional information about the following: (1) dietary supplements (vitamins, minerals, etc); (2) coffee and tea consumption; and (3) major dietary change in the last 2 years. Data about alcohol consumption are regularly gathered in the SHCS. The FFQ interviews were conducted by phone by a trained staff and were collected using the REDCap electronic data capture tool [17] hosted at University Hospital Zürich.
Statistical Analysis
To assess the dietary patterns, first, all food items were converted into daily consumption. Then, Ward error sum of squares hierarchical clustering method [18, 19] was used with 1-Kendall’s tau correlation matrix as an input. Based on the classification output, the 4 largest and most general food clusters were extracted, and for each person the sum of the consumption frequency of food items that belong to each of the 4 clusters was calculated separately. In other words, each person received 4 score variables (pattern I to pattern IV) that reflected the cumulative consumption frequency of the food items for each pattern.
Linear mixed-effects model with per center random intercept was used to assess the correlation between dietary patterns and total cholesterol. The closest available total cholesterol measurement to the dietary assessment was used regardless of fasting state. Fasting state during the measurement was adjusted for in the multivariable models. Two multivariable models were explored: (1) the minimally adjusted model, only for age, sex, risk group, physical activity, ART, lipid-lowering drugs, and fasting state; and (2) the model that was adjusted for the covariates in model I plus additional demographical and clinical covariates (ethnicity, university education, living alone, smoking, depression, diabetes, hypertension, number of years on ART, recent dietary change, multivitamin supplement, Omega 3 supplement, protein powder supplement) (Figure 4). Because the cumulative consumption scores were skewed to the right, they were ln(x+1) transformed. Benjamini-Hochberg adjustment for multiple testing was performed for the correlation matrix (Figure 2) but not for the multivariable analysis, due to limited power. Statistical analysis was performed with R (version 3.3.2).
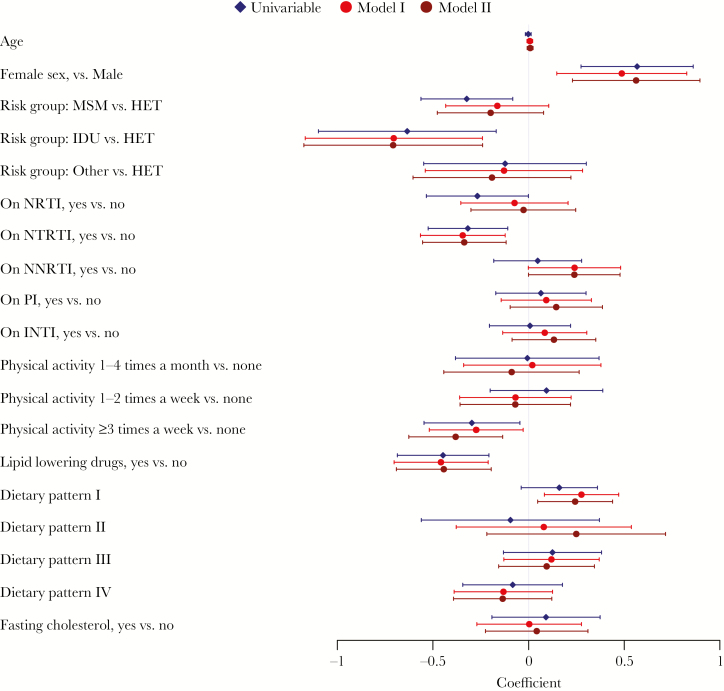
Figure 4.
Factors that correlate with total cholesterol, univariable and 2 linear mixed-effect models. Model I was adjusted for all the covariates in the figure. Model II was adjusted for all covariates in the figure and additionally for ethnicity, university education, living alone, smoking, depression, diabetes, hypertension, number of years on antiretroviral therapy, recent dietary change, multivitamin supplement, Omega 3 supplement, protein powder supplement. Dietary patterns consumption frequencies were ln(x+1) transformed. Pattern I: meat, refined/milled grains, carbonated beverages, coffee. Pattern II: organ meats, poultry, fish/seafood, alcohol. Pattern III: whole grains, dairy products, eggs, leafy green vegetables, other vegetables (raw and cooked), legumes/nuts/seeds, potatoes, boiled/mashed, pickled food, fruits, tea (black/green). Pattern IV: pizza, deep fried foods, salty snacks, ice cream/pudding, desserts/sweet snacks, confectionary sugars/syrups, fruit juice/drinks. Abbreviations: ART, antiretroviral therapy; HET, heterosexual; IDU, injecting drug users; INTI, integrase inhibitor; MSM, men who have sex with men; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; NTRTI, nucleotide reverse-transcriptase inhibitor; PI, protease inhibitor.
Figure 2.
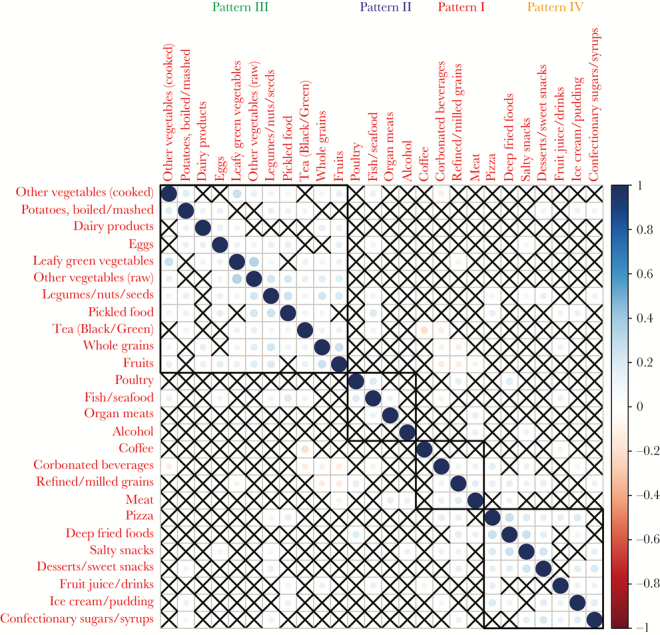
Correlation matrix of the examined food items. Squares with “X” are not statistically significant after adjustment for multiple testing.
RESULTS
Patients
In total, from June 2016 until October 2017, of 539 eligible participants 395 (73.3%) were interviewed for the study, the remaining either refused to participate (n = 31, 5.8%), could not be reached on at least 5 different occasions (n = 111, 20.6%), or provided incomplete response (n = 2). The median age of the participants was 55.7 years (interquartile range [IQR], 52.2–60.8), the majority were male (85.6%, 338 of 395), of white ethnicity (94.4%, 373 of 395), and belonged to the men-who-have-sex-with-men (MSM) risk group (59%, 233 of 395) (Table 1). A total of 11.6% (46 of 395) were obese and 32.4% (128 of 395) were overweight. The median time on ART was 14.2 years (IQR, 8.8–20.4), and the median most recent CD4 value was 648 cells/µL (IQR, 494.5–816.0). The vast majority of patients (95.9%, 379 of 395) were virally suppressed with HIV viral load below 50 copies/mL.
Table 1.
Patient Characteristics
| Characteristics | Overall |
|---|---|
| n | 395 |
| Age (median [IQR]) | 55.7 [52.2–60.8] |
| Sex, female (%) | 57 (14.4) |
| Ethnicity (%) | |
| White | 373 (94.4) |
| Black | 15 (3.8) |
| Hispanic | 7 (1.8) |
| Risk Group (%) | |
| Heterosexual | 108 (27.3) |
| Men who have sex with men | 233 (59.0) |
| Injecting drug users | 24 (6.1) |
| Other | 30 (7.6) |
| University education, yes (%) | 55 (13.9) |
| Living alone, yes (%) | 162 (41.0) |
| Current smoking, yes (%) | 126 (31.9) |
| BMI (%) | |
| Normal (≥18.5–25) | 212 (53.7) |
| Overweight (≥25–30) | 128 (32.4) |
| Obese (≥30) | 46 (11.6) |
| Underweight (<18.5) | 9 (2.3) |
| Depression, yes (%) | 59 (14.9) |
| Diabetes, yes (%) | 23 (5.8) |
| Hypertension, yes (%) | 135 (34.2) |
| Lipid-lowering drugs, yes (%) | 98 (24.8) |
| Virally suppressed, yes (%) | 379 (95.9) |
| CD4 (median [IQR]) | 648.0 [494.5–816.0] |
| Years on ART (median [IQR]) | 14.2 [8.8–20.4] |
| On NRTI, yes (%) | 320 (81.0) |
| On NTRTI, yes (%) | 192 (48.6) |
| On NNRTI, yes (%) | 117 (29.6) |
| On PI, yes (%) | 107 (27.1) |
| On INTI, yes (%) | 227 (57.5) |
| Physical activity (%) | |
| Never | 124 (31.4) |
| 1–4 times a month | 40 (10.1) |
| 1–2 times a week | 81 (20.5) |
| ≥3 times a week | 150 (38.0) |
| Dietary Change in the Last 2 Years (%) | |
| No | 360 (91.1) |
| Became vegetarian | 3 (0.8) |
| Other change | 32 (8.1) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; INTI, integrase inhibitor; IQR, interquartile range; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; NTRTI, nucleotide reverse-transcriptase inhibitor; PI, protease inhibitor.
Dietary Patterns
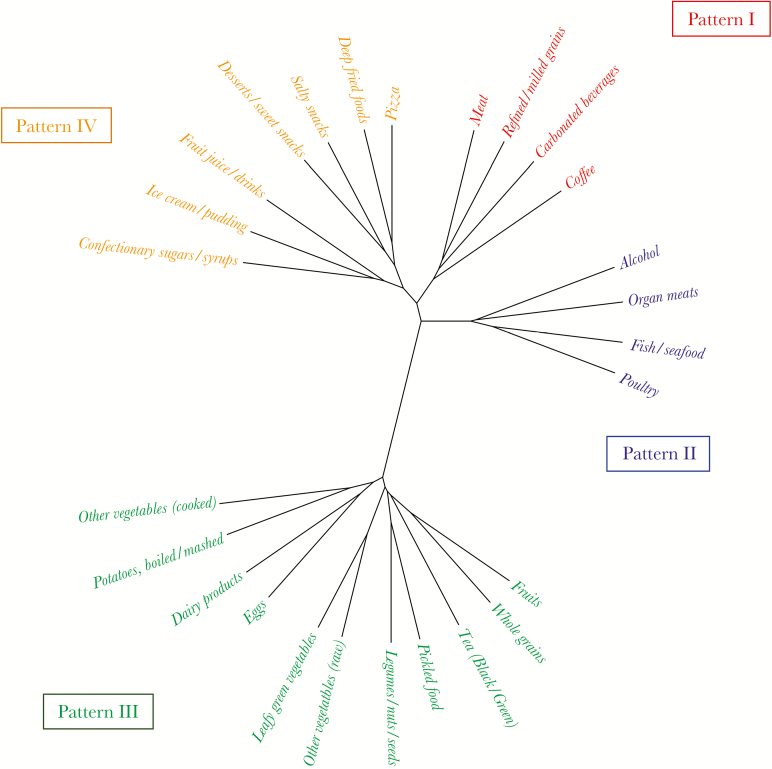
First, we aimed to explore which food groups correlate and cluster with each other. In other words, when patients consume one food group, which other food groups they are likely to consume as well (positive association) or on the other hand to avoid (negative association). Hierarchical clustering analysis suggested 4 distinct metapatterns (Figure 1, Figure 2): (Pattern I) “Meat,” “Refined/ milled grains,” “Carbonated beverages,” “Coffee” - This pattern positively correlated with smoking, with 21.4% (22 of 103) of smokers in the lowest quartile and 45.8% (44 of 96) of smokers in the highest quartile (Fisher’s exact test, P < .001) (Supplementary Table 1); (Pattern II) “Organ meats,” “Poultry,” “Fish/seafood,” “Alcohol” - Pattern II negatively correlated with female sex, with 26.7% (27 of 101) females in the lowest quartile and 8.2% (8 of 97) in the highest consumption quartile (Fisher’s exact test, P = .001) (Supplementary Table 2); (Pattern III) “Whole grains,” “Dairy products,” “Eggs,” “Leafy green vegetables,” “Other vegetables (raw),” “Other vegetables (cooked),” “Legumes/nuts/seeds,” “Potatoes, boiled/mashed,” “Pickled food,” “Fruits,” “Tea (Black/Green)” - Pattern III was positively associated with university education with 9.1% (9 of 99) having university education in the lowest quartile versus 22.2% (22 of 99) in the highest (Fisher’s exact test, P = .018) and was negatively associated with smoking with 45.5% (45 of 99) smokers in the lowest quartile and 20.2% (20 of 99) in the highest consumption quartile (Fisher’s exact test, P < .001) (Supplementary Table 3); (Pattern IV) “Pizza,” “Deep fried foods,” “Salty snacks,” “Ice cream/pudding,” “Desserts/sweet snacks,” “Confectionary sugars/syrups,” “Fruit juice/drinks” - This pattern was negatively associated with diabetes, hypertension, and lipid-lowering drugs (Fisher’s exact test, P = .016, .011, and .014, respectively), suggesting that patients diagnosed with these conditions tend to avoid junk food (Supplementary Table 4).
Figure 1.
Four main dietary patterns as determined by hierarchical clustering.
Noteworthy pairwise negative associations (Figure 2) were as follows: patients who consumed fruits more frequently consumed less frequently meat, refined/milled grains, and carbonated beverages. Coffee and tea were also negatively correlated (Kendall’s tau −0.19, adjusted P < .001), suggesting that when it comes to regular consumption, some patients tend to prefer either one or the other. Next, we explored separately the frequency of meat consumption; only 3.3% (13 of 395) of the participants reported to completely avoid meat and organ meats, and only 5 patients were lacto-ovo-vegetarian (1.3%, 5 of 395) avoiding meat, organ meats, poultry, and fish.
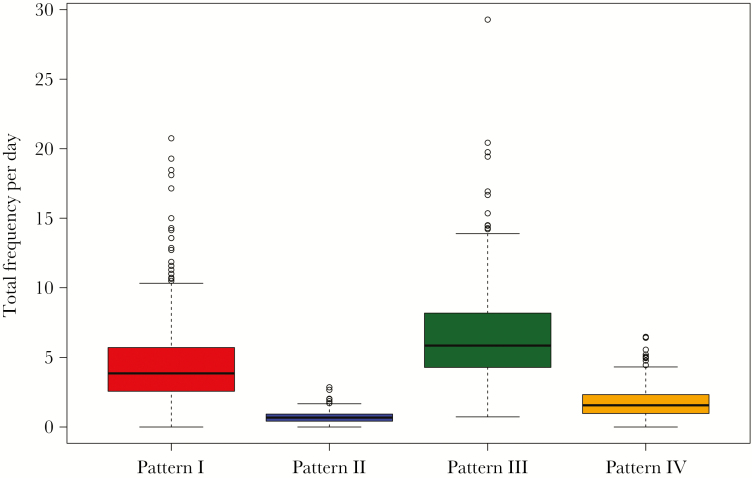
Examining the total daily consumption frequency stratified by pattern (Figure 3) shows that the most frequently consumed pattern is the apparently healthier pattern III (mean = 6.5, standard deviation [sd] = 3.3), followed by pattern I (mean = 4.6, sd = 3.1), pattern IV (mean = 1.8, sd = 1.1), and pattern II (mean = 0.7, sd = 0.4).
Figure 3.
Boxplot of total daily consumption stratified by dietary patterns. Pattern I: meat, refined/milled grains, carbonated beverages, coffee. Pattern II: organ meats, poultry, fish/seafood, alcohol. Pattern III: whole grains, dairy products, eggs, leafy green vegetables, other vegetables (raw and cooked), legumes/nuts/seeds, potatoes, boiled/mashed, pickled food, fruits, tea (black/green). Pattern IV: pizza, deep fried foods, salty snacks, ice cream/pudding, desserts/sweet snacks, confectionary sugars/syrups, fruit juice/drinks.
Physical Activity
Thirty-one percent (124 of 395) of the patients reported not to perform any leisure physical activity. Ten percent (40 of 395) reported physical activity frequency of 1 to 4 times a month, 21% (81 of 395) reported physical activity 1 to 2 times a week, and the largest group (38%, 150 of 395) reported physical activity 3 or more times per week.
Total Cholesterol
The median total cholesterol value was 5 mmol/L (sd = 1.1). Forty percent (158 of 395) of participants were above the cutoff of 5.2 mmol/L, and 13.1% (51 of 395) were above the cutoff of 6.2 mmol/L (Supplementary Figure 1). Lipid-lowering drugs were prescribed in 24.8% of the participants (98 of 395).
Factors Associated With Total Cholesterol
Next, we examined the factors that correlate with total cholesterol. In the first multivariable model (Model I; Figure 4), physical activity at least 3 times a week compared with none (β = −0.274; 95% confidence interval [CI], −0.519 to −0.029), lipid-lowering drugs (β = −0.458; 95% CI, −0.703 to −0.212), ART including a nucleotide reverse-transcriptase inhibitor (NtRTI) (β = −0.344; 95% CI, −0.565 to −0.123), and intravenous drug users (IDU) risk group (β = −0.705; 95% CI, −1.168 to −0.242) were negatively correlated with total cholesterol. On the other hand, female sex (β = 0.487; 95% CI, 0.147 to 0.827) and dietary pattern I (“Meat,” “Refined/ milled grains,” “Carbonated beverages,” “Coffee”)—but not the other 3 patterns—positively correlated with total cholesterol (β = 0.276; 95% CI, 0.082–0.471; P = .007).
After additionally adjusting for a wide range of demographic and clinical covariates (Model II; Figure 4), lipid-lowering drugs (β = −0.443; 95% CI, −0.691 to −0.196), NtRTI (β = −0.336; 95% CI, −0.554 to −0.118), and regular physical activity (β = −0.381; 95% CI, −0.626 to −0.136) remained negatively correlated with total cholesterol, in addition to black ethnicity (β = −0.967; 95% CI, −1.524 to −0.410), IDU risk group (β = −0.708; 95% CI, −1.175 to −0.241), living alone (β = −0.236; 95% CI, −0.436 to −0.035), and taking a multivitamin (β = −0.378; 95% CI, −0.730 to −0.027). On the other hand, female sex (β = 0.562; 95% CI, 0.229–0.896) and dietary pattern I (β = 0.243; 95% CI, 0.047–0.439; P = .02) remained positively and significantly correlated with total cholesterol even after adjustment for the additional covariates in Model II.
Sensitivity Analysis
As a sensitivity analysis, we repeated the multivariable analysis (Model I excluding lipid-lowering drugs) separately for patients on and off lipid-lowering drugs (Supplementary Table 5). For patients on lipid-lowering drugs, frequent physical activity (β = −0.732; 95% CI, −1.166 to −0.232), NtRTI (β = −0.546; 95% CI, −0.980 to −0.076), and IDU risk group (β = −1.148; 95% CI, −2.023 to −0.278) negatively correlated with total cholesterol. None of the covariates, including dietary pattern I (β = 0.352; 95% CI, −0.039 to 0.747; P = .115), significantly positively correlated with total cholesterol among patients on lipid-lowering drugs; however, the limited statistical power of this subgroup analysis should be taken into account.
Among patients not on lipid-lowering drugs, none of the covariates were significantly negatively associated with total cholesterol. On the other hand, dietary pattern I and female sex remained significantly positively correlated with total cholesterol in people not on lipid-lowering drugs (β = 0.234, 95% CI = 0.011–0.457, P = .047 and β = 0.574, 95% CI = 0.198–0.949, P = .004, respectively).
High-Density Lipoproteins and Triglycerides
Finally, to obtain a more complete picture on factors associated with dyslipidemia in general, we also explored the fully adjusted model with high-density lipoproteins (HDL) and triglycerides as outcomes. For HDL, age (β = 0.012; 95% CI, 0.006–0.019) and female sex (β = 0.474; 95% CI, 0.331–0.615) showed a positive association, whereas having hypertension (β = −0.132; 95% CI, −0.227 to −0.036) and ART regimen with protease inhibitors (β = −0.121; 95% CI, −0.224 to −0.018) exhibited a negative correlation. None of the remaining variables, including physical activity and the examined 4 dietary patterns, showed any significant correlation. For triglycerides, diabetes (β = 0.730; 95% CI, 0.144–1.367), hypertension (β = 0.371; 95% CI, 0.045–0.690), nonnucleoside reverse-transcriptase inhibitors (β = 0.480; 95% CI, 0.131–0.822), protease inhibitors (β = 0.695; 95% CI, 0.342–1.038), and integrase inhibitors (β = 0.589; 95% CI, 0.259–0.891) showed positive association, whereas age (β = −0.024; 95% CI, −0.045 to −0.001), female sex (β = −0.554; 95% CI, −1.009 to −0.047), MSM risk group (β = −0.455; 95% CI, −0.836 to −0.036), IDU risk group (β = −1.213; 95% CI, −1.886 to −0.540), living alone (β = −0.332; 95% CI, −0.630 to −0.051), and physical activity of at least 3 times per week (β = −0.389; 95% CI, −0.764 to −0.058) were negatively associated with triglycerides. In contrast to physical activity, none of the dietary patterns exhibited a significant association with triglycerides in our fully adjusted model.
DISCUSSION
In this analysis of HIV-positive individuals ≥45 years old predominantly on suppressive ART, we found several factors that were independently correlated with total cholesterol: female sex and the frequency of combined consumption of meat, refined/milled grains, carbonated beverages, and coffee (dietary pattern I) showed positive correlation. On the other hand, regular physical activity, lipid-lowering drugs, an ART regimen containing tenofovir, and black ethnicity exhibited a negative correlation. In the analysis restricted to patients not on lipid-lowering drugs, total cholesterol remained associated with dietary pattern I (meat, refined/milled grains, carbonated beverages, coffee), supporting current guidelines that encourage implementation of lifestyle and dietary changes before statin initiation [12]. In participants on lipid-lowering drugs, there was an additional benefit of physical activity, beyond the effect of lipid-lowering drugs.
It has been previously shown that people living with HIV consume more saturated fat and cholesterol compared with HIV-negative individuals [20]. It was also demonstrated that tackling HIV dyslipidemia with diet is feasible [10]. In a systematic review and meta-analysis of the effects of dietary intervention on HIV dyslipidemia, dietary intervention reduced total cholesterol by −0.36 mmol/L compared with placebo/control [21]. However, no study was performed on a predominantly aging HIV-positive population.
Several previous studies in the general population demonstrated associations between individual food items that comprise pattern I in our study and total cholesterol or cardiovascular risk. In a prospective cohort study of 31546 high-risk individuals from 40 countries, meat, poultry, and egg consumption were independently correlated with composite outcome of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for congestive heart failure [15]. Although in our analysis meat indeed clustered in pattern I, poultry and eggs clustered in different patterns.
Our results are biologically plausible because most meat products contain saturated fat that increases cholesterol [22]. In addition, almost all meat products contain cholesterol by itself [23, 24]. Frequency of coffee consumption also clustered with pattern I and is also supported by previous works. In a meta-analysis of 14 clinical trials, a dose-response relation between coffee consumption and both total cholesterol and low-density lipoprotein cholesterol was identified [25]. The effect was weak for filtered coffee; however, most of the coffee that is consumed in Switzerland is unfiltered.
The observation that pattern III that contained vegetables, legumes, and fruits positively correlated with higher education and negatively correlated with smoking is consistent with the well established association between education and health literacy [26]. This might also suggest that patients with low education could benefit most from dietary counseling.
The beneficial effect of regular exercise on cholesterol is well established. For example, in a randomized controlled trial from the UK, in which participants were asked to maintain their normal dietary habits, high-intensity exercise program for 24 weeks resulted in significant decreases in total cholesterol (mean change, −0.55 ± 0.81 mmol/L) [27]. Forty percent of our sample reported physical activity of 1 to 4 times a month or never, hence not meeting the World Health Organization (WHO) guidelines of 150 minutes of moderate-intensity aerobic physical activity throughout the week [28]. This suggests that there is considerable room for improvement in this area, especially because the benefits of physical activity extend beyond cardiovascular risk factors and also include prevention of dementia [29], depression, and anxiety [30]. Data about the benefits of physical activity for HIV dyslipidemia is scarce [31], especially in aging HIV-positive population.
The strengths of our study include the use of a population-based cohort, the SHCS, with a large array of prospectively collected clinical and laboratory data, enabling us to adjust for many important clinical and demographic potential confounders. Patients from 3 different study centers across Switzerland were included, hence increasing generalizability. Moreover, nutrition data were assessed by a well validated questionnaire used in large studies in the general population.
Our study also has limitations. Inherent to cross-sectional studies, there is a lack of temporality [32]. Due to the observational study design, and in light of the fact that there are many potential confounders associated with dietary patterns, physical activity and total cholesterol levels, we cannot rule-out residual confounding for which we could not account. Another limitation is that the FFQ measured frequency but not quantity of consumption [15]. Finally, due to collinearity between food items within the same consumption pattern, it was not possible to examine the effect of single food items. Nevertheless, our results are in line with large cohort studies, randomized controlled trials, and meta-analysis [15, 25, 27].
Future studies are needed to illuminate the effect of type of fat intake (saturated fat, monounsaturated fat, and polyunsaturated fat) on dyslipidemia and on the association between dietary patterns and hard endpoints such as cardiovascular morbidity and mortality. The most beneficial type of physical activity in terms of adherence and cardiovascular benefit for this population is also yet to be determined.
CONCLUSIONS
In summary, our cross-sectional study suggests that there are independent associations between certain dietary patterns and physical activity with total cholesterol. Physical activity should be substantially scaled up in this population to meet WHO guidelines and to achieve cardiovascular and other health benefits. The reported dietary patterns pave the way for further investigations in prospective cohort studies and randomized controlled trials.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Members of the Swiss HIV Cohort Study
V. Aubert, M. Battegay, E. Bernasconi, J. Böni, D. L. Braun, H. C. Bucher, A. Calmy, M. Cavassini, A. Ciuffi, G. Dollenmaier, M. Egger, L. Elzi, J. Fehr, J. Fellay, H. Furrer (Chairman of the Clinical and Laboratory Committee), C. A. Fux, H. F. Günthard (President of the SHCS), D. Haerry (Deputy of “Positive Council”), B. Hasse, H. H. Hirsch, M. Hoffmann, I. Hösli, C. Kahlert, L. Kaiser, O. Keiser, T. Klimkait, R. D. Kouyos, H. Kovari, B. Ledergerber, G. Martinetti, B. Martinez de Tejada, C. Marzolini, K. J. Metzner, N. Müller, D. Nicca, G. Pantaleo, P. Paioni, A. Rauch (Chairman of the Scientific Board), C. Rudin (Chairman of the Mother & Child Substudy), A. U. Scherrer (Head of Data Centre), P. Schmid, R. Speck, M. Stöckle, P. Tarr, A. Trkola, P. Vernazza, G. Wandeler, R. Weber, S. Yerly.
Acknowledgments
We thank Professors Mahshid Dehghan and Salim Yusuf and McMaster University for the Food-Frequency Questionnaire.
Financial support. This study was funded within the framework of the Swiss HIV Cohort Study ([SHCS] project no. 801), supported by the Swiss National Science Foundation (grant no. 148522), and by the SHCS research foundation. The data are gathered by the Five Swiss University Hospitals, 2 Cantonal Hospitals, 15 affiliated hospitals, and 36 private physicians (listed in http://www.shcs.ch/180-health-care-providers).
Contributor Information
Swiss HIV Cohort Study:
V Aubert, M Battegay, E Bernasconi, J Böni, D L Braun, H C Bucher, A Calmy, M Cavassini, A Ciuffi, G Dollenmaier, M Egger, L Elzi, J Fehr, J Fellay, H Furrer, C A Fux, H F Günthard, D Haerry, B Hasse, H H Hirsch, M Hoffmann, I Hösli, C Kahlert, L Kaiser, O Keiser, T Klimkait, R D Kouyos, H Kovari, B Ledergerber, G Martinetti, B Martinez de Tejada, C Marzolini, K J Metzner, N Müller, D Nicca, G Pantaleo, P Paioni, A Rauch, C Rudin, A U Scherrer, P Schmid, R Speck, M Stöckle, P Tarr, A Trkola, P Vernazza, G Wandeler, R Weber, and S Yerly
References
- 1. Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS 2016; 11:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verschuren WM, Jacobs DR, Bloemberg BP, et al. . Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA 1995; 274:131–6. [PubMed] [Google Scholar]
- 3. Peters SA, Singhateh Y, Mackay D, et al. . Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: a systematic review and meta-analysis. Atherosclerosis 2016; 248:123–31. [DOI] [PubMed] [Google Scholar]
- 4. Lo J. Dyslipidemia and lipid management in HIV-infected patients. Curr Opin Endocrinol Diabetes Obes 2011; 18:144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sculier D, Toutous-Trellu L, Verolet C, et al. . Lipohypertrophy and metabolic disorders in HIV patients on antiretroviral therapy: a systematic multidisciplinary clinical approach. J Int AIDS Soc 2014; 17(4 Suppl 3):19559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stein JH, Hsue PY. Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA 2012; 308:405–6. [DOI] [PubMed] [Google Scholar]
- 7. Vachiat A, McCutcheon K, Tsabedze N, et al. . HIV and ischemic heart disease. J Am Coll Cardiol 2017; 69:73–82. [DOI] [PubMed] [Google Scholar]
- 8. Aberg JA, Gallant JE, Ghanem KG, et al. . Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–34. [DOI] [PubMed] [Google Scholar]
- 9. Gleason LJ, Luque AE, Shah K. Polypharmacy in the HIV-infected older adult population. Clin Interv Aging 2013; 8:749–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lazzaretti RK, Kuhmmer R, Sprinz E, et al. . Dietary intervention prevents dyslipidemia associated with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected individuals: a randomized trial. J Am Coll Cardiol 2012; 59:979–88. [DOI] [PubMed] [Google Scholar]
- 11. Loonam CR, Mullen A. Nutrition and the HIV-associated lipodystrophy syndrome. Nutr Res Rev 2012; 25:267–87. [DOI] [PubMed] [Google Scholar]
- 12. EACS Guidelines. Available at: http://www.eacsociety.org/guidelines/eacs- guidelines/eacs-guidelines.html. Accessed 14 December 2017. [Google Scholar]
- 13. Schoeni-Affolter F, Ledergerber B, Rickenbach M, et al. . Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol 2010; 39:1179–89. [DOI] [PubMed] [Google Scholar]
- 14. Iqbal R, Anand S, Ounpuu S, et al. . Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation 2008; 118:1929–37. [DOI] [PubMed] [Google Scholar]
- 15. Dehghan M, Mente A, Teo KK, et al. . Relationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31546 high-risk individuals from 40 countries. Circulation 2012; 126:2705–12. [DOI] [PubMed] [Google Scholar]
- 16. Smyth A, Dehghan M, O’Donnell M, et al. . Healthy eating and reduced risk of cognitive decline: a cohort from 40 countries. Neurology 2015; 84:2258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Thielke R, et al. . Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ward JH. Hierarchical grouping to optimize an objective function. J Am Stat Assoc 1963; 58:236–44. [Google Scholar]
- 19. Murtagh F, Legendre P. Ward’s hierarchical agglomerative clustering method: which algorithms implement Ward’s criterion?J Classif 2014; 31:274–95. [Google Scholar]
- 20. Joy T, Keogh HM, Hadigan C, et al. . Dietary fat intake and relationship to serum lipid levels in HIV-infected patients with metabolic abnormalities in the HAART era. AIDS 2007; 21:1591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stradling C, Chen YF, Russell T, et al. . The effects of dietary intervention on HIV dyslipidaemia: a systematic review and meta-analysis. PLoS One 2012; 7:e38121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clarke R, Frost C, Collins R, et al. . Dietary lipids and blood cholesterol: quantitative meta-analysis of metabolic ward studies. BMJ 1997; 314:112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chizzolini R, Zanardi E, Dorigoni V, Ghidini S. Calorific value and cholesterol content of normal and low-fat meat and meat products. Trends Food Sci Technol 1999; 10:119–28. [Google Scholar]
- 24. Piironen V, Toivo J, Lampi AM. New data for cholesterol contents in meat, fish, milk, eggs and their products consumed in Finland. J Food Comp Anal 2002; 15:705–13. [Google Scholar]
- 25. Jee SH, He J, Appel LJ, et al. . Coffee consumption and serum lipids: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol 2001; 153:353–62. [DOI] [PubMed] [Google Scholar]
- 26. Zimmerman EB, Woolf SH, Haley A.. Understanding the Relationship Between Education and Health: a Review of the Evidence and an Examination of Community Perspectives | Agency for Healthcare Research & Quality. 2015. Available at: https://www.ahrq.gov/professionals/education/curriculum-tools/population-health/zimmerman.html. Accessed 14 December 2017. [Google Scholar]
- 27. O’Donovan G, Owen A, Bird SR, et al. . Changes in cardiorespiratory fitness and coronary heart disease risk factors following 24 wk of moderate- or high-intensity exercise of equal energy cost. J Appl Physiol (1985) 2005; 98:1619–25. [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization. Physical Activity and Adults. Available at: http://www.who.int/dietphysicalactivity/factsheet_adults/en/. Accessed 10 October 2017. [Google Scholar]
- 29. Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med 2009; 39:3–11. [DOI] [PubMed] [Google Scholar]
- 30. Rebar AL, Stanton R, Geard D, et al. . A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev 2015; 9:366–78. [DOI] [PubMed] [Google Scholar]
- 31. Mendes EL, Ribeiro Andaki AC, Brito CJ, et al. . Beneficial effects of physical activity in an HIV-infected woman with lipodystrophy: a case report. J Med Case Rep 2011; 5:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hill AB. The environment and disease: association or causation?Proc R Soc Med 1965; 58:295–300. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.