Abstract
The specific spatial distribution and habitat association—strongly influenced by environmental factors or competitive interactions—are major issues in ecology and conservation. We located and georeferenced nesting sites of five cliff-nesting raptors (Egyptian vulture Neophron percnopterus [a locally extinct species], common buzzard Buteo buteo, osprey Pandion haliaetus, common kestrel Falco tinnunculus, Barbary falcon Falco peregrinus pelegrinoides), and common raven Corvus corax on one of the most biodiverse hotspot within the Canary Islands (Teno, Tenerife). We used generalized linear models to evaluate the factors affecting abundance, richness, and intra- and interspecific interactions. Raptor abundance increased with slope, shrub-covered area, and habitat diversity, and decreased with altitude, and forested and grassed areas. Richness increased with slope and decreased with altitude. Threatened species (osprey, Barbary falcon, and raven) occupied cliffs farther away from houses and roads, and more rugged areas than the non-threatened species. The models suggested that the probability of cliff occupation by buzzards, falcons, and ravens depended only on inter-specific interactions. Buzzard occupation increased with the distance to the nearest raven and kestrel nests, whereas falcons and ravens seek proximity to each other. Teno holds between 75% and 100% of the insular breeding populations of the most endangered species (osprey and raven), indicating the high conservation value of this area. Our study suggests that the preservation of rugged terrains and areas of low human pressure are key factors for raptor conservation and provide basic knowledge on the community structure and habitat associations to develop appropriated management actions for these fragile island populations.
Keywords: Canary Islands, competitive interactions, human disturbances, habitat, threatened species
Understanding the patterns and processes underlying the structure of communities and its habitat association are major issues in ecology and conservation biology (Morrison et al. 2006). Ecologically, the spatial distribution and habitat associations of animals result from the combination of environmental factors (i.e., either biotic or abiotic) and intrinsic processes related to population dynamics and intra- and interspecific interactions (Martin 2001; Preston et al. 2008). Given its position in the food chain, top predators are highly sensitive to these factors and processes, making of them an excellent model to study factors affecting spatial distribution of animals.
As predators, raptors often exist at low densities, exhibit low reproductive rates, and require large foraging areas and healthy prey populations (Newton 1979). Thus, population demography can be modulated by inter- and intraspecific interactions such as competition for food resources or nesting sites (Katzner et al. 2003; Hakkarainen et al. 2004; Martínez et al. 2008); and usually, large species are very sensitive to human disturbances during breeding so they seek refuge in rugged, isolated, or protected areas (Richardson and Miller 1997; Rodríguez et al. 2013; Krüger et al. 2015). They are considered umbrella species as their protection acts upon many other species, so areas with high density of breeding raptors support higher biodiversity levels than ones with low density of raptors (Murphy and Noon 1992; Sergio et al. 2008). For all these reasons, raptors are usually used by conservationists for environmental awareness campaigns (Chiweshe 2007; Curti and Valdez 2009), in the planning of protected areas (Dunk et al. 2006), as bioindicators of environmental health (Rodríguez-Estrella et al. 1998; Hilty and Merenlender 2000) or as surrogates of biodiversity (Burgas et al. 2014).
In general, oceanic islands hold less diverse communities than mainland areas (Whittaker and Fernández-Palacios 2007). For raptors, diversity on islands is frequently high in comparison with other taxonomic groups because their good flight abilities allow them colonizing remote islands, but as top predators, they also need particular ecological conditions for the settlement to be successful (Donázar et al. 2005). Island raptor populations usually present several life-history traits of the so-called “insular syndrome”, that is, density compensation, wider niche breath, lower breeding rates, higher survival, or lack of migratory behavior (Thibault et al. 1992; Thiollay 1998; Donázar et al. 2002; Carrillo and González-Dávila 2009; Sanz-Aguilar et al. 2015). Small and isolated insular populations are at a high risk of extinction due to environmental, stochastic, demographic (e.g., inbreeding, low breeding rates, or too little immigration), or human-related threats (McKinney 1997; Lande 1998; White and Kiff 2000; Bretagnolle et al. 2004; Donázar et al. 2005). On the Macaronesian archipelagos, raptor populations seem to be more prone to disappear on islands with high human density due to higher frequency of fatalities with artificial structures, direct persecution, or habitat alteration (Donázar et al. 2005; Rodríguez et al. 2010a; Hille and Collar 2011). Hence, current rates of bird of prey extinction for the Canarian and Cape Verde archipelagos are 29% and 43%, respectively (Donázar et al. 2005). Only some raptor populations, mainly the most threatened, are systematically monitored on the Macaronesian archipelagos (Donázar et al. 2002; Palma et al. 2004; Siverio 2006; Gangoso et al. 2015), and quantitative information on raptor communities, their distributions, and habitat associations are scarce (but see Gangoso 2006). In fact, only a few studies quantifying breeding habitat features or nest characteristics of particular species are available for the Canary Islands (see Carrillo and González-Dávila 2005; Gangoso 2006; Rodríguez and Siverio 2006; Rodríguez et al. 2007, 2010b, 2013; Gangoso et al. 2015).
In this paper, we survey the cliff-nesting raptor community of Teno massif (hereafter Teno) situated in Tenerife (Canary Islands), that constitute one of the most biodiverse protected areas within the European Union (Sundseth 2005), where still many endangered and/or exclusive species of plants and animals (land snails, insects, lizards, and birds) persist (Reyes-Betancort et al. 2008; Martín 2010; Rodríguez et al. 2014). We provide population sizes, compare breeding habitat features among species, and analyze factors affecting abundance and diversity of six species of raptors and the common raven. By doing that, we assess the factors making this protected area a significant wildlife refuge of international significance (Sundseth 2005). This basic knowledge on particular island populations is essential to develop effective conservation and management actions to them due to the aforementioned differences in life-history traits respect to their mainland counterparts (Sutherland et al. 2004; Whittingham et al. 2007).
Materials and Methods
Study area
The Canary Islands are a volcanic archipelago located 100 km off the Atlantic coast of north-west Africa and comprised of seven major islands. Tenerife Island is the largest one (2,034 km2 and up to 3,718 m a.s.l.), and is situated in the central part of the archipelago (28°20.00′N–16°51.04′W, Figure 1). The study was conducted in Teno, a rugged mountainous zone characterized by big seacliffs and deep ravines located in the northwest of Tenerife (about 146 km2 including some near coastal areas and an altitudinal range of 0–1,350 m, Figure 1). The majority of this area is protected and cataloged as Rural Park under the Canary Islands environment law. The area holds a great diversity of vegetation influenced by north-easterly humid trade winds, altitude, and orientation (Del Arco et al. 2006). The coastal zones are covered by sparse and xeric vegetation, by plantations (especially banana) or human settlements. Three types of forest associated to climatic and geographic characteristics occur at different altitudes and orientations: 1) the endangered thermophilous forest at up to 200 m a.s.l. in slopes oriented to the North and between 500 and 900 m a.s.l. in the Southern ones, 2) the laurel forest in the North faces at 350–1,300 m a.s.l., and 3) small representations of pine woodlands at the highest elevations (Del Arco et al. 2006). Some areas at high altitudes have been deforested to create pasture lands for domestic livestock, mainly goats (Rodríguez et al. 2014).
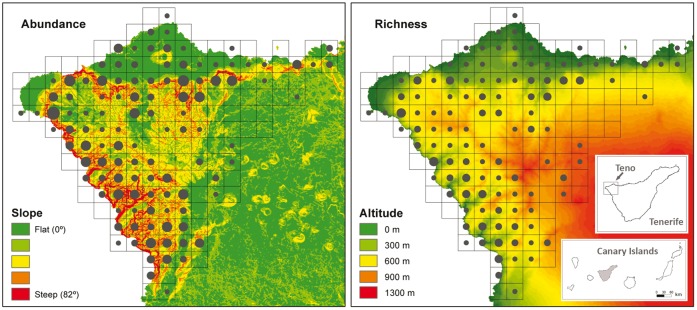
Figure 1.
Abundance (number of nesting-sites) and richness (number of species) of cliff-nesting raptors and the common raven in Teno, Tenerife, Canary Islands, according to 1 × 1 km grid. Abundance ranged between 0 (without point) and to 8 pairs (the largest points) and richness between 0 (without point) to 4 species (the largest points).
Target species
The Canarian diurnal breeding raptor community is currently composed by 7 species, the majority of them considered endemic subspecies (Egyptian vulture Neophron percnopterus majorensis, common buzzard Buteo buteo insularum, Macaronesian sparrowhawk Accipiter nisus granti, osprey Pandion haliaetus, common kestrel Falco tinnunculus [subspecies canariensis and dacotiae], Eleonora’s falcon Falco eleonorae and Barbary falcon Falco peregrinus pelegrinoides). The red kite Milvus milvus also bred on the archipelago, but became extinct in the 1960s (Madroño et al. 2004; Rodríguez et al. 2014). At least 2 breeding attempts of the black kite Milvus migrans have been also recently recorded on Gran Canaria (Trujillo 2009). With the exception of the Egyptian vulture (extinct since 1985 on the island) and the Eleonora’s falcon, all of these species breed regularly on Tenerife (Lorenzo 2007). The breeding raptor community of Teno is currently composed by five sedentary species: four basically cliff nesters (Table 1) and the Macaronesian sparrowhawk, excluded from this study since it is an obligate tree-nester. Given the Canarian common raven Corvus corax canariensis is a strict cliff nester in Tenerife (Siverio et al. 2007), we considered it as an ecological equivalent of raptors in our analyses. Finally, the recognizable nest sites of the extinct Egyptian vulture were also included, although all results are provided including and excluding this species (see Field procedures). Four out of the 6 studied species, if we also consider the Egyptian vulture, are threatened in the Canaries (Table 1).
Table 1.
Distribution, IUCN category of threat according to Madroño et al. (2004), estimated population size (according to different studies and years), density (pairs/100 km2), territory spacing (NND in km), and regularity of territory dispersion (G values) of the breeding cliff-nesting raptors and common raven of Teno, Tenerife, Canary Islands
| Species | Distribution | Biogeog. range | IUCN Category | Breeding pairs in |
Density | NND | G | P | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Canary Island | Tenerife | Teno (%)a | ||||||||
| Egyptian vulture Neophron percnopterus majorensis | L,F | Ce | CE | 60b | Extinct | 16* (-) | 11.0 | 0.83 ± 0.42 | 0.62 | 0.001 |
| Common buzzard Buteo buteo insularum | All except L | Ce | NT | 430–445c | 170–180c | 33 (18–19) | 22.6 | 1.12 ± 0.56 | 0.63 | 0.001 |
| Osprey Pandion haliaetus | L,T,G,H | — | CE | 14d | 4d | 4 (100) | 2.7 | 1.48 ± 1.29 | 0.40 | 0.000 |
| Common kestrel Falco tinnunculus canariensis | Gc,T,P,G,H | Me | LC | 7,110–8,820c | 1,100–1,300c | 197 (15–18) | 134.9 | 0.41 ± 0.21 | 0.57 | 0.002 |
| Barbary falcon Falco peregrinus pelegrinoides | All | — | EN | 128–132e | 35e | 11 (31) | 7.5 | 1.95 ± 0.56 | 0.87 | 0.001 |
| Common raven Corvus corax canariensis | All | Ce | EN | <400c | 12f | 9 (75) | 6.2 | 1.34 ± 0.55 | 0.75 | 0.001 |
Notes: L = Lanzarote including the northern islets, F = Fuerteventura, Gc = Gran Canaria, T = Tenerife, P = La Palma, G = La Gomera, H = El Hierro, Ce = Canarian endemism, Me = Macaronesian endemism, CE = Critically Endangered, EN = Endangered, VU = Vulnerable, NT = Near Threatened, LC = Least Concern, *number of recognizable nests. aPresent study. bRamírez et al. (2014). cLorenzo (2007). dSiverio (2008). eSiverio et al. (2009). fSiverio et al. (2010b).
Field procedures
During the breeding seasons (February–May) of 2005–2010, all cliffs in our study area were inspected for established breeding pairs. The presence of a nesting site was assumed when displaying or perched adults were present at a nest, or when recently used perches, territorial defensive behavior, and/or juveniles were recorded on cliffs. The locations of territories (nesting sites) were georeferenced considering the nest or the most suitable site judged capable to hold a nest. Coordinates were obtained using a hand hold Garmin GPS unit, 1:25,000 scale topographic maps or using a Geographic Information System (GIS). The locations of osprey, falcon, and raven territories are referred to the year 2008. Buzzards’ data correspond to the 2007 season (Rodríguez et al. 2010b), and for the most abundant species, the common kestrels, nest locations were obtained during 2005–2010 given that the most remote and rugged sectors were surveyed at least 1 year to cover all available habitats for this species. We are aware that raptor density may strongly fluctuate among years due to variability in climate or feeding resources (Newton 1979), but prey availability and climatic stability in the Canary Islands produce no large annual variation in raptor breeding densities (see Siverio 2006; Siverio et al. 2007; Rodríguez et al. 2010b; Siverio et al. 2010a). We may have missed a few nest sites, especially of the abundant kestrels, but we are confident that such omissions represent less than 5% of total nesting sites. Despite these drawbacks, our data constitute an important piece of information for the Canaries, and we are convinced they represent a good approximation of the spatial distribution of the cliff nesting raptors at the community level.
The Egyptian vulture is now extinct in Tenerife, but many of its old nests are still recognized by the large amount of its characteristic white dropping remains on the basaltic dark rocks and the rests of nest material (Rodríguez et al. 2014; personal observation). Although some nesting sites may be currently unrecognizable and the raptor community could have substantially changed since the extinction of this species in the 1980s, information on these nesting sites is still very valuable as it is the only one available for this extinct species in the Western Canaries, and it may be useful for conservation actions or future reintroduction programmes. For all the above, our analyses were conducted both including and excluding the information concerning the Egyptian vulture. Given that occupation dates of Egyptian vulture nest-sites are unknown we assumed that all sites were concurrently occupied.
Data analysis
We used six variables to describe nesting sites: 1) cliff height (m); 2) altitude (m); 3) steepness, measured as the difference of maximum and minimum altitude in a radius of 500 m (an arbitrarily selected value close to the mean distance—410 m—between territories of the most common species, the common kestrel; Supplementary Table S1) from the nest site; 4) distance to the nearest road (m); 5) distance to the nearest inhabited house (m); and 6) habitat diversity, as the Shannon Diversity Index of the proportions of land covered by forest, shrubs, grasses, urban areas, and sea in a 1.5-km radius circle from the nesting cliff (Krebs 1999). Variables were extracted using GIS, a Digital Elevation Model (DEM; cell size = 25 × 25 m, vertical resolution = 1 m; Digital Atlas of Tenerife, Cabildo de Tenerife) and 1:20,000 maps of vegetation (Del Arco et al. 2006). To test potential specific differences in nesting-site characteristics, we used Kruskal–Wallis tests considering the six previous variables, and after that, pairwise permutation tests as post hoc tests. P values were computed by Monte Carlo resampling (9,999 replications). Given that type I error rate may be inflated due to multiple comparisons, the P value was adjusted by the false discovery rate (Mangiafico 2015). The small number of osprey nests precluded statistical comparisons.
We calculated both intra- and interspecific nearest-neighbor distances (NND) of each nesting site (Supplementary Table S1). The dispersion pattern of nesting sites was estimated by means of the G-Statistic, that is, the ratio between geometric and arithmetic means of the squared NND. Values approaching 1 (>0.65) indicate a high degree of regularity, and those close to 0 randomness (Brown 1975). Deviation from randomness toward regularity of nest spacing was evaluated by means of the test proposed by Clark and Evans (1954).
To evaluate the potential effects of inter- or intraspecific competition, we tested the null hypothesis that specific nest sites were randomly distributed within the study area (Martínez et al. 2008). We used generalized linear models (GLMs) with a complementary log–log link function and binomial errors. For each species, we constructed a model, coding all nesting sites in binary form (1/0) according to the holder specific identity (response variable) and considering the NNDs of conspecifics, buzzards, kestrels, falcons and ravens as explanatory variables (see Martínez et al. 2008 for procedure details]. Egyptian vultures and ospreys were not considered as they are extinct and rare, respectively. Given the excess of ‘0’ in relation to ‘1’ in the response variables (except for kestrels), we employed the complementary log–log link function (Zuur et al. 2009). We performed multimodel inference according to corrected Akaike’s information criterion (AICc; see below). To assess collinearity among predictors, we used two pairwise diagnostic tools: correlation matrix and variance inflation factors (VIFs). All Pearson correlation coefficients were <0.49, and VIFs <1.7, indicating no collinearity issues.
To study density, we made a grid of 1 × 1 km, and counted the number of territories (abundance) and species (richness) for each cell (n = 172 cells). Additionally, we extracted the mean altitude, mean slope, and mean curvature for each cell using the DEM (1600 pixels per cell) and the Surface functions of the Spatial Analyst tools of ArcToolbox. For each pixel, slope function calculates the maximum change in elevation from that pixel to its 8 neighbors, whereas curvature is the second derivative of the surface. Positive or negative curvature indicates the surface is upwardly convex or concave at the pixel, respectively (for details see Spatial Analyst tools, ArcToolbox, ArcGIS). We also calculated for each cell the percentage of land covered by forest, shrubs, grasses, and urban areas using the vegetation maps (Del Arco et al. 2006). Finally, the Shannon Diversity Index (Krebs 1999) was used to calculate the habitat diversity in each cell. Cells with more than 50% of sea were excluded from analyses, reducing our sample size to 143 cells. Geographical analyses were conducted in ArcGIS v10 (Environmental Systems Research Institute, Redlands, CA) and QGIS v2.16.3 (Open Source Geospatial Foundation Project, http://qgis.osgeo.org).
To analyze the factors affecting abundance and richness (response variables) in 1 × 1 km cells, we used multimodel inference of GLMs with Poisson error distributions and log link functions, and with 8 explanatory variables (mean altitude, mean slope, mean curvature, proportion of land covered by forests, shrubs, grasslands and urban areas, and habitat diversity). Because kestrels composed the majority of nesting sites, we repeated the multimodel inference using 3 abundance response variables: 1) total raptors (number of all raptor nesting sites occurring in each cell); 2) kestrels (number of kestrel nesting sites); and 3) raptors excluding kestrels (number of all raptor nesting sites, excluding those occupied by kestrels). Correlation matrix and VIFs indicated lack of multicollinearity. All Pearson correlation coefficients and VIFs were lower than 0.58 and 3.5, respectively. Given that spatial dependence of observations, that is, values of a variable in proximate cells are more similar or dissimilar than expected for cells randomly distributed, may be a statistical problem when modeling spatial distributions, we examined the extent of spatial correlation both for response variables and for averaged residuals of the multimodel inferences by using the Moran’s index.
Multimodel inference allowed to identify the best possible models based on AICc and to rank all independent variables according to their influence on the two response variables (Burnham and Anderson 2002). The candidate models in the final selection, that is, models within 2 AICc units from the best model, and their Akaike weight of evidence (w) were used to estimate averaged regression coefficients (Bartoń 2013). Thus, the explanatory variables were ranked by importance, that is, sum of their w over all competing models (the closest to 1, the highest importance).
Models were fitted and selected in R (version 3.3.2) using the glm function. We used the package MuMIn for procedures of the multimodel inference method (Bartoń 2013). VIFs and Moran’s indexes were determined using the functions vif and Moran.I of the R-packages car and ape (Paradis et al. 2004; Fox and Weisberg 2011).
Results
Community structure and habitat features
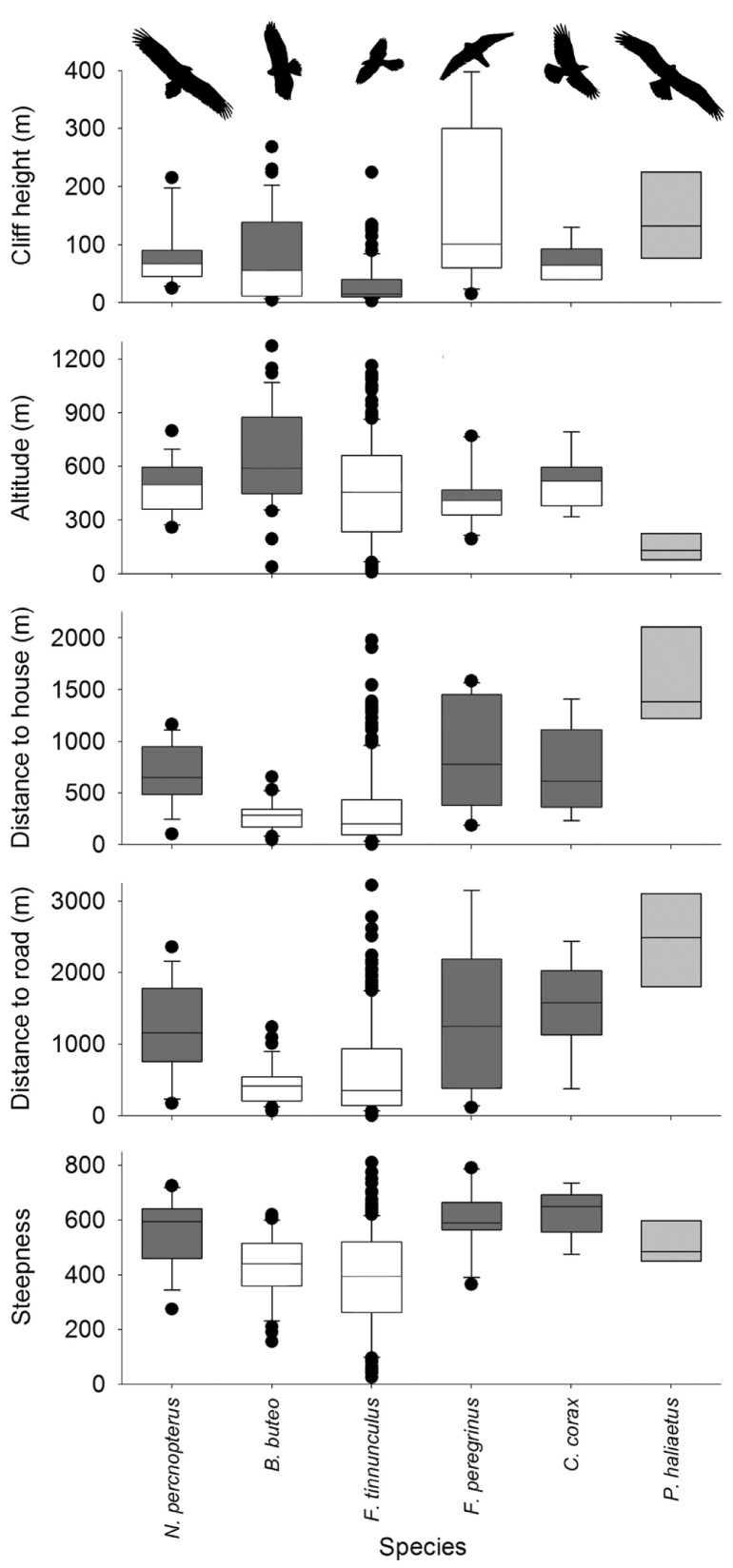
A total of 270 breeding territories of 6 species were mapped. The kestrel was the most abundant species with 197 territories and the osprey the scarcest one, with only 4 breeding pairs (see Table 1 for the number of nest-sites for each species). All nesting sites were located on natural cliffs, excepting 21 kestrel nests on man-made structures (abandon quarries, n = 11; concrete walls, n = 5; nest-box in electric pylon, n = 1) and trees or palm-trees (Phoenix canariensis, n = 2; Pinus canariensis, n = 1; Washingtonia robusta, n = 1); and three (9.1%) buzzard nests on trees (P. canariensis, n = 2, P. radiata, n = 1). Excluding habitat diversity around nesting sites (Shannon Index), all variables describing nesting sites varied significantly among species (Kruskall–Wallis tests; cliff height χ2 = 53.24, P < 0.01; altitude χ2 = 11.55, P < 0.02; steepness χ2 = 39.93, P < 0.01; distance to house χ2 = 38.79, P < 0.01; distance to road χ2 = 31.62, P < 0.01; habitat diversity χ2 = 3.48, P = 0.47). Kestrels and buzzards occupied cliffs located closer to houses and on flatter areas than falcons, ravens, and ospreys. Kestrels occupied the lowest cliffs, while falcons occupied the highest ones. The buzzard nesting sites were at higher altitude than kestrel sites (Figure 2; Supplementary Table S2).
Figure 2.
Box plots displaying variation on habitat variables for the cliff-nesting raptors and the common raven in Teno, Tenerife, Canary Islands. The line within boxes indicated the median, the bottom and top of the box represent the first and third quartiles, and the whiskers extend 1.5 times the interquartile range. Black dots represent outliers, and box color (white or dark gray) specifies significant differences according to results of pairwise permutation tests (see Supplementary Table S2). Osprey boxes were light-gray colored (not included in the statistical analyses).
Intra- and interspecific interactions
All species showed relative high G-statistic values, indicating some degree of regular distribution of territories that deviated significantly from randomness in all cases (Table 1). The same pattern was obtained when all the species were considered together (G = 0.45, P = 0.003; excluding Egyptian vulture G = 0.46, P = 0.003). As a whole, density of breeding territories was 184.9 pairs/100 km2 (excluding Egyptian vulture =174.0 pairs/100 km2); and mean NND was 0.34 ± 0.23 km (excluding Egyptian vulture = 0.36 ± 0.23 km). The GLMs of distance to the nearest neighbors indicated that interspecific interactions, both positive and negative, were more important than intraspecific ones. However, the small effect sizes and the low proportion of explained deviance make that these results should be treated with caution. The multimodel inference suggest that: 1) the probability of buzzard occupation increased with the distance to raven nesting sites; 2) falcons preferred to breed close to kestrels; and 3) ravens settled close to falcons (Table 2; Supplementary Tables S1 and S3). For kestrels, the multimodel inference did not retain any NND explanatory variable, that is, the null model obtained the lowest AICc, indicating that distances to the nearest neighbors do not influence the distribution of kestrel nesting sites (Supplementary Table S3).
Table 2.
Multimodel inference results for GLMs estimating the probability of occupation of nest sites and using the NNDs as explanatory variables
| Species | Explained deviance (%) | Term | Importance | Estimate | SE | Lower CI | Upper CI |
|---|---|---|---|---|---|---|---|
| B. buteo | 4.5 | NNDCc | 1 | 0.00011 | 0.00004 | 0.00002 | 0.00019 |
| NNDFt | 0.63 | 0.00063 | 0.00064 | −0.00008 | 0.00206 | ||
| NNDBb | 0.18 | −0.00002 | 0.00011 | −0.00060 | 0.00036 | ||
| F. tinnunculus | 0 | NNDBb | 0.22 | −0.02063 | 0.06271 | −0.00030 | 0.00011 |
| NNDCc | 0.19 | −0.00275 | 0.01163 | −0.00006 | 0.00003 | ||
| NNDFp | 0.16 | −0.00354 | 0.02705 | −0.00001 | 0.00001 | ||
| F. peregrinus | 14 | NNDFt | 1 | −0.00610 | 0.00232 | −0.01067 | −0.00153 |
| NNDCc | 0.65 | −0.00016 | 0.00019 | −0.00062 | 0.00012 | ||
| NNDBb | 0.42 | 0.00020 | 0.00034 | −0.00029 | 0.00123 | ||
| NNDFp | 0.31 | 0.00016 | 0.00033 | −0.00027 | 0.00131 | ||
| C. corax | 20 | NNDFp | 1 | −0.00134 | 0.00059 | −0.00250 | −0.00018 |
| NNDBb | 0.52 | 0.00036 | 0.00047 | −0.00017 | 0.00156 | ||
| NNDCc | 0.5 | −0.00012 | 0.00019 | −0.00066 | 0.00016 | ||
| NNDFt | 0.25 | −0.00053 | 0.00148 | −0.00666 | 0.00248 |
Notes: Variable response was coded as binary (0 = nest sites occupied by other raptor different to the focused species; 1 = nest sites occupied by the focused species). NND subscripts refer to Buteo buteo (Bb), Falco tinnunculus (Ft), Falco peregrinus (Fp) and Corvus corax (Cc). Importance indicates the sum of Akaike weight over all competing models (the closest to 1, the highest importance). Explanatory variables whose confidence intervals do not overlap with 0 are indicated in bold.
Factors affecting abundance and richness
Considering all the species, the models of abundance highlighted the negative influence of altitude, proportion of land covered by forests and grasslands, and the positive influence of slope (Figure 1), proportion of land covered by shrubs and habitat diversity. The models excluding the Egyptian vulture selected the same variables except the land covered by grasses. The models without the kestrel territories indicated the importance of slope and habitat diversity. Kestrel density was higher in cells with low forest cover, abundant shrub cover, and high habitat heterogeneity (Table 3 and Supplementary Tables S4 and S5). Richness was higher in the cells with lower altitude and with high values of slope, both when including or excluding Egyptian vulture nesting sites (Figure 1; Table 4 and Supplementary Table S4). Residuals for models excluding kestrels or kestrel models were spatially autocorrelated and thus these models should be taken with caution (Supplementary Table S6).
Table 3.
Multimodel inference results for number of nest-sites (referred to 1 × 1 km grid cells) of cliff-nesting raptors and the common raven in Teno, Tenerife, Canary Islands
| Variables | All species | All species without Egyptian vulture | All species without common kestrel | All species without common kestrel-Egyptian vulture | Common kestrel |
|---|---|---|---|---|---|
| Estimate ± SE | Estimate ± SE | Estimate ± SE | Estimate ± SE | Estimate ± SE | |
| Intercept | −0.516 ± 0.363 | −0.551 ± 0.367 | −3.588 ± 0.828* | −4.567 ± 0.844 | −0.315 ± 0.416 |
| Mean altitude | −0.001 ± 0.000* | −0.001 ± 0.000* | −0.001 ± 0.001 | −0.001 ± 0.001 | −0.001 ± 0.000 |
| Mean slope | 0.028 ± 0.008* | 0.026 ± 0.008* | 0.083 ± 0.014* | 0.09 ± 0.016* | 0.016 ± 0.009 |
| Mean curvature | 0.885 ± 0.715 | 0.620 ± 0.759 | 0.720 ± 1.146 | −0.792 ± 1.375 | 1.125 ± 0.916 |
| Land covered by forest | −0.012 ± 0.005* | −0.011 ± 0.005* | 0.004 ± 0.006 | 0.009 ± 0.006 | −0.028 ± 0.008* |
| Land covered by shrubs | 0.010 ± 0.004* | 0.010 ± 0.004* | 0.005 ± 0.006 | — | 0.012 ± 0.006* |
| Land covered by grass | −0.020 ± 0.010* | −0.018 ± 0.010 | −0.029 ± 0.022 | −0.021 ± 0.022 | −0.020 ± 0.011 |
| Land covered by houses | −0.02 ± 0.012 | −0.019 ± 0.012 | −0.095 ± 0.057 | −0.082 ± 0.056 | −0.015 ± 0.012 |
| Habitat diversity (Shannon) | 1.527 ± 0.626* | 1.463 ± 0.645* | 2.599 ± 1.046* | 3.337 ± 1.093* | 1.501 ± 0.758* |
Notes: Averaged coefficient estimates and standard errors (SE) are given. In bold values that represent maximum importance for that variable (sum of weight of evidence = 1); * = model-averaged coefficients whose confidence interval do not overlap with 0. See Supplementary Tables S4 and S5 for further details.
Table 4.
Multimodel inference results for richness (referred to 1 × 1 km grid cells) of cliff-nesting raptors and the common raven in Teno, Tenerife, Canary Islands
| Variables | All species | All species without Egyptian vulture |
|---|---|---|
| Estimate ± SE | Estimate ± SE | |
| Intercept | −0.476 ± 0.266 | −0.433 ± 0.278 |
| Mean altitude | −0.001 ± 0.000* | −0.001 ± 0.000* |
| Mean slope | 0.043 ± 0.000* | 0.039 ± 0.007* |
| Mean curvature | 0.446 ± 0.894 | — |
| Land covered by forest | −0.004 ± 0.004 | −0.001 ± 0.001 |
| Land covered by shrubs | 0.003 ± 0.003 | 0.001 ± 0.001 |
| Land covered by grass | −0.006 ± 0.010 | −0.001 ± 0.004 |
| Land covered by houses | −0.010 ± 0.013 | −0.001 ± 0.005 |
| Land diversity (Shannon) | 0.198 ± 0.566 | 0.050 ± 0.248 |
Notes: Averaged coefficient estimates and standard errors (SE) are given. In bold values that represent maximum importance (sum of weight of evidence = 1); * = model-averaged coefficients whose confidence interval do not overlap with 0. See Supplementary Tables S4 and S5 for further details.
Discussion
Community structure
Our models selected landscape diversity as an important variable explaining raptor abundance. More diverse habitats can hold higher diversity and prey abundance; and therefore are more suitable for foraging or nesting (e.g., Anderson 2001; Palomino and Carrascal 2007; Poirazidis et al. 2007). Coexisting raptors are expected to select different prey or to segregate foraging areas spatially and temporally according to the species-specific life-history traits (see Gliwicz 2008; Olsen et al. 2010; Kendall et al. 2012). In this sense, raptor guilds are usually composed by several mammal or bird eater specialists and at least one scavenging species (White and Cade 1971; Poole and Bromley 1988; Clouet et al. 2000; Aumann 2001; Jenkins and Van Zyl 2005) as occurs also in Teno: one bird-eater specialist (falcon), two mammal-lizard-eater specialists (buzzard and kestrel), one fish-eater specialist (osprey), and one omnivorous and scavenging species (raven).
Breeding density of raptors on Teno (174 breeding pairs/100 km2) is higher than in other continental areas worldwide (see White and Cade 1971; Donázar et al. 1989; Clouet et al. 2000; Aumann 2001; Jenkins and Van Zyl 2005). These results are highly conditioned by the most abundant species, the kestrel (134.9 breeding pairs/100 km2). In Europe, kestrel densities varies between 3 and 200 pairs/100 km2 depending on the extension of study areas, usually large ones include poor quality habitat areas or low availability of nesting sites (Village 1990). Taking into account the negative relationship between forest and kestrel density (Table 3) and the low proportion of land covered by forests in Teno, as well as the extremely high availability of cavities and holes on cliffs suitable for nesting, kestrel numbers could be overrepresented in our study area with respect to the rest of the island (estimated density on the entire island is 55.1–63.9 pairs/100 km2 while in Teno there are at least 134.9 pairs/100 km2; Table 1).
Habitat features
With the exception of landscape diversity, all variables describing nesting cliffs were significantly different among at least two species (Figure 2). Specific choice of breeding habitat features must be related with hunting behavior and tolerance of human disturbance. Worldwide peregrine falcons select the most dominant cliffs with respect to the surrounding (e.g., Brambilla et al. 2006; Rodríguez et al. 2007), likely providing better hunting opportunities. Pairs occupying higher cliffs usually achieve greater hunting success rates (Ratcliffe 1993; Jenkins 2000). In our study area, as well as in other sites within the archipelago, falcons selected the highest cliffs in comparison to other raptor species (Rodríguez and Siverio 2006; Rodríguez et al. 2007; this study). On Tenerife, buzzard breeding density is positively related to forested areas (Rodríguez et al. 2010b), so as forests are usually located at 200–2,000 m a.s.l., it is reasonable that buzzards occupy the highest elevations zones in Teno. In contrast, kestrels occupied lower cliffs than falcons and at lower altitudes than buzzards. Kestrels hunt mainly by hovering in open habitats and one of their most abundant prey are the Canarian lizards Gallotia spp. which show high densities in the warmest, low-vegetated areas (Padilla et al. 2007). In fact, our models indicate that the presence of forest is the single variable influencing negatively kestrel density, probably related with the low abundance of lizards and difficulties for hunting in close habitats like forest. In general, only buzzards and kestrels occupy areas with low cliff availability and close to humans (roads and houses). The rest of the species are limited to the South-West of the massif where landscape is dominated by inaccessible huge ravines and seacliffs, what is supported by the relative high positive importance of slope in the richness models (Table 3).
The information gathered on the presence and habitat characteristics of the extinct Egyptian vulture in Teno represents the unique available assessment of its habitat features on the Western Canary Islands. Formerly distributed in all the islands excepting La Palma, the current Canarian population (ca 50 pairs) survives in the Eastern group of islands, that is, Fuerteventura, Lanzarote, and its associated islets, where, in addition to large cliffs, also low, foot-accessible hills, and volcanic craters are used for nesting (Donázar et al. 2002; Gangoso and Palacios 2005; Ramírez et al. 2014). In contrast, our results based on the observable nest-sites after 30 years of its extinction suggest that vultures only nested on high cliffs in remote (far away from human structures) and rugged areas. Thus, it seems that endemic Egyptian vulture subspecies show some level of plasticity for breeding, occupying higher cliffs when available.
Intra- and interspecific interactions
Many studies have demonstrated that competitive interactions, both intra- and interspecific, play an important role in the distribution and habitat selection of raptors (e.g., Katzner et al. 2003; Hakkarainen et al. 2004; Martínez et al. 2008). Even positive interactions among different species could influence the nesting-habitat choice in these predators (Sergio et al. 2004). Our analysis of the NNDs suggests the existence of both negative and positive interspecific relationships (Table 2). The probability of cliff occupation by buzzards increased with the distance to ravens maybe related to interspecific differences in habitat selection. Buzzards positively select forested areas (Rodríguez et al. 2010b), whereas the small raven population is mainly located in the unforested south-facing slopes of Teno. Competition for food resources could also explain these relationships with buzzards. For instance, kleptoparasitism by buzzards on kestrels and falcons has been observed in Teno (Siverio et al. 2008). The models for falcons and ravens indicated that at least the raven positively associates to the falcons. In this sense, two simultaneous studies analyzing factors affecting habitat choice by Peregrine Falcon conducted in two different, but nearby, European populations reported apparently contradictory results. One indicates that peregrine productivity increases with proximity to raven nests, suggesting that both species could be benefitted; the falcons, by getting vigilance of the territory and the ravens by getting protection against other species (Sergio et al. 2004). The other indicates that breeding success and productivity are lower for peregrines coexisting with ravens, especially on cliffs with ravens and rock climbers occurring simultaneously. Thus, raven predation on peregrine eggs/chicks may be favored by human disturbance (Brambilla et al. 2004). Another complementary explanation is that ravens could take advantage of food stocked by falcons on their nesting territories (Ratcliffe 1997) which has been observed multiple times in Teno (personal observation).
Conservation remarks
Our results highlight the high conservation value of Teno for birds of prey. It is a very important stronghold for the threatened studied raptors: 1) the unique ospreys breeding pairs of the island, which constitute more than 30% of the Canarian breeding population, are bound to the Teno coastal cliffs (Rodríguez et al. 2013); 2) the first Barbary falcon breeding pairs of Tenerife were discovered on Teno in the early 1990s, since then the insular population has spread through the island reaching more than 35 pairs at present (31% of them breeding in Teno; Siverio et al. 2009, 2010a); 3) the common raven was formerly distributed through the island, but during the last four decades it has suffered a sharp decline and the bulk of the breeding insular population survived restricted to Teno (Lorenzo 2007; Siverio et al. 2007). In addition, many endemic plants and invertebrates occur there (Reyes-Betancort et al. 2008; Martín 2010), and some vertebrates maintain their more important or unique insular breeding populations there, such as for example the Canarian spotted lizard Gallotia intermedia, the Manx shearwater Puffinus puffinus, or the rock sparrow Petronia petronia (Rodríguez et al. 2014). But what does it make of Teno an important area for wildlife and threatened raptors in particular? According to our analyses, the endangered species (i.e., ospreys, falcons, ravens, and the extinct Egyptian vulture) occupied cliffs farther away from houses and roads, and in areas more rugged than the non-threatened species (kestrels and buzzards). These findings suggest that the rugged terrain of Teno and the low human occupation are key factors for its conservation. Excepting some kestrel nests, all breeding territories are included in the Canarian Network of Natural Protected Areas (Teno Rural Park). However, raptors are not free of human threats. A tourist industry specialized in activities in natural environment and extreme sports, such as climbing, trekking, rappel, recreational sailing, sea kayak, and scuba diving, has emerged in the last decade (Rodríguez et al. 2014; personal observation). These activities are mainly centered on the beaches, seacliffs, and deep ravines of the South-West sector, coinciding with the most diverse areas for raptors. The continued presence of tourist boats close to osprey nests could limit the establishment of new pairs or lead to low productivity by hindering nest attendance or foraging (Richardson and Miller 1997). For example, the recently used osprey nests are located at higher positions than historical nests, that is, unoccupied since 1999 or earlier, which has been explained as a response to the increase of human disturbances (Rodríguez et al. 2013). Therefore, according to our results, several conservation actions must be implemented in Teno to guarantee the conservation of the threatened raptor species: a) to increase vigilance during critical periods to avoid nest disturbances, b) to strictly regulate the practice of recreational activities as sailing, rock climbing, rappel, or hiking, and c) to reinforce the monitoring programs and studies on behavior and breeding rates of these species.
Supplementary Material
Acknowledgment
We would like to thank to Nazaret M. Carrasco, Rut Martínez, Francisco M. González, José Juan Hernández, Aurelio J. Acevedo, Juan Curbelo, and the Rangers of Teno Natural Park (Cabildo Insular de Tenerife) for their help during field work. We are also grateful to Isabel Afán (EBD-CSIC) for her help with GIS analysis. Two anonymous referees and Dr Juan José Negro made important and useful recommendations to greatly improve the manuscript.
Funding
This study was funded by the Canary Islands' Ornithology and Natural History Group (GOHNIC). Some expenses for fieldwork were funded by the Ayuntamiento (city council) de Santiago del Teide and the Ayuntamiento de Buenavista del Norte.
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
References
- Anderson DL, 2001. Landscape heterogeneity and diurnal raptor diversity in Honduras: the role of indigenous shifting cultivation. Biotropica 33:511–519. [Google Scholar]
- Aumann T, 2001. The structure of raptor assemblages in riparian environments in the south-west of the northern territory, Australia. Emu101: 293–304. [Google Scholar]
- Bartoń K, 2013. MuMIn: Multi-Model Inference, version 1.9.0. R package.
- Brambilla M, Rubolini D, Guidali F, 2004. Rock climbing and raven Corvus corax occurrence depress breeding success of cliff-nesting peregrines Falco peregrinus. Ardeola 51:425–430. [Google Scholar]
- Brambilla M, Rubolini D, Guidali F, 2006. Factors affecting breeding habitat selection in a cliff-nesting peregrine Falco peregrinus population. Journal of Ornithology 147:428–435. [Google Scholar]
- Bretagnolle V, Inchausti P, Seguin J-F, Thibault J-C, 2004. Evaluation of the extinction risk and of conservation alternatives for a very small insular population: the bearded vulture Gypaetus barbatus in Corsica. Biological Conservation 120:19–30. [Google Scholar]
- Brown D, 1975. A test of randomness of nest spacing. Wildfowl 26:102–103. [Google Scholar]
- Burgas D, Byholm P, Parkkima T, 2014. Raptors as surrogates of biodiversity along a landscape gradient. Journal of Applied Ecology 51:786–794. [Google Scholar]
- Burnham KP, Anderson DR, 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer-Verlag. [Google Scholar]
- Carrillo J, González-Dávila E, 2005. Breeding biology and nest characteristics of the Eurasian kestrel in different environments on an Atlantic island. Ornis Fennica 82:55–62. [Google Scholar]
- Carrillo J, González-Dávila E, 2009. Latitudinal variation in breeding parameters of the common kestrel Falco tinnunculus. Ardeola 56:215–228. [Google Scholar]
- Chiweshe N, 2007. Black eagles and hyraxes: the two flagship species in the conservation of wildlife in the Matobo Hills, Zimbabwe. Ostrich 78:381–386. [Google Scholar]
- Clark PJ, Evans FC, 1954. Distance to nearest neighbor as a measure of spatial relationships in populations. Ecology 35:445–453. [Google Scholar]
- Clouet M, Barrau C, Goar J-L, 2000. The diurnal Afro-alpine raptor community of the Ethiopian Balé Highlands. Ostrich 71:380–384. [Google Scholar]
- Curti M, Valdez U, 2009. Incorporating community education in the strategy for Harpy conservation in Panama. Journal of Environmental Education 40:3–16. [Google Scholar]
- Del Arco M, Wildpret W, Pérez PL, Rodríguez O, Acebes JR. et al. , 2006. Mapa de Vegetación de Canarias. GRAFCAN: S/C de Tenerife.
- Donázar JA, Ceballos O, Fernández C, 1989. Factors influencing the distribution and abundance of seven cliff-nesting raptors: a multivariate study In: Meyburg B-U, Chancellor RD, editors. Raptors in the Modern World. Berlin: WWGBP; 545–552. [Google Scholar]
- Donázar JA, Palacios CJ, Gangoso L, Ceballos O, González MJ. et al. , 2002. Conservation status and limiting factors in the endangered population of Egyptian vulture Neophron percnopterus in the Canary Islands. Biological Conservation 307:89–97. [Google Scholar]
- Donázar JA, Gangoso L, Forero MG, Juste J, 2005. Presence, richness and extinction of bird of prey in the Mediterranean and Macaronesian Islands. Journal of Biogeography 32:1701–1713. [Google Scholar]
- Dunk JR, Zielinski WJ, Welsh HH, 2006. Evaluating reserves for species richness and representation in northern California. Diversity and Distributions 12:434–442. [Google Scholar]
- Fox J, Weisberg S, 2011. Multivariate Linear Models in R. 2nd edn Thousand Oaks: Sage Publications. [Google Scholar]
- Gangoso L, 2006. Insularidad y Conservación: el caso del alimoche, Neophron percnopterus.en Canarias [PhD thesis]. University of Seville. [Google Scholar]
- Gangoso L, Palacios C-J, 2005. Ground nesting by Egyptian vultures Neophron percnopterus in the Canary Islands. Journal of Raptor Research 39:186–189. [Google Scholar]
- Gangoso L, Afán I, Grande JM, Figuerola J, 2015. Sociospatial structuration of alternative breeding strategies in a color polymorphic raptor. Behavioral Ecology 26:1119–1130. [Google Scholar]
- Gliwicz J, 2008. Body size relationships between avian predators and their rodent prey in a North-American Sagebrush Community. Acta Ornithologica 43:151–158. [Google Scholar]
- Hakkarainen H, Mykrä S, Kurki S, Tornberg R, Jugell S, 2004. Competitive interactions among raptors in boreal forests. Oecologia 141:420–424. [DOI] [PubMed] [Google Scholar]
- Hille SM, Collar NJ, 2011. Status assessment of raptors in Cape Verde confirms a major crisis for scavengers. Oryx 45:217–224. [Google Scholar]
- Hilty J, Merenlender A, 2000. Faunal indicator taxa selection for monitoring ecosystem health. Biological Conservation 92:185–197. [Google Scholar]
- Jenkins AR, 2000. Hunting mode and success of African Peregrines Falco peregrinus minor: does nesting habitat quality affect foraging efficiency? Ibis 142:235–246. [Google Scholar]
- Jenkins AR, Van Zyl AJ, 2005. Conservation status and community structure of cliff-nesting raptors and ravens of the Cape Peninsula, South Africa. Ostrich 76:175–184. [Google Scholar]
- Katzner TE, Bragin EA, Knick ST, Smith AT, 2003. Coexistence in a multispecies assemblage of eagles in Central Asia. The Condor 105:538–551. [Google Scholar]
- Kendall C, Virani MZ, Kirui P, Thomsett S, Githiru M, 2012. Mechanisms of coexistence in vultures: understanding the patterns of vulture abundance at carcasses in Masai Mara National Reserve, Kenya. The Condor 114: 523–531.
- Krebs CJ, 1999. Ecological Methodology. Menlo Park: Addison Wesley Longman. [Google Scholar]
- Krüger SC, Simmons RE, Amar A, 2015. Anthropogenic activities influence the abandonment of bearded vulture Gypaetus barbatus territories in southern Africa. The Condor 117:94–107. [Google Scholar]
- Lande R, 1998. Anthropogenic, ecological and genetic factors in extinction and conservation. Researches on Population Ecology 40:259–269. [Google Scholar]
- Lorenzo JA, 2007. Atlas de las aves nidificantes en el archipiélago canario, 1997–2003. Madrid: Dirección General de Conservación de la Naturaleza-SEO/BirdLife. [Google Scholar]
- Madroño A, González C, Atienza JC, 2004. Libro Rojo de las Aves de España. Madrid: Dirección General para la Biodiversidad–SEO/BirdLife. [Google Scholar]
- Mangiafico SS, 2015. An R companion for the handbook of biological statistics. Version 1.2.0. Available at rcompanion.org/rcompanion/.
- Martin TE, 2001. Abiotic vs biotic influences on habitat selection of coexisting species: climate change impacts? Ecology 82:175–188. [Google Scholar]
- Martín JL, 2010. Atlas de Biodiversidad de Canarias S/C de Tenerife: Ed. Gobierno de Canarias.
- Martínez JE, Martínez JA, Zuberogoitia I, Zabala J, Redpath SM. et al. , 2008. The effect of intra- and interspecific interactions on the large-scale distribution of cliff-nesting raptors. Ornis Fennica 85:13–21. [Google Scholar]
- McKinney ML, 1997. Extinction vulnerability and selectivity: combining ecological and paleontological views. Annual Review of Ecology and Systematics 28:495–516. [Google Scholar]
- Morrison ML, Marcot BG, Mannan RW, 2006. Wildlife–Habitat Relationship: Concepts and Applications. 3rd edn Washington (DC): Island Press. [Google Scholar]
- Murphy DD, Noon BR, 1992. Integrating scientific methods with habitat conservation planning: reserve design for northern spotted owls. Ecological Applications 2:3–17. [DOI] [PubMed] [Google Scholar]
- Newton I, 1979. Population Ecology of Raptors. London: T & AD Poyser. [Google Scholar]
- Olsen J, Judge D, Fuentes E, 2010. Diets of wedge-tailed eagles Aquila audax and little eagle Hieraaetus morphnoides breeding near Canberra, Australia. Journal of Raptor Research 44:50–61. [Google Scholar]
- Padilla DP, Nogales M, Marrero P, 2007. Prey size selection of insular lizards by two sympatric predatory bird species. Acta Ornithologica 42:167–172. [Google Scholar]
- Palma L, Ferrera J, Cangarto R, Vaz Pinto P, 2004. Current status of the osprey in the Cape Verde Islands. Journal of Raptor Research 38:141–147. [Google Scholar]
- Palomino D, Carrascal LM, 2007. Habitat associations of a raptor community in a mosaic landscape of Central Spain under urban development. Landscape and Urban Planning 83:268–274. [Google Scholar]
- Paradis E, Claude J, Strimmer K, 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. [DOI] [PubMed] [Google Scholar]
- Poirazidis K, Goutner V, Tsachalidis E, Kati V, 2007. Comparison of nest-site selection patterns of different sympatric raptor species as a tool for their conservation. Animal Biodiversity and Conservation 30:131–145. [Google Scholar]
- Poole KG, Bromley RG, 1988. Interrelationships within a raptor guild in the central Canadian Arctic. Canadian Journal of Zoology 66:2275–2282. [Google Scholar]
- Preston KL, Rotenberry JT, Redak RA, Allen MF, 2008. Habitat shifts of endangered species under altered climate conditions: importance of biotic interactions. Global Change Biology 14:2501–2515. [Google Scholar]
- Ramírez J, Roldán J, Moreno W, 2014. El guirre recupera lentamente sus territorios en las Canarias orientales. Quercus 346:32–37. [Google Scholar]
- Ratcliffe DA, 1993. The Peregrine Falcon. 2nd edn London: T & AD Poyser. [Google Scholar]
- Ratcliffe DA, 1997. The Raven: A Natural History in Ireland and Britain. London: T & AD Poyser. [Google Scholar]
- Reyes-Betancort A, Santos Guerra A, Guma IR, Humphries CJ, Carine MA, 2008. Diversity, rarity and the evolution and conservation of the Canary Islands endemic flora. Anales del Jardín Botánico de Madrid 65:25–45. [Google Scholar]
- Richardson CT, Miller CK, 1997. Recommendations for protecting raptors from human disturbance: a review. Wildlife Society Bulletin 25:634–638. [Google Scholar]
- Rodríguez-Estrella R, Donázar JA, Hiraldo F, 1998. Raptors as indicators of environmental change in the scrub habitat of Baja California Sur, Mexico. Conservation Biology 12:921–925. [Google Scholar]
- Rodríguez B, Siverio M, 2006. Density and breeding habitat characteristics of an insular population of Barbary falcon Falco peregrinus pelegrinoides El Hierro, Canary Islands. Ardeola 53:325–331. [Google Scholar]
- Rodríguez B, Siverio M, Rodríguez A, Siverio F, 2007. Density, habitat selection and breeding success of an insular population of Barbary falcon falco peregrinus pelegrinoides. Ardea 95:213–223. [Google Scholar]
- Rodríguez B, Rodríguez A, Siverio F, Siverio M, 2010a. Causes of raptor admissions to a wildlife rehabilitation center in Tenerife (Canary Islands). Journal of Raptor Research 44:30–39. [Google Scholar]
- Rodríguez B, Siverio F, Rodríguez A, Siverio M, Hernández JJ, Figuerola J, 2010b. Density, habitat selection and breeding biology of common buzzards Buteo buteo in an insular environment. Bird Study 57:75–83. [Google Scholar]
- Rodríguez B, Rodríguez A, Siverio M, Siverio F, 2013. Conservation implications of past and present nesting habitat selection of the endangered osprey Pandion haliaetus population of the Canary Islands. Ibis 155:891–897. [Google Scholar]
- Rodríguez B, Siverio F, Siverio M, Rodríguez A, Barone R, 2014. Los Vertebrados Terrestres de Teno. Catálogo ilustrado y comentado. Buenavista del Norte: GOHNIC. [Google Scholar]
- Sanz-Aguilar A, de Pablo F, Donázar JA, 2015. Age-dependent survival of island vs. mainland populations of two avian scavengers: delving into migration costs. Oecologia 179:405–414. [DOI] [PubMed] [Google Scholar]
- Sergio F, Rizzolli F, Marchesi L, Pedrini P, 2004. The importance of interspecific interactions for breeding site selection: Peregrine falcons seek proximity to raven nests. Ecography 27:818–826. [Google Scholar]
- Sergio F, Caro T, Brown D, Clucas B, Hunter J. et al. , 2008. Top predators as conservation tools: ecological rationale, assumptions, and efficacy. Annual Review of Ecology, Evolution, and Systematics 39:1–19. [Google Scholar]
- Siverio M, 2006. Population status and breeding biology of osprey Pandion haliaetus in Tenerife, Canary Islands (1997–2004). Alauda 74:413–419. [Google Scholar]
- Siverio M, 2008. El águila pescadora en Canarias In: Triay R, Siverio M, editors. El águila pescadora en España, Población en 2008 y método de censo. Madrid: Sociedad Española de Ornitología, 20–39. [Google Scholar]
- Siverio M, Siverio F, Rodríguez B, 2007. Annual variation and breeding success of a threatened insular population of common raven Corvus corax, Tenerife (Canary Islands). Vogelwelt 128:197–201. [Google Scholar]
- Siverio F, Rodríguez A, Padilla DP, 2008. Kleptoparasitism by Eurasian buzzard Buteo buteo on two Falco species. Journal of Raptor Research 42:62–68. [Google Scholar]
- Siverio M, Rodríguez B, Siverio F, 2009. El halcón tagarote Falco peregrinus pelegrinoides en las islas Canarias In: Del Moral JC, editor. El Halcón Peregrino en España. Madrid: Sociedad Española de Ornitología, 52–58. [Google Scholar]
- Siverio M, Siverio F, Rodríguez B, Rodríguez A, 2010a. Long-term monitoring of an insular population of Barbary falcon Falco peregrinus pelegrinoides. Ostrich 82:225–230. [Google Scholar]
- Siverio M, González EI, Siverio F, 2010b. Population size and status of common raven Corvus corax on the central-western islands of the Canarian archipelago. Vieraea 38:123–132. [Google Scholar]
- Sundseth K, 2005. Natura 2000 in the Macaronesian Region. Luxembourg: European Commission. [Google Scholar]
- Sutherland WJ, Pullin AS, Dolman PM, Knight TM, 2004. The need for evidence-based conservation. Trend in Ecology and Conservation 19:305–308. [DOI] [PubMed] [Google Scholar]
- Thibault J-C, Patrimonio O, Torre J, 1992. Does the diurnal raptor community of Corsica, west Mediterranean show insular characteristics? Journal of Biogeography 19:363–373. [Google Scholar]
- Thiollay J-M, 1998. Distributional patterns and insular biogeography of South Asian raptor communities. Journal of Biogeography 25:57–72. [Google Scholar]
- Trujillo D, 2009. El milano negro logra reproducirse en Canarias. Quercus 286:48–49. [Google Scholar]
- Village A, 1990. The Kestrel. London: T & AD Poyser. [Google Scholar]
- White CM, Cade TJ, 1971. Cliff-nesting raptors and ravens along the Colville River in arctic Alaska. Living Bird 10:107–150. [Google Scholar]
- White CM, Kiff LF, 2000. Biodiversity, island raptors, and species concepts In: Chancellor RD, Meyburg B-U, editors. Raptors at Risk. Berlin: WWGBP, 633–652. [Google Scholar]
- Whittaker RJ, Fernández-Palacios J-M, 2007. Island Biogeography. 2nd edn. Oxford: Oxford University Press. [Google Scholar]
- Whittingham MJ, Krebs JR, Swetnam RD, Vickery JA, Wilson JD. et al. , 2007. Should conservation strategies consider spatial generality? Farmland birds show regional not national patterns of habitat association. Ecology Letters 10:25–35. [DOI] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM, 2009. Mixed Effects Models and Extensions in Ecology with R. New York: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.