A 14-year-old girl was seen in her local emergency department following multiple syncopal episodes with loss of consciousness following 1 week of severe vaginal bleeding. She was tachycardic and had orthostatic hypotension and a haemoglobin of 50 g/L. She was treated with multiple blood transfusions, tranexamic acid and oral contraceptive (OCP) tablets. Her menarche occurred 3 months earlier, and she had three normal cycles before presentation.
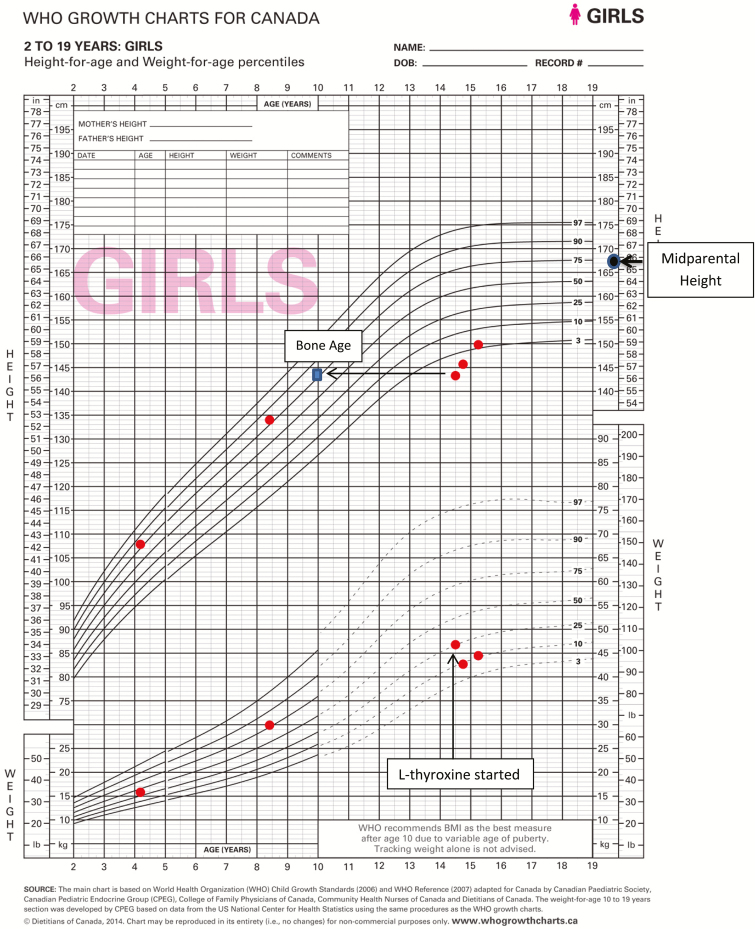
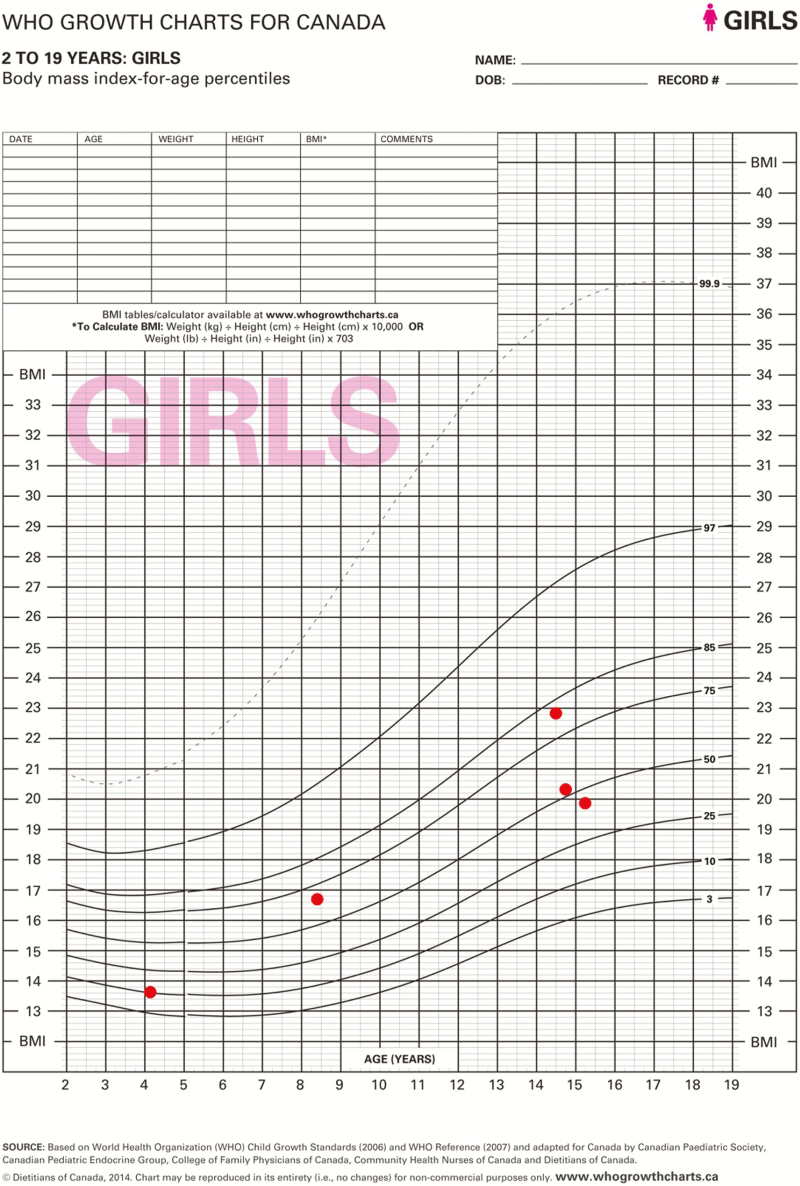
She weighed 46.9 kg (25th–50th percentile); her height was 143.3 cm (<<3rd percentile) and well below mid-parental height (167.6 cm, 75th percentile). Her BMI Z-score was 0.92 (80th percentile). She had mild proximal muscle weakness and delayed deep tendon reflexes. Her hair was dry and she had edema of her face and extremities. She had no thyromegaly, acanthosis nigricans or hypopigmentation. She was Tanner Stage III for breast development and early Tanner II for pubic hair development and had no axillary hair.
Investigations revealed normal coagulation studies, liver function, creatinine, glucose and electrolytes. Her beta-human chorionic gonadotropin (BHCG) screen was negative. Her transabdominal ultrasound showed clots in the uterus, no evidence of ovarian cysts and a moderate amount of free fluid in the pelvis. She had a normal echocardiogram.
Further history and testing revealed the diagnosis.
CASE DIAGNOSIS: AUTOIMMUNE THYROIDITIS
There was no personal or family history of any abnormal bleeding. Her family history was significant for hypothyroidism in her mother and maternal grandmother, and for thyroid cancer in her mother. Her functional inquiry was significant for cold intolerance, weakness and poor linear growth of at least 1-year duration. During this period she was seen once for knee pain by her family doctor. No anthropomorphic measurements were taken.
Blood work revealed a thyroid-stimulating hormone (TSH) of >100 mIU/L (0.47–4.00 mIU/L) and a free-thyroxine (FT4) of 1.57 pmol/L (10 to 17.6 pmol/L) with positive antithyroid peroxidase of 85 kIU/L (<35 kIU/L). Her follicle stimulating hormone (FSH) was 3 IU/L and luteinizing hormone (LH) was <1 IU/L. Her bone age was delayed at 10 years. She had a normal karyotype and negative celiac screen. Her cortisol was normal, her insulin-like growth factor was normal for her bone age and her prolactin was mildly increased at 35 µg/L (normal < 24 µg/L).
Due to the severity of her symptoms, the adult endocrinologist started her on l-thyroxine 200 mcg/day. The dose was subsequently decreased to 75 mcg/day as it was recognized that children with long standing severe hypothyroidism should be started on low doses of l-thyroxine with the aim of correcting TSH slowly to avoid idiopathic intracranial hypertension. A starting dose of a quarter of the target dose is typically recommended. She was discharged from hospital 4 days later. One month later, she was seen by a paediatric endocrinologist and her F T4 and TSH levels had normalized and her OCP pill was discontinued to prevent rapidly advancing her bone age and premature closure of her epiphyseal plates. At her 3-month follow-up, she reported having regular menstrual cycles, normal energy and no cold intolerance. She had normal hair, normal reflexes and no muscle pain or weakness. Her height had increased by 1.8 cm, and her weight decreased by 4.1 kg (Figures 1 and 2). Her breast development was stable, but her pubic hair had progressed to Tanner Stage III.
Figure 1.
Figure 2.
Hypothyroidism in children classically presents with short stature and delayed puberty, along with other common symptoms such as continued weight gain, proximal muscle weakness, cold intolerance, hair and nail changes, constipation and fatigue. A rare presentation of longstanding severe hypothyroidism is the Van Wyk–Grumbach syndrome. This is characterized by precocious breast development with a delayed bone age, galactorrhea, multicystic ovaries and absent or little sexual hair development (1). It can also result in excessive and irregular breakthrough menstrual bleeding. Boys with this syndrome present with macro-orchidism without signs of androgen dependent hair growth.
The precise mechanism by which hypothyroidism causes incomplete isosexual precocious puberty remains unclear. Van Wyk and Grumbach initially proposed that thyroid releasing hormone (TRH) elevations caused by the lack of negative feedback on the pituitary from the thyroid hormone deficiency resulted in an ‘overlap’ in pituitary hormone secretion. The TRH would increase not only TSH but gonadotropins as well (1). However, this did not explain the FSH dominant clinical picture (i.e., isolated breast development, menstruation and follicular cysts without signs of virilization). In vitro studies later showed that supraphysiologic TSH levels could act directly on FSH receptors in a dose dependent fashion (2). While this remains the most accepted theory, TRH induced elevations in prolactin have also been implicated and are thought to increase ovarian sensitivity to circulating levels of gonadotropins. However, high prolactin levels are not always found in this syndrome, and the clinical features are reversed only by thyroxine treatment and not by prolactin lowering agents such as bromocriptine. In addition, ovarian hyperstimulation is not seen in girls with prolactinomas.
CLINICAL PEARLS
Incomplete isosexual precocious puberty with excessive or irregular menstrual bleeding is a rare presentation of prolonged juvenile hypothyroidism and should be considered as part of the differential diagnosis in children with short stature, delayed bone age and signs of advancing puberty.
There is a normal progression through puberty along with the resumption of appropriate growth velocity following treatment with levothyroxine and normalization of TSH levels.
While treatment with estrogen may be needed in the short term to stabilize dysfunctional uterine bleeding, once TSH levels have normalized and are no longer abnormally stimulated the FSH receptor any exogenous estrogen should be discontinued to optimize final adult height.
References
- 1. Van Wyk JJ, Grumbach MM. Syndrome of precocious menstruation and galactorrhea in juvenile hypothyroidism: An example of hormonal overlap in pituitary feedback. J Pediatr 1960;57:416–35. [Google Scholar]
- 2. Anasti JN, Flack MR, Froehlich J, Nelson LM, Nisula BC. A potential novel mechanism for precocious puberty in juvenile hypothyroidism. J Clin Endocrinol Metab 1995;80:276–9. [DOI] [PubMed] [Google Scholar]