Abstract
We previously showed that fetal exposure to maternal type 1 diabetes (T1D) is associated with altered glucose-stimulated insulin secretion in adult offspring. Here, we investigated whether this β-cell defect displays a sex dimorphism. Twenty-nine adult nondiabetic offspring of T1D mothers (ODMs) were compared with 29 nondiabetic offspring of T1D fathers. We measured early insulin secretion in response to oral glucose and insulin secretion rate in response to intravenous glucose ramping. Insulin sensitivity and body composition were assessed by a euglycemic, hyperinsulinemic clamp and dual-energy X-ray absorptiometry, respectively. In response to oral glucose, male and female ODMs displayed a reduced insulin secretion. In contrast, in response to graded intravenous glucose infusion, only female ODMs (not males) exhibited decreased insulin secretion. There was no defect in response to combined intravenous arginine and glucose, suggesting that male and female ODMs exhibit a functional β-cell defect rather than a reduced β-cell mass. In conclusion, fetal exposure to maternal diabetes predisposes to β-cell dysfunction in adult male and female offspring. This β-cell defect is characterized by a sexual dimorphism following intravenous glucose stimulation.
Keywords: β-cell function, fetal programing, gestational diabetes, glucose homeostasis, pregnancy, sex difference
Insulin secretion was evaluated in adults who have been exposed in utero to maternal T1D. Only women (not men) exhibited decreased insulin secretion in response to intravenous glucose.
Increasing evidence demonstrates that alteration in intrauterine environment programs the development of chronic diseases later in life [1]. For example, we previously showed that fetal exposure to maternal diabetes is associated to altered glucose-stimulated insulin secretion at an adult age [2, 3]. However, there are fundamental aspects of the control of glucose homeostasis that are regulated differently in males and females [4]. Indeed, the prevalence of prediabetic syndromes such as impaired fasting glucose is more prevalent in men, whereas impaired glucose tolerance is more prevalent in women, which is reviewed in Mauvais-Jarvis [4]. Whether fetal exposure to maternal diabetes predisposes to alteration in insulin secretion in a sexually dimorphic manner is unknown. To address this issue, we studied oral and intravenous (IV) glucose-stimulated insulin secretion in adult male and female offspring following fetal exposure to maternal diabetes.
1. Subjects and Methods
Fifty-eight (27 males, 31 females), aged ≥18 years, offspring of type 1 diabetic (T1D) subjects (as defined by the American Diabetes Association), born at least two years after parental diagnosis of diabetes were included in the study [5, 6]. These offspring had no diabetes, no family history of type 2 diabetes, and were free from immune marker of T1D. Offspring exposed to maternal T1D during pregnancy (ODM) were compared with offspring of T1D fathers (ODF) as controls. The study was approved by the ethical committee of Paris Saint-Louis Hospital, and all participants gave written informed consent.
At the first visit, a 75-g oral glucose tolerance test was performed after a 12-hour overnight fast with blood samples collected at times 0, 30, and 120 minutes for glucose and insulin measurement. Percent body fat was measured by dual-energy X-ray absorptiometer (LUNAR iDXA, ME+ 200027, GE Health Care) and fat mass, and fat-free mass were calculated.
Within a week after the first visit, we performed a 100-minute euglycemic hyperinsulinemic clamp at 80 mU/m2/min insulin infusion to measure insulin-stimulated glucose disposal rate (M value), whereas blood glucose was clamped at 100 mg/dL (5.56 mol/L) [3, 7]. The clamp was followed by a graded IV glucose infusion (glucose ramping) consisting of four consecutive 40-minute IV infusion periods of 20% glucose at 4, 8, 12, and 16 mg/kg of body weight per minute. Then, the 20% glucose infusion rate was adjusted to obtain a blood glucose level of approximately 400 mg/dL (22 mmol/L). Glucose infusion was then maintained and an IV bolus of 5 g of arginine chlorhydrate was administered in 45 seconds, with venous blood sample collection before and 2, 3, 4, 5, 10, and 15 minutes after the bolus.
A. Calculations
The insulinogenic index (Δinsulin0-30/Δglucose0-30) was used to estimate early insulin secretion during oral glucose tolerance test. Insulin secretion rate (ISR) during glucose ramping was calculated by deconvolution of the C-peptide [8] and plotted against the corresponding mean glucose concentration. The dose-response effect of glucose on ISR and glucagon secretion inhibition during glucose ramping was estimated by comparing the slope of ISR or glucagon levels as a function of glucose levels. The incremental area under the curve (AUCI) of insulin and glucagon levels during the first 5 minutes (AUCI-5) and the whole 15 minutes (AUCI-15) of the arginine test were calculated using the trapezoidal rule. We also determined the acute insulin and glucagon responses to arginine as the mean of the 2-, 3-, 4-, and 5-minute plasma levels minus the basal (prearginine) plasma concentration [9]. The M value was calculated according to DeFronzo et al., after accounting for interindividual differences in glucose space, and was expressed in milligrams per kilogram of fat-free mass per minute [10]. We also calculated the insulin sensitivity index as the ratio of M value to the mean insulin concentration during the last 20 minutes of the euglycemic hyperinsulinemic clamp.
Statistical analysis was performed using R 2.6.2 (The R Foundation for Statistical Computing, Vienna, Austria). The relationship between ISR or glucagon and glucose levels during glucose ramping was analyzed using mixed-model analysis of covariance. Fixed effects in the model included body mass index, a linear and a quadratic effect for glucose levels, a fetal exposure group effect (ODMs vs ODFs), a sex effect, and their interactions. Random subject effects (intercept and slopes) were added to the models. In response to arginine, adjustment for prestimulus insulin and glucose levels was done. All tests were two-sided and the level of significance was set at P < 0.05.
2. Results
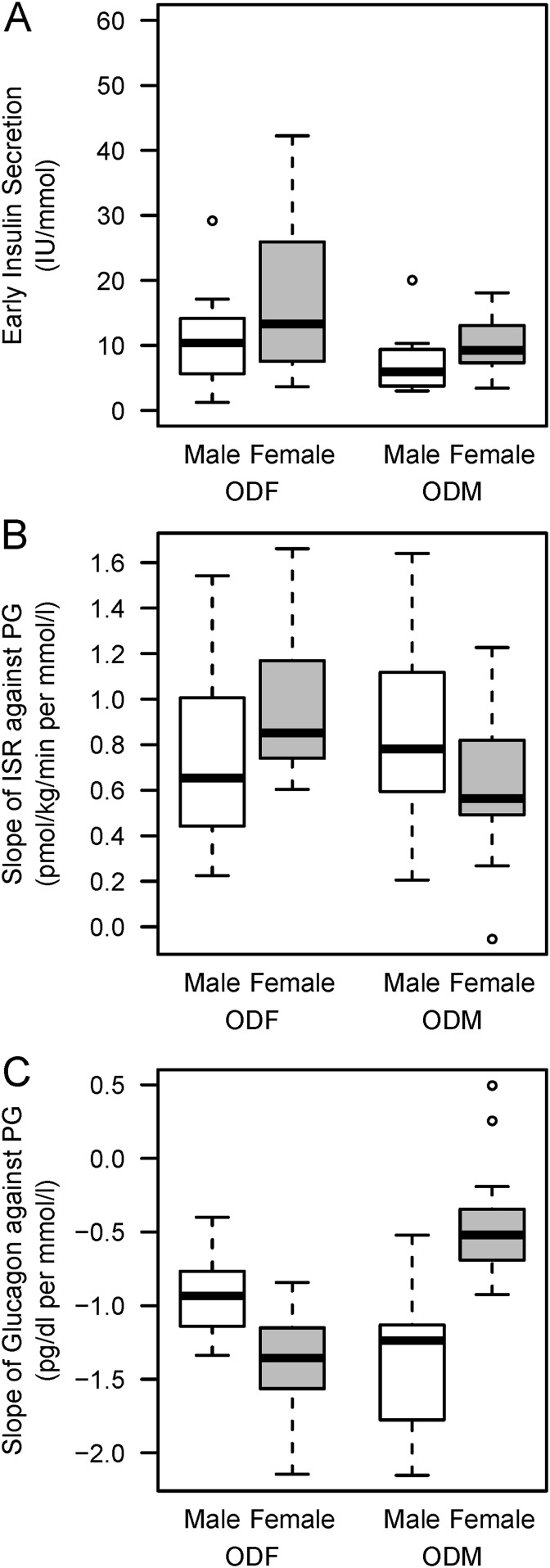
The characteristics of the offspring studied and their parents are shown in Table 1. There was no substantial difference between ODMs and ODFs with respect to anthropometric variables, routine laboratory tests, and insulin sensitivity. In response to oral glucose, the early insulin secretion was decreased in ODMs compared with ODFs (Table 2, Fig. 1A; P = 0.035). During the graded IV glucose infusion, the ISR was similar in ODMs and ODFs (Table 2). However, a significant interaction between group and offspring sex was found for the slope of ISR against plasma glucose (P = 0.03), indicating that ISR was lower and slower in female ODMs compared with female ODFs (Fig. 1B). During the graded IV glucose infusion, female ODMs also tended to exhibit reduced glucagon suppression by glucose compared with female ODFs (P = 0.07; Fig. 1C). In contrast, in response to combined glucose and arginine, insulin and glucagon secretion were similar in ODFs and ODMs with no sex effect (Table 2).
Table 1.
Participant Characteristics by Group and Sex
| ODF (n = 29) | ODM (n = 29) | ||||
|---|---|---|---|---|---|
| Male (n = 14) | Female (n = 15) | Male (n = 13) | Female (n = 16) | P a | |
| Diabetic parent characteristics | |||||
| Current age, mean (SD), y | 57.0 (6.2) | 57.7 (7.4) | 53.1 (6.1) | 55.2 (7.2) | 0.083 |
| Age at diabetes onset, mean (SD), y | 16.7 (8.5) | 19.3 (8.6) | 16.7 (8.7) | 14.4 (7.4) | 0.26 |
| Age at offspring birth, mean (SD), y | 30.4 (5.7) | 31.8 (3.7) | 27.5 (3.4) | 28.2 (4.1) | 0.006 |
| Current body-mass index, mean (SD), kg/m2 | 26.6 (4.5) | 24.2 (2.8) | 24.7 (5.1) | 25.8 (3.1) | 0.90 |
| Nephropathy, no. (%) | 3 (21) | 3 (21) | 1 (8) | 3 (20) | 0.51 |
| Retinopathy, no. (%) | 13 (93) | 12 (80) | 9 (69) | 11 (73) | 0.18 |
| Macroangiopathy, no. (%) | 6 (43) | 0 (0) | 3 (23) | 2 (14) | 0.83 |
| Birth data | |||||
| Preterm delivery, no. (%) | 0 (0) | 0 (0) | 6 (50) | 4 (25) | 0.001b |
| Birthweight, mean (SD), g | 3568 (523) | 3249 (408) | 3253 (848) | 3306 (490) | 0.56 |
| Offspring clinical characteristics | |||||
| Age, mean (SD), y | 26.6 (5.3) | 25.9 (6.9) | 25.4 (6.1) | 26.2 (6.6) | 0.82 |
| Body mass index, mean (SD), kg/m2 | 23.0 (2.5) | 22.4 (3.1) | 23.4 (3.6) | 22.9 (3.0) | 0.57 |
| SBP, mean (SD), mm Hg | 125 (11) | 117 (16) | 126 (14) | 120 (14) | 0.56 |
| DBP, mean (SD), mm Hg | 67 (9) | 66 (11) | 72 (7) | 66 (10) | 0.39 |
| Body fat, mean (SD), % | 19.0 (6.1) | 29.6 (5.6) | 19.5 (5.9) | 31.8 (6.3) | 0.41 |
| Waist circumference, mean (SD) cm | 84.1 (7.5) | 77.2 (7.8) | 82.4 (11.1) | 74.4 (6.0) | 0.35 |
| Offspring biological characteristics | |||||
| Serum creatinine, mean (SD), µmol/L | 81 (12) | 68 (9) | 87 (8) | 64 (7) | 0.71 |
| Uricemia, mean (SD), µmol/L | 343 (74) | 248 (70) | 342 (50) | 238 (50) | 0.71 |
| Total cholesterol, mean (SD), mmol/L | 4.4 (1.0) | 4.6 (0.8) | 5.4 (1.2) | 4.6 (0.7) | 0.097 |
| Triglycerides, mean (SD), mmol/L | 0.87 (0.40) | 1.05 (0.44) | 1.12 (0.44) | 0.86 (0.30) | 0.88 |
| LDL, median (IQR), mmol/L | 2.7 (2.1 to 3.1) | 2.8 (2.3 to 3.2) | 3.4 (2.7 to 4.4) | 2.5 (2.1 to 3.0) | 0.14 |
| HDL, median (IQR), mmol/L | 1.4 (1.3 to 1.6) | 1.5 (1.3 to 1.9) | 1.5 (1.3 to 1.7) | 1.6 (1.5 to 1.7) | 0.15 |
Abbreviations: DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; SD, standard deviation.
P value for group effect adjusted for sex.
Adjusted for sex and M value.
Table 2.
Metabolic Results by Group and Sex
| ODF (n = 29) | ODM (n = 29) | ||||
|---|---|---|---|---|---|
| Male (n = 14) | Female (n = 15) | Male (n = 13) | Female (n =16) | P a | |
| Oral glucose tolerance test | |||||
| Fasting plasma glucose, mean (SD), mmol/L | 4.7 (0.3) | 4.5 (0.3) | 4.8 (0.5) | 4.4 (0.3) | 0.73 |
| 2-h plasma glucose, mean (SD), mmol/L | 5.4 (0.9) | 6.0 (1.3) | 6.1 (1.0) | 6.1 (1.8) | 0.25 |
| Fasting plasma insulin, median (IQR), µU/mL | 3.1 (2.4–4.4) | 4.9 (3.4–6.8) | 3.2 (3.1–4.8) | 5.2 (3.2–6.0) | 0.88 |
| 2-h plasma insulin, median (IQR), µU/mL | 15 (10.2–18.7) | 29.2 (20.4–45.9) | 16.0 (11.6–22.3) | 22.4 (18.1–35.6) | 0.95 |
| Early insulin secretion, median (IQR), µU/mmol | 10.4 (5.9–14.2) | 13.3 (7.6–25.9) | 6.0 (3.8–9.4) | 9.3 (7.3–13.1) | 0.035b |
| Clamp study | |||||
| Adjusted M value, mean (SD), mg/kg/min | 11.9 (2.2) | 11.5 (2.8) | 10.9 (2.6) | 11.9 (3.1) | 0.74 |
| Steady-state plasma insulin, median (IQR), µU/mL | 104.5 (97.9–115.7) | 115.3 (100.8–135.1) | 109.6 (101.6–113) | 115.9 (100.2–124.7) | 0.99 |
| Insulin-sensitivity index, mean (SD) | 0.11 (0.03) | 0.11 (0.04) | 0.10 (0.02) | 0.11 (0.03) | 0.46 |
| Glucose ramping | |||||
| Insulin secretion rate, mean (SD), pmol/kg/min | 11.2 (5.9) | 15.2 (5.1) | 12.2 (4.4) | 12.5 (4.5) | 0.45 |
| Arginine test | |||||
| Plasma glucose pretest, mean (SD), mmol/L | 21.5 (3.4) | 19.8 (3.0) | 21.7 (2.9) | 22.6 (2.0) | 0.055 |
| Plasma insulin pretest, median (IQR), µU/mL | 57.6 (27.5–85.4) | 88.2 (48.7–159.6) | 39.6 (25.2–93.8) | 46.9 (32.6–74.7) | 0.13 |
| Plasma glucagon pretest, mean (SD), pg/mL | 119.8 (38.5) | 97.8 (30.5) | 108.9 (46.9) | 102.7 (40.6) | 0.79 |
| Acute insulin response, median (IQR), µU/mL | 235.1 (116.4–302.9) | 168.5 (98.6–218.6) | 222.2 (172.1–369.1) | 230 (152.8–273.6) | 0.44 |
| Acute glucagon response, median (IQR), pg/mL | 27.5 (15.2–34.6) | 29.8 (10–34.3) | 31.4 (12.9–38.4) | 23.2 (10.3 to 30.5) | 0.37 |
| Insulin AUCI-5, median (IQR), µU/mL | 1408 (598–1766) | 1061 (830–1660) | 957 (716–1930) | 1151 (742–1497) | 0.37 |
| Glucagon AUCI-5, median (IQR), pg/mL | 683 (599–856) | 584 (541–675) | 628 (610–734) | 594 (511–722) | 0.19 |
| Insulin AUCI-15, median (IQR), µU/mL | 3215 (1527–4291) | 2476 (1999–4708) | 2268 (1821–5070) | 2802 (1802–3601) | 0.34 |
| Glucagon AUCI-15, median (IQR), pg/mL | 1685 (1592–2337) | 1702 (1542–1931) | 1710 (1549–2013) | 1685 (1472–2057) | 0.26 |
P value for group effect adjusted for sex.
Adjusted for sex and M value.
Figure 1.
(A) Early insulin secretion in response to oral glucose. (B) ISR and (C) glucagon secretion suppression in response to IV glucose ramping in male and female ODM and ODF. The early insulin secretion was decreased in ODMs compared with ODFs (P = 0.035). The slopes of ISR against plasma glucose was reduced in female ODM compared with ODF (P = 0.003), and the slope of glucagon levels against plasma glucose tended to be reduced in female ODM (P = 0.07). Data are represented as boxplot showing median and interquartile range. PG, plasma glucose.
3. Discussion
The main finding of the current study is that, in response to oral glucose, males and females exposed in utero to maternal diabetes exhibit a reduced insulin secretion compared with nonexposed controls. We speculate that these alterations in insulin secretion likely reflect a defect in β-cell function, without alteration in β-cell mass, because insulin secretion in response to the combination of arginine and glucose, which in theory reflects β-cell mass [11, 12], was unaffected. In contrast, in response to graded IV glucose infusion, only females (not males) exposed in utero to maternal diabetes, exhibit decreased insulin secretion. Therefore, in males, the insulin secretory defect in response to oral glucose is no more present during IV glucose infusion. In response to IV glucose, insulin secretion is mostly dependent on the glucose stimulus of the islet, whereas, in response to oral glucose, insulin secretion is also dependent on gut incretins acting on the gut-brain-islet axis [13–15]. Together, our results lead us to speculate that, in male and female offspring exposed in utero to maternal diabetes, the gut incretin effect may be altered. In contrast, in male offspring exposed in utero to maternal diabetes, the direct β-cell stimulation of insulin secretion by glucose is preserved. In these males, we also speculate the newly identified insulinotropic action of testosterone via the androgen receptor in β cells [16] may compensate for the insulin secretory defect during IV glucose stimulation. Indeed, in absence of β-cell androgen receptors, male mice have a defective parenteral glucose-induced insulin secretion.
Our data confirm our previous findings that fetal exposure to maternal T1D diabetes is associated with a reduced insulin secretion in response to oral glucose independent of adiposity and insulin resistance [3]. In contrast, teenage offspring of T1D mothers have been reported to display insulin resistance without decreased insulin secretion in response to oral glucose compared with teenagers from the general population [17]; however, they were heavier than their controls and younger than in our study. In addition the disposition index, the product of insulin sensitivity with insulin secretion was lower, suggesting that the teenage offspring of T1D mothers also had β-cell failure.
In this study, a control group of offspring from the general population was not included. Considering the importance of environmental factors in glucose homeostasis, we believe that offspring of T1D fathers were a more representative control group (for offspring of T1D mothers), because the presence of one diabetic parent in a family may induce specific nutritional behaviors affecting the whole family, which would not be observed if the control group comprised a nondiabetic parent from the general population. We acknowledge that we could not quantify retrospectively the level of glycemic control because a majority of pregnancies occurred before 1980, a time when neither glycated hemoglobin nor blood glucose self-monitoring was available for diabetes care. However, the lack of frank macrosomia or microsomia and the birth weights not being different between groups suggest optimized blood glucose control.
In conclusion, fetal exposure to maternal diabetes predisposes to β-cell dysfunction in male and female adult offspring. However, this β-cell defect is characterized by a sexual dimorphism following IV glucose stimulation, suggesting that males are protected against or can compensate for the deleterious effect of maternal hyperglycemia on fetal β cells.
Acknowledgments
The authors thank the study participants, their parents, and their doctors (Hervé Leblanc and Thierry Gabreau, Lariboisière Hospital, Paris, France; Louise Morbois-Trabut and Camille Deybach, Montsouris Institute, Paris, France).
Financial Support: This study was supported by an institutional grant (PHRC AOR 04032; principal investigator, J.-F.G.) from Assistance Publique – Hôpitaux de Paris and by ASSERADT (a nonprofit patient association). C.a.K. was supported by grants from the International Society of Endocrinology and the Association Diabète Risque Vasculaire. F.M.-J. was supported by National Institutes of Health Grants DK074970 and DK107444), the American Diabetes Association (7-13-BS-101), and a VA Merit Review Award (BX003725). This study was also partially funded by National Institutes of Health Grant 2P30DK072476 at Pennington Biomedical Research Center (NORC).
Author Contributions: J.-F.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. J.-F.G. also designed the study, recruited the volunteers, undertook the metabolic assessment, contributed to the analysis of data, and wrote the first draft of the manuscript. M.M. and J.-P.R. contributed to the design of the study and the volunteers’ recruitment, contributed to the analysis of data, and reviewed the manuscript. R.P. carried out the statistical analyses and reviewed the manuscript. L.S.F., C.a.K., R.R., S.-P.C., S.H., and E.L. contributed to the clinical assessment, the recruitment of the volunteers, the interpretation of data, and reviewed the manuscript. F.I. and P.B. carried out the biological assays, contributed to the interpretation of data, and reviewed the manuscript. G.V. and E.R. contributed to the analysis and interpretation of data and reviewed the manuscript. F.M.-J. contributed to the analysis and interpretation of data as well as writing and review of the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AUCI
incremental area under the curve
- ISR
insulin secretion rate
- IV
intravenous
- ODF
offspring of T1D fathers
- ODM
offspring of T1D mothers
- T1D
type 1 diabetes.
References and Notes
- 1. Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4(2b, 2B):611–624. [DOI] [PubMed] [Google Scholar]
- 2. Gautier JF, Wilson C, Weyer C, Mott D, Knowler WC, Cavaghan M, Polonsky KS, Bogardus C, Pratley RE. Low acute insulin secretory responses in adult offspring of people with early onset type 2 diabetes. Diabetes. 2001;50(8):1828–1833. [DOI] [PubMed] [Google Scholar]
- 3. Sobngwi E, Boudou P, Mauvais-Jarvis F, Leblanc H, Velho G, Vexiau P, Porcher R, Hadjadj S, Pratley R, Tataranni PA, Calvo F, Gautier JF. Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet. 2003;361(9372):1861–1865. [DOI] [PubMed] [Google Scholar]
- 4. Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015;6(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abi Khalil C, Travert F, Fetita S, Rouzet F, Porcher R, Riveline JP, Hadjadj S, Larger E, Roussel R, Vexiau P, Le Guludec D, Gautier JF, Marre M. Fetal exposure to maternal type 1 diabetes is associated with renal dysfunction at adult age. Diabetes. 2010;59(10):2631–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gautier JF, Porcher R, Abi Khalil C, Bellili-Munoz N, Fetita LS, Travert F, Choukem SP, Riveline JP, Hadjadj S, Larger E, Boudou P, Blondeau B, Roussel R, Ferré P, Ravussin E, Rouzet F, Marre M. Kidney dysfunction in adult offspring exposed in utero to type 1 diabetes is associated with alterations in genome-wide DNA methylation. PLoS One. 2015;10(8):e0134654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choukem SP, Sobngwi E, Fetita LS, Boudou P, De Kerviler E, Boirie Y, Hainault I, Vexiau P, Mauvais-Jarvis F, Calvo F, Gautier JF. Multitissue insulin resistance despite near-normoglycemic remission in Africans with ketosis-prone diabetes. Diabetes Care. 2008;31(12):2332–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hovorka R, Soons PA, Young MA. ISEC: a program to calculate insulin secretion. Comput Methods Programs Biomed. 1996;50(3):253–264. [DOI] [PubMed] [Google Scholar]
- 9. Larsson H, Ahrén B. Glucose-dependent arginine stimulation test for characterization of islet function: studies on reproducibility and priming effect of arginine. Diabetologia. 1998;41(7):772–777. [DOI] [PubMed] [Google Scholar]
- 10. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. [DOI] [PubMed] [Google Scholar]
- 11. Seaquist ER, Robertson RP. Effects of hemipancreatectomy on pancreatic alpha and beta cell function in healthy human donors. J Clin Invest. 1992;89(6):1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Teuscher AU, Kendall DM, Smets YF, Leone JP, Sutherland DE, Robertson RP. Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes. 1998;47(3):324–330. [DOI] [PubMed] [Google Scholar]
- 13. Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65(1):34–43. [DOI] [PubMed] [Google Scholar]
- 14. Smith EP, An Z, Wagner C, Lewis AG, Cohen EB, Li B, Mahbod P, Sandoval D, Perez-Tilve D, Tamarina N, Philipson LH, Stoffers DA, Seeley RJ, D’Alessio DA. The role of β cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab. 2014;19(6):1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donath MY, Burcelin R. GLP-1 effects on islets: hormonal, neuronal, or paracrine? Diabetes Care. 2013;36(Suppl 2):S145–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Navarro G, Xu W, Jacobson DA, Wicksteed B, Allard C, Zhang G, De Gendt K, Kim SH, Wu H, Zhang H, Verhoeven G, Katzenellenbogen JA, Mauvais-Jarvis F. Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metab. 2016;23(5):837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vlachová Z, Bytoft B, Knorr S, Clausen TD, Jensen RB, Mathiesen ER, Højlund K, Ovesen P, Beck-Nielsen H, Gravholt CH, Damm P, Jensen DM. Increased metabolic risk in adolescent offspring of mothers with type 1 diabetes: the EPICOM study. Diabetologia. 2015;58(7):1454–1463. [DOI] [PubMed] [Google Scholar]