Abstract
Context
Spexin is a newly identified neuropeptide that is involved in satiety control, glucose, and lipids metabolism. It has also been related to human diseases, such as obesity and type 2 diabetes. However, whether spexin changes with age or not is still unclear.
Objective
The aim of this study is to investigate the relationship between circulating spexin levels and age and to study their interaction effects on body mass index (BMI), fasting glucose, and -lipids.
Design and Participants
This is a cross-sectional study, including 68 healthy adult women whose ages are in a wide range (minimum: 23; median: 38.5; maximum: 64).
Outcome Measures
The serum spexin levels were measured by an enzyme-linked immunosorbent assay. Fasting glucose, total cholesterol, triglycerides (TG), alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, urea, and creatinine were measured by routine biochemical test. Shapiro-Wilk’s test, Spearman and Pearson correlation analyses, χ2 test, and two-way analysis of variance were used to interpret the data.
Results
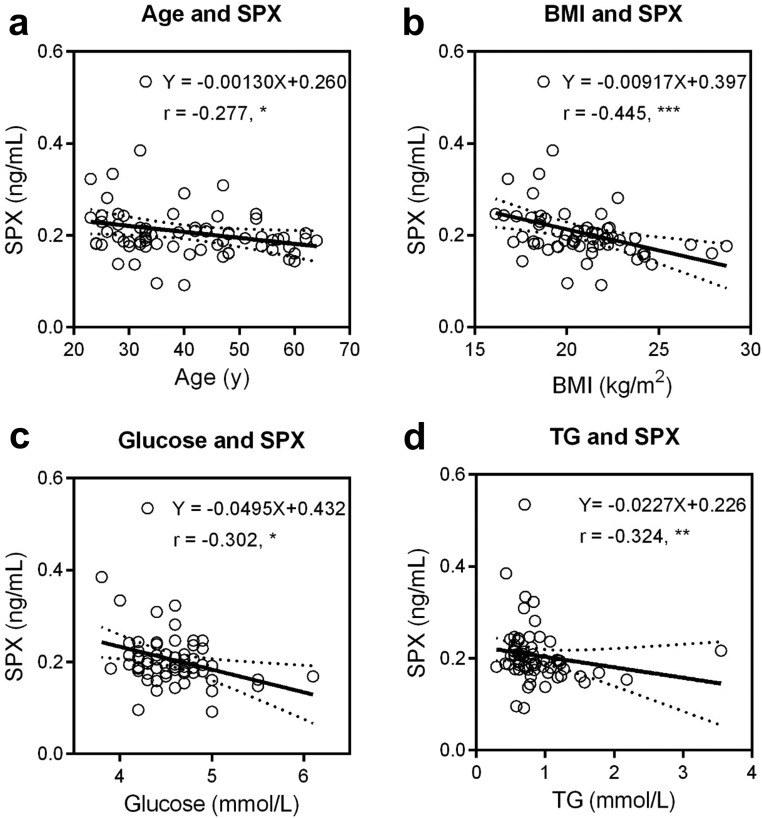
Serum spexin levels are significantly correlated with age (Spearman r = −0.277, P = 0.022), BMI (Spearman r = −0.445, P < 0.001), fasting glucose (Spearman r = −0.302, P = 0.014), and TG (Spearman r = −0.324, P = 0.008). Spexin levels independently predict the risk of high BMI and high fasting glucose. No interaction effects of spexin and age on BMI and fasting glucose were found.
Conclusions
Circulating spexin levels decrease with age, suggesting a possible role of this peptide in aging-related functions and disorders. Further investigations are needed to expand the clinical significance of this finding.
Keywords: age, body mass index, fasting glucose, healthy adult women, spexin, triglycerides
Circulating spexin levels negatively correlate with age and independently predict risk of high body mass index and fasting glucose in healthy adult women.
Spexin is a newly identified neuropeptide that was discovered by bioinformatics approaches [1, 2]. The cognate receptors of spexin and galanin receptors 2 and 3 (GALR2 and 3) were discovered in 2014 [3]. Expressed centrally and peripherally [4–7], spexin has been implicated in the regulation of satiety/food intake [8–12], gastrointestinal motility [1, 13], energy metabolism [12], and glucose/lipid metabolism [7]. It has also been suggested as a role in the control of adrenocortical cell proliferation [5], cardiovascular and renal function [14], endocrine [15], nociception [14, 16], and reproduction [15]. Spexin has been related to a number of human disorders, including obesity [17–22], type 2 diabetes mellitus (T2DM) [7, 19], fatty liver disease [19, 23], constipation [13], anxiety [24, 25], and depression [26].
Although a number of animal studies have been performed to investigate the biological functions of spexin in fish and rodents, the clinical significances of this neuropeptide, however, are still largely unknown. In 2014, Walewski et al. [17] reported that Ch12orf39, gene-encoding spexin, was remarkably downregulated (14.9-fold) in adult obese patient fat compared with the healthy human samples. In parallel, the circulating spexin levels were also decreased in obese human subjects and negatively correlated with the circulating leptin levels (Non-linear, r = −0.797) [17]. Walewski et al. [17] also demonstrated that daily spexin treatment led to reduced food intake and body weight loss in diet-induced obesity (DIO) mice/rats, possibly through inhibiting the long-chain fatty acid uptake into the adipocytes. This study suggested that spexin is an adipocyte-secreted, body-weight control factor. Approximately 1 year later, Gu et al. [7] found that serum spexin levels were significantly reduced in T2DM patients and correlated with blood glucose (Pearson r = −0.686) and triglycerides (TG; Pearson r = −0.236), indicating that spexin may also play a role in glucose and lipid metabolism. Subsequent to these pioneering works, Kumar et al. [18] reported that compared with the normal-weight subjects, circulating spexin levels were significantly reduced in obese children but did not correlate with blood glucose, insulin, or other adipokines. Strict reverse association between body mass index (BMI) and serum spexin levels were observed [18]. The same group also suggested that spexin could play a role in regulating satiety and some cardiovascular risk factors in obese children [21]. Controversially, the proposed role of spexin in childhood obesity was questioned by another group, who found that spexin did not correlate with any of the body composition, fitness, or blood measurements [20]. Very recently, Kolodziejskii et al. [22] confirmed that serum spexin levels were reduced in obese women and negatively correlated with BMI and insulin. They also found that spexin negatively correlated with glucagon and active ghrelin and positively correlated with obestatin, glucagon-like peptide-1, adiponectin, and orexin A [22].
To date, five groups worldwide have independently explored the role of spexin as metabolic regulator of body weight/glucose in obese adult/children or T2DM patients, and four of them have suggested that spexin could play an important role in obesity and T2DM. As both of these two diseases are associated with age [27, 28], we wondered if spexin would change with age or not. To that end, we collected blood samples from 68 healthy adult women of which age is in a relatively wide range (from 18 to 65). Very interestingly, we found that the circulating spexin levels were negatively correlated with age (Spearman r = −0.277, P = 0.022). The main and interaction effects of age and spexin on the BMI, fasting glucose, and lipids of subjects were also investigated.
1. Methods
A. Healthy Subjects and Serum Samples
Sixty-eight healthy women were recruited in the Clinical Division at the School of Chinese Medicine, Hong Kong Baptist University, Hong Kong, China. The recruiting criteria for a healthy population include inclusion criteria: (1) age of 18 to 65 years (inclusive); (2) no medical history of metabolic disorders, cardiovascular diseases (CVDs), neurodegenerative diseases, and gastrointestinal diseases; (3) normal hepatic, renal, and bowel functions within 3 years; (4) no drug-taking history for chronic diseases, metabolic diseases, CVD, and cerebrovascular diseases, psychiatric illness, disease of the immune system, and other serious diseases within 1 year; and (5) written, informed consent; and exclusion criteria: (1) pregnancy or breast-feeding; (2) surgical histories of gallbladder removal, gastrointestinal tract, and cerebral cranium; and (3) use of medications known to influence gastrointestinal transit, blood pressure, and fat. This study was approved by the Hong Kong Baptist University Ethics Committee on the Use of Human Subjects for Teaching and Research (approval no. HASC/16-17/0027). All participants signed an informed consent form for the project.
B. Biochemical Measurement
Several biochemical indexes, including fasting glucose, total cholesterol (TC), TG, alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, and creatinine, were tested at Chan & Hou Medical Laboratories (Hong Kong, China). Serum spexin levels were measured by using the enzyme-linked immunosorbent assay kit (cat. no. EK-023-81 CE; Research Resource Identifier: AB_2722531; Phoenix Pharmaceuticals, Belmont, CA).
C. Statistical Analysis
Descriptive statistics of all clinical characteristics and serum spexin levels were calculated with all subjects. Shapiro-Wilk’s test was performed to validate the normality of all variables. Since the distributions of most variables were significantly different from normal distribution (Supplemental Fig. S1 and Supplemental Table S1), continuous variables were summarized by median [interquartile range (IQR)]. The Spearman ranking correlations and Pearson correlation analyses were performed for age/spexin and remaining variables. Ordinal groups of spexin were formed based on tertiles, whereas the ordinal groups of age, BMI, fasting glucose, and TG were formed by using median, tertiles, and quantiles. The χ2 test was used to probe the statistical significance over these categorical variables. We also performed two-way analysis of variance (ANOVA) analysis to test the main and interaction effects of age and spexin on variables including BMI, fasting glucose, and TG. All statistical tests were two tailed, and the level of significance was set to P = 0.05. Software packages R 3.1.2 (https://www.r-project.org/), Prism 6 (GraphPad Software, La Jolla, CA), and SPSS 19 (IBM, Armonk, NY) were used for all analysis.
2. Results
A. Clinical Characteristics of Study Subjects
Demographics, clinical characteristics, and spexin concentration of the participants were analyzed and summarized in Table 1. The median and IQR age of the recruited female population was 38.5 (30.0 to 48.0) years, with median spexin concentration 0.195 (0.177 to 0.219) ng/mL. In this case, the median BMI and fasting glucose level were 20.570 (18.695 to 22.209) kg/m2 and 4.50 (4.225 to 4.775) mM, respectively. The median and IQR TC was 4.875 (4.360 to 5.580) mM, with median TG level at 0.750 (0.613 to 0.995) mM. All the other characteristics were within physical examination health range.
Table 1.
Characteristics of Study Subjects
| Characteristics | Median | Q1 | Q3 |
|---|---|---|---|
| Age, y | 38.500 | 30.000 | 48.000 |
| Spexin, ng/mL | 0.195 | 0.177 | 0.219 |
| BMI, kg/m2 | 20.570 | 18.695 | 22.209 |
| Glucose, mM | 4.500 | 4.225 | 4.775 |
| TC, mM | 4.875 | 4.360 | 5.580 |
| TG, mM | 0.750 | 0.613 | 0.995 |
| ALP, U/L | 58.500 | 52.000 | 71.000 |
| ALT, U/L | 14.000 | 10.000 | 18.000 |
| AST, U/L | 22.500 | 20.000 | 26.000 |
| Urea, mM | 4.100 | 3.500 | 5.075 |
| Creatinine, µM | 58.000 | 53.000 | 62.000 |
Abbreviation: Q, quantile.
B. Correlation Analysis Between Age/Spexin and Other Characteristics
Spearman’s ranking correlation analyses were performed in the included female subjects. Significant positive correlations were observed between age and BMI (Spearman r = 0.366, P = 0.003), fasting glucose (Spearman r = 0.304, P = 0.013), TC (Spearman r = 0.401, P < 0.001), TG (Spearman r = 0.366, P = 0.003), ALP (Spearman r = 0.423, P < 0.001), ALT (Spearman r = 0.539, P < 0.001), AST (Spearman r = 0.545, P < 0.001), and urea (Spearman r = 0.398, P < 0.001). In contrast, negative correlation was found between age and spexin concentration (Spearman r = −0.277, P = 0.022; Table 2). The spexin levels decrease slowly with age, and the slope is approximately −1.3 pg · mL−1/y (95% confidence interval: −2.5 to −0.03 pg · mL−1/y). As shown in Fig. 1 and Table 2, serum spexin levels were also negatively correlated with BMI (Spearman r = −0.445, P < 0.001), fasting glucose (Spearman r = −0.302, P = 0.014), and TG (Spearman r = 0.324, P = 0.008). Furthermore, the other characteristics, including TC, ALP, ALT, AST, urea, and creatinine, did not show correlations with spexin levels. To solidify these findings, Pearson correlation analysis was also performed. In this case, the age was shown with a consistent positive correlation between age and BMI, fasting glucose, TC, TG, ALP, ALT, AST, and urea. A reproducible negative correlation between spexin and age, fasting glucose, and BMI was noticed. However, by Pearson correlation analysis, significant correlation between spexin and urea (Pearson r = −0.277, P = 0.024) but not TG (Pearson r = −0.168, P = 0.178) was recorded (Supplemental Table S2). The correlation plots of age/spexin with other characteristics were shown in Supplemental Figs. S2 and S3.
Table 2.
Paired Correlation Analysis Between All Characteristics and Age/Spexin
| Characteristics | Correlationsa | |||
|---|---|---|---|---|
| Age, y | Spexin, ng/mL | |||
| r | P | r | P | |
| Age, y | NA | NA | −0.277 | 0.022 |
| Spexin, ng/mL | −0.277 | 0.022 | NA | NA |
| BMI, kg/m2 | 0.366 | 0.003 | −0.445 | <0.001 |
| Glucose, mM | 0.304 | 0.013 | −0.302 | 0.014 |
| TC, mM | 0.401 | <0.001 | −0.186 | 0.135 |
| TG, mM | 0.366 | 0.003 | −0.324 | 0.008 |
| ALP, U/L | 0.423 | <0.001 | −0.067 | 0.593 |
| ALT, U/L | 0.539 | <0.001 | −0.118 | 0.348 |
| AST, U/L | 0.545 | <0.001 | −0.072 | 0.567 |
| Urea, mM | 0.398 | <0.001 | −0.223 | 0.073 |
| Creatinine, µM | −0.030 | 0.813 | 0.011 | 0.932 |
Abbreviation: NA, not available.
Spearman ranking correlation (r) analyses were performed in Prism 6. The r and P values were recorded. P is in bold if the statistical test is significant (P < 0.05).
Figure 1.
The correlations between spexin (SPX) and (a) age, (b) BMI, (c) fasting glucose, and (d) TG. For each pair of variables, linear regression was performed, and the best-fit equation was presented. The linear fitting equation is shown as Y = a*X + b, where independent variable X is Age, BMI, Glucose, and TG, while dependent variable Y is SPX. The Spearman ranking correlation coefficient (r) and associated statistical significance were also shown: *P < 0.05, **P < 0.01, ***P < 0.001.
The distributions of female subjects with different spexin levels (grouped by tertiles) by age, BMI, fasting glucose, and TG, based on ordinal categorical groups, were described in Supplemental Tables S3–S6. In each of the three ordinal variables (median splits, tertiles, and quartiles), the number of subjects with maximum spexin level decreased as the age increased, with the P values of 0.197, 0.097, and 0.239 in age median splits, tertiles, and quartiles, respectively. Obviously, the distribution of subjects showed strictly reverse correlation between spexin and BMI in all of the ordinal variables, with the P values of 0.048, 0.003, and 0.035 in age median splits, tertiles, and quartiles, respectively. Similar patterns were also noticed in the case of fasting glucose and TG, although statistically not significant.
C. Correlations Between Spexin and BMI, Fasting Glucose, and TG Were Independent of Age
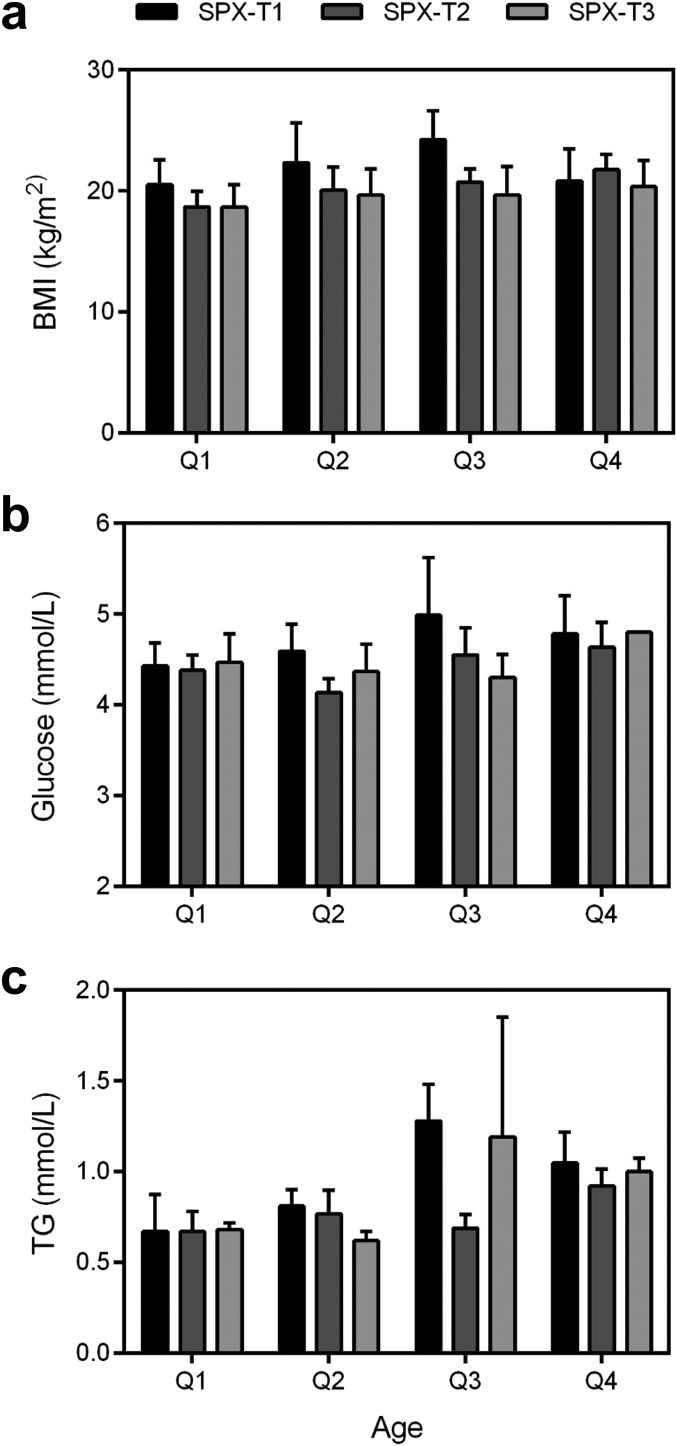
To investigate the main and interaction effects of age and spexin on BMI, fasting glucose, and TG, the female subjects were grouped by age quantiles and spexin tertiles. The quantiles of age (in years) are quantile (Q)1 (23, 30), Q2 (30, 38), Q3 (39, 48), and Q4 (48, 64). The tertiles of spexin (in nanograms per milliliter) are tertile (T)1 (0.092, 0.181), T2 (0.182, 0.209), and T3 (0.210, 0.535). The main and interaction effects were analyzed by ANOVA analysis, where F is the statistics of F-test. As shown in Table 3 and Fig. 2, the main effects of age on BMI, fasting glucose, and TG were 2.457 (P = 0.073), 3.419 (P = 0.024), and 2.332 (P = 0.084), respectively. The significant main effect of spexin on BMI (F = 5.727, P = 0.006) and fasting glucose (F = 3.382, P = 0.041) was observed, whereas the main effect of spexin on TG was shown to be 0.823 (P = 0.445). Furthermore, no significant interaction effects of age and spexin on BMI, fasting glucose, and TG were found, suggesting that the correlations between spexin and BMI, fasting glucose, and TG were age independent.
Table 3.
The Main and Interaction Effectsa of Age and Spexin on BMI, Fasting Glucose, and TG
| Ordinal Variables and Groups (Age, y) | Characteristics | Age | Spexin | Age and Spexin | |||
|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | ||
| Quartiles Q1 (23, 30) Q2 (30, 38) Q3 (39, 48) Q4 (48, 64) | BMI | 2.457 | 0.073 | 5.727 | 0.006 | 1.450 | 0.214 |
| Glucose | 3.419 | 0.024 | 3.382 | 0.041 | 1.360 | 0.247 | |
| TG | 2.332 | 0.084 | 0.823 | 0.445 | 0.576 | 0.748 | |
| Tertiles T1 (23, 32) T2 (33, 46) T3 (47,64) | BMI | 1.181 | 0.315 | 6.636 | 0.003 | 1.231 | 0.308 |
| Glucose | 3.048 | 0.055 | 4.306 | 0.018 | 0.641 | 0.654 | |
| TG | 1.699 | 0.192 | 0.908 | 0.409 | 0.304 | 0.874 | |
| Median splits M1 (23, 38) M2 (39, 64) | BMI | 4.385 | 0.041 | 7.366 | 0.010 | 0.466 | 0.630 |
| Glucose | 7.726 | 0.007 | 4.921 | 0.010 | 1.617 | 0.207 | |
| TG | 8.730 | 0.004 | 0.916 | 0.406 | 0.939 | 0.397 | |
Two-way ANOVA was used to analyze the main and interaction effects in SPSS 19. Spexin (ng/ml) was categorized into tertiles [T1 (0.092, 0.181), T2 (0.182, 0.209), and T3 (0.210, 0.535)], whereas age was categorized into quantiles (Q1, Q2, Q3 and Q4), tertiles (T1, T2 and T3), and median splits (M1 and M2). F is the statistics of F-test resulted from the ANOVA analysis. P is in bold if the statistical test is significant (P < 0.05).
Figure 2.
The (a) BMI, (b) fasting glucose, and (c) TG of subjects grouped by age quantiles and spexin (SPX) tertiles. The quantiles of age (in years) are Q1 (23, 30), Q2 (30, 38), Q3 (39, 48), and Q4 (48, 64). The tertiles of spexin (in nanograms per milliliter) are T1 (0.092, 0.181), T2 (0.182, 0.209), and T3 (0.210, 0.535).
3. Discussion
In this study, we found that circulating spexin is significantly correlated with age in healthy adult women. It is estimated that every 10 years, the average circulating spexin level reduces ~13 pg/mL. However, such correlation was not observed by Gu et al. [7] and Kumar et al. [18]. We argue that as spexin decreases slowly with age, long duration is needed to observe the trend of negative correlation; in the case of a cross-sectional study, a wide age range seems to be necessary. However, in both aforementioned studies, the age range was relatively narrow. In the study of Gu et al. [7], the ages of T2DM and healthy populations were 53.4 ± 8.5 years (means ± standard deviation) and 51.1 ± 9.1 years, whereas in the Kumar et al. [18] study, only children with the age of 15.3 ± 0.26 years were included. In contrast, the average age of subjects in our study is 40.2 ± 12.0 years, and the age is almost equally distributed in all groups, from 23 to 64 years. To confirm our hypothesis, we analyzed the age-spexin correlation in each age quantile group; not surprisingly, no substantial correlations were found (data not shown). Furthermore, the abnormal conditions, such as T2DM and obesity, could also make it difficult to find the correlations between age and circulating spexin, as the pathological conditions may suppress the expression of spexin.
In current study, the median and IQR of serum spexin concentration was 0.195 (0.177 to 0.219) ng/ml in healthy adult women, which is consistent with our previous report in the mixed adult population in Hong Kong [13]. In normal-weight children, the serum spexin level was shown to be 0.44 (0.33 to 0.62) ng/ml [18, 21]. The discrepancy of circulating spexin levels in the healthy adult women and normal children indirectly supports the notion that spexin decreases with age. To elaborate further the significance of spexin in aging, larger cross-sectional studies, including both men and women from young to old, will be needed. It could also be better to perform a longitudinal study to investigate the change of spexin from childhood to adulthood in a specific population, with more aging-related indices examined.
The underlying mechanism that causes decreasing circulating spexin with age will be of great interest to the scientific community. As a result of the strict reverse association between BMI and circulating spexin levels, it is possible that spexin decreases with age simply because of weight gain. To test such hypothesis, we analyzed the main and interaction effects of age and BMI on spexin levels by a grouped correlation analysis (Supplemental Fig. S4) and two-way ANOVA (Supplemental Table S7). Very interestingly, significant correlation (Spearman r = −0.484, P = 0.012) between age and spexin can be found in the BMI Q1 group but not in Q2, Q3, and Q4 groups (Supplemental Fig. S4). Similar trends were also observed when BMI ordinal groups were formed by tertiles and median splits (Supplemental Fig. S4). These results suggest that the effect of age on spexin levels could conditionally but not totally depend on BMI. It is also well known that the endocrine system undergoes major alterations during aging to affect the majority of body functions [29]. As one ages, the circulating levels of some hormones are unchanged—some increase, and some decrease—which may be caused by alterations in hormonal networks and concomitant hormonal deficits/excess [29]. In humans, spexin is highly expressed in adrenal gland, kidney, visceral fat, and digestive organs [7]. Aging may reduce kidney tubular epithelial cell proliferation [30] and cause the decline of adrenal hormonal and steroidal secretion [31]. It is possible that the production of spexin declines with age because of degeneration of the adrenal gland and kidney and other organs. Meanwhile, other possibilities causing decreased circulating spexin may also exist.
Extensive distribution of spexin, GALR2, and GALR3, in various tissues from the central nervous system to peripheral tissues [4, 7, 8, 32], indicates that spexin might exert multiple biofunctions. The proposed spexin receptor, GALR2, and GALR3 have been shown to be involved in mood disorder, such as seizure [33], anxiety [34, 35], and depression [34], suggesting the possible functions of spexin in the nervous system. The finding that GALR2 agonist AR-M1896 mediated a protective effect on rat basal forebrain septal neurons from Aβ neurotoxicity [36] provides indirect evidence for the involvement of spexin in the pathogenesis of Alzheimer’s disease, which is closely associated with aging. Currently, three important bio-functions of spexin have been highlighted in the regulation of appetite, body weight, and blood glucose. In clinical observations, serum spexin levels were found to be negatively correlated with BMI in obese subjects [18, 22] and blood glucose in T2DM patients [7]. In the obese DIO mice model without T2DM, spexin treatment effectively caused body-weight loss, improved glucose tolerance, and decreased insulin resistance [19]. Moreover, spexin gene expression was downregulated 14.9-fold in the human obese omental and subcutaneous fat, and spexin treatment inhibits the long-chain fatty acid uptake into the adipocytes [17]. In the current study, we demonstrated that circulating spexin levels showed expected negative correlation with BMI and fasting glucose in healthy adult women, proving that spexin may be one of the risk factors predicting obesity and diabetes. We also found that the BMI and blood glucose were positively correlated with age, which is consistent with the well-known fact that there is an age-associated increase in the prevalence of obesity and T2DM [37–40]. Combined together, the interplay among age, spexin, and BMI/blood glucose suggests a complex regulatory network. Interestingly, there were no significant interaction effects of age and spexin on BMI and fasting glucose, suggesting that the role of spexin in regulating body weight and blood glucose is independent of aging. However, we cannot exclude the possibility that spexin is involved in development of age-related obesity and diabetes.
Clinically, a large proportion of patients with obesity and/or T2DM have high TG levels [41, 42], which is associated with increased risk of CVD [43]. In the current study, we found that circulating spexin levels negatively correlated with the serum TG (P = 0.008) level in the healthy women population. In the obese DIO mice model without T2DM, the hepatic level of TG was dramatically increased, and the elevated total lipids could be significantly reversed by spexin treatment [19]. These results may interpret the recent finding that obese children with low spexin and high leptin manifested a greater risk for CVD [21].
In conclusion, circulating spexin levels decrease with age and negatively correlate with BMI, fasting glucose, and TG. As no interaction effects of spexin and age on BMI and fasting glucose were found, spexin may independently predict the risk of high BMI and high fasting glucose. The close correlation between spexin and age suggests the possible role of this peptide in aging-related functions and disorders, which need to be explored further by additional clinical studies and mechanistic investigations to elucidate the role of spexin in aging.
Supplementary Material
Acknowledgments
The authors thank the members of the Clinical Division Research Group, including Ziwan Ning, Lixiang Zhai, Wei Yang, Lin Lu, Liang Dai, and Zhen Yang, for their great help and support.
Financial Support: This study was supported by National Natural Science Foundation of China (project no. 31660335), Hong Kong Baptist University Matching Proof-of-Concept Fund (MPCF-002-2017/18), and Faculty Research Grant (project nos. FRG2/14-15/001 and FRG2/16-17/070).
Disclosure Summary: All authors have reviewed the manuscript and declare no competing financial interests.
Glossary
Abbreviations:
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- AST
aspartate aminotransferase
- BMI
body mass index
- CVD
cardiovascular disease
- DIO
diet-induced obesity
- GALR2/3
galanin receptor 2/3
- IQR
interquartile range
- Q
quantile
- T
tertile
- T2DM
type 2 diabetes mellitus
- TC
total cholesterol
- TG
triglycerides.
References and Notes
- 1. Mirabeau O, Perlas E, Severini C, Audero E, Gascuel O, Possenti R, Birney E, Rosenthal N, Gross C. Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res. 2007;17(3):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sonmez K, Zaveri NT, Kerman IA, Burke S, Neal CR, Xie X, Watson SJ, Toll L. Evolutionary sequence modeling for discovery of peptide hormones. PLOS Comput Biol. 2009;5(1):e1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim DK, Yun S, Son GH, Hwang JI, Park CR, Kim JI, Kim K, Vaudry H, Seong JY. Coevolution of the spexin/galanin/kisspeptin family: spexin activates galanin receptor type II and III. Endocrinology. 2014;155(5):1864–1873. [DOI] [PubMed] [Google Scholar]
- 4. Porzionato A, Rucinski M, Macchi V, Stecco C, Malendowicz LK, De Caro R. Spexin expression in normal rat tissues. J Histochem Cytochem. 2010;58(9):825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rucinski M, Porzionato A, Ziolkowska A, Szyszka M, Macchi V, De Caro R, Malendowicz LK. Expression of the spexin gene in the rat adrenal gland and evidences suggesting that spexin inhibits adrenocortical cell proliferation. Peptides. 2010;31(4):676–682. [DOI] [PubMed] [Google Scholar]
- 6. Porzionato A, Rucinski M, Macchi V, Stecco C, Sarasin G, Sfriso MM, Di Giulio C, Malendowicz LK, De Caro R. Spexin is expressed in the carotid body and is upregulated by postnatal hyperoxia exposure. Adv Exp Med Biol. 2012;758:207–213. [DOI] [PubMed] [Google Scholar]
- 7. Gu L, Ma Y, Gu M, Zhang Y, Yan S, Li N, Wang Y, Ding X, Yin J, Fan N, Peng Y. Spexin peptide is expressed in human endocrine and epithelial tissues and reduced after glucose load in type 2 diabetes. Peptides. 2015;71:232–239. [DOI] [PubMed] [Google Scholar]
- 8. Wong MK, Sze KH, Chen T, Cho CK, Law HC, Chu IK, Wong AO. Goldfish spexin: solution structure and novel function as a satiety factor in feeding control. Am J Physiol Endocrinol Metab. 2013;305(3):E348–E366. [DOI] [PubMed] [Google Scholar]
- 9. Ma A, He M, Bai J, Wong MK, Ko WK, Wong AO. Dual role of insulin in spexin regulation: functional link between food intake and spexin expression in a fish model. Endocrinology. 2017;158(3):560–577. [DOI] [PubMed] [Google Scholar]
- 10. Zheng B, Li S, Liu Y, Li Y, Chen H, Tang H, Liu X, Lin H, Zhang Y, Cheng CHK. Spexin suppress food intake in zebrafish: evidence from gene knockout study. Sci Rep. 2017;7(1):14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu H, Lin F, Chen H, Liu J, Gao Y, Zhang X, Hao J, Chen D, Yuan D, Wang T, Li Z. Ya-fish (Schizothorax prenanti) spexin: identification, tissue distribution and mRNA expression responses to periprandial and fasting. Fish Physiol Biochem. 2016;42(1):39–49. [DOI] [PubMed] [Google Scholar]
- 12. Li S, Liu Q, Xiao L, Chen H, Li G, Zhang Y, Lin H. Molecular cloning and functional characterization of spexin in orange-spotted grouper (Epinephelus coioides). Comp Biochem Physiol B Biochem Mol Biol. 2016;196-197:85–91. [DOI] [PubMed] [Google Scholar]
- 13. Lin CY, Zhang M, Huang T, Yang LL, Fu HB, Zhao L, Zhong LL, Mu HX, Shi XK, Leung CF, Fan BM, Jiang M, Lu AP, Zhu LX, Bian ZX. Spexin enhances bowel movement through activating L-type voltage-dependent calcium channel via galanin receptor 2 in mice. Sci Rep. 2015;5(1):12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toll L, Khroyan TV, Sonmez K, Ozawa A, Lindberg I, McLaughlin JP, Eans SO, Shahien AA, Kapusta DR. Peptides derived from the prohormone proNPQ/spexin are potent central modulators of cardiovascular and renal function and nociception. FASEB J. 2012;26(2):947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Li S, Qi X, Zhou W, Liu X, Lin H, Zhang Y, Cheng CH. A novel neuropeptide in suppressing luteinizing hormone release in goldfish, Carassius auratus. Mol Cell Endocrinol. 2013;374(1-2):65–72. [DOI] [PubMed] [Google Scholar]
- 16. Moazen P, Taherianfard M, Ahmadi Soleimani M, Norozpor M. Synergistic effect of spexin and progesterone on pain sensitivity attenuation in ovariectomized rats. Clin Exp Pharmacol Physiol. 2018;45(4):349–354. [DOI] [PubMed] [Google Scholar]
- 17. Walewski JL, Ge F, Lobdell H IV, Levin N, Schwartz GJ, Vasselli JR, Pomp A, Dakin G, Berk PD. Spexin is a novel human peptide that reduces adipocyte uptake of long chain fatty acids and causes weight loss in rodents with diet-induced obesity. Obesity (Silver Spring). 2014;22(7):1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar S, Hossain J, Nader N, Aguirre R, Sriram S, Balagopal PB. Decreased circulating levels of spexin in obese children. J Clin Endocrinol Metab. 2016;101(7):2931–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ge JF, Walewski JL, Anglade D, Berk PD. Regulation of hepatocellular fatty acid uptake in mouse models of fatty liver disease with and without functional leptin signaling: roles of NfKB and SREBP-1C and the effects of spexin. Semin Liver Dis. 2016;36(4):360–372. [DOI] [PubMed] [Google Scholar]
- 20. Hodges SK, Teague AM, Dasari PS, Short KR. Effect of obesity and type 2 diabetes, and glucose ingestion on circulating spexin concentration in adolescents. Pediatr Diabetes. 2018;19(2):212–216. [DOI] [PubMed] [Google Scholar]
- 21. Kumar S, Hossain MJ, Javed A, Kullo IJ, Balagopal PB. doi: 10.1111/ijpo.12249. Relationship of circulating spexin with markers of cardiovascular disease: a pilot study in adolescents with obesity [published online ahead of print 18 October 2017]. Pediatr Obes. doi: 10.1111/ijpo.12249. [DOI] [PMC free article] [PubMed]
- 22. Kolodziejskii PA, Pruszynska-Oszmalek E, Korek E, Sassek M, Szczepankiewicz D, Kaczmarek P, Nogowski L, Maćkowiak P, Nowak KW, Krauss H, Strowski MZ. Serum levels of spexin and kisspeptin negatively correlate with obesity and insulin resistance in women. Physiol Res. 2018;67(1):45–56. [DOI] [PubMed] [Google Scholar]
- 23. Berk PD, Verna EC. Nonalcoholic fatty liver disease: lipids and insulin resistance. Clin Liver Dis. 2016;20(2):245–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reyes-Alcaraz A, Lee YN, Son GH, Kim NH, Kim DK, Yun S, Kim DH, Hwang JI, Seong JY. Development of spexin-based human galanin receptor type II-specific agonists with increased stability in serum and anxiolytic effect in mice. Sci Rep. 2016;6(1):21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pałasz A, Janas-Kozik M, Borrow A, Arias-Carrión O, Worthington JJ. The potential role of the novel hypothalamic neuropeptides nesfatin-1, phoenixin, spexin and kisspeptin in the pathogenesis of anxiety and anorexia nervosa. Neurochem Int. 2018;113:120–136. [DOI] [PubMed] [Google Scholar]
- 26. Pałasz A, Suszka-Świtek A, Filipczyk Ł, Bogus K, Rojczyk E, Worthington J, Krzystanek M, Wiaderkiewicz R. Escitalopram affects spexin expression in the rat hypothalamus, hippocampus and striatum. Pharmacol Rep. 2016;68(6):1326–1331. [DOI] [PubMed] [Google Scholar]
- 27. Tzanetakou IP, Katsilambros NL, Benetos A, Mikhailidis DP, Perrea DN. “Is obesity linked to aging?”: adipose tissue and the role of telomeres. Ageing Res Rev. 2012;11(2):220–229. [DOI] [PubMed] [Google Scholar]
- 28. Alexander CM, Landsman PB, Grundy SM. The influence of age and body mass index on the metabolic syndrome and its components. Diabetes Obes Metab. 2008;10(3):246–250. [DOI] [PubMed] [Google Scholar]
- 29. Diamanti-Kandarakis E, Dattilo M, Macut D, Duntas L, Gonos ES, Goulis DG, Gantenbein CK, Kapetanou M, Koukkou E, Lambrinoudaki I, Michalaki M, Eftekhari-Nader S, Pasquali R, Peppa M, Tzanela M, Vassilatou E, Vryonidou A; Combo Endo Team: 2016 . Mechanisms in endocrinology: aging and anti-aging: a combo-endocrinology overview. Eur J Endocrinol. 2017;176(6):R283–R308. [DOI] [PubMed] [Google Scholar]
- 30. Schmitt R, Marlier A, Cantley LG. Zag expression during aging suppresses proliferation after kidney injury. J Am Soc Nephrol. 2008;19(12):2375–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neuhaus JF, Baris OR, Kittelmann A, Becker K, Rothschild MA, Wiesner RJ. Catecholamine metabolism induces mitochondrial DNA deletions and leads to severe adrenal degeneration during aging. Neuroendocrinology. 2017;104(1):72–84. [DOI] [PubMed] [Google Scholar]
- 32. Waters SM, Krause JE. Distribution of galanin-1, -2 and -3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience. 2000;95(1):265–271. [DOI] [PubMed] [Google Scholar]
- 33. Mazarati A, Lu X, Kilk K, Langel U, Wasterlain C, Bartfai T. Galanin type 2 receptors regulate neuronal survival, susceptibility to seizures and seizure-induced neurogenesis in the dentate gyrus. Eur J Neurosci. 2004;19(12):3235–3244. [DOI] [PubMed] [Google Scholar]
- 34. Swanson CJ, Blackburn TP, Zhang X, Zheng K, Xu ZQ, Hökfelt T, Wolinsky TD, Konkel MJ, Chen H, Zhong H, Walker MW, Craig DA, Gerald CP, Branchek TA. Anxiolytic- and antidepressant-like profiles of the galanin-3 receptor (Gal3) antagonists SNAP 37889 and SNAP 398299 [published erratums appear in Proc Natl Acad Sci USA 2006;103(14):5632 and (40):14978]. Proc Natl Acad Sci USA. 2005;102(48):17489–17494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brunner SM, Farzi A, Locker F, Holub BS, Drexel M, Reichmann F, Lang AA, Mayr JA, Vilches JJ, Navarro X, Lang R, Sperk G, Holzer P, Kofler B. GAL3 receptor KO mice exhibit an anxiety-like phenotype. Proc Natl Acad Sci USA. 2014;111(19):7138–7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ding X, MacTavish D, Kar S, Jhamandas JH. Galanin attenuates beta-amyloid (Abeta) toxicity in rat cholinergic basal forebrain neurons. Neurobiol Dis. 2006;21(2):413–420. [DOI] [PubMed] [Google Scholar]
- 37. Sakurai T, Iimuro S, Araki A, Umegaki H, Ohashi Y, Yokono K, Ito H. Age-associated increase in abdominal obesity and insulin resistance, and usefulness of AHA/NHLBI definition of metabolic syndrome for predicting cardiovascular disease in Japanese elderly with type 2 diabetes mellitus. Gerontology. 2010;56(2):141–149. [DOI] [PubMed] [Google Scholar]
- 38. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–1029. [DOI] [PubMed] [Google Scholar]
- 39. Ladabaum U, Mannalithara A, Myer PA, Singh G. Obesity, abdominal obesity, physical activity, and caloric intake in US adults: 1988 to 2010. Am J Med. 2014;127(8):717–727.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stout MB, Justice JN, Nicklas BJ, Kirkland JL. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda). 2017;32(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S.; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and MetabolismCouncil on Arteriosclerosis, Thrombosis and Vascular BiologyCouncil on Cardiovascular NursingCouncil on the Kidney in Cardiovascular Disease . Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–2333. [DOI] [PubMed] [Google Scholar]
- 42. Kushner PA, Cobble ME. Hypertriglyceridemia: the importance of identifying patients at risk. Postgrad Med. 2016;128(8):848–858. [DOI] [PubMed] [Google Scholar]
- 43. Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Nordestgaard BG, Ray KK, Reiner Z, Taskinen MR, Tokgözoglu L, Tybjærg-Hansen A, Watts GF; European Atherosclerosis Society Consensus Panel . Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32(11):1345–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.