Abstract
Luteinizing hormone/chorionic gonadotropin receptor (LHR) expression in the ovary is regulated by a messenger RNA (mRNA) binding protein, which specifically binds to the coding region of LHR mRNA. We have shown that miR-122, a short noncoding RNA, mediates LHR mRNA levels by modulating the expression of LHR mRNA–binding protein (LRBP) through the regulation of sterol regulatory element binding protein (SREBP) activation. The present results show that miR-122 regulates LRBP levels by increasing the processing of SREBP through the degradation of Insig1, the anchoring protein of SREBP. We present evidence showing that mRNA and protein levels of Insig1 undergo a time-dependent increase following the treatment of rat granulosa cells with follicle-stimulating hormone (FSH), which leads to a decrease in LRBP levels. Furthermore, overexpression of miR-122 using an adenoviral vector (AdmiR-122) abolished FSH-induced increases in Insig1 mRNA and protein. We further confirmed the role of Insig1 by showing that inhibition of Insig1 using a specific small interfering RNA prior to FSH treatment resulted in the abrogation of LHR upregulation. Silencing of Insig1 also reversed FSH-mediated decreases in SREBP and LRBP activation. These results show that decreased levels of miR-122 increase Insig1 and suppress SREBP processing in response to FSH stimulation of rat granulosa cells.
miR-122 regulates LHR expression by suppressing Insig1, thereby increasing the activation of the transcription factor SREBP, which causes an increase in the expression of the binding protein LRBP.
Luteinizing hormone/chorionic gonadotropin receptor (LHR) plays an important role in the regulation of ovarian function, including steroidogenesis, oocyte maturation, and ovulation (1–4). Understanding the mechanisms of LHR expression in the ovaries has been a focus of our laboratory. Our past studies have shown that the changes in LHR expression during the ovarian cycle in response to constantly changing hormonal milieu occur, at least in part, due to posttranscriptional mechanisms that control the steady-state levels of LHR expression through mechanisms that regulate the stability of LHR messenger RNA (mRNA). The rapid decline in LHR mRNA levels in response to conditions that mimic a preovulatory luteinizing hormone surge is controlled by the selective binding of a protein—designated LHR mRNA–binding protein (LRBP) (5–8). The changes in both the expression and LHR mRNA–binding activity of LRBP have been demonstrated in both rodent and human ovaries (7, 9). Most important, LRBP expression and its function show a reciprocal relationship with LHR levels (7, 8). The ability of LRBP to cause accelerated degradation of LHR mRNA has been demonstrated in a reconstituted LHR mRNA decay assay system (7, 8). Characterization of LRBP revealed that, like many mRNA binding proteins (10–13), LRBP—later identified as mevalonate kinase—was a metabolic enzyme involved in cholesterol biosynthesis (8). Further studies discovered that a noncoding RNA designated as miR-122 plays a key role in increasing LRBP through its ability to activate sterol regulatory element binding protein (SREBP) (14–16). SREBP is a transcription factor that has been known to regulate cholesterol and fatty acid metabolism. The current study focused on the mechanism by which miR-122 regulates SREBP-mediated LRBP expression in the context of the regulation of LHR expression in the ovary. SREBP exists in an inactive state in the endoplasmic reticulum (ER) in association with another protein—sterol cleavage activating protein (SCAP). The translocation of the SCAP-SREBP complex from the ER to the Golgi, where SREBP undergoes proteolytic cleavage into its active form, is prevented through its association with an anchoring protein called Insig1 (17). Insig1 is an ER membrane–resident protein with six transmembrane helices. Insig exists in two isoforms, Insig1 and Insig2 (18). In animal cells, in addition to causing sterol-regulated ER retention of the SCAP-SREBP complex, it has been shown to play a role in sterol-dependent degradation of 3-hydroxy-3-methylglutaryl–coenzyme A reductase (19). The role of Insig1 in SREBP activation has been established previously (20–24). Because Insig1 is predicted to be one of the targets of miR-122 (www.microrna.org) and its expression is inversely related to SREBP activation (20), we hypothesized that Insig1 might serve as the target of miR-122 in the ovaries.
We hypothesized that miR-122 might inhibit the expression of Insig1 in the ER and that this decrease might accelerate SREBP processing by proteolytic cleavage following translocation to the Golgi. The increased expression of SREBP might increase the transcription of members of the sterol regulatory element–containing gene family, including LRBP. Our results show that miR-122 increases SREBP and LRBP activation causing LHR downregulation while inhibition of miR-122 increases LHR expression by increasing the association of SREBP with Insig1.
Materials and Methods
Materials
Ovine follicle-stimulating hormone (FSH) (NIDDK-oFSH-20) and purified human chorionic gonadotropin (hCG) were purchased from Dr. A. F. Parlow (National Hormone and Peptide Program, Torrance, CA). 17 β-Estradiol (E2) was from Sigma-Aldrich (St. Louis, MO). EDTA-free protease inhibitor mixture tablets were purchased from Roche Applied Science (Indianapolis, IN). Real-time polymerase chain reaction (PCR) primers for LHR, LRBP [mevalonate kinase (MVK)], Insig1, and 18S ribosomal RNA (TaqMan Assay-on-Demand Gene Expression Products) were purchased from Applied Biosystems/Life Technologies (Foster City, CA). Pierce BCA protein assay kit was purchased from Thermo Fisher Scientific (Waltham, MA). RNase inhibitor (RNasin) was purchased from Promega (Madison, WI). Adenoviral vector containing a rat miR-122 insert, Ad-miRA-rno-mir-122 (AdmiR-122), was purchased from ABM (Richmond, Canada) and amplified and titrated [1 × 1012 plaque-forming unit (PFU)] at the University of Michigan vector core. An adenovirus expressing a scramble pre-miR with a green fluorescent protein reporter (AdGFP; 1 × 1011 PFU) was obtained from the University of Michigan vector core and was used as a control. Because LRBP was identified as MVK, anti–N-terminal mevalonate kinase immunoglobulin G was raised against the first 15 N-terminal amino acids of MVK (MLSEVLLVSAPGKVI); this antibody is referred to as the LRBP [Research Resource Identifier (RRID): AB_2722589] antibody in the text. SREBP-1a mouse monoclonal antibody (RRID: AB_2722590), which recognizes the active fragment of SREBP-1a, was raised against amino acids 301 to 407 of human SREBP-1a (ATCC, Manassas, VA). Antibodies against β-tubulin (RRID: AB_477580) and Insig1 (RRID: AB_2126794) were from Sigma-Aldrich and Santa Cruz Biotechnology (Dallas, TX), respectively. The Super Signal West Femto chemiluminescence kit and anti-rabbit/anti-mouse immunoglobulin G conjugated to horseradish peroxidase were obtained from Pierce (Rockford, IL). BCA reagent was purchased from GE Healthcare Life Sciences.
Isolation of rat granulosa cells
Rat granulosa cells were isolated as described previously (25). Briefly, 23-day-old female rats were injected subcutaneously with 1.5 mg E2 for 3 consecutive days. On day 4, rats were euthanized and their ovaries collected. Ovaries were cleared from the surrounding fat and punctured with 25-gauge needles. Cells were collected in phenol red–free Dulbecco’s modified Eagle medium (DMEM)–F12 containing 0.2% bovine serum albumin, 10 mM HEPES, and 6.8 mM EGTA; incubated for 15 minutes at 37°C under 95% O2/5% CO2; and centrifuged at 250 × g for 5 minutes. The pellets were suspended in a solution containing 0.5 M sucrose, 0.2% bovine serum albumin, and 1.8 mM EGTA in DMEM-F12 and incubated for 5 minutes at 37°C. After incubation, the suspension was diluted with 3 volumes DMEM-F12, centrifuged at 250 × g, and treated sequentially with trypsin (20 μg/mL) for 1 minute, 300 μg/mL soybean trypsin inhibitor for 5 minutes, and deoxyribonuclease I (100 μg/mL) for 5 minutes at 37°C to remove dead cells. The cells were then rinsed twice with serum-free media and suspended in DMEM-F12. The cell number was then determined, and cell viability was examined by the trypan blue exclusion method. Cells were cultured in serum-free DMEM-F12 media supplemented with 20 mM HEPES (pH 7.4), 4 mM glutamine, 100 IU penicillin/mL, and 100 μg/mL streptomycin. Before seeding, the culture dishes were coated with 10% fetal calf serum for 2 hours at 37°C and washed with DMEM-F12. Granulosa cells were cultured in 60-mm dishes in duplicates. The experiments were repeated three to four times with consistent results. The cells were routinely examined for any detectable changes in morphology. The cell viability was analyzed using the trypan blue dye exclusion assay to verify that the treatments did not adversely affect the quality of the granulosa cells. Animal handling and treatments were conducted in accordance with the accepted standards of humane animal care, as outlined in the Ethical Guidelines of the University of Michigan, and were reviewed and approved by the University Committee on Use and Care of Animals.
Treatment of rat granulosa cells with adenovirus constructs
Rat granulosa cells were infected with adenoviral constructs (AdmiR-122 or AdGFP) diluted in 3 μL serum-free DMEM for 24 hours. On the basis of previous studies (15), we chose the optimum dose of 1 × 109 PFU (200 multiplicity of infection) and a 24-hour incubation period for our studies. After 24 hours of infection, the growth medium was replaced, and the cells were incubated with FSH and E2 (FE) in fresh media for different time periods [2 (FE-2h), 4 (FE-4h), and 6 (FE-6h) hours].
Real-time (quantitative) PCR analysis
Total RNAs were reverse transcribed and subjected to real-time PCR quantitation as per the manufacturer’s instructions (Applied Biosystems). The fold change in gene expression was calculated using the comparative cycle threshold (ΔΔCt) method with 18S ribosomal RNA as the internal control, as described previously (26).
Western blot analysis
Cell lysates in radioimmunoprecipitation assay (RIPA) buffer were subjected to Western blot analysis as previously described (26). Briefly, proteins were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (10%) and transferred to a nitrocellulose membrane. The membranes were blocked in 5% fat-free skimmed milk in Tris-buffered saline–Tween-20 buffer, pH 7.4, for 1 hour at room temperature and then incubated overnight at 4°C with primary antibody in 5% fat-free milk/Tris-buffered saline–Tween-20 buffer. The membranes were then incubated with appropriate horseradish peroxidase–conjugated secondary antibodies for 1 hour at room temperature. Detection of signals was performed with an enhanced Western blotting detection system. Protein loading was normalized by reprobing the same blots with antibody against tubulin. The scanned images of the blot were quantified using ImageJ (National Institutes of Health, Bethesda, MD) software.
Transfection of rat granulosa cells with small interfering RNA
Rat granulosa cells were transfected with small interfering RNA (siRNA) targeted against Insig1 or with control siRNA (SC 37007; Santa Cruz Biotechnology) using the Amaxa Cell Line Nucleofector Kit V, following the instructions of the manufacturer (Amaxa, Cologne, Germany) as previously described in detail (26). After transfection, the cells were cultured on 35-mm dishes; after 48 hours, the media were changed to normal growth medium. Transfection efficiency was calculated by performing real-time PCR analysis of the cultured cells and analyzing the changes in the Insig1 mRNA levels.
Statistical analysis
Statistical analysis was carried out using one-way analysis of variance followed by the Tukey multiple comparison test. Values were considered statistically significant for P < 0.05.
Results
Insig1 expression is increased in rat granulosa cells in response to FSH treatment
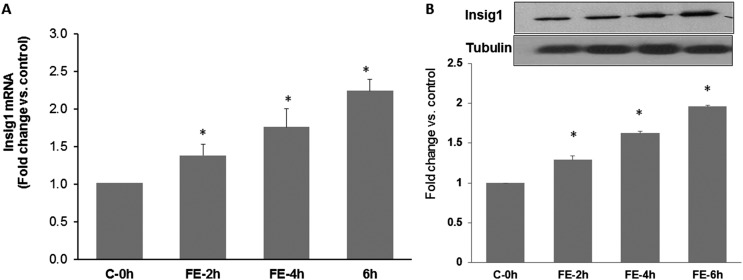
We first determined the changes in the expression of Insig1 during FSH treatment by conducting a time course study to analyze the levels of Insig1 mRNA and protein using real-time PCR and Western blot analyses, respectively. The results, as represented in Fig. 1A and 1B, conclusively show that the expressions of Insig1 and miR-122 follow a reciprocal relationship during LHR upregulation. The mRNA expression of Insig1 was increased at 2 hours (1.38 ± 0.15-fold vs control, P < 0.05; Fig. 1A) and further increased with time, reaching a maximum at 6 hours (2.24 ± 0.15-fold vs control, P < 0.05) after FSH treatment. A similar trend was also observed in the protein levels, as represented in Fig. 1B (fold change vs control: FE-2h, 1.38 ± 0.05; FE-4h, 1.65 ± 0.02; FE-6h, 1.95 ± 0.01; P < 0.05). This is in agreement with our previous studies, where we showed decreased expression of miR-122 during this time period (15). This reciprocal relationship between the expressions of miR-122 and Insig1 supported the notion that Insig1 could be a target of miR-122 in the rat granulosa cells.
Figure 1.
Time-dependent increase in Insig1 mRNA and protein by FSH and E2 treatment in isolated rat granulosa cells. Rat granulosa cells were isolated from immature female rats injected subcutaneously with estradiol (1.5 mg) for 3 consecutive days. Cells were cultured in serum-free media for 24 hours and then treated with FSH (50 ng/mL) and E2 (1 nM) for 2, 4, and 6 hours. (A) Total RNAs were isolated and reverse transcribed, and the resulting complementary DNAs were subjected to real-time PCR quantitation using specific primers and probes for Insig1. The graph represents changes in mRNA levels normalized to 18S ribosomal RNA and shown as fold change vs control. (B) S10 fractions were prepared using RIPA buffer. Equal amounts of protein from the S10 fractions were subjected to Western blot analysis using Insig1 antibody, followed by stripping and reprobing with tubulin antibody. The graph represents the quantitative analysis of the bands in the autoradiogram. Error bars represent mean ± standard error. *P < 0.05 vs C-0h; n = 4. C-0h, control at 0 hours.
Overexpression of miR-122 inhibited FSH-mediated increases in Insig1 expression
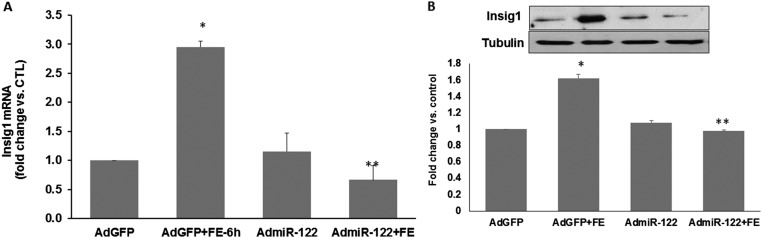
To further confirm the role of Insig1 in the miR-122–mediated regulation of LHR in the ovaries, we examined whether the increases in Insig1 during FSH-mediated LHR upregulation can be inhibited by overexpressing miR-122. This was accomplished by using adenoviral vector–mediated transfection of rat granulosa cells with miR-122 to determine what effect overexpression of miR-122 produces on Insig1 levels. Real-time PCR analysis of Insig1 mRNA levels after treatment with FSH and E2 in the presence and absence of AdmiR-122 showed that overexpression of miR-122 significantly inhibited FSH-mediated increases in Insig1 expression (Fig. 2A). As shown in the figure, there was a 2.96-fold increase (P < 0.05) in the levels of Insig1 mRNA in the FSH-treated samples compared with control; however, this was inhibited significantly by AdmiR-122 (0.67-fold vs control, P < 0.05). This was also confirmed using Western blot analysis. AdmiR-122 significantly suppressed the FSH-induced increases in the protein expression of Insig1 (Fig. 2B; fold change vs AdGFP: AdGFP + FE, 1.68 ± 0.03; AdmiR-122 + FE, 0.98 ± 0.06; P < 0.05 vs AdGFP + FE). These results confirmed our hypothesis that miR-122 targets Insig1 mRNA in the ovaries and that LHR upregulation during follicle growth is probably facilitated by increasing Insig1 levels.
Figure 2.
Adenovirus-mediated overexpression of miR-122 reverses FSH-mediated increases in Insig1. Rat granulosa cells were isolated from immature female rats injected subcutaneously with estradiol (1.5 mg) for 3 consecutive days. Cells were pretreated with AdmiR-122 (1 × 109 PFU) or AdGFP (1 × 109 PFU) for 24 hours. Media were replaced after 24 hours and cells were treated with FSH (50 ng/mL) and E2 (1 nM) for 6 hours. (A) Total RNAs were isolated and reverse transcribed, and the resulting complementary DNAs were subjected to real-time PCR quantitation using specific primers and probes for LHR. The graph represents changes in mRNA levels normalized to 18S ribosomal RNA and shown as fold change vs control. (B) S10 fractions were prepared using RIPA buffer. Equal amounts of protein from the S10 fractions were subjected to Western blot analysis using Insig1 antibody, followed by stripping and reprobing with tubulin antibody. The graph represents quantitative analysis of the bands in the autoradiogram. Error bars represent mean ± standard error. *P < 0.05 vs AdGFP; **P < 0.05 vs AdGFP + FE; n = 4. CTL, control.
miR-122 overexpression reversed FSH-induced decreases in SREBP activation and LRBP expression
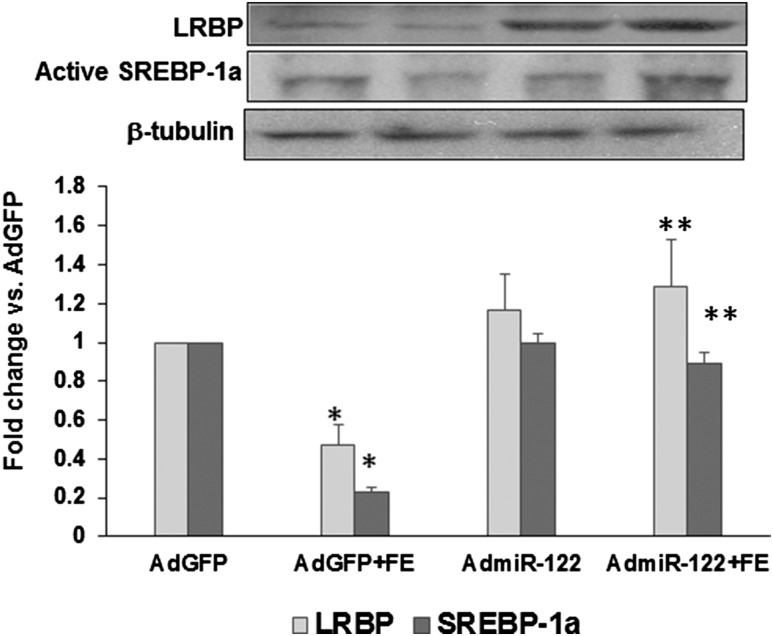
The changes in SREBP and LRBP expression following overexpression of miR-122 were examined by pretreating the cells with AdmiR-122 to increase miR-122 prior to treatment with FSH and E2, then analyzing the levels of active SREBP and LRBP in the cell lysates. Active SREBP was detected as a distinct band corresponding to 68 kDa in the Western blot analysis. The band density was quantified using image analysis software as described in the methods. The results showed that, as expected, there was a decrease in the levels of active SREBP-1a following FSH + E2 treatment, as shown in Fig. 3. This increase was reversed in the presence of AdmiR-122 (lane 2, fold change vs AdGFP: AdGFP + FE, 0.22 ± 0.02; AdmiR-122 + FE, 0.9 ± 0.04; P < 0.05 vs AdGFP + FE). Concurrently, the FSH-mediated decrease in the expression of LRBP was also inhibited by AdmiR-122 treatment (lane 1; fold change vs AdGFP: AdGFP + FE, 0.4 ± 0.1; AdmiR-122 + FE, 1.2 ± 0.2; P < 0.05 vs AdGFP + FE). These results supported our hypothesis that miR-122 initiates a series of events that lead to an increase in LRBP levels that decrease LHR expression.
Figure 3.
FSH-mediated decreases in the protein expressions of active SREBP and LRBP were inhibited by the overexpression of miR-122. Rat granulosa cells were isolated from immature female rats injected subcutaneously with estradiol (1.5 mg) for 3 consecutive days. Cells were pretreated with AdmiR-122 (1 × 109 PFU) or AdGFP (1 × 109 PFU) for 24 hours. Media were replaced after 24 hours and cells were treated with FSH (50 ng/mL) and E2 (1 nM) for 6 hours. S10 fractions were prepared using RIPA buffer. Equal amounts of protein from the S10 fractions were subjected to Western blot analysis using LRBP antibody, followed by stripping and reprobing with SREBP-1a, and tubulin antibodies. The graph represents a quantitative analysis of the bands in the autoradiogram. Error bars represent mean ± standard error. *P < 0.05 vs AdGFP; **P < 0.05 vs AdGFP + FE; n = 4.
Silencing Insig1 expression reversed FSH-mediated decreases in SREBP activation and LRBP expression
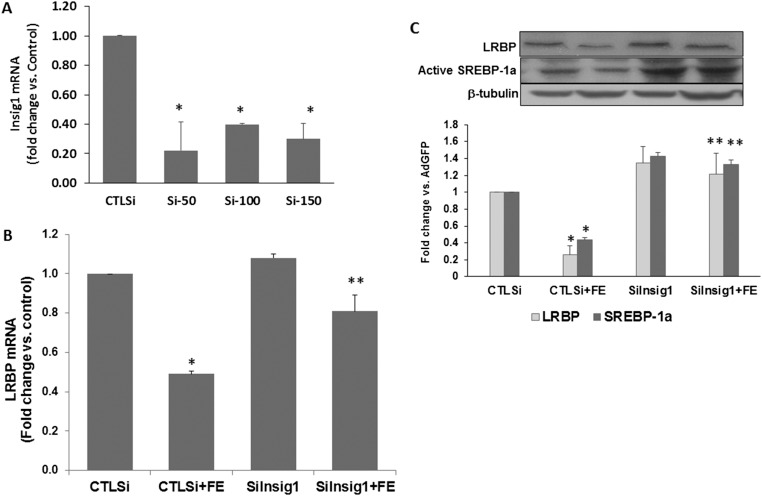
The preceding results were further confirmed by testing the effect siRNA-mediated silencing of Insig1 would have on FSH-induced LHR expression. Rat granulosa cells were transfected with siRNA against Insig1, and the resultant depletion of Insig1 was verified using real-time PCR (Fig. 4A). As shown in the figure, all three doses (50-, 100-, and 150-nM final concentrations) silenced the expression of Insig1 mRNA significantly compared with control. For further studies, we chose 50 nM siRNA to suppress Insig1 [fold change vs control siRNA (CTLsi), 0.22 ± 0.19, P < 0.05]. Rat granulosa cells were treated with FSH and E2 48 hours after exposing cultures to siRNA treatment. Cell lysates were analyzed for the expression of LRBP mRNA and protein, as well as SREBP activation. As expected, LRBP mRNA level was decreased (15) in response to FSH treatment (Fig. 4B, CTLsi + FE, 0.49-fold vs control). The inhibition of Insig1 abrogated this decrease [Insig1 siRNA (siInsig1) + FE, 0.81-fold vs control; P < 0.05 vs CTLsi + FE]. The Western blot results showing the bands representing active SREBP-1a and LRBP are shown in Fig. 4C. As indicated in the figure, LRBP protein expression showed the same trend as LRBP mRNA. siInsig1 inhibited FSH-mediated decreases in LRBP protein. As expected, silencing Insig1 resulted in an increase of SREBP activation in the presence and absence of FSH. These studies provide convincing evidence for the role of Insig1 in the miR-122–SREBP–LRBP pathway, which in turn controls LHR expression.
Figure 4.
Insig1 silencing reversed FSH-mediated decreases in SREBP activation and LRBP expression during LHR upregulation. Rat granulosa cells were isolated from immature female rats injected subcutaneously with estradiol (1.5 mg) for 3 consecutive days. Cells were transfected with 50 nM negative CTLsi or 50 (Si-50), 100 (Si-100), or 150 (Si-150) nM Insig1 small interfering RNA (siIngsig1) and cultured for 48 hours. One group of siRNA (50 nM)–transfected cells was then treated with FSH (50 ng/mL) and E2 (1 nM) for 6 hours. Total RNAs were isolated and reverse transcribed, and the resulting complementary DNAs were subjected to real-time PCR quantitation using specific primers and probes for (A) Insig1 or (B) LRBP. The graph represents changes in mRNA levels normalized to 18S ribosomal RNA and shown as fold change vs control. (C) S10 fractions were prepared using RIPA buffer. Equal amounts of protein from the S10 fractions were subjected to Western blot analysis using LRBP antibody, followed by stripping and reprobing with SREBP-1a and tubulin antibodies. The graph represents a quantitative analysis of the bands in the autoradiogram. Error bars represent mean ± standard error. *P < 0.05 vs CTLsi; **P < 0.05 vs CTLsi + FE; n = 4.
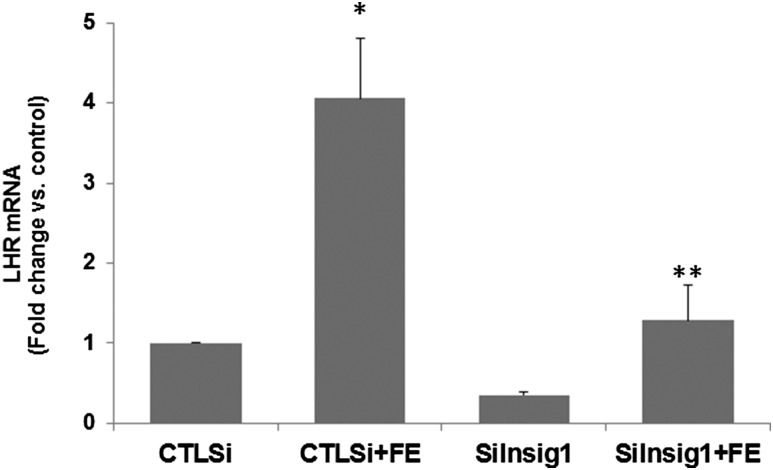
FSH-mediated LHR upregulation was inhibited by Insig1 silencing
Results of the analysis of LHR expression following FSH treatment in the presence and absence of Insig siRNA are represented in Fig. 5. The upregulation of LHR brought about by FSH treatment was completely abrogated by inhibiting Insig1 (fold change vs CTLsi: CTLsi + FE, 4.06 ± 0.79; siInsig1 + FE, 1.29 ± 0.44; P < 0.05 vs CTLsi + FE). This result establishes the role of Insig1 in the regulation of LH receptor expression in the ovaries.
Figure 5.
Insig1 silencing inhibited FSH-mediated LHR upregulation. Rat granulosa cells were isolated from immature female rats injected subcutaneously with estradiol (1.5 mg) for 3 consecutive days. Cells were transfected with 50 nM of either negative CTLsi or siInsig1 and cultured for 48 hours. Cells were then treated with FSH (50 ng/mL) and E2 (1 nM) for 24 hours. Total RNAs were isolated and reverse transcribed, and the resulting complementary DNAs were subjected to real-time PCR quantitation using specific primers and probes for LHR. The graph represents changes in mRNA levels normalized to 18S ribosomal RNA and shown as fold change vs control. Error bars represent mean ± standard error. *P < 0.05 vs CTLsi; **P < 0.05 vs CTLsi + FE; n = 4.
Role of miR-122 in LHR downregulation
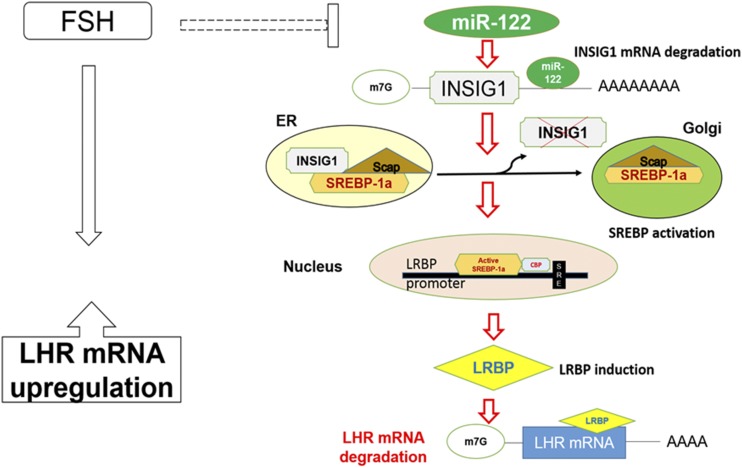
The mechanism of SREBP processing, including localization in different cellular compartments, has been well established (20–24, 27, 28). The model presented in Fig. 6 is based on this information as well as our studies (14–16, 29). During LH/hCG-induced LHR downregulation, miR-122 expression is increased, leading to a decrease in the expression of Insig1. By silencing Insig1, the SREBP-SCAP complex translocates freely from the ER to the Golgi (27), where SREBP undergoes proteolytic cleavage to produce its active form. Active SREBP binds to the promoter region of LRBP (14), resulting in an enhanced expression of LRBP protein, which in turn binds to LHR mRNA and induces its degradation (30). During FSH-induced LHR upregulation, this pathway is suppressed by inhibiting miR-122 expression (15).
Figure 6.
Schematic model depicting the role of miR-122 in the regulation of LHR in rat granulosa cells. The model depicted in the figure represents how miR-122 regulates LHR expression by modulating the levels of its binding protein LRBP in the ovaries. The dashed line indicates that several steps might be involved in this process. miR-122 directly targets Insig1 mRNA and induces its degradation, thereby decreasing its expression and removing its inhibitory effect on SREBP activation. SREBP processing in different intracellular organelles has been established previously (27, 28). During FSH-mediated upregulation of LHR, expression of miR-122 is suppressed, thereby inhibiting the LRBP-mediated degradation pathway and facilitating increases in LHR mRNA expression.
Discussion
As described earlier, we have established that LHR expression is regulated, at least in part, through a posttranscriptional mechanism mediated by an RNA binding protein. Furthermore, the expression of this RNA binding protein, characterized as being mevalonate kinase, has been shown to be regulated by miR-122. Although miR-122 was initially identified as a liver-specific noncoding RNA, its presence has now been established in several nonhepatic tissues, including the ovary (16, 31). In the ovary, we have for the first time shown, to our knowledge, that miR-122 regulates LHR levels by inducing LRBP expression through the activation of the transcription factor SREBP (14, 16). During LH/hCG-induced downregulation of LHR, miR-122 expression shows an increase and leads to the activation of the SREBP-LRBP pathway (16). Conversely, during FSH-induced LHR upregulation, miR-122 expression is suppressed (15), thereby inhibiting the LRBP-mediated LHR degradation pathway. The current study shows the missing link between miR-122 and SREBP activation. We examined the possibility that Insig1 could serve as a direct target of miR-122 to regulate LRBP levels and influence LHR expression. Using adenoviral overexpression and siRNA-mediated silencing approaches, we have provided evidence to show that miR-122 increases SREBP-mediated LRBP induction in ovaries by targeting Insig1 mRNA.
The crucial role played by Insig proteins in lipid metabolism is well documented in the literature. Knockout of Insig1 has been shown to cause cholesterol and triglyceride overaccumulation in the mouse liver (32). Furthermore, overexpression of Insig1 has been shown to inhibit SREBP activation (21). It has been shown that Insig1 mediates cholesterol biosynthesis through its sterol-dependent binding to SCAP and β-hydroxy β-methylglutaryl–CoA reductase proteins (24, 33). In addition, Insig1 has been shown to inhibit the exit of the SCAP-SREBP complex from the ER to the Golgi (23, 24, 34), thereby negatively regulating β-hydroxy β-methylglutaryl–CoA reductase transcription by suppressing activation of SREBP (19, 35). In agreement with this, our past studies have shown that SREBP-1a binds to the promoter region of LRBP/MVK, leading to an increase in its expression during ligand-mediated LHR downregulation (14). The inhibition of SREBPs was also shown to inhibit hCG-mediated LRBP/MVK induction (14).
We present evidence showing that miR-122 activates SREBPs in the ovary by directly targeting Insig1. Reports in the literature show other microRNAs (miRNAs) targeting Insig1 mRNA. For example, insulin signaling in adipose tissue has been modulated by miR-29 through Insig1 (36). However, ours is the first study, to our knowledge, to show the regulation of Insig1 by miR-122. It is not understood at the present time how miR-122 decreases Insig1 mRNA levels. Based on a recent study showing downregulation of the translation of the 1.4-kb Insig1 isoform mRNA by a miR-122 precursor that resulted in the downregulation of Insig1 protein abundance (37), it is possible that a similar mechanism might also operate in this system. Additional studies are needed to understand the molecular details involved in the regulation of Insig1 expression by miR-122.
Based on the various reports, it appears that RNA -binding proteins are regulated by miRNAs to fine-tune gene expression. Reports in the literature show the regulation of RNA binding proteins by miRNAs, with potential functional consequences that are similar to those in the current study. For example, the RNA binding protein Musashi 1 (Msi1), which acts at the translation level to control stem cell fate, nervous system development, and tumorigenesis, has been shown to be regulated by a class of tumor suppressor miRNAs (miR-34a, miR-101, miR-128, miR-137, and miR-138) in glioblastoma and medulloblastoma cells (38–40). These miRNAs cooperatively bind to the 3′UTR of Musashi, thereby negatively affecting the proliferation of glioblastoma cells. Similarly, a regulatory axis involving at least three miRNAs (miR-24, miR-221, and miR-222) and one RNA binding protein (Dnd1) that all converge onto p27Kip1 regulation in tumor cell lines has also been a topic of interest (41). However, miR-122 increases the expression of LRBP indirectly by targeting Insig1, which affects SREBP levels. This connection is important, because the LHR mRNA–binding protein LRBP is also an enzyme in the cholesterol biosynthetic pathway, which highlights the intriguing possibility that posttranscriptional regulation of the luteinizing hormone receptor and sterol metabolism are linked in regulating steroid hormone biosynthesis by the ovary. Although sterol metabolism is important in supplying cholesterol, the precursor of steroid hormones, LHR generates this signal for regulating steroid hormone production.
In summary, the current study shows that by regulating the expression of miR-122, Insig1 expression is altered in the ovaries, as depicted in Fig. 6. This leads to changes in the processing of SREBP, which, in turn, regulates LRBP. Because LRBP negatively affects LHR expression, an increase or decrease in miR-122 levels decreases or increases LHR expression. It is well established that LHR undergoes dynamic changes during different phases of the ovarian cycle and that appropriate levels of LHR expression are crucial for supporting key reproductive processes such as ovulation and corpus luteum function. This study provides new insights, to our knowledge, into the mechanism by which LHR expression is regulated in the ovary by the miR-122–LRBP cascade.
Acknowledgments
We appreciate Ms. Sarah Block for her help in the careful reading of and editorial assistance with the manuscript.
Financial Support: This work was supported by National Institutes of Health Grant R01 HD06656-41.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AdGFP
adenovirus expressing a scramble pre-miR with a green fluorescent protein reporter
- CTLsi
control small interfering RNA
- DMEM
Dulbecco’s modified Eagle medium
- E2
17 β-estradiol
- ER
endoplasmic reticulum
- FE
follicle-stimulating hormone and 17 β-estradiol
- FE-2h
2-hour incubation with follicle-stimulating hormone and 17 β-estradiol
- FE-4h
4-hour incubation with follicle-stimulating hormone and 17 β-estradiol
- FE-6h
6-hour incubation with follicle-stimulating hormone and 17 β-estradiol
- FSH
follicle-stimulating hormone
- hCG
human chorionic gonadotropin
- LHR
luteinizing hormone/chorionic gonadotropin receptor
- LRBP
luteinizing hormone receptor messenger RNA–binding protein
- mRNA
messenger RNA
- miRNA
microRNA
- MVK
mevalonate kinase
- PCR
polymerase chain reaction
- PFU
plaque-forming unit
- RIPA
radioimmunoprecipitation assay
- RRID
Research Resource Identifier
- SCAP
sterol cleavage activating protein
- siInsig1
Insig1 small interfering RNA
- siRNA
small interfering RNA
- SREBP
sterol regulatory element binding protein
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| β-Tubulin | Monoclonal anti–β-tubulin (clone 2-28-33) | Sigma, T 5293 | Mouse; monoclonal | 1:10,000 | AB_477580 | |
| SREBP-1a | Amino acids 301–407 of human SREBP1 | SREBP-1a | ATCC, Manassas, VA | Mouse; monoclonal | 1:1000 | AB_2722590 |
| MVK | First 15 N-terminal amino acids of MVK (MLSEVLLVSAPGKVI) | LRBP | Pacific Immunology | Rabbit; polyclonal | 1:500 | AB_2722589 |
| Insig1 | Insig-1 (M-15) | Santa Cruz Biotechnology, sc-51103 | Goat; polyclonal | 1:1000 | AB_2126794 |
References
- 1. Menon KMJ, Clouser CL, Nair AK. Gonadotropin receptors: role of post-translational modifications and post-transcriptional regulation. Endocrine. 2005;26(3):249–258. [DOI] [PubMed] [Google Scholar]
- 2. Menon KMJ, Munshi UM, Clouser CL, Nair AK. Regulation of luteinizing hormone/human chorionic gonadotropin receptor expression: a perspective. Biol Reprod. 2004;70(4):861–866. [DOI] [PubMed] [Google Scholar]
- 3. Ascoli M, Puett D. The gonadotropin hormones and their receptors In: Strauss JF III, Barbieri RL, eds. Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 6th ed.Amsterdam: Elsevier; 2009:35–55. [Google Scholar]
- 4. Dufau ML. The luteinizing hormone receptor. Annu Rev Physiol. 1998;60(1):461–496. [DOI] [PubMed] [Google Scholar]
- 5. Kash JC, Menon KMJ. Identification of a hormonally regulated luteinizing hormone/human chorionic gonadotropin receptor mRNA binding protein: increased mRNA binding during receptor down-regulation. J Biol Chem. 1998;273(17):10658–10664. [DOI] [PubMed] [Google Scholar]
- 6. Kash JC, Menon KMJ. Sequence-specific binding of a hormonally regulated mRNA binding protein to cytidine-rich sequences in the lutropin receptor open reading frame. Biochemistry. 1999;38(51):16889–16897. [DOI] [PubMed] [Google Scholar]
- 7. Nair AK, Kash JC, Peegel H, Menon KMJ. Post-transcriptional regulation of luteinizing hormone receptor mRNA in the ovary by a novel mRNA-binding protein. J Biol Chem. 2002;277(24):21468–21473. [DOI] [PubMed] [Google Scholar]
- 8. Nair AK, Menon KMJ. Isolation and characterization of a novel trans-factor for luteinizing hormone receptor mRNA from ovary. J Biol Chem. 2004;279(15):14937–14944. [DOI] [PubMed] [Google Scholar]
- 9. Nair AK, Peegel H, Menon KMJ. The role of luteinizing hormone/human chorionic gonadotropin receptor-specific mRNA binding protein in regulating receptor expression in human ovarian granulosa cells. J Clin Endocrinol Metab. 2006;91(6):2239–2243. [DOI] [PubMed] [Google Scholar]
- 10. Klausner RD, Rouault TA. A double life: cytosolic aconitase as a regulatory RNA binding protein. Mol Biol Cell. 1993;4(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klausner RD, Rouault TA, Harford JB. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993;72(1):19–28. [DOI] [PubMed] [Google Scholar]
- 12. Rouault TA, Stout CD, Kaptain S, Harford JB, Klausner RD. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991;64(5):881–883. [DOI] [PubMed] [Google Scholar]
- 13. Hentze MW, Kühn LC. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci USA. 1996;93(16):8175–8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Menon B, Gulappa T, Menon KMJ. miR-122 regulates LH receptor expression by activating sterol response element binding protein in rat ovaries. Endocrinology. 2015;156(9):3370–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Menon B, Gulappa T, Menon KMJ. Molecular regulation of LHCGR expression by miR-122 during follicle growth in the rat ovary. Mol Cell Endocrinol. 2017;442:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Menon B, Sinden J, Franzo-Romain M, Botta RB, Menon KMJ. Regulation of LH receptor mRNA binding protein by miR-122 in rat ovaries. Endocrinology. 2013;154(12):4826–4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong XY, Tang SQ, Chen JD. Dual functions of Insig proteins in cholesterol homeostasis. Lipids Health Dis. 2012;11(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sever N, Song BL, Yabe D, Goldstein JL, Brown MS, DeBose-Boyd RA. Insig-dependent ubiquitination and degradation of mammalian 3-hydroxy-3-methylglutaryl-CoA reductase stimulated by sterols and geranylgeraniol. J Biol Chem. 2003;278(52):52479–52490. [DOI] [PubMed] [Google Scholar]
- 20. Boden G, Salehi S, Cheung P, Homko C, Song W, Loveland-Jones C, Jayarajan S. Comparison of in vivo effects of insulin on SREBP-1c activation and INSIG-1/2 in rat liver and human and rat adipose tissue. Obesity (Silver Spring). 2013;21(6):1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Engelking LJ, Kuriyama H, Hammer RE, Horton JD, Brown MS, Goldstein JL, Liang G. Overexpression of Insig-1 in the livers of transgenic mice inhibits SREBP processing and reduces insulin-stimulated lipogenesis. J Clin Invest. 2004;113(8):1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dobrosotskaya IY, Goldstein JL, Brown MS, Rawson RB. Reconstitution of sterol-regulated endoplasmic reticulum-to-Golgi transport of SREBP-2 in insect cells by co-expression of mammalian SCAP and Insigs. J Biol Chem. 2003;278(37):35837–35843. [DOI] [PubMed] [Google Scholar]
- 23. Sun LP, Li L, Goldstein JL, Brown MS. Insig required for sterol-mediated inhibition of Scap/SREBP binding to COPII proteins in vitro. J Biol Chem. 2005;280(28):26483–26490. [DOI] [PubMed] [Google Scholar]
- 24. Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R, Goldstein JL, Brown MS. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 2002;110(4):489–500. [DOI] [PubMed] [Google Scholar]
- 25. Pradeep PK, Li X, Peegel H, Menon KMJ. Dihydrotestosterone inhibits granulosa cell proliferation by decreasing the cyclin D2 mRNA expression and cell cycle arrest at G1 phase. Endocrinology. 2002;143(8):2930–2935. [DOI] [PubMed] [Google Scholar]
- 26. Menon B, Franzo-Romain M, Damanpour S, Menon KMJ. Luteinizing hormone receptor mRNA down-regulation is mediated through ERK-dependent induction of RNA binding protein. Mol Endocrinol. 2011;25(2):282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daemen S, Kutmon M, Evelo CT. A pathway approach to investigate the function and regulation of SREBPs. Genes Nutr. 2013;8(3):289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsuda M, Korn BS, Hammer RE, Moon YA, Komuro R, Horton JD, Goldstein JL, Brown MS, Shimomura I. SREBP cleavage-activating protein (SCAP) is required for increased lipid synthesis in liver induced by cholesterol deprivation and insulin elevation. Genes Dev. 2001;15(10):1206–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Menon KMJ, Nair AK, Wang L, Peegel H. Regulation of luteinizing hormone receptor mRNA expression by a specific RNA binding protein in the ovary. Mol Cell Endocrinol. 2007;260-262:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Menon KMJ, Nair AK, Wang L. A novel post-transcriptional mechanism of regulation of luteinizing hormone receptor expression by an RNA binding protein from the ovary. Mol Cell Endocrinol. 2006;246(1–2):135–141. [DOI] [PubMed] [Google Scholar]
- 31. Sirotkin AV, Kisová G, Brenaut P, Ovcharenko D, Grossmann R, Mlyncek M. Involvement of microRNA Mir15a in control of human ovarian granulosa cell proliferation, apoptosis, steroidogenesis, and response to FSH. MicroRNA. 2014;3(1):29–36. [DOI] [PubMed] [Google Scholar]
- 32. Engelking LJ, Liang G, Hammer RE, Takaishi K, Kuriyama H, Evers BM, Li WP, Horton JD, Goldstein JL, Brown MS. Schoenheimer effect explained—feedback regulation of cholesterol synthesis in mice mediated by Insig proteins. J Clin Invest. 2005;115(9):2489–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sever N, Yang T, Brown MS, Goldstein JL, DeBose-Boyd RA. Accelerated degradation of HMG CoA reductase mediated by binding of insig-1 to its sterol-sensing domain. Mol Cell. 2003;11(1):25–33. [DOI] [PubMed] [Google Scholar]
- 34. Yabe D, Komuro R, Liang G, Goldstein JL, Brown MS. Liver-specific mRNA for Insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc Natl Acad Sci USA. 2003;100(6):3155–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Espenshade PJ, Hughes AL. Regulation of sterol synthesis in eukaryotes. Annu Rev Genet. 2007;41(1):401–427. [DOI] [PubMed] [Google Scholar]
- 36. He A, Zhu L, Gupta N, Chang Y, Fang F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol. 2007;21(11):2785–2794. [DOI] [PubMed] [Google Scholar]
- 37. Norman KL, Chen TC, Zeiner G, Sarnow P. Precursor microRNA-122 inhibits synthesis of Insig1 isoform mRNA by modulating polyadenylation site usage. RNA. 2017;23(12):1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vo DT, Abdelmohsen K, Martindale JL, Qiao M, Tominaga K, Burton TL, Gelfond JA, Brenner AJ, Patel V, Trageser D, Scheffler B, Gorospe M, Penalva LO. The oncogenic RNA-binding protein Musashi1 is regulated by HuR via mRNA translation and stability in glioblastoma cells. Mol Cancer Res. 2012;10(1):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vo DT, Qiao M, Smith AD, Burns SC, Brenner AJ, Penalva LO. The oncogenic RNA-binding protein Musashi1 is regulated by tumor suppressor miRNAs. RNA Biol. 2014;8(5):817–828. [DOI] [PubMed] [Google Scholar]
- 40. Glazer RI, Vo DT, Penalva LO. Musashi1: an RBP with versatile functions in normal and cancer stem cells. Front Biosci. 2012;17(1):54–64. [DOI] [PubMed] [Google Scholar]
- 41. Kedde M, van Kouwenhove M, Zwart W, Oude Vrielink JA, Elkon R, Agami R. A Pumilio-induced RNA structure switch in p27-3′ UTR controls miR-221 and miR-222 accessibility. Nat Cell Biol. 2010;12(10):1014–1020. [DOI] [PubMed] [Google Scholar]