Abstract
The skin, a self-regulating protective barrier organ, is empowered with sensory and computing capabilities to counteract the environmental stressors to maintain and restore disrupted cutaneous homeostasis. These complex functions are coordinated by a cutaneous neuro-endocrine system that also communicates in a bidirectional fashion with the central nervous, endocrine, and immune systems, all acting in concert to control body homeostasis. Although UV energy has played an important role in the origin and evolution of life, UV absorption by the skin not only triggers mechanisms that defend skin integrity and regulate global homeostasis but also induces skin pathology (e.g., cancer, aging, autoimmune responses). These effects are secondary to the transduction of UV electromagnetic energy into chemical, hormonal, and neural signals, defined by the nature of the chromophores and tissue compartments receiving specific UV wavelength. UV radiation can upregulate local neuroendocrine axes, with UVB being markedly more efficient than UVA. The locally induced cytokines, corticotropin-releasing hormone, urocortins, proopiomelanocortin-peptides, enkephalins, or others can be released into circulation to exert systemic effects, including activation of the central hypothalamic-pituitary-adrenal axis, opioidogenic effects, and immunosuppression, independent of vitamin D synthesis. Similar effects are seen after exposure of the eyes and skin to UV, through which UVB activates hypothalamic paraventricular and arcuate nuclei and exerts very rapid stimulatory effects on the brain. Thus, UV touches the brain and central neuroendocrine system to reset body homeostasis. This invites multiple therapeutic applications of UV radiation, for example, in the management of autoimmune and mood disorders, addiction, and obesity.
UV energy triggers skin-protective responses against stress, coordinated by the cutaneous-neuroendocrine system, and activates central neuroendocrine system pathways that regulate global homeostasis.
The sensory and computing capabilities of the skin are designed to control cutaneous and body homeostasis (1). Its structure, composed of epidermis, dermis, dermal adipose layer, and hypodermis (subcutaneous fat) with adnexal structures, innervation, vascular system, (neuro-) endocrine, immunological, pigmentary and metabolic activities, and bidirectional interaction of skin with the microbiome all serve the skin’s central function as self-regulating protective barrier organ against an essentially hostile environment (1–3). Because this crucial interface organ also engages in thermoregulation, energy storage, and social communication (1, 4–8), these complex functions require coordination by both the local and the central neuroendocrine system (1, 8).
Given that UV light is a key determinant of life on Earth and that mammals exclusively come into contact with UV via their integument and eyes (9), it is timely to reconsider the multiple different levels on which UV impacts via these two biological ports of entry not only on skin biology and pathology, but also on the organism as a whole—well beyond cutaneous physiology and pathology. Although the beneficial effect of UVB on vitamin D is well known (10–12) and therefore only discussed cursorily here, this review focuses on exploring the role of UV and, to a lesser degree, of visible light (VIS) in the regulation of central nervous system (CNS) and endocrine gland functions and overall body homeostasis.
Why Is UV Important?
The electromagnetic energy of solar radiation reaching Earth’s surface encompasses infrared (700 nm to 1 mm), visible (400 to 700 nm), and UV (290 to 400 nm; shorter wavelengths, including UVC, are filtered by atmosphere), representing 53%, 44%, and 3%, respectively, of the ground level spectrum radiation of the sun in zenith (13, 14).
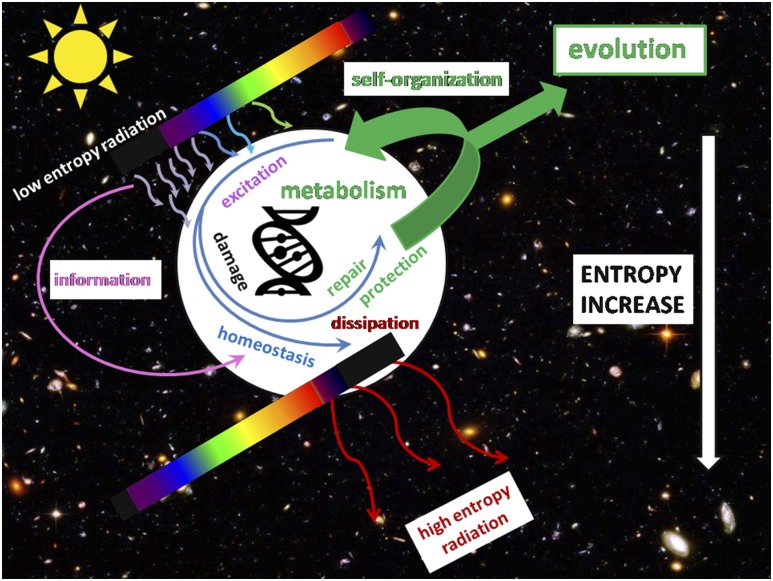
The biologically highly active UV spectra have played a fundamental role in the origin of life on Earth when simple organic molecules harnessed its energy and converted it into high-energy chemical bonds to generate molecular complexity (15–17), perhaps initiating self-organization patterns (18) (Fig. 1). In this context, it is important to mention that energy of UV is comparable to energy of covalent bonds. For instance, the carbonyl n→π* transition (λ = 280 nm) demands energy of ∼4 eV (i.e., ∼400 kJ/mol). It means that any electron excitement of a molecule with UV is enough to generate or break a covalent bond. In a biological system, it is also a supplemental way to support the cell with energy, while in the beginning of biogenesis—perhaps an important or even the only way. Under constant pressure and volume, this must lead to an increase of the total enthalpy of the system. The net negative change in the free enthalpy (Gibbs free energy) is the primary condition for the spontaneousness of a thermodynamic process. Such a process may have led to an increase of the inner-level organization (the self-organization) in the system. Therefore, it is not surprising that UV together with VIS has shaped biological evolution according to the laws of thermodynamics and promoted a diversity of organisms and species, including humans (16, 20, 21). Thus, the basic photochemical processes have not only defined the nature of biological responses but have also determined biological evolution according to the thermodynamic laws (Fig. 1). An example is the production of vitamin D2 from ergosterol (present in some of the earliest phytoplankton life forms) and vitamin D3 from 7-dehydrocholesterol after UVB-induced carbon-carbon bond cleavage (22).
Figure 1.
UV radiation as an initiator and driver of biological evolution. According to the second thermodynamic law, any self-organization must be driven by an irreversible process of energy flow in the form of low-entropy radiation from a container of high temperature (the Sun), and dissipate it in the form of high-entropy radiation in a container of low temperature (the space). UV radiation is enough to drive chemical reactions independently on metabolism and to damage the cell. DNA effectively dissipates the energy of excitation (19) and can store genetic information including only a limited number of UV-induced mutations. The cell also gets information on UV to set off the processes of repair and photoprotection, thus maintaining homeostasis, preserving enthalpy, and facilitating evolution. (The background: Hubble Deep Field; NASA, https://www.nasa.gov/. The DNA icon: PngTree, https://pngtree.com/free-icons.)
Electromagnetic Energy of Solar Light Is Transformed Into Chemical Energy
The biologically relevant spectra of UV, which constitute major cutaneous stressors, include UVC (200 to 280 nm), UVB (280 to 320 nm), and UVA (320 to 400 nm) (1, 13, 23, 24). UVC is profoundly mutagenic, lethal, and is absorbed by the stratum corneum after irradiation by artificial light sources (24). UVB, while representing only a small fraction of Sun energy reaching Earth in the form of UV (5% of the total UV energy; i.e., 0.03 × 0.05 = 0.0015 = 0.15% of the total solar radiation energy reaching Earth’s surface), is very efficient at exerting biological effects and is absorbed mostly by the upper layers of the human epidermis, but also penetrates to the papillary dermis (13, 23). UVA has better penetration reaching the reticular dermis but has 1000 times lower efficiency at inducing biological effects (expressed as the minimal erythema dose) than UVB (25, 26). VIS deeply penetrates the skin reaching the hypodermis but it apparently does not inflict substantial photodamage, possibly due to a relatively poor absorption in the epidermis (9, 13, 23). The wavelengths of solar light reaching the Earth have similar photon energy (eV): VIS: 1.65 to 3.1, UVA = 3.10 to 3.94, UVB = 3.94 to 4.43 and UVC = 4.43 to 12.4 (http://www.spacewx.com/pdf/SET_21348_2004.pdf). This indicates that magnitude of biological response is defined by nature of the chromophore and quantum mechanics of electron excitation.
In this context, most of biologically relevant chromophores such as compounds with a benzene ring including aromatic amino acids, biogenic amines, or proteins containing corresponding amino acids, pyrimidines, and purines with their derivatives alone or in nucleic acids, trans-urocanic acid (UCA), quinones, melatonin, indoles, melanin monomers, polymers and precursors, unsaturated lipids and 7-dehydrocholesterol as examples, absorb UVC and UVB, with surprisingly high absorption spectra in the UVC range (1, 10, 12, 13, 17, 20, 21, 23, 24, 27–29). Thus, the UVC signature may be a molecular record of the past, although UVB is highly relevant to the evolution of the integumental structures represented by the skin (1). In this context, UVB absorption by chromophores with their structural transformation to yield biologically relevant effects are of great importance (1, 13, 27, 30). In contrast, UVA, although it is weakly absorbed by DNA and by limited cellular chromophores (including NADH, reduced form of NADP, riboflavin, porphyrins), has the phenotypic effects that are mainly secondary to oxidative changes in the cells generated by reactive oxygen species (13, 23, 27). Another example is generation of nitric oxide (NO) from nitrosoglutathione (31) and photoreactivity of nitroxyl NO− (32).
As it relates to VIS, its main retinal chromophore, in conjunction with opsin, is involved in phototransduction necessary for the vision and/or regulation of circadian rhythm (13). The flavins and pterines harvest shorter wavelengths of VIS and in conjunction with cryptochromes are involved in the photoreception and phototransduction and may affect circadian rhythm (13). Photogeneration of NO from nitrosoglutathione may contribute to the overall homeostasis of the organism, as NO, depending on the dose and the microenvironment, may act as a parahormonal regulator or neuromodulator/neurotransmitter, or a factor of oxidative/nitrosative stress (33). Concerning light-driven circadian rhythm, it can include induction of protective mechanisms necessary to counteract UV-induced damages during exposure to daylight, and for restoration of UV-disturbed homeostasis during night.
The Skin-Brain Axis Displays Major Neuroendocrine Activity
The skin is a recognized target for neuroendocrine signals delivered from circulation (hormones and neurohormones) or via nerve endings (neurotransmitters, neuropeptides, neurotrophins) that act on both resident and circulating cells of the skin through activation of specific membrane-bound and/or nuclear receptors (8, 34, 35). Examples of classical hormones or neuromediators generated by the central endocrine system regulating functions of the skin are adrenocorticotropic hormone (ACTH), glucocorticoids, mineralocorticoids, sex hormones, thyroid hormones, growth hormone, prolactin, various neuropeptides, neurotransmitters, and biogenic amines.
The phenotypic consequences of the signals exchanged between endocrine organs and the skin as well as between the brain, spinal cord, and the skin have been a subject of extensive reviews (1, 5, 8, 34–39) and thus will not be discussed in-depth here.
The skin operates as fully functional peripheral neuroendocrine organ
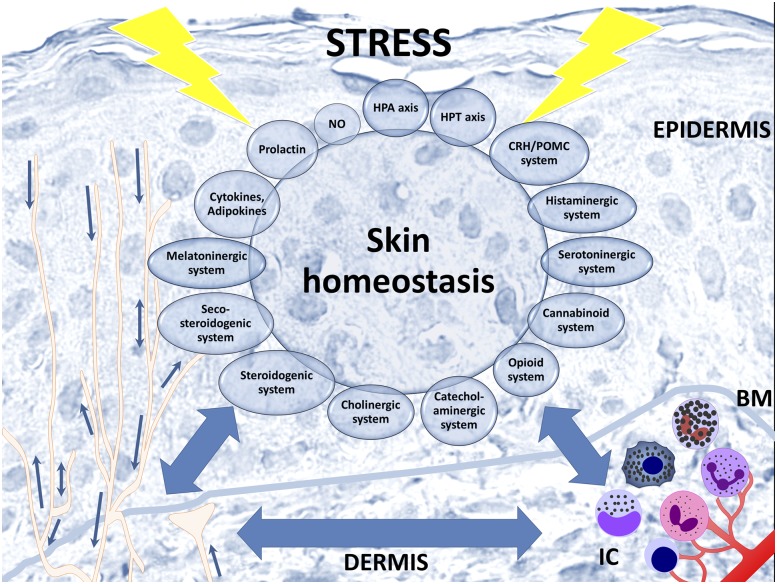
Because the initial recognition of the skin as the neuroendocrine organ involved in local stress responses that have systemic implications (8, 34), a substantial evidence has accumulated documenting the intracutaneous use of the same mediators and signal transduction pathways as the ones operating in the central neuroendocrine system [reviewed in (1, 4, 34–42)]. They include capabilities to produce on site variety of steroids (35, 42, 43), proopiomelanocortin (POMC)-derived peptides, corticotropin-releasing hormone (CRH), urocortins (34, 37), proenkephalin-derived peptides (1, 41, 44), endocannabinoids (45), catecholamines (46, 47), serotonin (48), melatonin (30, 49), thyroid-releasing hormone, thyroid-stimulating hormone, thyroid hormones (50, 51), acetylcholine (47, 52), oxytocin (53), adipokines (54), prolactin (55), growth hormone (56), and neurotrophins (57, 58). The complex interactions between these neuro-messengers and corresponding receptors in mammalian skin typically follow central regulatory paradigms of the hypothalamic-pituitary-adrenal (HPA) (59) and hypothalamic-pituitary-thyroid axes (50, 51, 54), prolactin (4, 60), or corticosteroidogenic (1, 36, 40, 61), cholinergic (52), opioid (41), biogenic amines (47, 48), melatonin (49, 62), and endocannabinoid (45) circuitries, complete with positive and negative feedback loops (Fig. 2). These interactions occur in addition to vitamin D formation and its activation through canonical (11, 12) and noncanonical pathways (43, 63, 64).
Figure 2.
Skin neuroendocrine system. The skin neuroendocrine system integrates locally and centrally produced classical neuroendocrine or endocrine signaling molecules, thus providing a natural platform of interaction between internal organs and environment. To respond to a variety of internal and external signals, skin cells not only are sensitive to neurohormonal regulation but also produce elements of the HPA axis or hypothalamic-pituitary-thyroid axis, as well as other neuropeptides, biogenic amines, serotonin, melatonin, NO, opioids, cannabinoids, catecholamines, acetylcholine, steroids, secosteroids, and growth factors adipokines and cytokines. Skin’s neuroendocrine system comprises epidermal and dermal cells including resident immune cells, nerve endings, and sensory receptors in the skin and its appendages. BM, basement membrane; IC, immune cells; HPT, hypothalamic-pituitary-thyroid.
The skin immune system communicates with this diffuse neuroendocrine system in a bidirectional fashion using the same neuroendocrine messengers, cytokines, and cognate receptors, presumably to protect the local skin homeostasis against external stressors (1, 4, 8, 37). Release of soluble neuro-endocrine-immune factors into the circulation can exert systemic, endocrine, and CNS effects, as is impressively illustrated by UV radiation (UVR)–induced β-endorphin (1, 65–67) and CRH (66, 68) releases from the skin, whereas immune cells that have been UV stimulated in the skin can act as cellular “second messengers” of the cutaneous neuro-endocrine-immune system to impact on global organismal homeostasis (Fig. 2).
NO and its donors (nitrosothiols) represent important hormonelike regulators of skin homeostasis; at the same time, NO is a mediator of the immunological, melanogenic, and neurologic effects of UV (69, 70). NO may be produced in both enzymatic and nonenzymatic fashion. There are three types of NO synthases encoded in various loci (71), and expressed in various skin cells according to the skin status (normal vs inflamed) (72) and the predominant phase of hair follicle cycling (73, 74). When produced in high amounts, NO itself becomes a nonspecific proinflammatory effector, and an important effector of oxidative/nitrosative stress (e.g., by generation of peroxynitrite) (75).
NO may affect melanogenesis in both normal and malignant melanocytes (76, 77) and it potently regulates local blood flow in a paracrine manner (70). These NO-related effects strongly depend on UV. Importantly, blood-borne nitrosothiols such as nitrosoglutathione nonenzymatically release high amounts of NO upon action of UVA and short-wave VIS (31, 78). Thus, nitrosothiols serve as transient NO storage molecules and NO transport vehicles to distant body sites, executing the endocrine way of NO action. Under physiological conditions, Cu,Zn-dependent superoxide dismutase may reversely reduce NO to the nitroxyl anion NO− (79). The latter is a chromophore for UV, and primarily undergoes phototransition to the singlet nitroxyl 1NO− isoelectronic with singlet oxygen 1O2 (80).
Neuroendocrine communication within the skin-brain axis is bidirectional
Most skin components are innervated by the autonomic and/or somatosensory nerve fibers that transmit signals from and to the brain via spinal cord, cranial nerves, the sympathetic chain (paravertebral), and parasympathetic ganglia (8, 38). Moreover, direct spino-cutaneous neural reflexes are established that do not require direct input from the brain (38). The classical sensory activities and routes of signal transmission including touch, pain, itch, temperature, stretch, and vibration are well established (38). Similarly, the descending pathways involved in the regulation of skin functions such as thermoregulation, sweat gland functions, blood flow, and other adnexal functions are also relatively well defined (38).
Although the importance of psychological factors in dermatology has long been recognized (81–83), psychodermatology as a field has recently witnessed a renaissance (84–87), not the least through improved understanding of how perceived (psychoemotional) stress aggravates or triggers skin pathology (e.g., via the induction of neurogenic inflammation) (1, 5, 37, 38, 88–94). This growing body of knowledge is complemented by more recent insights into nonclassical skin sensory activities that encompass the sensing of defined biological (95–97) and physicochemical insults/stimuli, such as olfactory receptors or TRP mediated signals, VIS, and UV, which have been considered in skin physiology only relatively recently (1, 98–100).
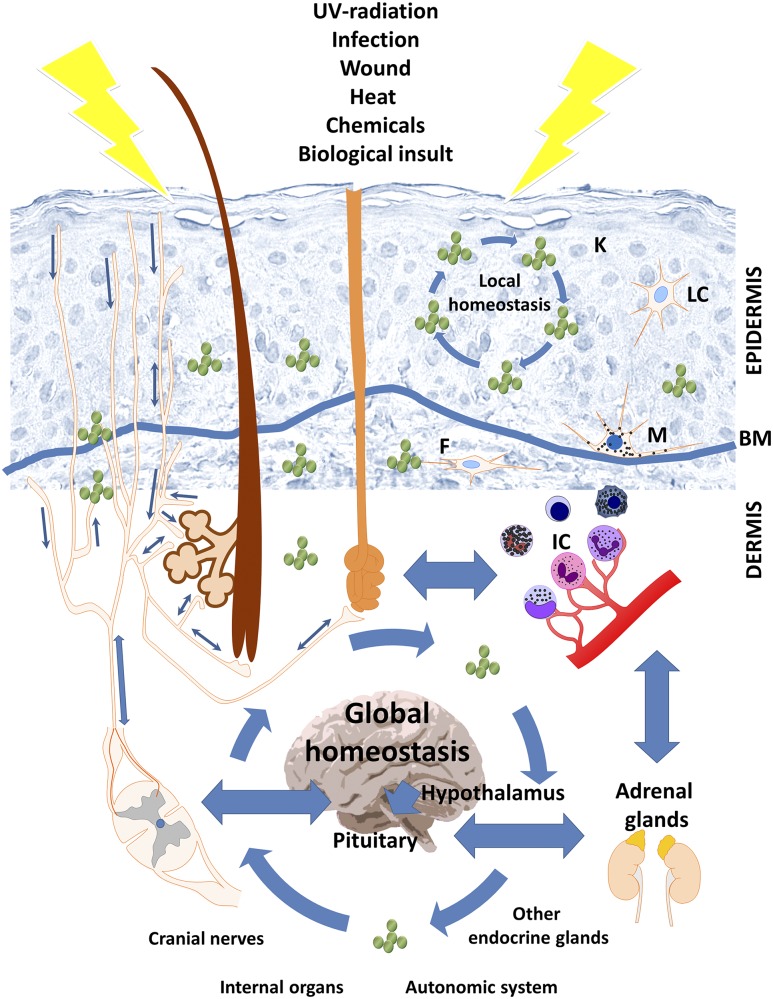
Somatosensory nerves not only deliver signals from and to the brain through spinal cord or cranial nerves, but also form local networks transmitting signals between different skin compartments, adnexal structures, and the hypodermis, using ante- and retrograde reflexes, as discussed previously (8) (Fig. 3). For example, sensory nerve endings penetrate distal epidermal cell layers until immediately below the level of stratum corneum and innervate Merkel cells in both the epidermis, namely in tactile discs (Pinkus Haarscheiben), and the outer root sheath of the hair follicle, and densely innervated the bulge stem cell region of hair follicles (101–103). Thus, disturbances in the upper epidermis and hair follicle epithelium can be sensed and translated into electrical impulses that are rapidly transmitted to the spinal cord or via retrograde reflex arches leading to enhanced intracutaneous release of neuropeptides, neurotransmitters, and/or neurotrophins from sensory nerve fibers as proposed originally (8).
Figure 3.
The interaction between the skin’s endocrine system and central neuroendocrine system in stress response. The skin stress response system can activate central neuroendocrine responses with its direct homeostatic, metabolic, and phenotypic consequences. The crosstalk between local and central elements of stress response is maintained by bidirectional neuronal stimulation through naked nerve endings in the epidermis and innervation of adnexal structures (hair follicles; sebaceous, eccrine, and apocrine glands; arrector pili muscle). Active neuroendocrine mediators could also be directly secreted in response to stimuli by neurons, epidermal keratinocytes, melanocytes, and Langerhans cells, as well as dermal residing mast cells, macrophages, and fibroblasts or infiltrating lymphocytes and granulocytes. Furthermore, the circulatory system provides an additional route for an exchange of signaling molecules between the skin’s neuroendocrine system and central endocrine organs, including elements of the HPA axis. Constant exchange of neuroendocrine mediators between skin and other organs is responsible for the maintenance of local and global homeostasis and skin response to external and internal signals. BM, basement membrane; F, fibrocytes/fibroblasts; IC, immune cells; K, keratinocytes; LC, Langerhans cells; M, melanocytes.
These sensory innervation pathways can also activate nerve fiber-associated mast cells that appear to play a key role as “central switchboards” of neurogenic inflammation, setting off a proinflammatory chain of neurogenic skin inflammation events that also impacts on other intracutaneous immunocytes (104–107), and can even have systemic immunomodulatory effects (Fig. 3). Interestingly, it is known that the skin generates electrical fields under conditions of wound healing and that, vice versa, electrical stimulation can promote both wound healing and the induction of neural differentiation markers (108).
Therefore, it is also conceivable that local activation of the endothelium of the papillary dermis plexus might transmit an electric current to the deep dermal or hypodermal plexuses, because these plexus form one continuum resulting in phenotypic activities and/or release of soluble mediators (Fig. 3).
UVR regulates body homeostasis via activation of the central neuroendocrine system
Because the original concept that mammalian skin harbors peripheral equivalent of the central HPA (cHPA) (109), which was further developed into the concept that HPA organization was first developed in the integument during evolution and was only later adapted by the central neuroendocrine system (110), substantial evidence has accumulated that strongly supports the notion that skin and its appendages are using molecular elements of the HPA to coordinate responses against stressors [reviewed in (4, 5, 37, 59, 104, 111–116)].
UVR stimulates both the intracutaneous and the cHPA axis
However, although on the central level, all regulatory elements of the HPA (hypothalamus, pituitary, adrenal glands, cytokine signaling) are anatomically separated and have a linear hierarchy (117, 118), these exist in close proximity to each other and often even within the same epithelial cells of mammalian skin, thus securing nonlinear interactions that are evolutionary conserved (1, 4, 5, 37, 110). Therefore, in principle, the local CRH/urocortin (119–121), POMC-signaling axes including the POMC-derived peptides such as α–melanocyte-stimulating hormone (MSH), β-endorphin, and ACTH (34, 122), and the cutaneous steroidogenic systems (43, 61, 123) all can act both individually and in concert to regulate local tissue homeostasis (4, 35, 37). A disruption in these interactions may result in skin pathology, as has recently been demonstrated for psoriasis (1, 5, 36, 37, 104, 111, 112, 124–126).
UVB can upregulate α-MSH receptor (MC1R) expression and activity (127, 128), POMC expression, and POMC peptide production including that of α-MSH, β-endorphin, and ACTH (127, 129–131), presumably to regulate mammalian skin pigmentation, to protect skin from UV induced injury, and to modulate skin immune responses (1, 34, 122, 128, 132–135). UVB also stimulates CRH and urocortin production (131, 136–138) and changes CRH receptor type 1 (CRH-R1) expression pattern and activity (139–141), all to restore local homeostasis and to build protection against UVB damage (1, 37, 142). However, only recently it has been demonstrated that UV can simultaneously stimulate all elements of the cHPA including glucosteroidogenesis (66, 131, 136, 143). This stimulation is dependent on wavelength with the shortest spectrum UVC having the strongest effect, followed by UVB, whereas UVA has no or minor effects restricted to increases of CRH and β-endorphin peptides (131, 136).
Importantly, the exposure of shaved back skin of C57BL/6 mice to UVB significantly stimulates cHPA axis activity already 12 and 24 hours after irradiation (66). Moreover, UVB enhances skin and plasma levels of CRH, urocortin, β-endorphin, ACTH, and corticosterone levels, along with simultaneous stimulation of CRH gene and protein expression in the paraventricular nucleus of the hypothalamus and of MC2R, StAR (steroidogenic acute regulatory protein), and CYP11B1 genes in adrenals. Because hypophysectomy abolishes UVB stimulation of plasma, but not of skin corticosterone levels, and has no effect on UVB stimulation of CRH and urocortin, this documents that the regulation of body homeostasis by UVB through the cHPA axis requires an intact pituitary for systemic effects.
This systemic stimulation of POMC peptides and corticosteroidogenesis by UVB provides a plausible endocrine mechanistic explanation of the well-known phenomenon of systemic immunosuppression by UVB (144), which was traditionally thought to be secondary to changes in the keratinocyte cytokine signaling milieu and a result of immunosuppressive T-cell activities (14, 144, 145). These systemic immunosuppressive effects appear to be independent from the UVB-induced production of vitamin D (145, 146). As a consequence, stimulation of the local and systemic HPA axes or their individual elements (POMC signaling and steroidogenesis), which encompass both chemical mediators, and stimulation of cognate receptors must play an important but long neglected role in intracutaneous or systemic immunosuppression.
In parallel, the Hiramoto group (147–150) reported remarkable systemic effect of exposing the eyes of C57BL6 mice to UVB, which increased the serum levels of α-MSH (147–149), ACTH, CRH, and urocortin 2 (149, 150) within hours after the UVB exposure. Hiramoto et al. (147) proposed that this systemic neuroendocrine UVB effect is mediated through stimulation of the hypothalamic pituitary axis in an NO-dependent manner through the ciliary (parasympathetic) ganglia involving the first branch of the trigeminal nerve (ophthalmic) but not through the optic nerve. Thus, UVB can activate cHPA or its elements both through the skin and/or via the eye (98). Furthermore, irradiation of the eye by UVA led to the stimulation of systemic α-MSH, ACTH, and β-endorphin with downstream immunosuppressive effects (150–152), with signal transmission to the brain involving optic but not trigeminal nerve (151). Thus, even eye-transmitted systemic neuroendocrine effects, at least to some extent, can be regulated by cutaneous structures that impact the ocular transmission of UVB energy.
In this context, one should not forget that UVB energy can be absorbed by lobular, fornical-orbital, and tarsal conjunctiva and by adjacent connective tissue as well as posterior and intermarginal cutaneous elements of the eyelid, all of which represent integumental structures (98).
Interestingly, irradiation of the ear also enhances POMC-derived α-MSH blood levels (147–149), consistent with UVB activation of central POMC activity through the skin (66–68). Our own data also indicate involvement of neural signal transmission from the skin to the CNS with partial deviation from classical HPA paradigms, as documented by the rapid stimulation of brain and plasma CRH, β-endorphin, ACTH, and corticosterone levels 30 and/or 90 minutes after UVB exposure, with hypophysectomy having no effect on UVB-induced increases of systemic corticosterone (68). In summary, UVR can thus regulate entire or selected elements of the cHPA axis through neural and humoral mechanisms that are wavelength-dependent and defined by anatomical structures sensing UV energy.
Stimulation of the central POMC and related systems exerts downstream homeostatic effects
Recently, the hypothesis that UV can even regulate internal organ functions through the brain or spinal cord reflexes (8) has received experimental support (67, 68). Specifically, exposure of back skin to the UVB stimulated the expression of Pomc and MC4R messenger RNAs in the hypothalamus with concomitant inhibition of AgRP in a time- and dose-dependent fashion. The effect on Pomc was seen as early as 1 hour after exposure to UVB (67). These changes in gene expression were accompanied by more α-MSH and MC4R-immunoreactive neurons in the arcuate nucleus as well as by increased brain and plasma levels of α-MSH and β-endorphin (67). It was suggested that this route of stimulation might link UVB with the central regulation of body metabolism and feeding behavior (67).
This surprising discovery was supplemented by the finding that UVB-exposure rapidly (i.e., after 30 and 90 minutes) increases of CRH and β-endorphin levels in the brain and plasma; furthermore, ACTH and corticosterone plasma levels were quickly raised, as were adrenal StAR and CYP11B1 gene expression (68). Thus, UVB can also rapidly activate the POMC system in the brain, followed by the release of centrally derived POMC peptides into the circulation, in the manner that is separate and distinct from the HPA axis.
The UVB reception in the skin not only activated central neuroendocrine pathways, but also induced rapid systemic immunosuppressive effects, which showed an extended duration, as illustrated by inhibition of Th1 and Th2 activities in the spleen 30 and/or 90 minutes after UVB exposure that lasted for at least 24 hours (68). The concomitant rapid stimulation of systemic CRH, ACTH, β-endorphin, and corticosterone, accompanied by rapid immunosuppressive effects on splenocytes appeared to be independent of the cHPA axis (68). The proposed mechanism includes rapid transmission of the signal via the ascending and descending autonomic nervous system pathways of the spinal cord (e.g., preganglionic intermediolateral nucleus) to influence several nodes of the brains’ autonomic control network (e.g., amygdala, hypothalamus, periaqueductal gray matter, raphe nuclei, and selected brainstem nuclei) and subsequently, may have result in an activation of corticosteroidogenesis in adrenal cortex and sympathetic inhibition of immune activity in the spleen (68).
The Hiramoto group (147–157) has also shown that exposure of the eye to the UV not only activates central (pituitary) POMC-signaling system in a wavelength-dependent manner (UVB vs UVA) that uses NO signaling, but also has distinct functional effects on internal organs and the skin depending on times postradiation varying substantially between 6 hours (151, 152), several days (149, 153), and 20 weeks (157) after UVR-exposure. These authors showed stimulation of cutaneous melanocytes by UVB-induced α-MSH (147, 149), downregulation of cutaneous Langerhans cells by UVA (151), deterioration of dextran sodium sulfate-induced ulcerative colitis by UVB and its improvement by UVA (150), and amelioration of atopic dermatitis by UVA (156). Moreover, they reported amelioration of colon carcinoma induced by azoxymethane and dextran sodium sulfate through UVA stimulation of β-endorphin and methionine-enkephalin (157) and modulation of mucosal intestinal functions by UVA (153).
The proposed mechanism for these UV-induced effects involves activation of the hypothalamic-pituitary (147–149, 151) or HPA axis (152). Although the requirement for intact optic nerve function in UVA induced signal transmission (151) suggests a critical involvement of the retina in this process, lack of such requirement for UVB (147) indicates an involvement of integumental structures connected with the eye (e.g., conjunctiva and eyelid) (98). Interestingly, repeated UVB exposure of the skin can negatively affect hippocampal neurogenesis and synaptic plasticity along with HPA axis activation (158).
UV stimulates the opioidogenic system
The UVB stimulated β-endorphin levels in the skin (66, 131, 143), plasma (65–68), and brain (66–68) can be linked to the phenomenon of “UVB addiction” (65, 159, 160) as well as to nociceptive and other behavioral effects (1, 65, 67). It has been hypothesized that these UVB-induced, β-endorphin-dependent behavioral and POMC pigmentary activities are regulated by p53 (65, 161). This requires independent confirmation, because there is no p53 responsive region in the POMC gene and because C57BL6 mice (i.e., model used in these studies) produces eumelanin constitutively without requirement of POMC (162, 163). In any case, that UVB can stimulate β-endorphin production not only in the skin (66, 131, 143), but also in the brain shortly (68) or after longer time periods post exposure (66, 67) identifies an exciting area for future research into how light “touches” the brain.
UVA also stimulates β-endorphin in the skin (131) and colon with concomitant increases of methionine-enkephalin and attenuation of colonic carcinogenesis (157). This not only documents that UVA can exert an opioidogenic effect (1, 41) but also that this may have selected beneficial health effects (157). Concerning enkephalins, it has been demonstrated that UVB can stimulate proenkephalin gene expression in skin cells in a dose- and time-dependent manner (44).
UVB exerts indirect effects via vitamin D actions
The pleiotropic effects of vitamin D including endocrine activities are well documented and extensively reviewed (10–12, 164, 165). The effects of active forms of vitamin D on brain functions including regulation of biogenic amines levels (166, 167) and neurosteroidal activity (168) are being gradually appreciated. Recent evidence documents an involvement of vitamin D in the upregulation of tryptophan hydroxylase type 2, with enhancement of brain serotonin exerting complex behavioral effects (169–171). Furthermore, it has been demonstrated that 1,25(OH)2D3, and recently discovered noncalcemic vitamin D analogs (43, 172, 173), can stimulate the expression of CRH, urocortins, and POMC, and their receptors, CRHR1, CRHR2, MC1R, MC2R, MC3R, and MC4R in human skin (174). Thus, active metabolites of vitamin D could contribute indirectly to the UVB induced behavioral effects or may impact the stimulation of the HPA and/or CRH and POMC-signaling systems in the periphery or at the central levels.
Major Open Questions and How to Address Them
One of the most intriguing and challenging current questions is whether human skin can sense and transform VIS into organized local and central responses represents a challenging question, because of the central function of the eye in the photoreception and central regulation of circadian rhythm.
The skin has eyelike photosensory system
One can argue that such system should operate as a conserved mechanism employed by invertebrates and lower vertebrates who use melanopsin and invertebrates opsins for light sensitivity outside the eye (175–177). This mechanism could overlap with the cutaneous UVR reception system as previously predicted (128, 178). In fact, experimental evidence is accumulating for the establishment of cutaneous photosensory mechanisms composed of different light-sensitive opsins, namely rhodopsin, melanopsin, neuropsin, and encephalopsin and photosensitive circadian clock proteins (179–183). Activation of these photosensory systems in skin and skin cells by visible or UVA light leads to measurable phenotypic effects (184–186) of which the best characterized is melanin pigmentation (180, 187–189). For example, it has been proposed that UV phototransduction in melanocytes involves a retinal-dependent signaling cascade that involves the activation of a Gαq/11-dependent phosphoinositide cascade, which resembles phototransduction in the eye (190). This UV reception mechanism involves the activation of transient receptor potential A1 (TRPA1) ion channels (190–192).
Perhaps, the greatest experimental challenge in dissecting this cutaneous photosensing system is how to distinguish VIS from UV effects and to define the relevant pathways, because UV lamps contain also VIS spectra. It also relates to the regulation of the local circadian rhythm and its communication with the local neuroendocrine systems (193) including serotonino-melatoninergic pathways (30, 48, 49). Furthermore, the skin is rich in pterines, light chromophores, and has an efficient system for their de novo synthesis and recycling, which are important not only for local phenotypic effects and homeostasis but also for the local synthesis of biogenic amines (194–198). This raises additional questions on the wavelength-dependent detection of solar light energy by the skin with its translation into precise signal transduction pathways affecting local and systemic neuroendocrine systems. In addition, the recently described discovery shows the prosurvival effect of infrared A spectrum of solar radiation on UV-damaged human melanocytes (199, 200). Also melanocytes and keratinocytes show distinctive responses to UVR (201), and different spectra of light have different effects on free radical formation and lipid composition in the skin (202).
How absorption of the UV energy by the skin is translated into central neuroendocrine activities
Another key problem is how to dissect direct UVR effects from indirect ones and their further downstream signaling. The direct effects are secondary to the absorption of UV energy by specific chromophore(s) in a wavelength-dependent manner, followed by conformational changes of the interacting protein (light receptors). The latter effects also include the direct activation of sensory nerve endings via UV-induced changes in the physicochemical environment (e.g., pH, temperature, free radicals, local ion concentrations) of the exposed nerve ending or its close vicinity. Alternatively, depolarizing cell membrane damage could result in electrical impulses that are transmitted after reaching activating threshold (8, 178).
Interestingly, in planarians TRPA1 can be activated by reactive oxygen species indicating a conserved mechanism for animal nociception induced by physicochemical factors (203). Therefore, it is conceivable that the specificity of UV responses may be enhanced by the receptors coupled to ion channels, such as members of TRP family including TRPA1. In addition, UV can directly activate TRPA1 via the chromophore retinal in melanocytes (190–192), cells of neural crest origin.
Another important question is which chromophores have appropriate protein partners in skin cells or nerve endings that trigger signal transduction pathways, which then translate the frequency of electromagnetic energy into chemical messengers. In this scenario, the nature of the chromophore with its absorption of a defined frequency of electromagnetic radiation would define the specificity for UVB, UVA, or VIS responses. A classical example is 7-dehydrocholesterol, which after absorption of UVB photoisomerizes to previtamin D (10, 12). UCA is another chromophore, whose photoproduct, cis-UCA, although being a potent immunosuppressor (204), can also interact with serotonin receptors (205–207). Also, tryptophan photoproducts can interact with the aryl hydrocarbon receptor (208–210). Moreover, UVB absorption by DNA resulting in DNA damage concomitantly stimulates pigmentation and both expression and activity of POMC (211, 212) as an indirect effect of UVR (1). Finally, the contribution of lipids in UV transduction must not be underestimated in this context, given their paramount role in epidermal barrier functions (2, 3) and predominant absorption of short UVB wavelengths by the stratum corneum with lower penetration to stratum granulosum (9, 23, 24).
The indirect chemical messengers for UVR include local neurohormones produced and released by skin cells (e.g., ACTH, α-MSH, β-endorphin, CRH, urocortins, enkephalins, cytokines, steroids, and others) [reviewed in (1, 35, 37)]. Their local and systemic effects would be defined by cell types producing such messengers, and their spatial location including proximity to blood vessels and nerve endings. The former will secure entry of soluble mediators into the circulation (8), whereas the latter will include rapid transmission to the CNS once locally produced enkephalins, β-endorphin, α-MSH, serotonin, or other neurohormones bind to the receptors expressed on cutaneous nerve endings (8). It must be noted that sensory nerves express opioid, cannabinoid, MC1, serotonin, and other receptors for locally produced neurotransmitters and neuropeptides as well as cytokine receptors (38, 41, 96, 97, 104, 133). The future challenge is to map such receptor expression in sensory nerves in relation to their anatomical and spatial distribution within human skin, along with measuring the subsequent central input after intracutaneous receptor stimulation, preferably by noninvasive brain imaging techniques such as functional magnetic resonance imaging or transcranial magnetic stimulation (88).
The downstream signaling will include stimulation of endocrine organs (39) or systemic immune system (213–215) secondary to UV-induced entry of humoral signals to the circulation (CRH, urocortins, POMC-peptides, cytokines, serotonin, melatonin) or the organ activation via neural transmission through somatosensory and autonomic system with signals originating from or bypassing brain (1, 8, 30). The downstream signaling will be represented by endocrine activities of the pituitary, adrenal glands, and possibly other glands such as thyroid, pancreas, and gonads, which will regulate body homeostasis and metabolic activities. The final effectors will be internal organs (e.g., GI tract, liver, spleen, kidney, or lungs) with their changed activities secondary to UV exposure of the skin.
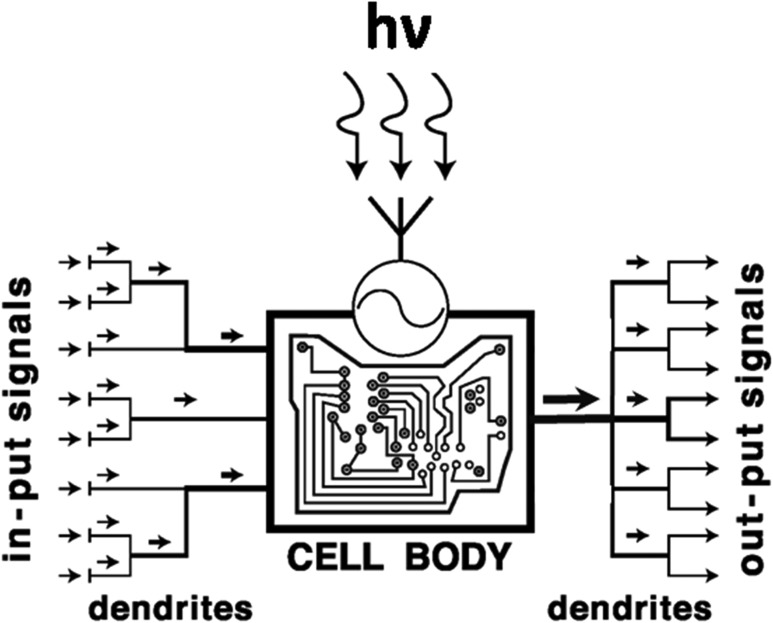
The final challenge is to define which skin cell type would act as the main system that senses and computes UV energy and then translates this into defined biological responses in the cutaneous neuroendocrine concert. Besides UV-sensitive keratinocytes, other obvious candidates are melanocytes that can sense UV and respond to it through melanogenesis, synthesis, and release of neurohormones and cytokines; moreover, melanocytes can engage in direct cell–cell contact via their UV-stimulated network of dendritic processes that communicate with multiple cellular targets as proposed previously (216–219) (Fig. 4). In the dermis, instead, UV-sensitive mast cells (221–223) may operate as master regulators that translate light energy into biological responses (104, 223). Given that, in murine skin with synchronized hair follicle cycling, numerous cutaneous functions, ranging from pigmentation via type IV immune and photoallergy responses to neuroendocrine activities and wound healing, show major hair cycle-associated fluctuations (4, 224–229), the hair follicles also impact profoundly on cutaneous responses to UVR.
Figure 4.
Melanocyte as the computing UV sensor, effector, and master regulator in the epidermal neuroendocrine concert. Melanocytes—melanin-producing cells with neuroendocrine capabilities (122, 218)—after receiving UV electromagnetic waves, decode them according to their frequency, translate the absorbed energy/information into biologically relevant signals and activities, and convey them to multiple effector targets (217, 218). The UV-induced formation of multiple dendrites (122, 128, 212, 220) allows them not only to amplify the biological effect (right, output) but also to enhance the capability of sensing UV-induced disturbances in epidermal homeostasis by collecting information from multiple structures at different, sometimes distant spaciotemporal locations (left, input). hv, a quantum of UV irradiation.
Afferent neuronal UV-induced signaling pathways may originate in skin
Although the sensory innervation of the skin is well characterized (1, 38, 230, 231), there is lack of satisfactory information on what type of sensory nerves are UV responsive, and where, how, and via which neural route UV-induced signals are transmitted. It is likely that signal transduction will be dependent on neuroanatomical differences in skin innervation (e.g., between head/neck, torso, and extremities). Another main challenge in this context is to determine which crucial coordinating brain structures are activated in addition to hypothalamus (66–68, 147, 151), including arcuate nucleus and paraventricular nucleus (66–68), and through which communicating network the UV-induced signals follow (98). It is also unclear which effector systems (sympathetic or parasympathetic) are activated and which UV-induced spinal or extraspinal neural circuits can bypass the brain to directly target immune organs such as the spleen (68) and other internal organs (98, 150, 153, 157). These questions remain open because experiments on mice, a nocturnal species with distinct neurobiological characteristics, may provide only limited information that is directly transferable to humans (1, 35, 37).
Conclusions and Future Directions
Because UV energy has shaped both biological evolution and homeostatic responses, it is not surprising that UV regulates global homeostasis after absorption and transduction of its electromagnetic energy into chemical, hormonal, and neural signals in a wavelength-dependent fashion, defined by its tissue penetration and the nature of the chromophores UVR interacts with. This homeostatic activity includes activation of the CNS and/or endocrine glands through neural transmission or chemical messengers originating in the skin. This type of regulation, although representing relics from earlier periods of biological evolution, follows precise neuroendocrine regulatory mechanisms of which examples are represented by HPA, CRH-POMC, opioidogenic, serotonin/melatoninergic, secosteroid/steroidogenic, or NO systems.
In dermatology, phototherapy is used to treat inflammatory, pigmentary, and other skin disorders (23, 87). Phototherapy includes direct use of UVB or UVA, psoralens with UVA, or various combinations thereof. An increasing range of dermatoses and cosmetic skin conditions may also be amenable to phototherapy with VIS (232–234). Thus, with the increasing application of UVR and VIS to human skin, it becomes ever-more important to understand how exactly UV and VIS light “touch” the brain along the routes synthesized in this review, and to which extent the clinically desired outcomes of UV therapy reflect secondary phenomena that result from resetting the body homeostasis through activation of central neuroendocrine pathways.
That phototherapy also may hold promise in the treatment of selected systemic autoimmune diseases such as rheumatoid arthritis, inflammatory bowel diseases, multiple sclerosis, and scleroderma (59, 66, 68, 150, 152) all of which renders the need to better understand the UV-skin/eye-brain axis discussed here even more pressing. This is further underscored by emerging evidence that UV therapy may also be used in treatment of chemical addiction and in mood disorders due to its opioidogenic effects (1, 44, 65–67), and that UV may even be employed to regulate body metabolism, food intake, and appetite via its effects on POMC, CRH, and agouti-related protein signaling (67, 98).
Thus, we have long entered into an exciting new territory of endocrinological research that may perhaps best be termed “photo-neuroendocrinology” and is just waiting to be systematically explored and therapeutically targeted.
Acknowledgments
We thank Dr. R. Slominski for English editing.
Financial Support: The writing of this mini-review was supported in part by Grants 1R01AR056666-01A2, 1R01AR071189–01A1, R21AR066505, and 1R01AR073004-01A1 (to A.T.S.) from the National Institutes of Health and by the National Institute for Health Research, Manchester Biomedical Research Centre (to R.P.). The Faculty of Biochemistry, Biophysics, and Biotechnology of the Jagiellonian University in Kraków is a partner of the Leading National Research Center (KNOW) supported by the Polish Ministry of Science and Higher Education. The article was partially supported by Grant KNOW 35p/10/2015 (to P.M.P.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACTH
adrenocorticotropic hormone
- cHPA
central hypothalamic-pituitary-adrenal
- CNS
central nervous system
- CRH
corticotropin-releasing hormone
- HPA
hypothalamic-pituitary-adrenal
- MSH
melanocyte-stimulating hormone
- NO
nitric oxide
- POMC
proopiomelanocortin
- TRPA1
transient receptor potential A1
- UCA
urocanic acid
- UVR
UV radiation
- VIS
visible light
References
- 1. Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elias PM. The skin barrier as an innate immune element. Semin Immunopathol. 2007;29(1):3–14. [DOI] [PubMed] [Google Scholar]
- 3. Feingold KR, Schmuth M, Elias PM. The regulation of permeability barrier homeostasis. J Invest Dermatol. 2007;127(7):1574–1576. [DOI] [PubMed] [Google Scholar]
- 4. Paus R, Langan EA, Vidali S, Ramot Y, Andersen B. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol Med. 2014;20(10):559–570. [DOI] [PubMed] [Google Scholar]
- 5. Paus R. The skin and endocrine disorder In: Griffiths CEM, Barker J, Bleiker T, Chalmers R, Creamer D, eds. Rook’s Textbook of Dermatology. Vol 4 9th ed.Oxford: Wiley-Blackwell; 2016:1–13. [Google Scholar]
- 6. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–139. [DOI] [PubMed] [Google Scholar]
- 7. Szöllősi AG, Oláh A, Bíró T, Tóth BI. Recent advances in the endocrinology of the sebaceous gland. Dermatoendocrinol. 2017;9(1):e1361576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21(5):457–487. [DOI] [PubMed] [Google Scholar]
- 9. Holick MF. Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res. 2016;36(3):1345–1356. [PubMed] [Google Scholar]
- 10. Holick MF, Vitamin D. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88(2):296–307. [DOI] [PubMed] [Google Scholar]
- 11. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 12. Bikle DD. Vitamin D: an ancient hormone. Exp Dermatol. 2011;20(1):7–13. [DOI] [PubMed] [Google Scholar]
- 13. Bjorn LO. Photobiology: The Science of Life and Light. 2nd ed. New York, NY: Springer; 2008. [Google Scholar]
- 14. Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem Photobiol. 2008;84(1):10–18. [DOI] [PubMed] [Google Scholar]
- 15. Patel BH, Percivalle C, Ritson DJ, Duffy CD, Sutherland JD. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat Chem. 2015;7(4):301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rapf RJ, Vaida V. Sunlight as an energetic driver in the synthesis of molecules necessary for life. Phys Chem Chem Phys. 2016;18(30):20067–20084. [DOI] [PubMed] [Google Scholar]
- 17. Dworkin J, Deamer D, Sandford S, Allamandola L. Self-assembling amphiphilic molecules: Synthesis in simulated interstellar/precometary ices. Proc Natl Acad Sci USA. 2001;98(3):815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kauffman SA. The Origins of Order: Self-Organization and Selection in Evolution. New York, NY: Oxford University Press; 1993. [Google Scholar]
- 19. Schultz T, Samoylova E, Radloff W, Hertel IV, Sobolewski AL, Domcke W. Efficient deactivation of a model base pair via excited-state hydrogen transfer. Science. 2004;306(5702):1765–1768. [DOI] [PubMed] [Google Scholar]
- 20. Raven JA, Cockell CS, De La Rocha CL. The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philos Trans R Soc Lond B Biol Sci. 2008;363(1504):2641–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Juzeniene A, Brekke P, Dahlback A, Andersson-Engels S, Reichrath J, Moan K, Holick MF, Grant WB, Moan J. Solar radiation and human health. Rep Prog Phys. 2011;74(6):066701. [Google Scholar]
- 22. Wacker M, Holick MF. Sunlight and vitamin D: a global perspective for health. Dermatoendocrinol. 2013;5(1):51–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gilchrest BA. Photodamage. Cambridge, MA: Blackwell Science, Inc; 1995. [Google Scholar]
- 24. Morison WL. Phototherapy and Photochemotherapy for Skin Disease. 3rd ed. Boca Raton, FL: CRC Press; 2005. [Google Scholar]
- 25. Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84(3):539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parrish JA, Jaenicke KF, Anderson RR. Erythema and melanogenesis action spectra of normal human skin. Photochem Photobiol. 1982;36(2):187–191. [DOI] [PubMed] [Google Scholar]
- 27. Young AR. Chromophores in human skin. Phys Med Biol. 1997;42(5):789–802. [DOI] [PubMed] [Google Scholar]
- 28. Cockell CS. Carbon biochemistry and the ultraviolet radiation environments of F, G, and K main sequence stars. Icarus. 1999;141(2):399–407. [Google Scholar]
- 29. Mulkidjanian AY, Cherepanov DA, Galperin MY. Survival of the fittest before the beginning of life: selection of the first oligonucleotide-like polymers by UV light. BMC Evol Biol. 2003;3(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slominski AT, Zmijewski MA, Semak I, Kim TK, Janjetovic Z, Slominski RM, Zmijewski JW. Melatonin, mitochondria, and the skin. Cell Mol Life Sci. 2017;74(21):3913–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sexton DJ, Muruganandam A, McKenney DJ, Mutus B. Visible light photochemical release of nitric oxide from S-nitrosoglutathione: potential photochemotherapeutic applications. Photochem Photobiol. 1994;59(4):463–467. [DOI] [PubMed] [Google Scholar]
- 32. Addison CC, Gamlen GA, Thompson R. The ultra-violet absorption spectra of sodium hyponitrite and sodium α-oxyhyponitrite: the analysis of mixtures with sodium nitrite and nitrate. J Chem Soc. 1952:338–345.
- 33. Serafim RA, Primi MC, Trossini GH, Ferreira EI. Nitric oxide: state of the art in drug design. Curr Med Chem. 2012;19(3):386–405. [DOI] [PubMed] [Google Scholar]
- 34. Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80(3):979–1020. [DOI] [PubMed] [Google Scholar]
- 35. Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids. 2015;103:72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jozic I, Stojadinovic O, Kirsner RS, Tomic-Canic M. Stressing the steroids in skin: paradox or fine-tuning? J Invest Dermatol. 2014;134(12):2869–2872. [DOI] [PubMed] [Google Scholar]
- 37. Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34(6):827–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86(4):1309–1379. [DOI] [PubMed] [Google Scholar]
- 39. Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams Textbook of Endocrinology 13th ed. Philadelphia, PA: Elsevier; 2016. [Google Scholar]
- 40. Zmijewski MA, Slominski AT. Neuroendocrinology of the skin: An overview and selective analysis. Dermatoendocrinol. 2011;3(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bigliardi PL, Dancik Y, Neumann C, Bigliardi-Qi M. Opioids and skin homeostasis, regeneration and ageing—what’s the evidence? Exp Dermatol. 2016;25(8):586–591. [DOI] [PubMed] [Google Scholar]
- 42. Nikolakis G, Stratakis CA, Kanaki T, Slominski A, Zouboulis CC. Skin steroidogenesis in health and disease. Rev Endocr Metab Disord. 2016;17(3):247–258. [DOI] [PubMed] [Google Scholar]
- 43. Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, Tuckey RC. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol. 2015;151:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Slominski AT, Zmijewski MA, Zbytek B, Brozyna AA, Granese J, Pisarchik A, Szczesniewski A, Tobin DJ. Regulated proenkephalin expression in human skin and cultured skin cells. J Invest Dermatol. 2011;131(3):613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bíró T, Tóth BI, Haskó G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30(8):411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schallreuter KU, Pittelkow MR, Swanson NN, Beazley WD, Körner C, Ehrke C, Büttner G. Altered catecholamine synthesis and degradation in the epidermis of patients with atopic eczema. Arch Dermatol Res. 1997;289(12):663–666. [DOI] [PubMed] [Google Scholar]
- 47. Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126(9):1948–1965. [DOI] [PubMed] [Google Scholar]
- 48. Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19(2):176–194. [DOI] [PubMed] [Google Scholar]
- 49. Slominski AT, Hardeland R, Zmijewski MA, Slominski RM, Reiter RJ, Paus R. Melatonin: a cutaneous perspective on its production, metabolism and functions. J Invest Dermatol. 2018;138(3):490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramot Y, Paus R. Harnessing neuroendocrine controls of keratin expression: a new therapeutic strategy for skin diseases? BioEssays. 2014;36(7):672–686. [DOI] [PubMed] [Google Scholar]
- 51. Slominski A, Wortsman J, Kohn L, Ain KB, Venkataraman GM, Pisarchik A, Chung JH, Giuliani C, Thornton M, Slugocki G, Tobin DJ. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J Invest Dermatol. 2002;119(6):1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grando SA. Cholinergic control of epidermal cohesion. Exp Dermatol. 2006;15(4):265–282. [DOI] [PubMed] [Google Scholar]
- 53. Deing V, Roggenkamp D, Kühnl J, Gruschka A, Stäb F, Wenck H, Bürkle A, Neufang G. Oxytocin modulates proliferation and stress responses of human skin cells: implications for atopic dermatitis. Exp Dermatol. 2013;22(6):399–405. [DOI] [PubMed] [Google Scholar]
- 54. Paus R. Exploring the “thyroid-skin connection”: concepts, questions, and clinical relevance. J Invest Dermatol. 2010;130(1):7–10. [DOI] [PubMed] [Google Scholar]
- 55. Foitzik K, Langan EA, Paus R. Prolactin and the skin: a dermatological perspective on an ancient pleiotropic peptide hormone. J Invest Dermatol. 2009;129(5):1071–1087. [DOI] [PubMed] [Google Scholar]
- 56. Slominski A, Malarkey WB, Wortsman J, Asa SL, Carlson A. Human skin expresses growth hormone but not the prolactin gene. J Lab Clin Med. 2000;136(6):476–481. [DOI] [PubMed] [Google Scholar]
- 57. Botchkarev VA, Yaar M, Peters EM, Raychaudhuri SP, Botchkareva NV, Marconi A, Raychaudhuri SK, Paus R, Pincelli C. Neurotrophins in skin biology and pathology. J Invest Dermatol. 2006;126(8):1719–1727. [DOI] [PubMed] [Google Scholar]
- 58. Truzzi F, Marconi A, Pincelli C. Neurotrophins in healthy and diseased skin. Dermatoendocrinol. 2011;3(1):32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265-266:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Langan EA, Ramot Y, Hanning A, Poeggeler B, Bíró T, Gaspar E, Funk W, Griffiths CE, Paus R. Thyrotropin-releasing hormone and oestrogen differentially regulate prolactin and prolactin receptor expression in female human skin and hair follicles in vitro. Br J Dermatol. 2010;162(5):1127–1131. [DOI] [PubMed] [Google Scholar]
- 61. Slominski AT, Manna PR, Tuckey RC. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity. Exp Dermatol. 2014;23(6):369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Slominski AT, Semak I, Fischer TW, Kim TK, Kleszczyński K, Hardeland R, Reiter RJ. Metabolism of melatonin in the skin: why is it important? Exp Dermatol. 2017;26(7):563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Slominski AT, Kim TK, Hobrath JV, Oak ASW, Tang EKY, Tieu EW, Li W, Tuckey RC, Jetten AM. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORα and RORγ. J Steroid Biochem Mol Biol. 2017;173:42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EK, Tuckey RC. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep. 2015;5(1):14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin β-endorphin mediates addiction to UV light. Cell. 2014;157(7):1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Skobowiat C, Slominski AT. UVB activates hypothalamic-pituitary-adrenal axis in C57BL/6 mice. J Invest Dermatol. 2015;135(6):1638–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Skobowiat C, Slominski AT. Ultraviolet B stimulates proopiomelanocortin signalling in the arcuate nucleus of the hypothalamus in mice. Exp Dermatol. 2016;25(2):120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Skobowiat C, Postlethwaite AE, Slominski AT. Skin exposure to ultraviolet B rapidly activates systemic neuroendocrine and immunosuppressive responses. Photochem Photobiol. 2017;93(4):1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Weller R. Nitric oxide: a key mediator in cutaneous physiology. Clin Exp Dermatol. 2003;28(5):511–514. [DOI] [PubMed] [Google Scholar]
- 70. Cals-Grierson MM, Ormerod AD. Nitric oxide function in the skin. Nitric Oxide. 2004;10(4):179–193. [DOI] [PubMed] [Google Scholar]
- 71. Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357(Pt 3):593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tsatmali M, Graham A, Szatkowski D, Ancans J, Manning P, McNeil CJ, Graham AM, Thody AJ. Alpha-melanocyte-stimulating hormone modulates nitric oxide production in melanocytes. J Invest Dermatol. 2000;114(3):520–526. [DOI] [PubMed] [Google Scholar]
- 73. Sowden HM, Naseem KM, Tobin DJ. Differential expression of nitric oxide synthases in human scalp epidermal and hair follicle pigmentary units: implications for regulation of melanogenesis. Br J Dermatol. 2005;153(2):301–309. [DOI] [PubMed] [Google Scholar]
- 74. Dippel E, Mayer B, Schönfelder G, Czarnetzki BM, Paus R. Distribution of constitutive nitric oxide synthase immunoreactivity and NADPH-diaphorase activity in murine telogen and anagen skin. J Invest Dermatol. 1994;103(1):112–115. [DOI] [PubMed] [Google Scholar]
- 75. Blough NV, Zafiriou OC. Reaction of superoxide with nitric oxide to form peroxonitrite in alkaline aqueous solution. Inorg Chem. 1985;24(22):3502–3504. [Google Scholar]
- 76. Roméro-Graillet C, Aberdam E, Clément M, Ortonne JP, Ballotti R. Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. J Clin Invest. 1997;99(4):635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yang Z, Misner B, Ji H, Poulos TL, Silverman RB, Meyskens FL, Yang S. Targeting nitric oxide signaling with nNOS inhibitors as a novel strategy for the therapy and prevention of human melanoma. Antioxid Redox Signal. 2013;19(5):433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Opländer C, Deck A, Volkmar CM, Kirsch M, Liebmann J, Born M, van Abeelen F, van Faassen EE, Kröncke KD, Windolf J, Suschek CV. Mechanism and biological relevance of blue-light (420-453 nm)-induced nonenzymatic nitric oxide generation from photolabile nitric oxide derivates in human skin in vitro and in vivo. Free Radic Biol Med. 2013;65:1363–1377. [DOI] [PubMed] [Google Scholar]
- 79. Murphy ME, Sies H. Reversible conversion of nitroxyl anion to nitric oxide by superoxide dismutase. Proc Natl Acad Sci USA. 1991;88(23):10860–10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shafirovich V, Lymar SV. Nitroxyl and its anion in aqueous solutions: spin states, protic equilibria, and reactivities toward oxygen and nitric oxide. Proc Natl Acad Sci USA. 2002;99(11):7340–7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Koo JY, Pham CT. Psychodermatology. Practical guidelines on pharmacotherapy. Arch Dermatol. 1992;128(3):381–388. [DOI] [PubMed] [Google Scholar]
- 82. Koo JY, Do JH, Lee CS. Psychodermatology. J Am Acad Dermatol. 2000;43(5 Pt 1):848–853. [DOI] [PubMed] [Google Scholar]
- 83. Misery L, Alexandre S, Dutray S, Chastaing M, Consoli SG, Audra H, Bauer D, Bertolus S, Callot V, Cardinaud F, Corrin E, Feton-Danou N, Malet R, Touboul S, Consoli SM. Functional itch disorder or psychogenic pruritus: suggested diagnosis criteria from the French psychodermatology group. Acta Derm Venereol. 2007;87(4):341–344. [DOI] [PubMed] [Google Scholar]
- 84. Leon A, Levin EC, Koo JY. Psychodermatology: an overview. Semin Cutan Med Surg. 2013;32(2):64–67. [DOI] [PubMed] [Google Scholar]
- 85. Gupta MA, Gupta AK. Current concepts in psychodermatology. Curr Psychiatry Rep. 2014;16(6):449. [DOI] [PubMed] [Google Scholar]
- 86. Jafferany M, Franca K. Psychodermatology: basics concepts. Acta Derm Venereol. 2016;96(217):35–37. [DOI] [PubMed] [Google Scholar]
- 87. Griffiths CEM, Barker J, Bleiker T, Chalmers R, Creamer D. Rook’s Textbook of Dermatology. 9th ed. Oxford: Wiley-Blackwell; 2016. [Google Scholar]
- 88. Mueller SM, Hogg S, Mueller JM, McKie S, Itin P, Reinhardt J, Griffiths CEM, Kleyn CE. Functional magnetic resonance imaging in dermatology: the skin, the brain and the invisible. Exp Dermatol. 2017;26(10):845–853. [DOI] [PubMed] [Google Scholar]
- 89. Paus R. Exploring the “brain-skin connection”: leads and lessons from the hair follicle. Curr Res Transl Med. 2016;64(4):207–214. [DOI] [PubMed] [Google Scholar]
- 90. Paus R, Schmelz M, Bíró T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest. 2006;116(5):1174–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006;27(1):32–39. [DOI] [PubMed] [Google Scholar]
- 92. Joachim RA, Kuhlmei A, Dinh QT, Handjiski B, Fischer T, Peters EM, Klapp BF, Paus R, Arck PC. Neuronal plasticity of the “brain-skin connection”: stress-triggered up-regulation of neuropeptides in dorsal root ganglia and skin via nerve growth factor-dependent pathways. J Mol Med (Berl). 2007;85(12):1369–1378. [DOI] [PubMed] [Google Scholar]
- 93. Kim D-H, Ryu Y, Hahm DH, Sohn BY, Shim I, Kwon OS, Chang S, Gwak YS, Kim MS, Kim JH, Lee BH, Jang EY, Zhao R, Chung JM, Yang CH, Kim HY. Acupuncture points can be identified as cutaneous neurogenic inflammatory spots. Sci Rep. 2017;7(1):15214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Peters EMJ, Müller Y, Snaga W, Fliege H, Reißhauer A, Schmidt-Rose T, Max H, Schweiger D, Rose M, Kruse J. Hair and stress: a pilot study of hair and cytokine balance alteration in healthy young women under major exam stress. PLoS One. 2017;12(4):e0175904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity [published correction appears in Immunity. 2015;43(4):830]. Immunity. 2015;43(3):515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510(7503):157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, Chen S, Trier AM, Xu AZ, Tripathi SV, Luo J, Gao X, Yang L, Hamilton SL, Wang PL, Brestoff JR, Council ML, Brasington R, Schaffer A, Brombacher F, Hsieh CS, Gereau RW, Miller MJ, Chen ZF, Hu H, Davidson S, Liu Q, Kim BS. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171(1):217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Slominski AT. Ultraviolet radiation (UVR) activates central neuro-endocrine-immune system. Photodermatol Photoimmunol Photomed. 2015;31(3):121–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tsai T, Veitinger S, Peek I, Busse D, Eckardt J, Vladimirova D, Jovancevic N, Wojcik S, Gisselmann G, Altmüller J, Ständer S, Luger T, Paus R, Cheret J, Hatt H. Two olfactory receptors-OR2A4/7 and OR51B5-differentially affect epidermal proliferation and differentiation. Exp Dermatol. 2017;26(1):58–65. [DOI] [PubMed] [Google Scholar]
- 100. Busse D, Kudella P, Grüning NM, Gisselmann G, Ständer S, Luger T, Jacobsen F, Steinsträßer L, Paus R, Gkogkolou P, Böhm M, Hatt H, Benecke H. A synthetic sandalwood odorant induces wound-healing processes in human keratinocytes via the olfactory receptor OR2AT4. J Invest Dermatol. 2014;134(11):2823–2832. [DOI] [PubMed] [Google Scholar]
- 101. Abels C. Intra-epidermal nerve fibres in human skin: back to the roots. Exp Dermatol. 2014;23(4):232–233. [DOI] [PubMed] [Google Scholar]
- 102. Xiao Y, Williams JS, Brownell I. Merkel cells and touch domes: more than mechanosensory functions? Exp Dermatol. 2014;23(10):692–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lloyd DM, McGlone FP, Yosipovitch G. Somatosensory pleasure circuit: from skin to brain and back. Exp Dermatol. 2015;24(5):321–324. [DOI] [PubMed] [Google Scholar]
- 104. Theoharides TC. Neuroendocrinology of mast cells: challenges and controversies. Exp Dermatol. 2017;26(9):751–759. [DOI] [PubMed] [Google Scholar]
- 105. Williams RM, Bienenstock J, Stead RH. Mast cells: the neuroimmune connection. Chem Immunol. 1995;61:208–235. [PubMed] [Google Scholar]
- 106. Marshall JS, Bienenstock J. The role of mast cells in inflammatory reactions of the airways, skin and intestine. Curr Opin Immunol. 1994;6(6):853–859. [DOI] [PubMed] [Google Scholar]
- 107. Theoharides TC. The mast cell: a neuroimmunoendocrine master player. Int J Tissue React. 1996;18(1):1–21. [PubMed] [Google Scholar]
- 108. Sebastian A, Volk SW, Halai P, Colthurst J, Paus R, Bayat A. Enhanced neurogenic biomarker expression and reinnervation in human acute skin wounds treated by electrical stimulation. J Invest Dermatol. 2017;137(3):737–747. [DOI] [PubMed] [Google Scholar]
- 109. Slominski A, Mihm MC. Potential mechanism of skin response to stress. Int J Dermatol. 1996;35(12):849–851. [DOI] [PubMed] [Google Scholar]
- 110. Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. J Clin Invest. 2007;117(11):3166–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Slominski AT, Brożyna AA, Tuckey RC. Cutaneous glucocorticoidogenesis and cortisol signaling are defective in psoriasis. J Invest Dermatol. 2017;137(8):1609–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hannen R, Udeh-Momoh C, Upton J, Wright M, Michael A, Gulati A, Rajpopat S, Clayton N, Halsall D, Burrin J, Flower R, Sevilla L, Latorre V, Frame J, Lightman S, Perez P, Philpott M. Dysfunctional skin-derived glucocorticoid synthesis is a pathogenic mechanism of psoriasis. J Invest Dermatol. 2017;137(8):1630–1637. [DOI] [PubMed] [Google Scholar]
- 113. Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, Tomic-Canic M. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286(12):10265–10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19(10):1332–1334. [DOI] [PubMed] [Google Scholar]
- 115. Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005;162(1-2):97–102. [DOI] [PubMed] [Google Scholar]
- 116. Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288(4):E701–E706. [DOI] [PubMed] [Google Scholar]
- 117. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381. [DOI] [PubMed] [Google Scholar]
- 118. Cawley NX, Li Z, Loh YP. 60 years of POMC: biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J Mol Endocrinol. 2016;56(4):T77–T97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Slominski A, Roloff B, Curry J, Dahiya M, Szczesniewski A, Wortsman J. The skin produces urocortin. J Clin Endocrinol Metab. 2000;85(2):815–823. [DOI] [PubMed] [Google Scholar]
- 120. Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145(2):941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ito N, Ito T, Betterman A, Paus R. The human hair bulb is a source and target of CRH. J Invest Dermatol. 2004;122(1):235–237. [DOI] [PubMed] [Google Scholar]
- 122. Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. [DOI] [PubMed] [Google Scholar]
- 123. Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, Li W, Janjetovic Z, Postlethwaite A, Zouboulis CC, Tuckey RC. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol. 2013;137:107–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sarkar MK, Kaplan N, Tsoi LC, Xing X, Liang Y, Swindell WR, Hoover P, Aravind M, Baida G, Clark M, Voorhees JJ, Nair RP, Elder JT, Budunova I, Getsios S, Gudjonsson JE. Endogenous glucocorticoid deficiency in psoriasis promotes inflammation and abnormal differentiation [published correction appears in J Invest Dermatol. 2017;137(12):2665]. J Invest Dermatol. 2017;137(7):1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kim JE, Cho BK, Cho DH, Park HJ. Expression of hypothalamic-pituitary-adrenal axis in common skin diseases: evidence of its association with stress-related disease activity. Acta Derm Venereol. 2013;93(4):387–393. [DOI] [PubMed] [Google Scholar]
- 126. Slominski A. On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. Br J Dermatol. 2009;160(2):229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chakraborty AK, Funasaka Y, Slominski A, Bolognia J, Sodi S, Ichihashi M, Pawelek JM. UV light and MSH receptors. Ann N Y Acad Sci. 1999;885(1):100–116. [DOI] [PubMed] [Google Scholar]
- 128. Pawelek JM, Chakraborty AK, Osber MP, Orlow SJ, Min KK, Rosenzweig KE, Bolognia JL. Molecular cascades in UV-induced melanogenesis: a central role for melanotropins? Pigment Cell Res. 1992;5(5 Pt 2):348–356. [DOI] [PubMed] [Google Scholar]
- 129. Scholzen TE, Kalden DH, Brzoska T, Fisbeck T, Fastrich M, Schiller M, Böhm M, Schwarz T, Armstrong CA, Ansel JC, Luger TA. Expression of proopiomelanocortin peptides in human dermal microvascular endothelial cells: evidence for a regulation by ultraviolet light and interleukin-1. J Invest Dermatol. 2000;115(6):1021–1028. [DOI] [PubMed] [Google Scholar]
- 130. Schiller M, Brzoska T, Böhm M, Metze D, Scholzen TE, Rougier A, Luger TA. Solar-simulated ultraviolet radiation-induced upregulation of the melanocortin-1 receptor, proopiomelanocortin, and alpha-melanocyte-stimulating hormone in human epidermis in vivo. J Invest Dermatol. 2004;122(2):468–476. [DOI] [PubMed] [Google Scholar]
- 131. Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301(3):E484–E493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Böhm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, Schwarz T, Schwarz A. Alpha-melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J Biol Chem. 2005;280(7):5795–5802. [DOI] [PubMed] [Google Scholar]
- 133. Böhm M, Luger TA, Tobin DJ, García-Borrón JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol. 2006;126(9):1966–1975. [DOI] [PubMed] [Google Scholar]
- 134. Kadekaro AL, Chen J, Yang J, Chen S, Jameson J, Swope VB, Cheng T, Kadakia M, Abdel-Malek Z. Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes. Mol Cancer Res. 2012;10(6):778–786. [DOI] [PubMed] [Google Scholar]
- 135. Abdel-Malek ZA, Kadekaro AL, Swope VB. Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res. 2010;23(2):171–186. [DOI] [PubMed] [Google Scholar]
- 136. Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol. 2013;168(3):595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Slominski A, Baker J, Ermak G, Chakraborty A, Pawelek J. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS Lett. 1996;399(1-2):175–176. [DOI] [PubMed] [Google Scholar]
- 138. Zbytek B, Wortsman J, Slominski A. Characterization of a ultraviolet B-induced corticotropin-releasing hormone-proopiomelanocortin system in human melanocytes. Mol Endocrinol. 2006;20(10):2539–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15(14):2754–2756. [DOI] [PubMed] [Google Scholar]
- 140. Zmijewski MA, Slominski AT. CRF1 receptor splicing in epidermal keratinocytes: potential biological role and environmental regulations. J Cell Physiol. 2009;218(3):593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, Tobin DJ. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11(1):2230–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zmijewski MA, Slominski AT. Emerging role of alternative splicing of CRF1 receptor in CRF signaling. Acta Biochim Pol. 2010;57(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- 143. Skobowiat C, Nejati R, Lu L, Williams RW, Slominski AT. Genetic variation of the cutaneous HPA axis: an analysis of UVB-induced differential responses. Gene. 2013;530(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kripke ML. Ultraviolet radiation and immunology: something new under the sun--presidential address. Cancer Res. 1994;54(23):6102–6105. [PubMed] [Google Scholar]
- 145. Schwarz A, Navid F, Sparwasser T, Clausen BE, Schwarz T. 1,25-dihydroxyvitamin D exerts similar immunosuppressive effects as UVR but is dispensable for local UVR-induced immunosuppression. J Invest Dermatol. 2012;132(12):2762–2769. [DOI] [PubMed] [Google Scholar]
- 146. Becklund BR, Severson KS, Vang SV, DeLuca HF. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc Natl Acad Sci USA. 2010;107(14):6418–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hiramoto K, Yanagihara N, Sato EF, Inoue M. Ultraviolet B irradiation of the eye activates a nitric oxide-dependent hypothalamopituitary proopiomelanocortin pathway and modulates functions of alpha-melanocyte-stimulating hormone-responsive cells. J Invest Dermatol. 2003;120(1):123–127. [DOI] [PubMed] [Google Scholar]
- 148. Hiramoto K, Sato EF. Ultraviolet B radiation to the eye induces pigmentation in the epidermis via the activation of the subunit gp91 phox of reduced nicotinamide adenine dinucleotide phosphate oxidase. Clin Exp Dermatol. 2012;37(1):65–67. [DOI] [PubMed] [Google Scholar]
- 149. Hiramoto K, Yamate Y, Kobayashi H, Ishii M, Sato EF, Inoue M. Ultraviolet B irradiation of the mouse eye induces pigmentation of the skin more strongly than does stress loading, by increasing the levels of prohormone convertase 2 and α-melanocyte-stimulating hormone. Clin Exp Dermatol. 2013;38(1):71–76. [DOI] [PubMed] [Google Scholar]
- 150. Hiramoto K, Yamate Y, Sato EF. The effects of ultraviolet eye irradiation on dextran sodium sulfate-induced ulcerative colitis in mice. Photochem Photobiol. 2016;92(5):728–734. [DOI] [PubMed] [Google Scholar]
- 151. Hiramoto K. Ultraviolet A irradiation of the eye activates a nitric oxide-dependent hypothalamo-pituitary pro-opiomelanocortin pathway and modulates the functions of Langerhans cells. J Dermatol. 2009;36(6):335–345. [DOI] [PubMed] [Google Scholar]
- 152. Hiramoto K, Jikumaru M, Yamate Y, Sato EF, Inoue M. Ultraviolet A irradiation of the eye induces immunomodulation of skin and intestine in mice via hypothalomo-pituitary-adrenal pathways. Arch Dermatol Res. 2009;301(3):239–244. [DOI] [PubMed] [Google Scholar]
- 153. Yamate Y, Hiramoto K, Kasahara E, Jikumaru M, Sato EF, Inoue J, Inoue M. Ultraviolet-A irradiation to the eye modulates intestinal mucosal functions and properties of mast cells in the mouse. Photochem Photobiol. 2011;87(1):191–198. [DOI] [PubMed] [Google Scholar]
- 154. Hiramoto K, Yamate Y, Kobayashi H, Ishii M. Long-term ultraviolet A irradiation of the eye induces photoaging of the skin in mice. Arch Dermatol Res. 2012;304(1):39–45. [DOI] [PubMed] [Google Scholar]
- 155. Hiramoto K, Yamate Y, Sugiyama D, Takahashi Y, Mafune E. Tranexamic acid suppresses ultraviolet B eye irradiation-induced melanocyte activation by decreasing the levels of prohormone convertase 2 and alpha-melanocyte-stimulating hormone. Photodermatol Photoimmunol Photomed. 2014;30(6):302–307. [DOI] [PubMed] [Google Scholar]
- 156. Hiramoto K, Yamate Y, Yokoyama S. Ultraviolet A eye irradiation ameliorates atopic dermatitis via p53 and clock gene proteins in NC/Nga mice. Photochem Photobiol. 2018;94(2):378–383. [DOI] [PubMed] [Google Scholar]
- 157. Hiramoto K, Yokoyama S, Yamate Y. Ultraviolet A eye irradiation ameliorates colon carcinoma induced by azoxymethane and dextran sodium sulfate through β-endorphin and methionine-enkephalin. Photodermatol Photoimmunol Photomed. 2017;33(2):84–91. [DOI] [PubMed] [Google Scholar]
- 158. Han M, Ban JJ, Bae JS, Shin CY, Lee DH, Chung JH. UV irradiation to mouse skin decreases hippocampal neurogenesis and synaptic protein expression via HPA axis activation. Sci Rep. 2017;7(1):15574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Nolan BV, Taylor SL, Liguori A, Feldman SR. Tanning as an addictive behavior: a literature review. Photodermatol Photoimmunol Photomed. 2009;25(1):12–19. [DOI] [PubMed] [Google Scholar]
- 160. Kourosh AS, Harrington CR, Adinoff B. Tanning as a behavioral addiction. Am J Drug Alcohol Abuse. 2010;36(5):284–290. [DOI] [PubMed] [Google Scholar]
- 161. Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D’Orazio J, Fung CY, Schanbacher CF, Granter SR, Fisher DE. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128(5):853–864. [DOI] [PubMed] [Google Scholar]
- 162. Slominski A, Plonka PM, Pisarchik A, Smart JL, Tolle V, Wortsman J, Low MJ. Preservation of eumelanin hair pigmentation in proopiomelanocortin-deficient mice on a nonagouti (a/a) genetic background. Endocrinology. 2005;146(3):1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Slominski A, Tobin DJ, Paus R. Does p53 regulate skin pigmentation by controlling proopiomelanocortin gene transcription? Pigment Cell Res. 2007;20(4):307–308, author reply 309–310. [DOI] [PubMed] [Google Scholar]
- 164. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Tekes K, Gyenge M, Folyovich A, Csaba G. Influence of neonatal vitamin A or vitamin D treatment on the concentration of biogenic amines and their metabolites in the adult rat brain. Horm Metab Res. 2009;41(4):277–280. [DOI] [PubMed] [Google Scholar]
- 167. Tekes K, Gyenge M, Hantos M, Csaba G. Transgenerational hormonal imprinting caused by vitamin A and vitamin D treatment of newborn rats. Alterations in the biogenic amine contents of the adult brain. Brain Dev. 2009;31(9):666–670. [DOI] [PubMed] [Google Scholar]
- 168. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. [DOI] [PubMed] [Google Scholar]
- 169. Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J. 2014;28(6):2398–2413. [DOI] [PubMed] [Google Scholar]
- 170. Kaneko I, Sabir MS, Dussik CM, Whitfield GK, Karrys A, Hsieh JC, Haussler MR, Meyer MB, Pike JW, Jurutka PW. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: implication for behavioral influences of vitamin D. FASEB J. 2015;29(9):4023–4035. [DOI] [PubMed] [Google Scholar]
- 171. Patrick RP, Ames BN. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015;29(6):2207–2222. [DOI] [PubMed] [Google Scholar]
- 172. Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272(16):4080–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zmijewski MA, Li W, Chen J, Kim TK, Zjawiony JK, Sweatman TW, Miller DD, Slominski AT. Synthesis and photochemical transformation of 3β,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2011;76(1-2):193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Wierzbicka JM, Żmijewski MA, Piotrowska A, Nedoszytko B, Lange M, Tuckey RC, Slominski AT. Bioactive forms of vitamin D selectively stimulate the skin analog of the hypothalamus-pituitary-adrenal axis in human epidermal keratinocytes. Mol Cell Endocrinol. 2016;437:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Arnheiter H. Evolutionary biology. Eyes viewed from the skin. Nature. 1998;391(6668):632–633. [DOI] [PubMed] [Google Scholar]
- 176. Lerner MR. Tools for investigating functional interactions between ligands and G-protein-coupled receptors. Trends Neurosci. 1994;17(4):142–146. [DOI] [PubMed] [Google Scholar]
- 177. Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: an opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95(1):340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Slominski A, Pawelek J. Animals under the sun: effects of ultraviolet radiation on mammalian skin. Clin Dermatol. 1998;16(4):503–515. [DOI] [PubMed] [Google Scholar]
- 179. Haltaufderhyde K, Ozdeslik RN, Wicks NL, Najera JA, Oancea E. Opsin expression in human epidermal skin. Photochem Photobiol. 2015;91(1):117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Kim HJ, Son ED, Jung JY, Choi H, Lee TR, Shin DW. Violet light down-regulates the expression of specific differentiation markers through Rhodopsin in normal human epidermal keratinocytes. PLoS One. 2013;8(9):e73678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Tsutsumi M, Ikeyama K, Denda S, Nakanishi J, Fuziwara S, Aoki H, Denda M. Expressions of rod and cone photoreceptor-like proteins in human epidermis. Exp Dermatol. 2009;18(6):567–570. [DOI] [PubMed] [Google Scholar]
- 182. Al-Nuaimi Y, Hardman JA, Bíró T, Haslam IS, Philpott MP, Tóth BI, Farjo N, Farjo B, Baier G, Watson REB, Grimaldi B, Kloepper JE, Paus R. A meeting of two chronobiological systems: circadian proteins Period1 and BMAL1 modulate the human hair cycle clock. J Invest Dermatol. 2014;134(3):610–619. [DOI] [PubMed] [Google Scholar]
- 183. Zanello SB, Jackson DM, Holick MF. Expression of the circadian clock genes clock and period1 in human skin. J Invest Dermatol. 2000;115(4):757–760. [DOI] [PubMed] [Google Scholar]
- 184. Liebel F, Kaur S, Ruvolo E, Kollias N, Southall MD. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132(7):1901–1907. [DOI] [PubMed] [Google Scholar]
- 185. Fischer MR, Abel M, Lopez Kostka S, Rudolph B, Becker D, von Stebut E. Blue light irradiation suppresses dendritic cells activation in vitro. Exp Dermatol. 2013;22(8):558–560. [DOI] [PubMed] [Google Scholar]
- 186. Toh PP, Bigliardi-Qi M, Yap AM, Sriram G, Bigliardi P. Expression of peropsin in human skin is related to phototransduction of violet light in keratinocytes. Exp Dermatol. 2016;25(12):1002–1005. [DOI] [PubMed] [Google Scholar]
- 187. de Assis LV, Moraes MN, da Silveira Cruz-Machado S, Castrucci AM. The effect of white light on normal and malignant murine melanocytes: a link between opsins, clock genes, and melanogenesis. Biochim Biophys Acta. 2016;1863(6 Pt A):1119–1133. [DOI] [PubMed] [Google Scholar]
- 188. Wicks NL, Chan JW, Najera JA, Ciriello JM, Oancea E. UVA phototransduction drives early melanin synthesis in human melanocytes. Curr Biol. 2011;21(22):1906–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Randhawa M, Seo I, Liebel F, Southall MD, Kollias N, Ruvolo E. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10(6):e0130949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Bellono NW, Najera JA, Oancea E. UV light activates a Gαq/11-coupled phototransduction pathway in human melanocytes. J Gen Physiol. 2014;143(2):203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Bellono NW, Kammel LG, Zimmerman AL, Oancea E. UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc Natl Acad Sci USA. 2013;110(6):2383–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Hu QM, Yi WJ, Su MY, Jiang S, Xu SZ, Lei TC. Induction of retinal-dependent calcium influx in human melanocytes by UVA or UVB radiation contributes to the stimulation of melanosome transfer. Cell Prolif. 2017;50(6):e12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Slominski AT, Hardeland R, Reiter RJ. When the circadian clock meets the melanin pigmentary system. J Invest Dermatol. 2015;135(4):943–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Schallreuter KU, Wood JM, Pittelkow MR, Gütlich M, Lemke KR, Rödl W, Swanson NN, Hitzemann K, Ziegler I. Regulation of melanin biosynthesis in the human epidermis by tetrahydrobiopterin. Science. 1994;263(5152):1444–1446. [DOI] [PubMed] [Google Scholar]
- 195. Schallreuter KU, Beazley WD, Hibberts NA, Tobin DJ, Paus R, Wood JM. Pterins in human hair follicle cells and in the synchronized murine hair cycle. J Invest Dermatol. 1998;111(4):545–550. [DOI] [PubMed] [Google Scholar]
- 196. Chavan B, Gillbro JM, Rokos H, Schallreuter KU. GTP cyclohydrolase feedback regulatory protein controls cofactor 6-tetrahydrobiopterin synthesis in the cytosol and in the nucleus of epidermal keratinocytes and melanocytes. J Invest Dermatol. 2006;126(11):2481–2489. [DOI] [PubMed] [Google Scholar]
- 197. Slominski A, Pisarchik A, Semak I, Sweatman T, Szczesniewski A, Wortsman J. Serotoninergic system in hamster skin. J Invest Dermatol. 2002;119(4):934–942. [DOI] [PubMed] [Google Scholar]
- 198. Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J, Szczesniewski A, Slugocki G, McNulty J, Kauser S, Tobin DJ, Jing C, Johansson O. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002;16(8):896–898. [DOI] [PubMed] [Google Scholar]
- 199. Skobowiat C, Slominski AT. Sun-derived infrared A and ultraviolet B radiation: allies or enemies in melanomagenesis? Exp Dermatol. 2016;25(10):760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Kimeswenger S, Schwarz A, Födinger D, Müller S, Pehamberger H, Schwarz T, Jantschitsch C. Infrared A radiation promotes survival of human melanocytes carrying ultraviolet radiation-induced DNA damage. Exp Dermatol. 2016;25(6):447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Sun X, Kim A, Nakatani M, Shen Y, Liu L. Distinctive molecular responses to ultraviolet radiation between keratinocytes and melanocytes. Exp Dermatol. 2016;25(9):708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Lohan SB, Müller R, Albrecht S, Mink K, Tscherch K, Ismaeel F, Lademann J, Rohn S, Meinke MC. Free radicals induced by sunlight in different spectral regions - in vivo versus ex vivo study. Exp Dermatol. 2016;25(5):380–385. [DOI] [PubMed] [Google Scholar]
- 203. Arenas OM, Zaharieva EE, Para A, Vásquez-Doorman C, Petersen CP, Gallio M. Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception. Nat Neurosci. 2017;20(12):1686–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Noonan FP, De Fabo EC. Immunosuppression by ultraviolet B radiation: initiation by urocanic acid. Immunol Today. 1992;13(7):250–254. [DOI] [PubMed] [Google Scholar]
- 205. Sreevidya CS, Khaskhely NM, Fukunaga A, Khaskina P, Ullrich SE. Inhibition of photocarcinogenesis by platelet-activating factor or serotonin receptor antagonists. Cancer Res. 2008;68(10):3978–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Walterscheid JP, Nghiem DX, Kazimi N, Nutt LK, McConkey DJ, Norval M, Ullrich SE. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc Natl Acad Sci USA. 2006;103(46):17420–17425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Ullrich SE. Sunlight and skin cancer: lessons from the immune system. Mol Carcinog. 2007;46(8):629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Wei YD, Rannug U, Rannug A. UV-induced CYP1A1 gene expression in human cells is mediated by tryptophan. Chem Biol Interact. 1999;118(2):127–140. [DOI] [PubMed] [Google Scholar]
- 209. Fritsche E, Schäfer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hübenthal U, Cline JE, Hajimiragha H, Schroeder P, Klotz LO, Rannug A, Fürst P, Hanenberg H, Abel J, Krutmann J. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci USA. 2007;104(21):8851–8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Rannug A, Fritsche E. The aryl hydrocarbon receptor and light. Biol Chem. 2006;387(9):1149–1157. [DOI] [PubMed] [Google Scholar]
- 211. Eller MS, Ostrom K, Gilchrest BA. DNA damage enhances melanogenesis. Proc Natl Acad Sci USA. 1996;93(3):1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Gilchrest BA, Park HY, Eller MS, Yaar M. Mechanisms of ultraviolet light-induced pigmentation. Photochem Photobiol. 1996;63(1):1–10. [DOI] [PubMed] [Google Scholar]
- 213. Blalock JE. The immune system as the sixth sense. J Intern Med. 2005;257(2):126–138. [DOI] [PubMed] [Google Scholar]
- 214. Blalock JE. The syntax of immune-neuroendocrine communication. Immunol Today. 1994;15(11):504–511. [DOI] [PubMed] [Google Scholar]
- 215. Blalock JE. Harnessing a neural-immune circuit to control inflammation and shock. J Exp Med. 2002;195(6):F25–F28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25(1):14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Slominski A, Paus R, Schadendorf D. Melanocytes as “sensory” and regulatory cells in the epidermis. J Theor Biol. 1993;164(1):103–120. [DOI] [PubMed] [Google Scholar]
- 218. Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol. 2009;18(9):760–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Slominski A, Paus R. Are L-tyrosine and L-dopa hormone-like bioregulators? J Theor Biol. 1990;143(1):123–138. [DOI] [PubMed] [Google Scholar]
- 220. Plonka PM, Passeron T, Brenner M, Tobin DJ, Shibahara S, Thomas A, Slominski A, Kadekaro AL, Hershkovitz D, Peters E, Nordlund JJ, Abdel-Malek Z, Takeda K, Paus R, Ortonne JP, Hearing VJ, Schallreuter KU. What are melanocytes really doing all day long...? Exp Dermatol. 2009;18(9):799–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Schweintzger NA, Bambach I, Reginato E, Mayer G, Limón-Flores AY, Ullrich SE, Byrne SN, Wolf P. Mast cells are required for phototolerance induction and scratching abatement. Exp Dermatol. 2015;24(7):491–496. [DOI] [PubMed] [Google Scholar]
- 222. Alard P, Niizeki H, Hanninen L, Streilein JW. Local ultraviolet B irradiation impairs contact hypersensitivity induction by triggering release of tumor necrosis factor-alpha from mast cells. Involvement of mast cells and Langerhans cells in susceptibility to ultraviolet B. J Invest Dermatol. 1999;113(6):983–990. [DOI] [PubMed] [Google Scholar]
- 223. Siiskonen H, Smorodchenko A, Krause K, Maurer M. Ultraviolet radiation and skin mast cells: Effects, mechanisms and relevance for skin diseases. Exp Dermatol. 2018;27(1):3–8. [DOI] [PubMed] [Google Scholar]
- 224. Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1, Suppl):90S–97S. [DOI] [PubMed] [Google Scholar]
- 225. Ansell DM, Kloepper JE, Thomason HA, Paus R, Hardman MJ. Exploring the “hair growth-wound healing connection”: anagen phase promotes wound re-epithelialization. J Invest Dermatol. 2011;131(2):518–528. [DOI] [PubMed] [Google Scholar]
- 226. Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Paus R, Nickoloff BJ, Ito T. A ‘hairy’ privilege. Trends Immunol. 2005;26(1):32–40. [DOI] [PubMed] [Google Scholar]
- 228. Tokura Y, Hofmann U, Müller-Röver S, Paus R, Wakita H, Yagi H, Seo N, Furukawa F, Takigawa M. Spontaneous hair follicle cycling may influence the development of murine contact photosensitivity by modulating keratinocyte cytokine production. Cell Immunol. 1997;178(2):172–179. [DOI] [PubMed] [Google Scholar]
- 229. Slominski A, Goodman-Snitkoff G, Maurer M, Paus R. Hair cycle-associated changes in splenocyte proliferation. In Vivo. 1997;11(1):101–102. [PubMed] [Google Scholar]
- 230. Kandel ER, Schwartz JH, Jessel TM, Siegelbaum SA, Hudspeth AJ. Principles of Neural Science 5th ed New York, NY: McGraw-Hill Education; 2013.
- 231. Kittaka H, Tominaga M. The molecular and cellular mechanisms of itch and the involvement of TRP channels in the peripheral sensory nervous system and skin. Allergol Int. 2017;66(1):22–30. [DOI] [PubMed] [Google Scholar]
- 232. Leong C, Bigliardi PL, Sriram G, Au VB, Connolly J, Bigliardi-Qi M. Physiological doses of red light induce IL-4 release in cocultures between human keratinocytes and immune cells. Photochem Photobiol. 2018;94(1):150–157. [DOI] [PubMed] [Google Scholar]
- 233. Jagdeo J, Austin E, Mamalis A, Wong C, Ho D, Siegel DM. Light-emitting diodes in dermatology: a systematic review of randomized controlled trials [published online ahead of print January 22, 2018]. Lasers Surg Med. 2018. [DOI] [PMC free article] [PubMed]
- 234. Garza ZCF, Born M, Hilbers PAJ, van Riel NAW, Liebmann J. Visible light therapy: molecular mechanisms and therapeutic opportunities [published online ahead of print July 27, 2017]. Curr Med Chem. 2017. [DOI] [PubMed]