Abstract
Regulation of adipogenesis is of major interest given that adipose tissue expansion and dysfunction are central to metabolic syndrome. Glucocorticoids (GCs) are important for adipogenesis in vitro. However, establishing a role for GCs in adipogenesis in vivo has been difficult. GC receptor (GR)‒null mice die at birth, a time at which wild-type (WT) mice do not have fully developed white adipose depots. We conducted several studies aimed at defining the role of GC signaling in adipogenesis in vitro and in vivo. First, we showed that GR-null mouse embryonic fibroblasts (MEFs) have compromised ability to form adipocytes in vitro, a phenotype that can be partially rescued with a peroxisome proliferator–activated receptor γ agonist. Next, we demonstrated that MEFs are capable of forming de novo fat pads in mice despite the absence of GR or circulating GCs [by bilateral adrenalectomy (ADX)]. However, ADX and GR-null fat pads and their associated adipocyte areas were smaller than those in controls. Second, using adipocyte-specific luciferase reporter mice, we identified adipocytes in both WT and GR-null embryonic day (E)18 mouse embryos. Lastly, positive perilipin staining in WT and GR-null E18 embryos confirmed the presence of early white inguinal and brown adipocytes. Taken together, these results provide compelling evidence that GCs and GR augment but are not required for the development of functional adipose tissue in vivo.
The role of glucocorticoid signaling in in vivo adipose development is unclear. We show that neither glucocorticoid receptor protein nor glucocorticoids are required for in vivo adipogenesis.
Obesity is associated with an increased risk of cardiovascular disease and type 2 diabetes mellitus and involves an expansion of adipose depots by an increase in cell size (hypertrophy) and cell number (hyperplasia) (1). The latter process requires the differentiation of preadipocytes within the stromal vascular fraction into mature brown or white adipocytes capable of serving as a lipid storage reservoir and regulator of energy homeostasis (2). Importantly, the expansion of fat mass by accumulation of small, metabolically healthy adipocytes may circumvent the development of insulin resistance and other complications of obesity (3, 4). Thus, an understanding of the mechanisms regulating adipogenesis is critical to the development of novel approaches to treat and prevent the development of dysfunctional adipocytes in the setting of obesity.
Adipogenesis is tightly regulated by the temporal expression of a number of transcription factors, including CCAAT enhancer–binding protein (C/EBP) β and δ, which drive the expression of peroxisome proliferator–activated receptor γ (PPARγ) and C/EBPα (5). This process has been studied extensively in vitro using mouse embryonic fibroblasts (MEFs) or immortalized preadipocytes, such as 3T3-L1 cells. These cells can be induced to differentiate with an adipogenic cocktail containing several hormonal stimuli, including dexamethasone (DEX), a synthetic glucocorticoid (GC). This treatment results in the accumulation of triglyceride (TG) and the formation of mature adipocytes. The metabolic effects of GC signaling are mediated by the GC receptor (GR), a member of the nuclear hormone receptor family of transcription factors.
GC signaling has been implicated in the regulation of in vitro adipogenesis on the basis of (1) the central role of DEX in inducing C/EBPδ expression in preadipocytes and (2) the role of GR, along with C/EBPβ, in promoting increased expression of PPARγ via epigenetic modifications that facilitate enhancer activity (6–8). In vitro adipogenesis is impaired in wild-type (WT) MEFs after pharmacologic antagonism of GR and in preadipocytes and MEFs lacking GR expression via short hairpin RNA‒mediated knockdown and isolation from GR-null mice, respectively (6, 9, 10). Moreover, the function of GR within the context of in vitro adipogenesis is dependent on DNA-binding activity, as MEFs expressing a GR allele incapable of dimerization and DNA-binding demonstrated a similar defect in adipogenesis in vitro (9).
Despite these in vitro findings, the role of GC signaling in in vivo adipogenesis is not well established because pups with global knockout of GR die soon after birth as a result of respiratory distress in the setting of lung immaturity (11). Recently, no defect in adipogenesis was shown in in vivo brown adipocyte development despite cell-specific knockout of GR in skeletal muscle and brown adipose tissue (BAT) precursors (12). Nevertheless, the role of GR in white adipose tissue (WAT) development has not been addressed.
We used several approaches to investigate the role of GR signaling in in vivo white adipocyte development. Importantly, we demonstrated that GR-null MEFs transplanted into WT mice and WT MEFs transplanted into adrenalectomized mice are capable of forming functional, albeit smaller, fat pads with reduced adipocyte size. Moreover, GR-GC signaling did not appear to significantly regulate the expression of adipocyte genes involved in endocrine function and lipid metabolism. These de novo fat pad studies are further supported by the presence of luciferase activity in the anatomic regions of a fat-specific luciferase reporter mouse correlating to early WAT depots in GR-null embryos, as well as the presence of perilipin-positive, early adipocytes within the inguinal WAT (IWAT) depots of GR-null embryonic day (E)18 embryos.
Material and Methods
Animals
Mice (Mus musculus) on the C57BL/6 background were used for generation of MEFs and MEF/fat pad transplant studies. The GR-null allele was generated by crossing actin-cre mice (13) with GRflox3/flox3 mice (14, 15), followed by breeding out the cre transgene. GR∆/∆ embryos were then produced by crossing GR∆/+ male and female mice. For isolation of MEFs, appropriate genotype was confirmed by a rapid genotyping protocol specific for deletion of the GR allele.
Fat-glow mice were generated by crossing adiponectin-cre mice (16) on the C57BL/6 background with stop-flox luciferase mice on the FVB/NJ background (17). GR-null fat-glow mice were generated by crossing male and female GR∆/+ mice with a combination of cre and luciferase transgenes.
Animals were maintained in a temperature-controlled room (22°C) on a 12-hour light, 12-hour dark cycle. All animal work was performed according to the policies of the Animal Studies Committee at Washington University in St. Louis, Missouri. All mice were under approved protocol and were provided appropriate care while undergoing research that complied with the standards in the guide and the Animal Welfare Act.
MEF isolation
MEFs were prepared as described previously (18). For in vitro adipocyte differentiation, MEFs were then diluted and plated. For subcutaneous injections, MEFs derived from the embryo of the appropriate genotype were pelleted by centrifugation at 1000 rpm for 5 minutes and then resuspended in ∼150 µL phosphate buffered saline (PBS). MEFs from one embryo were injected into one host.
In vitro adipogenesis assay
MEFs were plated in hi-glucose Dulbecco’s modified Eagle medium with 10% fetal bovine serum (HyClone) plus penicillin/streptomycin and allowed to grow to confluence (day 0), which occurred within 3 days of plating. On day 3, MEFs were treated with various components of an adipogenic cocktail, including DEX (1 μM), insulin (5 μg/mL), 3-isobutyl-1-methylxanthine (IBMX; 1 mM), and troglitazone (15 μM). On day 6, MEFs were then treated with insulin (5 μg/mL) ± troglitazone (15 μM), with the latter being included only when troglitazone was included in the original adipogenic cocktail. On day 9, the medium was changed to standard growth medium for an additional 2 to 3 days. Adipocytes were then fixed in 10% neutral buffered formalin, and neutral lipid content was assessed by Oil Red O (ORO) staining. Alternatively, RNA or TGs were extracted for downstream applications (see below).
In vivo MEF implantation
C57BL/6 mice were anesthetized via isoflurane administration. MEFs were delivered via subcutaneous injection to the tissue overlying the sternum in 150 µL of PBS. Fat pads were harvested at 8 weeks and preserved in either Trizol (Ambion) for RNA isolation or 10% neutral buffered formalin for histology. Adipocyte area was calculated using ImageJ software (National Institutes of Health) with the Adipocyte Tools macro.
TG assay
MEFs were differentiated, and TGs were extracted by addition of 1 mL of hexane:isopropanol (3:2). After evaporation, lipid was then resuspended in 200 μL of 1% Triton X-100 in chloroform. After an additional round of evaporation, solubilized lipid was then resuspended in 200 μL of water. TG levels were determined by Infinity Triglyceride Reagent (Thermo Fisher Scientific).
Adrenalectomy
Six-week-old male C57BL/6 mice were used for bilateral adrenalectomy (ADX). Ten days after surgery, staples were removed using isoflurane as anesthesia. Blood was collected from the tail vein 5 minutes after mice were awakened from anesthesia to elicit a peak in plasma levels of corticosterone (19). Serum corticosterone levels were measured by enzyme-linked immunosorbent assay (Arbor Assays). ADX mice required 1% NaCl drinking water because of mineralocorticoid deficiency.
In vivo imaging of fat-glow embryos
E18 fat-glow embryos were imaged using the IVIS in vivo imaging system (PerkinElmer). Pregnant female mice were injected with 150 mg/kg of luciferin (Xenogen) and anesthetized with isoflurane after 5 minutes for subsequent bioluminescent imaging. Pregnant female mice were then euthanized. Embryos were harvested, injected with an additional 50 μL of luciferin (15 mg/mL), and subjected to bioluminescent imaging.
Quantitative real-time polymerase chain reaction
Fat pads were homogenized using the TissueLyser II (Qiagen), and RNA was extracted using the RNeasy Lipid Tissue Mini Kit (Qiagen). For differentiated adipocytes in culture, RNA was extracted using TRIzol Reagent (Ambion). Any genomic DNA contamination was removed by TURBO DNA-free Kit (Thermo Fisher Scientific). Complementary DNA was prepared by using an iScript cDNA Synthesis Kit (Bio-Rad). Real-time polymerase chain reaction (RT-PCR) was performed by Power SYBR Green PCR Master Mix (Applied Biosystem) in a MicroAmp Optical 384-Well Reaction Plate (Applied Biosystem) using the ViiA 7 Real-Time PCR System (Applied Biosystem). Relative gene expression was determined by the ΔΔCt method using cyclophilin A as a reference gene. Primers were designed with qPrimer Depot. Forward and reverse primer sequences used are listed in Supplemental Table 1.
Immunofluorescence
For perilipin immunofluorescence, paraffin-embedded tissue sections from WT and GR-null E18 embryos were deparaffinized, rehydrated, and placed in citrate buffer, pH 6.0, at 90°C for 20 minutes for antigen retrieval. Sections were then permeabilized in 0.2% Triton X-100 in PBS and blocked in 10% donkey serum in 0.1M of Tris-HCl (pH 7.4), 0.15M NaCl, and 0.05% Tween 20 (TNT buffer). Slides were incubated overnight at 4°C with antiperilipin antibody (Progen) and diluted 1:400 in TNT/2.5% donkey serum, followed by incubation for 1 hour at room temperature with fluorochrome-conjugated secondary antibody anti‒guinea pig immunoglobulin G-Cy3 (Jackson), diluted 1:200 in TNT. Sections were then counterstained with 4′,6-diamidino-2-phenylindole (1 μg/mL), and slides were mounted in aqueous mounting medium. Digital images were obtained with an Olympus FV1200 confocal microscope.
Western blot analysis
The protein expression of GR in WT and GR-null MEFs was determined by Western blot analysis. Protein lysates were prepared in radioimmunoprecipitation assay buffer (Sigma) with protease/phosphatase inhibitor cocktail (Roche). Primary antibodies against GR [Research Resource Identifier (RRID): AB_11179072; Cell Signaling] or actin (RRID: AB_10694076; Cell Signaling) and secondary goat anti-rabbit horseradish peroxidase–conjugated antibody (Invitrogen) were used. Immunoreactivity was visualized by enhanced chemiluminescence detection (Pierce).
Reporter assay
A GR response element (GRE)‒luciferase reporter (specifically mouse mammary tumor virus–luciferase; kind gift of Keith Yamamoto) and SV40-renilla reporter were used to assess DEX-mediated GR transcriptional activity in WT and GR-null MEFs. MEFs were isolated as described previously, and transfections were carried out using TurboFect transfection reagent (Thermo Fisher Scientific). After a 4-hour incubation with DNA-TurboFect complexes, cells were supplied with fresh growth medium containing vehicle or 100 nM of DEX. Cells were harvested ∼24 hours after transfection in reporter lysis buffer (Promega). The Dual-Luciferase Reporter Assay System (Promega) was used to assay firefly and renilla luciferase. Firefly luciferase relative light units were normalized to renilla luciferase relative light units to obtain a normalized measure of GR transcriptional activity.
Statistical analysis
All data are presented as mean ± standard deviation and were analyzed by the unpaired two-tailed Student t test for comparisons of two groups and one-way analysis of variance or two-way analysis of variance for comparisons of multiple groups. P < 0.05 was considered significant.
Results
Validation of GR deletion in MEFs
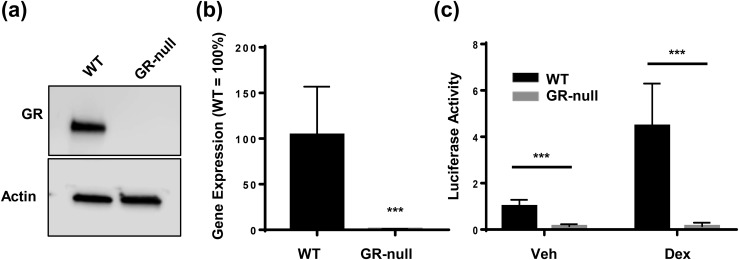
Because GR-null mice die in the early postnatal period as a result of impaired lung maturation (20), the role of GC signaling in adipocyte development has been difficult to dissect. Many of the models presented in this study rely on the isolation of GR-null (GR∆/∆) MEFs. Western blot analysis and quantitative RT-PCR demonstrated the absence of both GR transcript and protein in MEFs isolated from GR-null embryos [Fig. 1(a) and 1(b)]. To confirm the functional absence of GR in GR-null MEFs, we used GRE-luciferase reporter assays to measure GC-stimulated GR transactivation in WT and GR-null MEFs treated with either vehicle or 100 nM of DEX, a synthetic GC. In WT MEFs, DEX treatment resulted in an approximately fourfold increase in GR transactivation, whereas in GR-null MEFs, GR transactivation was abolished, thus confirming the absence of functional GR protein [Fig. 1(c)]. Basal levels of GRE-luciferase activity were also lower in GR-null cells than in WT cells.
Figure 1.
Validation of GR knockout in GR-null MEFs. (a) GR protein levels in WT and GR-null MEFs were assessed by Western blot. (b) Quantitative RT-PCR was performed to analyze GR expression in WT and GR-null MEFs. Relative gene expression was determined by the ΔΔCt method using cyclophilin A as a reference gene, and GR expression in WT MEFs was normalized to 100%. Data are represented as mean ± standard deviation (SD). (c) WT and GR-null MEFs were transfected with a GR-responsive luciferase reporter and SV40-renilla expression vector to control for transfection efficiency and then treated with vehicle or 100 nM DEX. Cell lysates were prepared 24 hours after transfection, after which luciferase activities were assayed. Data are represented as fold change in luciferase activity, for which the activity of WT MEFs treated with vehicle was normalized to 1. Data are represented as mean ± SD. ***P < 0.001. Veh, vehicle.
GR signaling enhanced in vitro adipogenesis
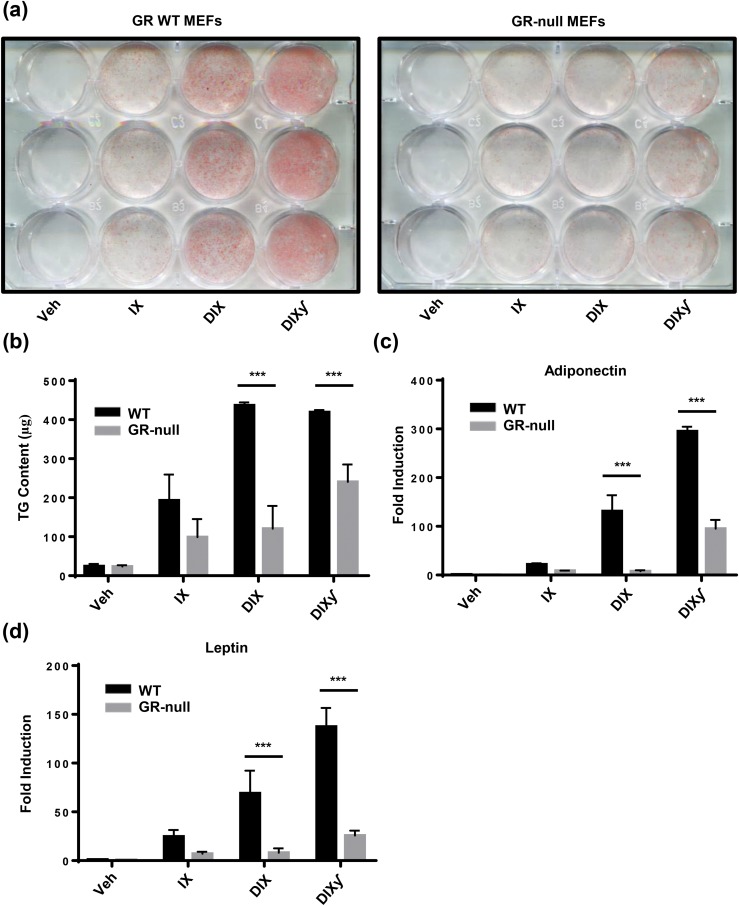
We treated WT and GR-null MEFs with variations of an adipogenic cocktail [insulin and IBMX (IX); DEX, insulin, and IBMX (DIX); DIX and troglitazone (DIXγ)] to determine the requirement of GC signaling in adipogenesis in vitro. At the end of the differentiation protocol (day 12), cultures were stained for neutral lipid content with ORO to assess for the presence of mature adipocytes [Fig. 2(a)], and TG content was measured by colorimetric assay with the Infinity Triglyceride Reagent [Fig. 2(b)]. With IX treatment, low-level adipogenesis occurred independently of GR status, and TG content did not differ significantly between the two genotypes. The addition of DEX to the cocktail (DIX) resulted in a substantial increase in ORO staining in WT MEFs, which correlated with an ∼2.2-fold increase in TG content. This finding was not observed in GR-null MEFs.
Figure 2.
GR-null MEFs demonstrated impaired adipogenesis in vitro. WT and GR-null MEFs were treated with variations of an adipogenic cocktail to induce in vitro differentiation. DEX (1 μM); insulin (5 μg/mL); IBMX (1 mM); PPARγ agonist (troglitazone; 15 μM). (a) Representative photograph of WT and GR-null MEFs. Lipid content was stained with ORO. (b) TG content was assessed using the Infinity Triglyceride Reagent. Data are represented as mean ± standard deviation (SD). (c, d) Quantitative RT-PCR was performed to analyze adiponectin and leptin expression in WT and GR-null MEFs. Relative gene expression was determined by the ΔΔCt method using cyclophilin A as a reference gene, and gene expression in WT MEFs treated with vehicle was normalized to 1. Data are represented as mean ± SD. ***P < 0.001. Veh, vehicle; γ, PPARγ agonist.
Lastly, the addition of troglitazone, a PPARγ agonist, to the cocktail (DIXγ) resulted in a small increase in ORO staining in WT MEFs treated with DIXγ compared with DIX, though this finding was not confirmed with measurement of TG content. Interestingly, an increase in ORO staining was observed in GR-null MEFs treated with DIXγ compared with DIX, which correlated with a twofold increase in TG content between these groups. A similar trend was observed when analyzing gene transcript levels of the adipokines adiponectin and leptin by quantitative RT-PCR [Fig. 2(c) and 2(d)]. There was a low level of expression of adiponectin in WT IX-treated cells, which was greatly increased with the addition of DEX to the WT culture only, and the final addition of TZD increased expression in both WT and GR-null cultures.
Taken together, these data suggest that low-level adipogenesis occurs in the absence of GR and GCs and that the augmentation of adipogenesis in the presence of GCs requires GR expression. Furthermore, adipogenesis may be accelerated in the absence of GR with the addition of a PPARγ agonist, which presumably results in activation of a more terminal step in the adipogenic cascade.
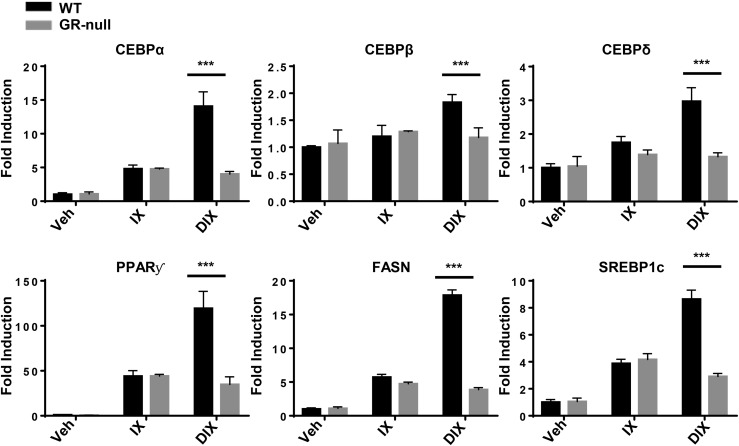
We next aimed to determine the consequences of GC-GR signaling blockade on the gene expression of major regulators of lipid metabolism [sterol regulatory element-binding transcription factor 1 (SREBP1c) and fatty acid synthase (FASN)] and adipocyte development (C/EBPα, C/EBPβ, C/EBPδ, and PPARγ) in MEFs at an early time point in the differentiation protocol. Therefore, WT and GR-null MEFs were subjected to the variations of the adipogenic cocktail noted previously. The protocol was terminated on day 6 (of 12), and RNA was extracted for measurement of gene transcript levels. Consistent with the observation that IX-treated MEFs demonstrated low levels of GR-independent adipogenesis [Fig. 2(a) and 2(b)], expression of terminal transcriptional regulators of adipogenesis (C/EBPα and PPARγ) and regulators of lipogenesis (FASN and SREBP1c) were significantly increased in IX-treated GR-null and WT MEFs (Fig. 3). The addition of DEX to the adipogenic cocktail (DIX) resulted in a GR-dependent increase in expression of both early (C/EBPα and C/EBPβ) and terminal (C/EBPα and PPARγ) transcriptional regulators of adipogenesis. As expected, expression levels of FASN and SREBP1c were upregulated in a concordant manner (Fig. 3).
Figure 3.
GR-null MEFs demonstrated diminished gene expression of lipogenic and adipogenic regulators. WT and GR-null MEFs were treated with variations of an adipogenic cocktail to induce in vitro differentiation. The study was terminated on day 6 of the protocol. Quantitative RT-PCR was performed to analyze gene expression in WT and GR-null MEFs. Relative gene expression was determined by the ΔΔCt method using cyclophilin A as a reference gene, and gene expression in WT MEFs treated with vehicle was normalized to 1. Data are represented as mean ± standard deviation. ***P < 0.001. Veh, vehicle.
MEFs are capable of fat pad formation in vivo in the absence of GR or GCs
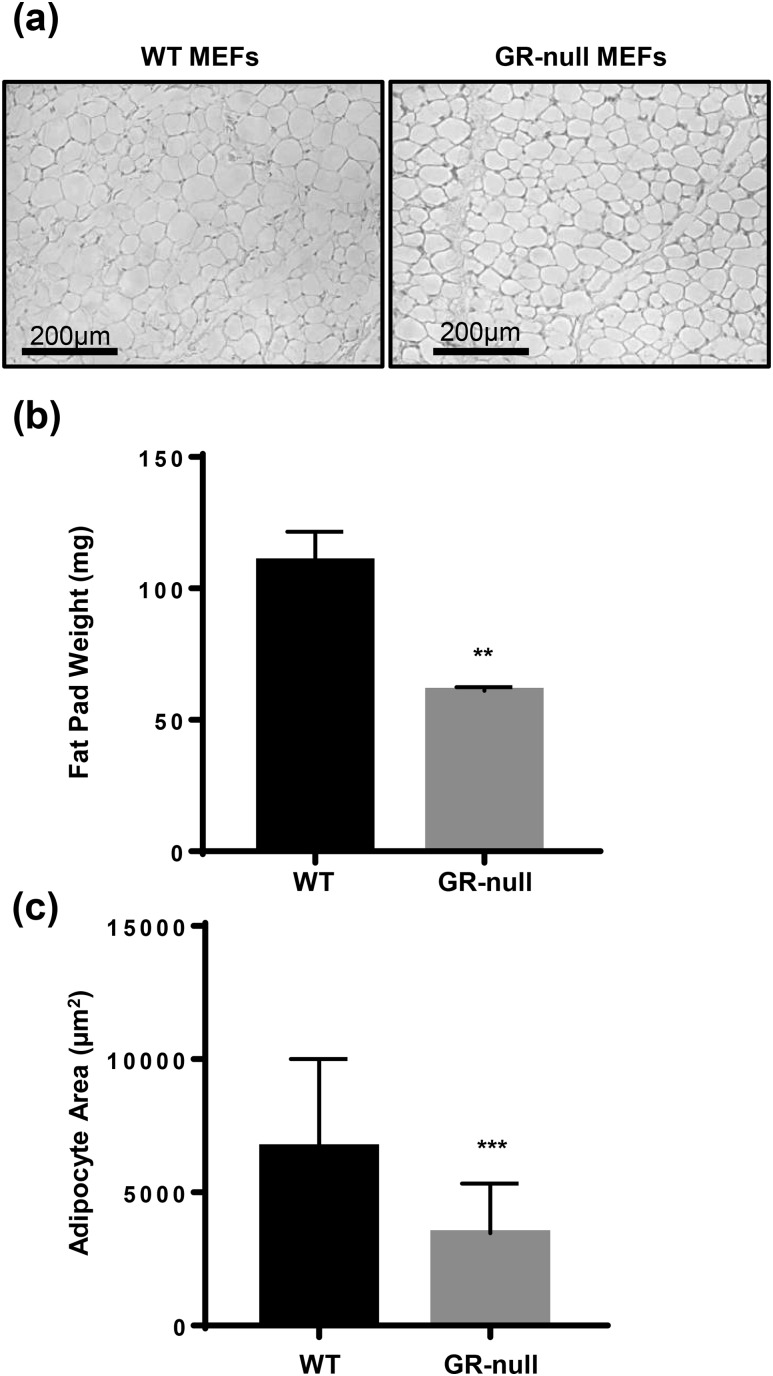
To test the role of GC-GR signaling in in vivo adipogenesis, we conducted two complementary experiments. In the first, WT and GR-null MEFs were isolated and injected into the subcutaneous tissue overlying the sternum of syngeneic C57BL/6 mice. Fat pads were harvested after 8 weeks. We observed fat pad development in 100% of animals injected with either WT or GR-null MEFs (n = 8 per group). Fat pads derived from GR-null and WT MEFs appeared histologically similar [Fig. 4 (a)], but GR-null fat pad weight and average adipocyte area were both reduced by ∼50% [Fig. 4(b) and 4(c)].
Figure 4.
GR-null MEFs retained the ability to form fat pads in vivo. (a) WT or GR-null MEFs were injected into subcutaneous tissue overlying the sternum of WT mice, and fat pad development was assessed at 8 weeks. Representative hematoxylin and eosin sections (20× power) of fat pads are shown (scale bar, 200 µm). (b) Fat pad weight was measured. Data are represented as mean ± standard deviation (SD). (c) Adipocyte size was assessed using ImageJ software. Data are represented as mean ± SD. **P < 0.01; ***P < 0.001.
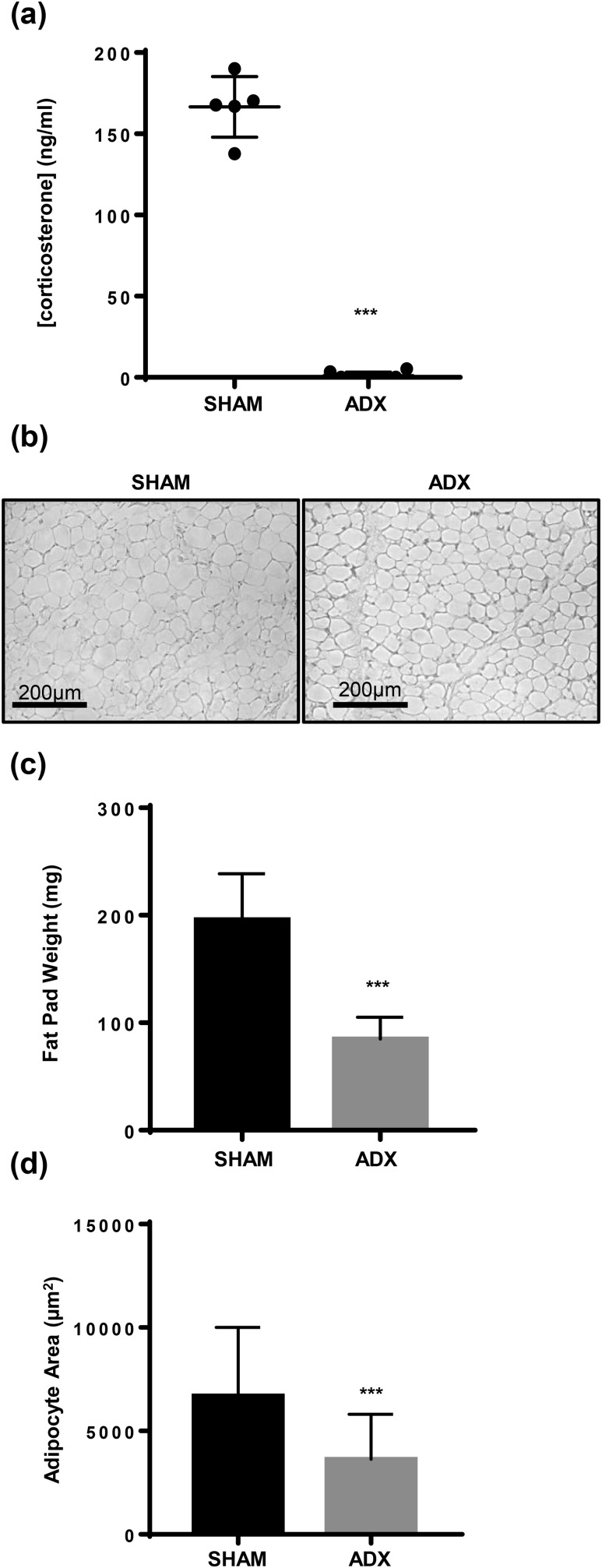
In the second experiment, we aimed to eliminate the ligand rather than the receptor. C57BL/6 mice underwent either sham procedure or ADX, and then WT MEFs were injected into the subcutaneous tissue overlying the sternum of syngeneic C57BL/6 mice 2 weeks later. Corticosterone is the primary circulating active GC in rodents, and plasma levels are high after awakening from isoflurane anesthesia (19). In sham operated animals, circulating corticosterone levels were 166.9 ng/mL after anesthesia administration, but circulating corticosterone was undetectable in ADX mice [Fig. 5(a)]. Consistent with our findings with GR-null‒derived fat pads, histologically similar fat pads developed in 100% of sham and ADX host animals (n = 5, sham; n = 8, ADX) [Fig. 5(b)]. In ADX animals, fat pad weight was reduced by 55% and adipocyte area was reduced by 52% [Fig. 5(c) and 5(d)].
Figure 5.
WT MEFs retained the ability to form fat pads in vivo despite ADX to eliminate circulating endogenous GCs. WT MEFs were injected into subcutaneous tissue overlying the sternum of sham (n = 5) and ADX (n = 8) mice, and fat pad development was assessed at 8 weeks. (a) Serum corticosterone levels were measured by enzyme-linked immunosorbent assay (Arbor Assays) 5 minutes after mice awakened from isoflurane anesthesia. Data are represented as mean ± standard deviation (SD). (b) Representative hematoxylin and eosin sections (20× power) of fat pads are shown (scale bar, 200 µm). (c) Fat pad weight was measured. Data are represented as mean ± SD. (d) Adipocyte size was assessed using ImageJ software. Data are represented as mean ± SD. ***P < 0.001.
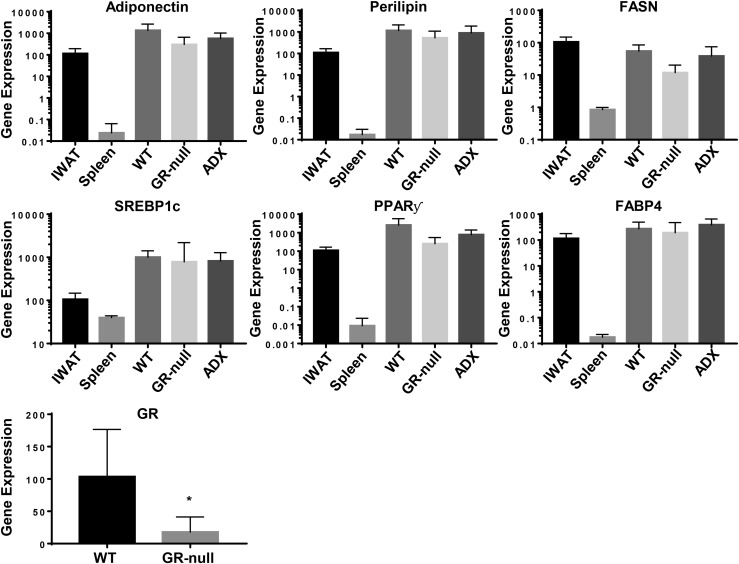
To better characterize these fat pads, we analyzed the expression of adipocyte-specific genes associated with adipose endocrine function and lipid metabolism, including PPARγ, FASN, SREBP1c, adiponectin, and perilipin. Target gene expression from IWAT served as a positive control and was normalized to 100%. Importantly, no significant difference in target gene expression was observed among endogenous IWAT, de novo fat pads derived from WT MEFs in ADX host animals, and de novo fat pads derived from GR-null MEFs and WT MEFs in WT host animals (Fig. 6). Of note, GR-null fat pads demonstrated low but detectable levels of GR expression compared with WT fat pads, most likely because of the presence of nonadipocyte cells derived from the WT host within the fat depot. Taken together, these data suggest that although intact GC-GR signaling may enhance MEF-derived de novo fat pad development, it is not essential for fat pad development or expression of genes critical for adipocyte function.
Figure 6.
Fats pads formed in the absence of intact GC signaling demonstrated similar expression of adipocyte-specific markers and transcription factors. RNA was isolated from IWAT; spleen; and WT, GR-null, and ADX fat pads. Quantitative RT-PCR was performed to analyze gene expression of adipocyte markers in WT and GR-null MEFs. Relative gene expression was determined by the ΔΔCt method using cyclophilin A as a reference gene, and gene expression in IWAT was normalized to 100%. Data are represented as mean ± standard deviation. *P < 0.05. FABP4, fatty acid–binding protein 4.
GR-null embryos demonstrated no defect in the development of primitive adipose depots
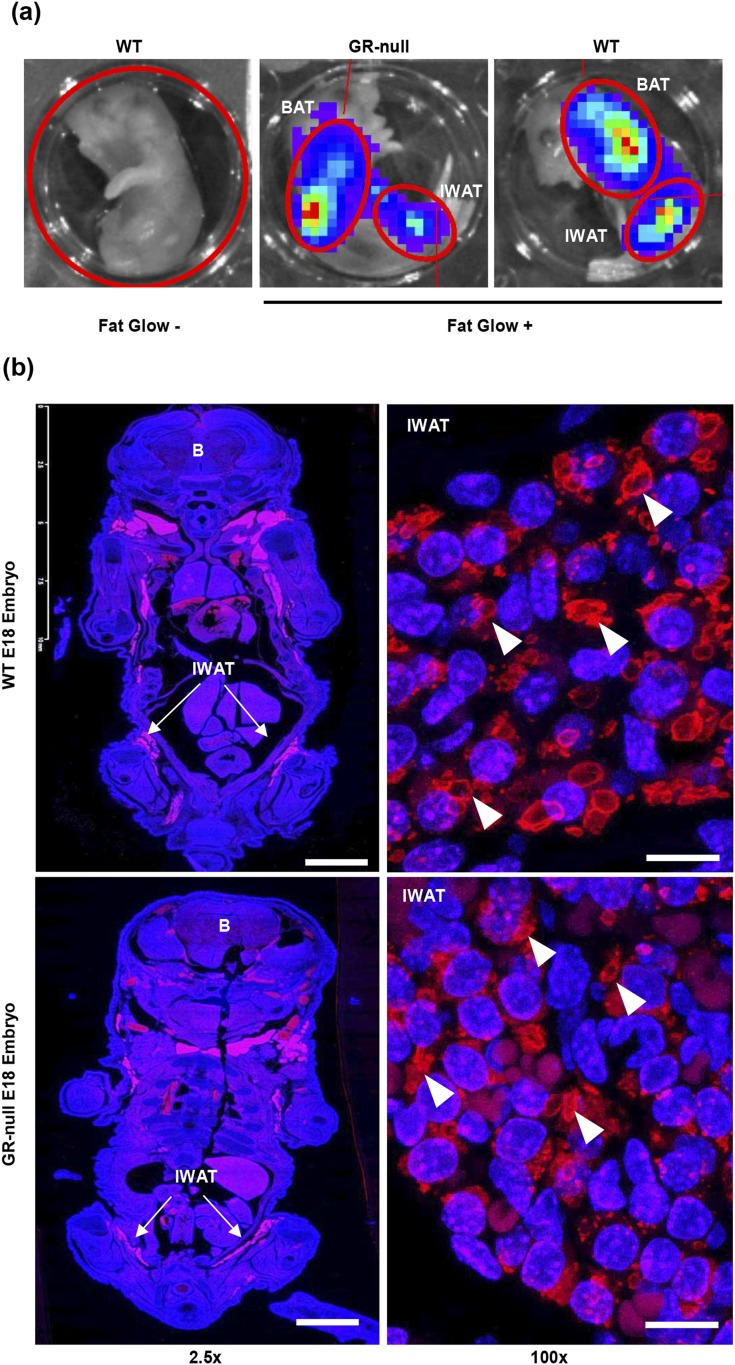
Next, we aimed to identify primitive WAT depots in E18 embryos to determine whether GR expression affects early adipocyte development. First, we used an adipocyte-specific luciferase reporter mouse (fat-glow mouse), in which cre-mediated excision of a floxed STOP cassette upstream of a luciferase reporter was targeted to the adipocyte by selective expression under the adiponectin promoter. Because adiponectin expression in mice was observed at E16.5 (21), we predicted that luciferase activity would be observed in early WAT and BAT depots. Fat-glow GR∆/+ mice were crossed with GR∆/+ mice to generate fat-glow GR+/+ and GR∆/∆ embryos. Embryos were harvested at day E18, and bioluminescence was measured after administration of luciferin. Fat-glow‒negative embryos demonstrated no luciferase activity. Consistent with our GR-null fat pad data, luciferase activity was observed in the anatomic locations corresponding to BAT and WAT depots in both WT and GR-null E18 embryos [Fig. 7(a)].
Figure 7.
Early fat pads were present in E18 embryos, independent of GR-status. (a) Bioluminescence was measured in WT and GR-null E18 fat-glow embryos after luciferin injection in the IVIS imaging system. In this model, cre-mediated excision of a floxed STOP cassette upstream of a luciferase reporter was targeted to the adipocyte by selective expression under the adiponectin promoter. Fat-glow‒negative embryos served as a negative control. (b) Coronal sections of fixed and paraffin-embedded WT and GR-null E18 embryos were stained for perilipin (red) to assess for the presence of early adipose depots. Representative images are shown at ×2.5 (scale bar, 2.5 mm) and ×100 (scale bar, 10 µm) power. Nuclei are stained with 4′,6-diamidino-2-phenylindole (blue). Arrowheads represent perilipin-positive lipid droplets. White arrows represent IWAT. B, brain.
Hong et al. (22) recently identified early lipid-lacking, perilipin-positive, and adiponectin-positive adipocytes in E16.5 mouse embryos. Therefore, to further assess the presence of adipocytes, WT and GR-null E18 embryos were fixed, embedded in paraffin, and sectioned along the coronal plane. Perilipin (PLIN1, a lipid droplet-associated protein specific to adipocytes) staining was assessed by immunofluorescence as previously performed by Hong et al. (22) and was found to be present in both BAT and early IWAT depots in WT embryos. Importantly, GR-null embryos also demonstrated perilipin-positive early adipocytes in similar numbers and pattern as WT embryos [Fig. 7(b)]. Taken together, these data confirm that GR expression is not required for the development of early BAT and WAT fat pads.
Discussion
Obesity is associated with increased risk of type 2 diabetes mellitus, metabolic syndrome, coronary artery disease, and some malignancies. Thus, an understanding of the mechanisms associated with adipose development and expansion is imperative. GC signaling has been implicated in fat development and function. In humans, clinical excess of exogenous and endogenous GCs drives the expansion of visceral adipose tissue, resulting in insulin resistance, while also resulting in wasting of peripheral subcutaneous adipose depots. Although the depot-specific action of GCs is not entirely understood, it appears to be in part related to enhanced activity of lipoprotein lipase activity, the availability of specific transcription factors within the visceral adipose depot, and the enhanced capacity of visceral adipose tissue to generate locally active GCs (23–25).
Further evidence for a critical role for GC signaling in adipose development has been largely based on in vitro adipogenesis assays, indicating a requirement of GCs to form mature adipocytes and impaired adipogenesis in GR-null MEFs and preadipocytes (9, 10, 26). Additional studies have noted the role of GC signaling in the induction of transcription factors required for adipogenesis (6–8). Assessment of the role of GR-GC signaling in in vivo embryonic adipogenesis has been difficult to study because of postembryonic lethality in GR-null mice (11). However, Park and Ge (12) recently deleted GR in vivo in brown adipocyte precursors and demonstrated no defect in BAT development. Nevertheless, the role of GR in embryonic WAT development in vivo remained unclear. Indeed, Wang et al. (27) demonstrated that the requirement for specific transcription factors in adipogenesis may vary with adipose depot as well as maturation status (adult vs embryonic), highlighting the need for completion of the studies outlined in this report.
Previous studies from our laboratory and others have demonstrated that the elimination of GR in mature adipocytes by expression of cre recombinase under the control of adiponectin, has minimal or no effect on fat mass expansion in chow- or high-fat diet‒fed animals (28–31), suggesting little contribution of GR in the regulation of adult fat mass expansion. This is an interesting point because deletion of other adipogenic mediators such as PPARγ and the insulin receptor in mature adipocytes (using the same adiponectin-cre driver) leads to severe loss of adipose tissue (32, 33). Therefore, to address the role of GR in embryonic development of WAT, we initially focused our efforts on the development of a tissue-specific knockout of GR in white adipocyte precursors by crossing GRflox3/flox3 mice (14, 15) with mice expressing cre recombinase under the control of pdgfRα (34), prx1 (35), or pparg (36, 37) regulatory elements.
Unfortunately, knockout of GR in WAT precursors was not successful, prompting us to test the effects of GR deletion utilizing several alternative in vivo methods. We first demonstrated that MEFs are able to form de novo fat pads despite lack of either GR or GCs. Although a reduction in fat pad size and adipocyte area in response to GR deletion and GC ablation was observed, WT and GR-null fat pads demonstrated strikingly similar transcriptional profiles. One potential drawback of the ADX experiment is that it is possible E14 MEFs are already committed to adipogenesis before transplantation. However, this caveat does not apply to the experiment transplanting GR-null MEFs. Because those experiments used an indirect model of adipogenesis, we aimed to determine if GR expression was required for development of early WAT depots in late embryos in situ. Indeed, the presence of luciferase activity in anatomic locations consistent with early IWAT and the presence of perilipin-positive, early inguinal adipocytes in GR-null embryos confirmed that WAT depots develop in the absence of GR expression. Thus, in this report, we demonstrated that GR expression is not required for the development of embryonic WAT depots and that adipocyte precursor cells are capable of forming fat pads in the absence of GC signaling.
These results contradict current dogma regarding the role of GC signaling in adipose development and necessitated the use of several complementary approaches because of the inability to eliminate GR in WAT precursors. Nevertheless, our findings are consistent with a recent report by Park and Ge (12), who successfully deleted GR in brown adipocyte precursors in vivo. The authors further demonstrated that after a prolonged in vitro adipogenesis protocol (21 days), the adipogenesis defect between WT and GR-null white and brown preadipocytes was attenuated, most likely because of an early role for GR in driving the expression of regulatory transcription factors 3 days after initiation of the adipogenesis protocol. In our studies, we confirmed a GR-dependent increase in the expression of C/EBPα, C/EBPβ, C/EBPδ, and PPARγ in response to DEX administration 6 days after initiation of the adipogenesis protocol. Thus, GC-GR signaling may not serve as a critical regulator of adipogenesis; instead, it may augment and accelerate the process, particularly during short-term experiments performed in vitro.
Fat pad size was reduced in response to both ADX and deletion of GR, despite an overall similar histological appearance between these distinct groups of fat pads. Adipocyte area was reduced as well, suggesting that a change in adipocyte size rather than number was responsible for the reduction in fat pad size. This finding would further argue against a defect in in vivo adipogenesis. Instead, the primary role of GC signaling in this process may be to regulate TG metabolism. It is well established that GCs are capable of regulating both lipolysis and lipogenesis and in some cases may simultaneously drive both processes within the same adipose depot (38–40). In the case of de novo fat pad formation, the absence of GC signaling may result in impaired lipogenesis, thus contributing to decreases in adipocyte area and fat pad size.
Lastly, it is worth considering a role for the mineralocorticoid receptor (MR), rather than GR, in driving adipose development. This possibility has been debated extensively in the literature, and results tend to differ on the basis of cell culture model. Caprio et al. (41) demonstrated very low levels of MR expression in 3T3-L1 preadipocytes. Moreover, expression increased with differentiation, and small interfering RNA–mediated knockdown of MR, but not GR, impaired differentiation of 3T3-L1 cells into mature adipocytes. On the other hand, Lee and Fried (10) demonstrated that in primary cultured human preadipocytes, MR levels did not change with differentiation, and small interfering RNA–mediated knockdown of GR, but not MR, blocked differentiation in preadipocytes.
Although the studies presented here demonstrate that GR is not required for adipogenesis in vivo, they do not directly address the role of MR in this process. GR-null embryos examined in the E18 imaging studies may in fact have elevated plasma corticosterone levels as a result of excessive adrenocorticotropic hormone production secondary to loss of GR-mediated negative feedback at the level of the hypothalamus and pituitary. Indeed, Cole et al. (20) demonstrated that corticosterone levels were approximately 2.5-fold higher in GR-null E18.5 mice than in WT littermates, suggesting significant dysregulation of the hypothalamic-pituitary-adrenal axis in these animals. Thus, corticosterone excess in GR-null E18 embryos may result in hyperactivation of MR, thereby contributing to embryonic adipogenesis in these GR-null embryos. However, if MR contributes significantly to in vivo adipogenesis, either as a primary regulator of the process or in a compensatory role in GR-null embryos, one would expect that elimination of corticosterone altogether would block adipogenesis. Importantly, our data do not support this hypothesis. In our de novo fat pad model in which we injected WT MEFs into ADX host animals, which lack the ligand for both MR and GR, fat pads developed in both sham and ADX animals, suggesting that co-elimination of GR and MR signaling is permissive for in vivo fat development. Further studies examining MR-null mice for the presence of adipose tissue will address this issue conclusively.
Supplementary Material
Acknowledgments
We thank Keith Yamamoto for mouse mammary tumor virus–luciferase plasmid and Drs. Clarissa Craft and Jesse Procknow for their technical assistance. In vivo imaging studies were performed with the assistance of Lynne Marsala [National Institutes of Health P50 CA094056 (Molecular Imaging Center) and NCI P30 CA091842 (Siteman Cancer Center Small Animal Cancer Imaging shared resource)]. The contents do not represent the views of the US Department of Veterans Affairs or the US government.
Financial Support: This work was funded by National Institutes of Health Grants DK106083 (to C.A.H.) and DE024178 (to E.L.S.). K.T.B. was supported by T32 Grant DK007120 to Washington University School of Medicine, the Endocrine Scholar’s Award, and the St. Louis Veterans Affairs Medical Center.
Author Contributions: K.T.B. designed and performed the experiments, interpreted the data, assembled the figures, and drafted the manuscript. I.H. assisted with performing experiments. E.L.S. assisted with immunofluorescence studies. C.A.H. conceived the study, performed the initial experiments, and directed the research. C.A.H. and E.L.S. critically reviewed the manuscript, which was approved by all authors.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ADX
adrenalectomy
- BAT
brown adipose tissue
- C/EBP
CCAAT enhancer–binding protein
- DEX
dexamethasone
- DIX
dexamethasone, insulin, and 3-isobutyl-1-methylxanthine
- DIXγ
dexamethasone, insulin, 3-isobutyl-1-methylxanthine, and troglitazone
- E
embryonic day
- FASN
fatty acid synthase
- GC
glucocorticoid
- GR
glucocorticoid receptor
- GRE
glucocorticoid receptor response element
- IBMX
3-isobutyl-1-methylxanthine
- IX
insulin and 3-isobutyl-1-methylxanthine
- IWAT
inguinal white adipose tissue
- MEF
mouse embryonic fibroblast
- MR
mineralocorticoid receptor
- ORO
Oil Red O
- PBS
phosphate-buffered saline
- PPARγ
peroxisome proliferator–activated receptor γ
- RRID
Research Resource Identifier
- RT-PCR
real-time polymerase chain reaction
- SREBP1c
sterol regulatory element-binding transcription factor 1
- TG
triglyceride
- TNT
Tris-HCl, NaCl, and Tween-20
- WT
wild-type
Appendix.
List of Antibodies
| Manufacturer, Catalog No. | RRID | |
|---|---|---|
| GR | Cell Signaling Technology, 3660S | AB_11179072 |
| Actin | Cell Signaling Technology, 5125S | AB_10694076 |
References
- 1. Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. J Cell Biol. 2015;208(5):501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12(11):722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts R, Hodson L, Dennis AL, Neville MJ, Humphreys SM, Harnden KE, Micklem KJ, Frayn KN. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52(5):882–890. [DOI] [PubMed] [Google Scholar]
- 4. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–783. [DOI] [PubMed] [Google Scholar]
- 5. Ali AT, Hochfeld WE, Myburgh R, Pepper MS. Adipocyte and adipogenesis. Eur J Cell Biol. 2013;92(6-7):229–236. [DOI] [PubMed] [Google Scholar]
- 6. Steger DJ, Grant GR, Schupp M, Tomaru T, Lefterova MI, Schug J, Manduchi E, Stoeckert CJ Jr, Lazar MA. Propagation of adipogenic signals through an epigenomic transition state. Genes Dev. 2010;24(10):1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5(9):1538–1552. [DOI] [PubMed] [Google Scholar]
- 8. Yeh WC, Cao Z, Classon M, McKnight SL. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9(2):168–181. [DOI] [PubMed] [Google Scholar]
- 9. Asada M, Rauch A, Shimizu H, Maruyama H, Miyaki S, Shibamori M, Kawasome H, Ishiyama H, Tuckermann J, Asahara H. DNA binding-dependent glucocorticoid receptor activity promotes adipogenesis via Krüppel-like factor 15 gene expression. Lab Invest. 2011;91(2):203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee MJ, Fried SK. The glucocorticoid receptor, not the mineralocorticoid receptor, plays the dominant role in adipogenesis and adipokine production in human adipocytes. Int J Obes. 2014;38(9):1228–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bird AD, McDougall AR, Seow B, Hooper SB, Cole TJ. Glucocorticoid regulation of lung development: lessons learned from conditional GR knockout mice. Mol Endocrinol. 2015;29(2):158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park YK, Ge K. Glucocorticoid receptor accelerates, but is dispensable for, adipogenesis. Mol Cell Biol. 2017;37(2):e00260-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewandoski M, Meyers EN, Martin GR. Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb Symp Quant Biol. 1997;62(1):159–168. [PubMed] [Google Scholar]
- 14. Mittelstadt PR, Monteiro JP, Ashwell JD. Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J Clin Invest. 2012;122(7):2384–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brewer JA, Khor B, Vogt SK, Muglia LM, Fujiwara H, Haegele KE, Sleckman BP, Muglia LJ. T-cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat Med. 2003;9(10):1318–1322. [DOI] [PubMed] [Google Scholar]
- 16. Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13(3):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Safran M, Kim WY, Kung AL, Horner JW, DePinho RA, Kaelin WG Jr. Mouse reporter strain for noninvasive bioluminescent imaging of cells that have undergone Cre-mediated recombination. Mol Imaging. 2003;2(4):297–302. [DOI] [PubMed] [Google Scholar]
- 18. Harris CA, Haas JT, Streeper RS, Stone SJ, Kumari M, Yang K, Han X, Brownell N, Gross RW, Zechner R, Farese RV Jr. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J Lipid Res. 2011;52(4):657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobsen KR, Kalliokoski O, Teilmann AC, Hau J, Abelson KS. The effect of isoflurane anaesthesia and vasectomy on circulating corticosterone and ACTH in BALB/c mice. Gen Comp Endocrinol. 2012;179(3):406–413. [DOI] [PubMed] [Google Scholar]
- 20. Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schütz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9(13):1608–1621. [DOI] [PubMed] [Google Scholar]
- 21. Fujimoto N, Matsuo N, Sumiyoshi H, Yamaguchi K, Saikawa T, Yoshimatsu H, Yoshioka H. Adiponectin is expressed in the brown adipose tissue and surrounding immature tissues in mouse embryos. Biochim Biophys Acta. 2005; 1731(1):1–12. [DOI] [PubMed] [Google Scholar]
- 22. Hong KY, Bae H, Park I, Park DY, Kim KH, Kubota Y, Cho ES, Kim H, Adams RH, Yoo OJ, Koh GY. Perilipin+ embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development. 2015;142(15):2623–2632. [DOI] [PubMed] [Google Scholar]
- 23. Rebuffé-Scrive M, Krotkiewski M, Elfverson J, Björntorp P. Muscle and adipose tissue morphology and metabolism in Cushing’s syndrome. J Clin Endocrinol Metab. 1988;67(6):1122–1128. [DOI] [PubMed] [Google Scholar]
- 24. Lindroos J, Husa J, Mitterer G, Haschemi A, Rauscher S, Haas R, Gröger M, Loewe R, Kohrgruber N, Schrögendorfer KF, Prager G, Beck H, Pospisilik JA, Zeyda M, Stulnig TM, Patsch W, Wagner O, Esterbauer H, Bilban M. Human but not mouse adipogenesis is critically dependent on LMO3. Cell Metab. 2013;18(1):62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing’s disease of the omentum”? Lancet. 1997;349(9060):1210–1213. [DOI] [PubMed] [Google Scholar]
- 26. Pantoja C, Huff JT, Yamamoto KR. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol Biol Cell. 2008;19(10):4032–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang QA, Tao C, Jiang L, Shao M, Ye R, Zhu Y, Gordillo R, Ali A, Lian Y, Holland WL, Gupta RK, Scherer PE. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat Cell Biol. 2015;17(9):1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whirledge S, DeFranco DB. Glucocorticoid signaling in health and disease: insights from tissue-specific GR knockout mice. Endocrinology. 2018;159(1):46–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bose SK, Hutson I, Harris CA. Hepatic glucocorticoid receptor plays a greater role than adipose GR in netabolic syndrome despite renal compensation. Endocrinology. 2016;157(12):4943–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Desarzens S, Faresse N. Adipocyte glucocorticoid receptor has a minor contribution in adipose tissue growth. J Endocrinol. 2016;230(1):1–11. [DOI] [PubMed] [Google Scholar]
- 31. Shen Y, Roh HC, Kumari M, Rosen ED. Adipocyte glucocorticoid receptor is important in lipolysis and insulin resistance due to exogenous steroids, but not insulin resistance caused by high fat feeding. Mol Metab. 2017;6(10):1150–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang F, Mullican SE, DiSpirito JR, Peed LC, Lazar MA. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proc Natl Acad Sci USA. 2013;110(46):18656–18661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qiang G, Whang Kong H, Xu S, Pham HA, Parlee SD, Burr AA, Gil V, Pang J, Hughes A, Gu X, Fantuzzi G, MacDougald OA, Liew CW. Lipodystrophy and severe metabolic dysfunction in mice with adipose tissue-specific insulin receptor ablation. Mol Metab. 2016;5(7):480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL. The transcriptome of retinal Müller glial cells. J Comp Neurol. 2008;509(2):225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33(2):77–80. [DOI] [PubMed] [Google Scholar]
- 36. Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA. 2002;99(16):10482–10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Macfarlane DP, Forbes S, Walker BR. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J Endocrinol. 2008;197(2):189–204. [DOI] [PubMed] [Google Scholar]
- 39. Yu CY, Mayba O, Lee JV, Tran J, Harris C, Speed TP, Wang JC. Genome-wide analysis of glucocorticoid receptor binding regions in adipocytes reveal gene network involved in triglyceride homeostasis. PLoS One. 2010;5(12):e15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harris C, Roohk DJ, Fitch M, Boudignon BM, Halloran BP, Hellerstein MK. Large increases in adipose triacylglycerol flux in Cushingoid CRH-Tg mice are explained by futile cycling. Am J Physiol Endocrinol Metab. 2013;304(3):E282–E293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Caprio M, Fève B, Claës A, Viengchareun S, Lombès M, Zennaro MC. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J. 2007;21(9):2185–2194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.