Abstract
The luteinizing hormone receptor (LHCGR) is expressed at low levels in mural granulosa cells and cumulus cells of antral follicles and is induced dramatically in granulosa cells but not in cumulus cells by follicle-stimulating hormone (FSH). Therefore, we hypothesized that FSH not only activates transcription factors controlling Lhcgr expression but also alters other events to permit and enhance Lhcgr expression in granulosa cells but not in cumulus cells. In granulosa cells, the level of DNA methylation in the Lhcgr promoter region was significantly decreased by equine chorionic gonadotropin (eCG) in vivo. However, in cumulus cells, hypermethylation of the Lhcgr promoter remained after eCG stimulation. eCG induced estrogen production from testosterone (T) and retinoic acid (RA) synthesis in granulosa cells. When either T or RA in the presence or absence of FSH was added to granulosa cell cultures, the combined treatment with FSH and RA induced demethylation of Lhcgr-promoter region and Lhcgr expression. FSH-dependent RA synthesis was negatively regulated by coculture of granulosa cells with denuded oocytes, suggesting that oocyte-secreted factors downregulate RA production in cumulus cells where Lhcgr expression was not induced. Strikingly, treatment of cultured cumulus-oocyte complexes with a SMAD inhibitor, SB431542, significantly induced RA production, demethylation of Lhcgr-promoter region, and Lhcgr expression in cumulus cells. These results indicate the demethylation of the Lhcgr-promoter region is mediated, at least in part, by RA synthesis and is a key mechanism regulating the cell type–specific differentiation during follicular development.
Oocytes negatively regulate retinoic acid synthesis in preovulatory follicles, which affects the expression of Lhcgr in follicular somatic cells via a DNA-demethylation–dependent mechanism.
The pituitary surge of luteinizing hormone (LH) acts on granulosa cells of preovulatory follicles to induce ovulation (1), whereas basal levels of LH act on theca cells of growing follicles to maintain androgen biosynthesis; androgen, in turn, is converted to estradiol in granulosa cells (2, 3). During the development of preovulatory follicles androgens, estradiol and follicle-stimulating hormone (FSH) promote the induction of LH receptors (LHCGRs) in mural granulosa cells that is obligatory for LH-induced cumulus cell-oocyte complex (COC) expansion, ovulation, and subsequent luteinization of granulosa cells (4).
LHCGR is a member of the G-protein coupling receptor family and is encoded by the Lhcgr gene (5). Lhcgr is constitutively expressed in theca cells of growing follicles and is selectively expressed in mural granulosa cells of preovulatory follicles but is not expressed in cumulus cells (6). The FSH-induced expression of LHCGR in granulosa cells is regulated by a PI 3-kinase–PKB pathway activation of β-catenin that binds a TCF3 site in the Lhcgr promoter (7). The Lhcgr promoter region also has Sp1 binding sites that affect the expression of Lhcgr not only in granulosa cells and theca cells but also in testicular Leydig cells (8), indicating that Sp1 binding sites may act as a basic regulator of Lhcgr gene expression, whereas the TCF3 region is a modifier to enhance Lhcgr gene expression in granulosa cells.
The transcription factor Sp1 has multiple phosphorylation sites that, when phosphorylated by PKA and PKC in response to FSH, lead to Sp1’s transcriptional activation in granulosa cells (9, 10). Moreover, either FSH or forskolin increase intracellular cyclic adenosine monophosphate levels, which rapidly stimulate the activity of Lhcgr promoter–luciferase reporter constructs in granulosa cells, indicating that the promoter is highly responsive to hormone induction (7). However, the induction of Lhcgr messenger RNA (mRNA) by FSH in granulosa cells in vitro or in vivo is not rapid and is only observed after >24 hours of FSH and equine chorionic gonadotropin (eCG) treatment (11, 12). Therefore, not only cell signaling pathways that activate the transcription factors β-catenin and Sp1 but also other unknown mechanisms appear to regulate Lhcgr expression to ensure its expression in granulosa cells of preovulatory follicles is coordinated with the timing of LH surge, thus ensuring female fertility.
In a previous study (13), we described the following three characteristics of de novo synthesized retinoic acid (RA): (1) It is produced by theca cells and granulosa cells during follicular development, especially in FSH-stimulated granulosa cells, due to the high expression of alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) family members; (2) acts on granulosa cells and theca cells by activating members of the nuclear receptor RA receptor (RAR) family, including RARγ; and (3) when suppressed by the injection of either ADH or ALDH inhibitors, de novo synthesized RA significantly reduced expression of Lhcgr in granulosa cells and suppressed ovulation. On the basis of these results, we hypothesized that RA controls the cell- and timing-specific expression of Lhcgr by indirect mechanisms that affect Lhcgr promoter activation.
One possible mechanism involves epigenetic regulation of Lhcgr promoter activity. Sp1 selectively binds to CG repeat sequences (CpG islands) that, if methylated, prevent Sp1 from binding to this target sequence, resulting in the suppression of gene expression (14). Zhu et al. (15) reported that the expression level of Lhcgr was associated with the methylation status of the lhcgr promoter region in ovaries of patients with polycystic ovary syndrome (PCOS). However, there is little information about what regulates the epigenetic status of the Lhcgr gene, especially the dynamic changes of methylation that occur in the Lhcgr promoter region in granulosa cells during follicular development. Because the RA-RAR pathway can act not only at the transcriptional level but also as a regulator of epigenetic events (16, 17), we investigated the kinetic changes and cell type–specific changes in the methylation status of the Lhcgr promoter region in distinct ovarian somatic cells during follicular development and ovulation.
Materials and Methods
Materials
eCG and human chorionic gonadotropin (hCG) were purchased from Asuka Seiyaku (Tokyo, Japan), Dulbecco’s modified Eagle medium (DMEM)/F12 medium and penicillin-streptomycin were purchased from Invitrogen (Carlsbad, CA), fetal calf serum (FCS) from Life Technologies (Grand Island, NY), oligonucleotide poly-(dT) from Invitrogen, and AMV reverse transcription from Promega Corporation (Madison, WI). Routine chemicals and reagents were obtained from Nacalai Chemical Company (Osaka, Japan) or Sigma-Aldrich (St. Louis, MO).
Animals
Immature female (3 weeks old) C57BL/6 mice were obtained from Charles River Laboratories Japan (Yokohama, Japan). Twenty-three day-old female mice were injected intraperitoneally with 4 IU of eCG to stimulate follicular growth; after 48 hours, they were injected with 5 IU of hCG to stimulate ovulation and luteinization. For pharmacological experiments, other immature mice were injected with 8 mg/kg 4-methylpyrazole (4MP; Sigma-Aldrich) two times every 24 hours. To analyze the functional activity of RA in ovaries, CD1 retinoic acid responsive element (RARE) reporter mice harboring an RA-responsive transgene (RARE-Hspa1b-LacZ; Jackson Laboratory, Bar Harbor, ME) were used in this study. The RARE reporter mice allow visualization of the distribution of RA signaling by X-Gal staining (18). Animals were housed under 12 hours of light:12 hours of dark in the Experiment Animal Center at Hiroshima University and provided food and water ad libitum. Animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as approved by the Animal Care and Use Committee at Hiroshima University.
RNA extraction
Total RNA was obtained from mouse granulosa cells, from cumulus cells in COCs, theca cells (remaining cells excluding granulosa cells from each ovarian follicle), granulosa cells cultured with or without germinal vesicle (GV)–stage oocytes or cultured COCs using RNAeasy Mini Kit (Qiagen Germantown, MD) according to the manufacturer's instruction. Total RNA (100 ng) was reverse transcribed using 500 ng of poly-dT (Invitrogen) and 0.25 U of avian myeloblastosis virus–reverse transcription (Promega) at 42°C for 75 minutes and at 95°C for 5 minutes.
Real-time polymerase chain reaction
Complementary DNA and primers were added to 15 μL total reaction volume of Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Polymerase chain reactions (PCRs) were then performed using the StepOne Real-Time PCR system (Applied Biosystems). Conditions were set to the following parameters: 10 minutes at 95°C, followed by 40 cycles each of 15 seconds at 95°C and 1 minute at 60°C to 64°C. The primer sets are shown in Supplemental Table 1. L19 was used as a control for reaction efficiency and variations in concentrations of mRNA in the original real-time PCR.
Genomic DNA extraction
Genomic DNA was obtained from mouse granulosa cells, COCs, theca cells (remaining cells excluding granulosa cells from each ovarian follicle), granulosa cells cultured with or without GV-stage oocytes or cultured COCs using QIAamp DNA Blood Mini Kit (Qiagen) according to the manufacturer's instruction. Genomic DNA (1 mg) was prepared for bisulfite sequence assay.
Bisulfite sequence assay
CpG islands in the putative Lhcgr promoter region were predicted by the MethPrimer database (http://www.urogene.org/methprimer/). Genomic DNA (1 mg) and 0.3 M NaOH (Nacalai) were added to the sodium bisulfite reaction mix using the EpiTect Bisulfite Kit (Qiagen), and then incubated at 37°C for 20 minutes. A program of bisulfite DNA conversion was set to the following parameters: 5 minutes at 95°C, 25 minutes at 60°C, 5 minutes at 95°C, 85 minutes at 60°C, 5 minutes at 95°C, 175 minutes at 60°C, 5 minutes at 95°C, and 120 minutes at 60°C.
After this reaction, the bisulfite-converted DNA was purified and then used for PCR using a specific primer set to amplify the sequences containing CpG islands in the Lhcgr promoter region. The primer sets are forward, 5′-GTGAGAGGGGAGGGTTGGAG-3′, and reverse, 5′-TTCAAGACCAACATTACCAA CACCA-3′. The PCR product was cloned into TOPO TA cloning vector (Invitrogen) for the sequence analysis. The sequence analysis was performed using BigDye Terminator version 3.1/1.1 Cycle Sequencing Kit (Applied Biosystems). Sequence analysis was performed on more than five colonies for each treatment. DNA nucleotide sequence was determined using the 3130/3130xl Genetic Analyzer (Applied Biosystems). The percentage of methylation of CpG sites in the Lhcgr promoter region was analyzed by the Quantification tool for Methylation Analysis (http://quma.cdb.riken.jp/).
Isolation and in vitro culture of granulosa cells
Granulosa cells were collected from ovaries of immature mice (3 weeks old) at 6 hours after injections of eCG. The cells were collected in a 15-mL tube and were centrifuged for 5 minutes at 750g. After the supernatant was removed, the cell pellet was suspended again in medium (DMEM/F12 containing penicillin and streptomycin; Invitrogen) and then the mixture was seeded onto an FCS-recoated 96-well plate. Granulosa cells were treated with 50 ng/mL FSH (National Institute of Diabetes and Digestive and Kidney Diseases, Torrance, CA) in the absence of FCS or in the presence of 1% FCS. Some granulosa cell wells were treated with testosterone (T; 10 ng/mL), estradiol 17β (1 μM), 5-aza-2ʹ-deoxycytidine (5azadC; 1 or 2 μM), or 1 μM RA (all from Sigma-Aldrich) in the presence or absence of FSH.
Isolation and in vitro culture of COCs
Ovaries of immature mice primed with eCG for 48 hours contain multiple preovulatory follicles. COCs were isolated from these follicles by needle puncture and collected by pipette. Groups of 50 COCs per well were cultured in DMEM/F12 medium containing 0.23 mM sodium pyruvate (Nacalai), 3 mg/mL bovine serum albumin (Sigma-Aldrich), 10 μM estradiol 17β (Sigma-Aldrich), and 0.2 mM of 3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich) in a four-well dish for 20 hours. IBMX was added to suppress the spontaneous oocyte maturation. Some COCs were cultured in medium containing 5 μM RA (Sigma-Aldrich) or 2 μM SB431542 (Cayman Chemical, MI) and 1% FCS.
Isolation and coculture of oocytes with granulosa cells
Granulosa cells were collected from ovaries of immature mice (3 weeks old) as described previously in the “Materials and Methods” section. GV-stage oocytes were collected from COCs isolated from each ovarian antral follicle by needle puncture. Granulosa cells and GV-stage oocytes were cocultured in a 96-well plate (HTS Transwell-96 Permeable Support with 8.0-µm Pore Polyester Membrane no. 3374; Corning Incorporated, Kennebunk, ME) for 48 hours and treated with 50 ng/mL FSH and 10 ng/mL T or 10 μM RA in the presence of 1% FCS and 0.2 mM IBMX. IBMX was added to suppress the spontaneous oocyte maturation because GV-stage oocytes strongly suppressed Lhcgr expression in granulosa cells.
Quantitative β-galactosidase activity assay
COCs, granulosa cells, theca cells (remaining cells excluding granulosa cells from each ovarian follicle), and granulosa cells cultured with or without GV-stage oocytes or cultured COCs were lysed with cell extract buffer [20 mM sodium phosphate solution (pH 7.2), 50 mM 2-mercaptoethanol solution, and 1 mM MgCl2]. Extracts were centrifuged at 15,000 rpm at 4°C for 5 minutes. The supernatant from each sample was used to measure protein concentrations. 10 μL of 30 mg/mL chlorophenol red-β-d-galactopyranoside (Roche Diagnostics, Indianapolis, IN)/double-distilled water was added to each 200 μg of cell extract and incubated at 37°C for 2 hours. The reactions were stopped by adding 100 μL of 1 M sodium carbonate. β-galactosidase activity was measured using a microplate reader to determine the amount of substrate converted at 595 nm.
Statistics
Statistical analyses of all data from three or four replicates for comparison were carried out by one-way analysis of variance followed by Fisher t test (Statview; Abacus Concepts, Berkeley, CA).
Results
DNA methylation levels in the Lhcgr promoter region differ in each type of ovarian somatic cell
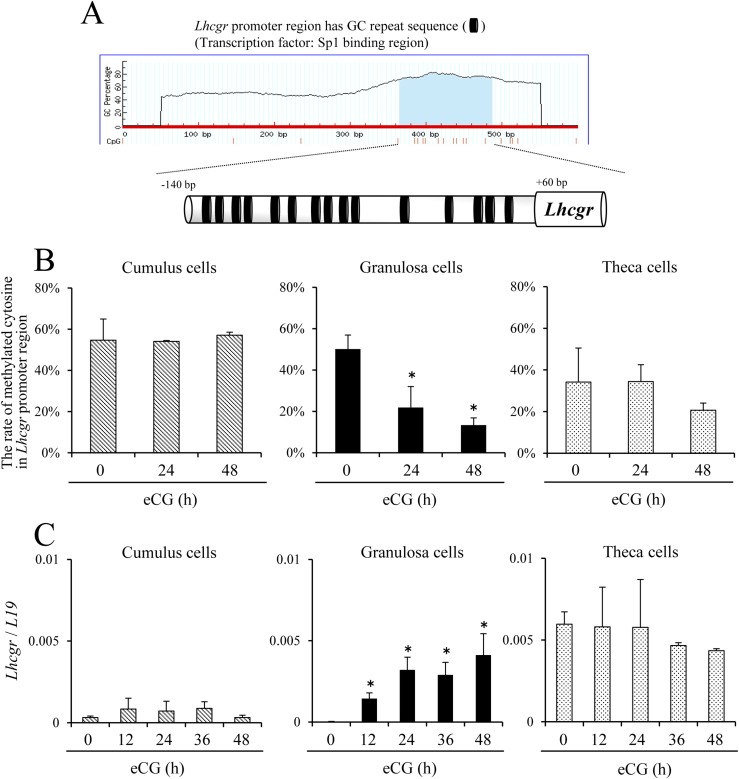
Using the MethPrimer database, 15 sites of GC-rich sequences (CpG islands) from −140 bp to +60 bp in the Lhcgr promoter region were identified (Fig. 1A). The degree of methylated CpG islands was calculated by using the Quantification tool for Methylation Analysis. In cumulus cells, >50% of cytosines in the CpG islands of the Lhcgr promoter region were methylated when cumulus cells were recovered from either immature mice or eCG-injected mice. Although almost a similar degree of Lhcgr promoter methylation (∼50%) was observed in granulosa cells as in cumulus cells before eCG injection, the degree of methylation was significantly decreased by eCG in a time-dependent manner, reaching <20% at 48 hours after hormone treatment. In theca cells, the degree of CpG island methylation in the Lhcgr promoter was ∼20% and this level was not significantly changed during eCG-induced follicular development (Fig. 1B).
Figure 1.
The relationship between the expression of Lhcgr mRNA and methylated CpG islands in the Lhcgr promoter region in eCG-stimulated ovarian somatic cells. (A) The position of GC repeat sequences (CpG islands) in the Lhcgr promoter region. CpG islands in putative Lhcgr promoter region were predicted by the MethPrimer database (http://www.urogene.org/methprimer/). (B) The kinetic changes of cytosine methylation in the Lhcgr promoter region in cumulus cells, granulosa cells and theca cells after eCG injection. Sequence analysis was performed on more than five colonies for each treatment. The studies were repeated three times. Values are given as mean ± standard error of the mean (SEM). Significant differences were observed between eCG at 0 hours and eCG-primed mice (P < 0.05). (C) The kinetic changes of the expression of Lhcgr in cumulus cells, granulosa cells, and theca cells after eCG injection. Levels of mRNA were normalized to that of L19. Values are presented as the mean ± SEM of three replicates. *P < 0.05.
The expression levels of Lhcgr in cumulus cells, granulosa cells, or theca cells were analyzed by real-time PCR. In cumulus cells, the expression of Lhcgr was not dramatically changed by eCG injection. In granulosa cells, the expression of Lhcgr was low, as in cumulus cells before eCG treatment, whereas the expression was significantly induced after hormone injection. In theca cells, Lhcgr expression was much higher than in cumulus cells or granulosa cells of immature mice but was not further increased by eCG injection (Fig. 1C).
De novo synthesized RA during follicular development induces DNA demethylation of the Lhcgr promoter region in granulosa cells
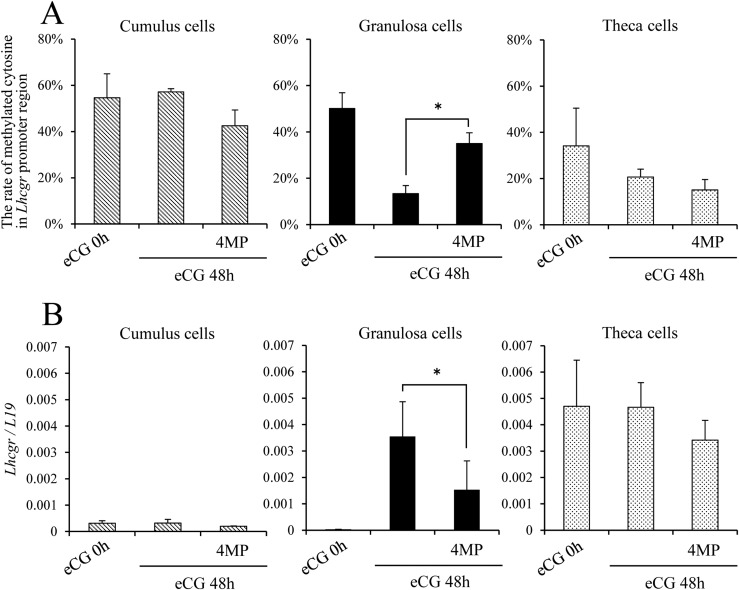
When the ADH inhibitor 4MP was coinjected with eCG into immature female mice, the level of DNA methylation in the Lhcgr promoter region was not affected in cumulus cells or theca cells. However, in granulosa cells, the eCG-induced demethylation of the Lhcgr promoter region was significantly blocked by the combined treatment with 4MP (Fig. 2A). Concomitantly with increased methylation of the Lhcgr promoter region in granulosa cells, eCG-induced Lhcgr expression was significantly suppressed by 4MP. The inhibitor did not alter methylation in cumulus cells and theca cells (Fig. 2B).
Figure 2.
RA synthesis is required for the induction of Lhcgr expression in granulosa cells (but not in cumulus cells and theca cells) and is related to DNA demethylation in the Lhcgr promoter region. (A) Changes in cytosine methylation in the Lhcgr promoter region in cumulus cells, granulosa cells, and theca cells at 48 hours after in vivo injection of eCG and/or the ADH inhibitor 4MP. Significant differences were observed between granulosa cells of eCG at 48 hours and those of eCG plus 4MP–primed mice. Sequence analysis was performed on more than five colonies for each treatment. The studies were repeated three times. Values are given as mean ± standard error of the mean (SEM). (B) The kinetic changes in the expression of Lhcgr in cumulus cells, granulosa cells, and theca cells after eCG and/or 4MP injection. Levels of mRNA were normalized to that of L19. Significant differences were observed between eCG-primed mice and eCG plus 4MP–primed mice (P < 0.05). Values are given as the mean ± SEM of three replicates. *P < 0.05.
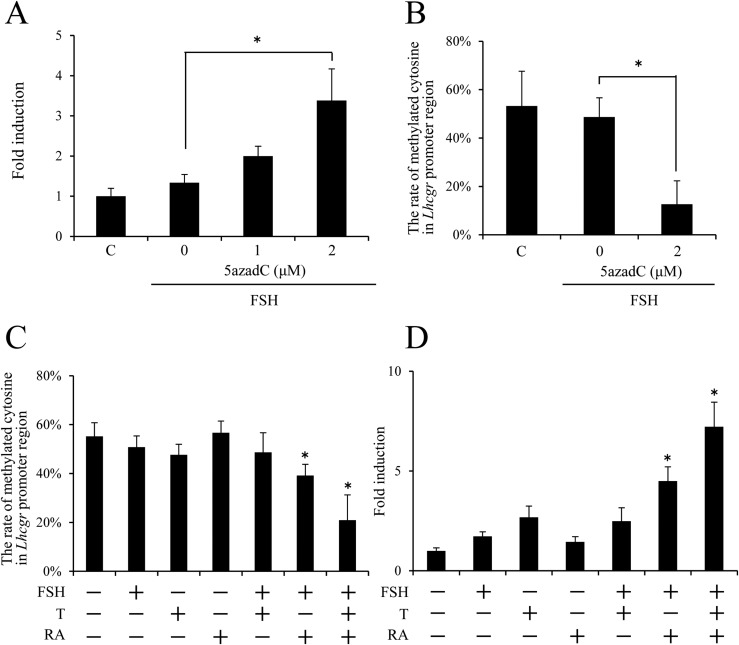
Lhcgr mRNA expression in granulosa cells depends on FSH stimulation and either treatment with DNA methyl transferase inhibitor or RA in culture
When undifferentiated granulosa cells were cultured with FSH and/or a DNA methyltransferase inhibitor, 5azadC, the combined treatment with FSH and 2 μM 5azadC for 48 hours significantly increased the level of Lhcgr mRNA as compared with that by either FSH or 5azadC treatment alone (Fig. 3A; Supplemental Fig. 2). Methylation of the Lhcgr promoter region was ∼50% in granulosa cells cultured for 48 hours in FSH alone. The addition of 2 μM 5azadC to FSH-containing medium significantly decreased the methylation of the Lhcgr promoter region to ∼15% (Fig. 3B).
Figure 3.
The effects of the methyltransferase inhibitor 5azadC or RA on Lhcgr expression and methylation of the Lhcgr promoter region in FSH-stimulated granulosa cells. (A) The effects of 5azadC on the Lhcgr expression in FSH-stimulated granulosa cells. Granulosa cells were collected from ovaries of immature mice and were cultured with FSH and the different concentrations of 5azadC in the absence of FCS for 48 hours. Levels of mRNA were normalized to that of L19. Values are given as mean ± standard error of the mean (SEM) of three replicates. (B) The effect of 5azadC on the methylation of the Lhcgr promoter region in FSH-stimulated granulosa cells. 5azadC (2 μM) significantly decreased the rate of methylated cytosine in the Lhcgr promoter region in FSH-stimulated granulosa cells (P < 0.05). Sequence analysis was performed on more than five colonies for each treatment of three replicates. Values are given as mean ± SEM. (C) The methylation of the Lhcgr promoter region in cultured granulosa cells. Significant differences were observed between the control group (without any hormones) and the treatment group of FSH plus RA or FSH plus RA plus T (P < 0.05). Sequence analysis was performed on more than five colonies for each treatment. The studies were repeated three times. Values are given as mean ± standard error of the mean (SEM). Granulosa cells were cultured with T (10 ng/mL). Granulosa cells were cultured with 50 ng/mL FSH. Granulosa cells were cultured with 1 μM RA. (D) The expression of Lhcgr in cultured granulosa cells. Significant differences were observed between the control group (without any hormones) and the treatment group of FSH plus RA or FSH plus RA plus T (P < 0.05). Levels of mRNA were normalized to that of L19. Values are given as mean ± SEM of three replicates. The value of the control group (without any hormones) was set as 1, and the data are presented as fold induction. Granulosa cells were cultured with 10 ng/mL T. FSH: Granulosa cells were cultured with FSH (50 ng/mL). RA: Granulosa cells were cultured with RA (1 μM). *P < 0.05.
Methylation of the Lhcgr promoter region was not significantly changed when granulosa cells were cultured in the presence of either FSH, T, or RA in the FCS-free condition. The combined treatment with FSH and T also did not significantly affect the methylation status. However, methylation of Lhcgr promoter was significantly decreased when cells were cultured with FSH plus RA and further reduced to ∼20% by the addition of T to the medium containing FSH and RA (Fig. 3C). The expression of Lhcgr was not significantly induced by FSH, RA, T, or FSH plus T treatment groups, whereas the addition of RA to FSH-containing medium or FSH plus T –containing medium significantly increased the level of Lhcgr mRNA (Fig. 3D).
When FCS was added to granulosa cell culture medium, FSH plus T treatment significantly increased Lhcgr expression (Supplemental Fig. 1A) and significantly reduced methylation of Lhcgr promoter region (Supplemental Fig. 1B) without RA treatment.
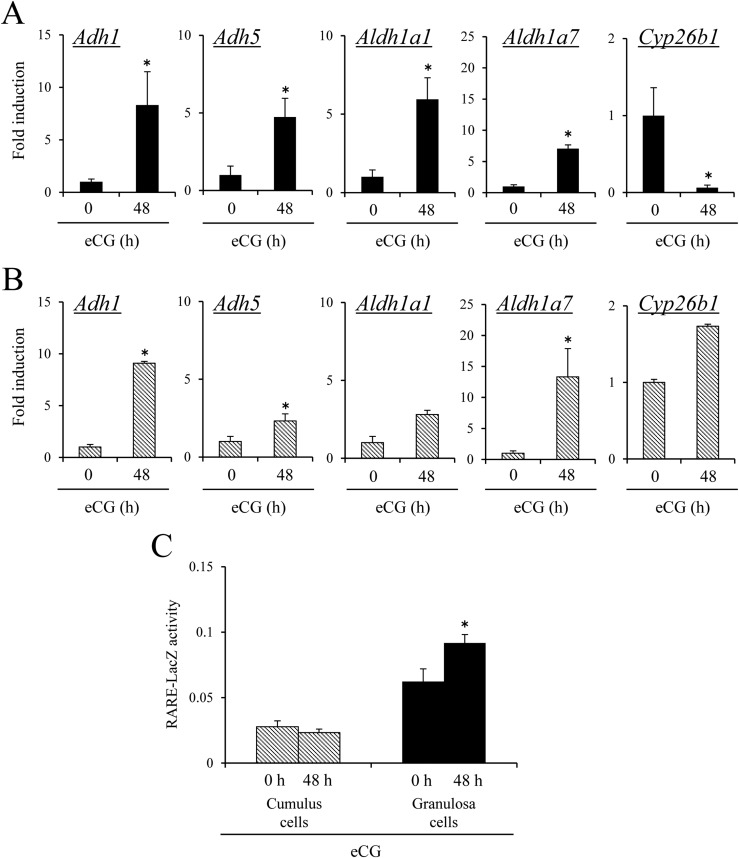
Relation of RA synthesis pathways and DNA methylation of the Lhcgr promoter region during follicular development
In granulosa cells, the expression of genes involved in RA synthesis, Aldh1a1, Adh1a7, Adh1 and Adh5 was significantly increased by eCG injections. The expression of Cyp26b1 (controlling RA degradation) was significantly decreased by eCG in granulosa cells (Fig. 4A). In cumulus cells, the expression levels of Aldh1a7, Adh1, and Adh5 were also significantly increased by eCG injection; however, a reduction of Cyp26b1 expression was not observed in cumulus cells (Fig. 4B). The level of de novo synthesized RA was examined by analyzing the RARE reporter mice. LacZ enzyme activity in granulosa cells, but not in cumulus cells, was significantly increased by eCG (Fig. 4C).
Figure 4.
The difference in RA synthesis and degradation enzymes between cumulus cells and granulosa cells. (A) The expression of Adh1, Adh5, Aldh1a1, Aldh1a7, and Cyp26b1 mRNA in granulosa cells of immature mice treated with or without eCG in vivo. The value of eCG at 0 hours in granulosa cells was set as 1, and the data are presented as fold induction. Values are given as mean ± standard error of the mean (SEM) of three replicates. Significant differences were observed in comparison with the value of eCG at 0 hours (P < 0.05). (B) The expression of Adh1, Adh5, Aldh1a1, Aldh1a7, and Cyp26b1 mRNA in cumulus cells of immature mice treated with or without eCG in vivo. The value of eCG at 0 hours in cumulus cells was set as 1, and the data are presented as fold induction. Values are given as mean ± SEM of three replicates. Significant differences were observed in comparison with the value of eCG at 0 hours (P < 0.05). (C) Activity of LacZ enzyme driven by RARE promoter in cumulus cells and granulosa cells of immature mice with or without 48-hour eCG treatment. Values are shown for RARE-LacZ activity at 595 nm after the protein samples were incubated for 2 hours at 37°C with chlorophenol red-β-d-galactopyranoside. Values are given as mean ± SEM of more than three replicates. eCG treatment significantly increased RARE-LacZ activity in granulosa cells. *P < 0.05.
The RA synthesis and demethylation of Lhcgr in granulosa cells during follicular development is regulated, in part, by factors coming from oocytes
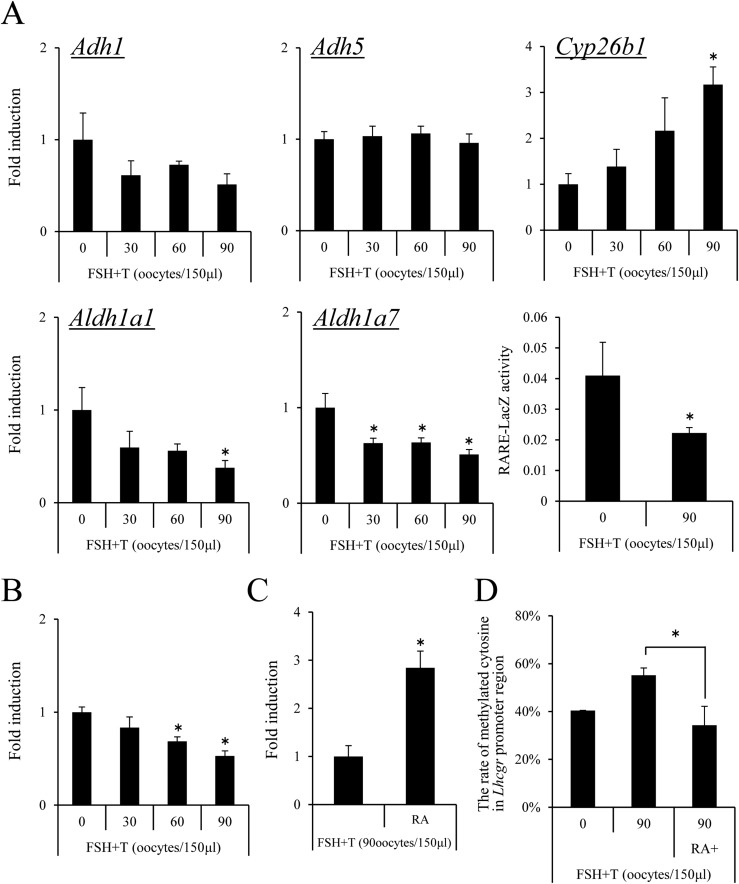
When granulosa cells were cocultured with the different numbers of GV-stage oocytes, increased numbers of GV-stage oocytes (60 to 90 oocytes per 150 μL of medium) significantly suppressed the expression of the Aldh1a1, Aldh1a7, and Lhcgr genes and significantly increased Cyp26b1 expression (Fig. 5A and 5B). LacZ enzyme activity of granulosa cells from RARE-LacZ mice was also significantly decreased by coculture with oocytes (Fig. 5A). The addition of RA overcame the negative effect of coculture with oocytes on Lhcgr expression in granulosa cells (Fig. 5C). Methylation of the Lhcgr promoter region was significantly increased by the coculture with oocytes, whereas the addition of RA overcame the negative effect (Fig. 5D).
Figure 5.
The negative effects of oocytes on the induction of Lhcgr expression occur via the regulation of methylation in the Lhcgr promoter region in granulosa cells. (A) The effect of oocytes on the expression of Adh1, Adh5, Aldh1a1, Alhd1a7, and Cyp26b1 in granulosa cells. Granulosa cells were collected from ovaries of immature mice after treatment with eCG for 6 hours and were cultured in the medium supplemented with FSH plus T in the presence of 1% FCS. GV-stage oocytes were collected from COCs. None, 30, 60, or 90 of GV-stage oocytes per 150 μL were cultured with granulosa cells in a 96-well plate. Values are given as mean ± standard error of the mean (SEM) of three replicates. The value of granulosa cells that were cultured without denuded oocytes was set as 1, and the data are presented as fold induction. FSH plus T: Granulosa cells were cultured with 50 ng/mL FSH and 10 ng/mL T for 48 hours. Significant differences were observed compared with those treated with no oocytes (P < 0.05). Activity of LacZ enzyme driven by the RARE promoter in granulosa cells cultured with 90 oocytes per 150 μL: Values are shown for RARE-LacZ activity at 595 nm after the protein samples were incubated for 2 hours at 37°C with chlorophenol red-β-d-galactopyranoside. Values are given as mean ± SEM of more than three replicates. Significant differences were observed compared with those treated with no oocytes (P < 0.05). (B) The effect of oocytes on the expression of Lhcgr in granulosa cells. Granulosa cells were collected from ovaries of immature mice after treatment with eCG for 6 hours, and were cultured in the medium supplemented with FSH plus T in the presence of 1% FCS. GV-stage oocytes were collected from COCs. None, 30, 60, or 90 GV-stage oocytes per 150 μL were cocultured with granulosa cells. Values are given as mean ± SEM of three replicates. The value of granulosa cells at 0 hours cultured without denuded oocytes was set as 1, and the data are presented as fold induction. FSH plus T : Granulosa cells were cultured with 50 ng/mL FSH and 10 ng/mL T for 48 hours. Significant differences were observed compared with cells treated with no oocytes (P < 0.05). (C) The additional treatment with RA rescued the negative effects of denuded oocytes on Lhcgr expression in granulosa cells. RA (1 μM) was added to the 150 μL of medium in which granulosa cells were cultured with 90 GV-stage oocytes. Significant differences were observed as compared with cells treated with 90 oocytes per 150 μL (P < 0.05). (D) The effect of oocytes on the methylation levels of Lhcgr promoter region in granulosa cells. Granulosa cells were collected from ovaries of immature mice, after treating with eCG for 6 hours, and were cultured in the medium supplemented with FSH plus T in the presence of 1% FCS. GV-stage oocytes were collected from COCs. GV-stage oocytes (90 per 150 μL of medium) were cultured with granulosa cells in a 96-well plate. Values are given as mean ± SEM of three replicates. FSH plus T: Granulosa cells were cultured with 50 ng/mL FSH and T10 ng/m T for 48 hours. RA (1 μM) was added to the in vitro culture of granulosa cells with 90 GV-stage oocytes per 150 μL of medium. Significant differences were observed as compared with cells treated with 90 oocytes per 150 μL of medium. *P < 0.05.
Treatments with RA or SMAD inhibitors induced Lhcgr expression in cumulus cells of COCs
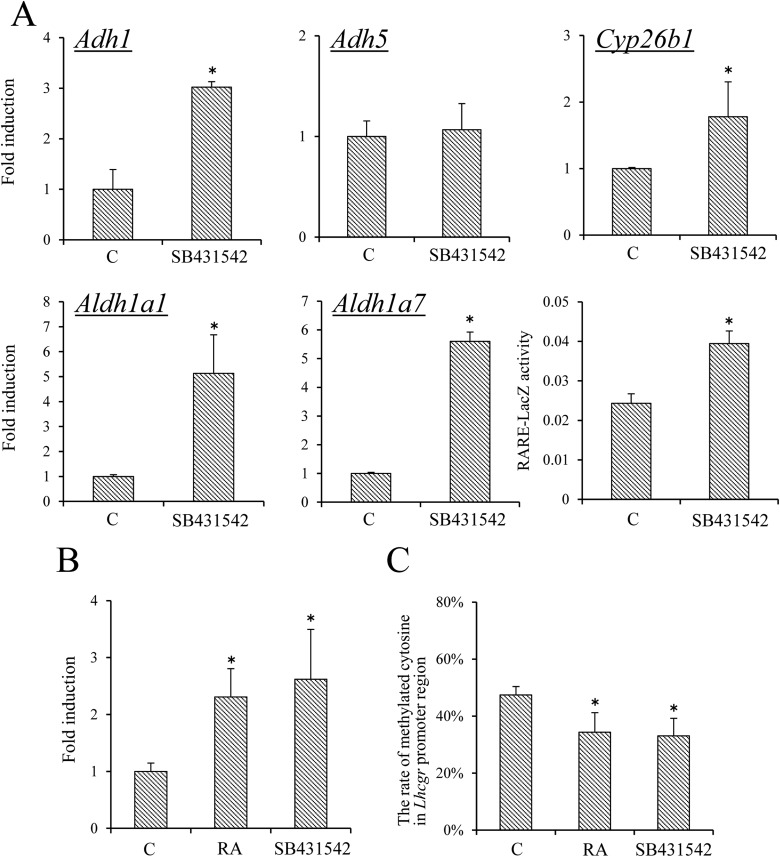
Oocytes release transforming growth factor–related factors (GDF9 and BMP15) that can activate intracellular SMAD pathway in cumulus cells and granulosa cells (19). When COCs were treated with the SMAD inhibitor SB431542, the expression levels of RA synthesizing enzymes (i.e., Adh1, Aldh1a1, and Aldh1a7) were significantly increased as compared with those in cumulus cells of control COCs. LacZ enzyme activity in cumulus cells of COCs in RARE-mice was also significantly increased by the SMAD inhibitor (Fig. 6A). Furthermore, SB431542 significantly increased the expression of Lhcgr in cumulus cells of COCs and this was associated with reduced methylation of the Lhcgr promoter region (Fig. 6C).
Figure 6.
The effect of RA or SMAD inhibitors on Lhcgr expression relative to Lhcgr promoter methylation in cumulus cells of COCs. (A) The effect of SB431542 on the expression of Adh1, Adh5, Aldh1a1, Aldh1a7, and Cyp26b1 in cumulus cells of COCs. COCs were collected from ovaries of immature mice primed with eCG for 48 hours. COCs (n = 50) were cultured in DMEM/F12 medium containing sodium pyruvate (0.23 mM), bovine serum albumin (BSA; 3 mg/mL), IBMX (0.2 mM), 1% FCS, and estradiol 17β (10 μM) for 20 hours. SB431542: COCs were cultured for 20 hours with SB431542 (2 μM). Levels of mRNA were normalized to that of L19. The value of controls (i.e., COCs cultured without SB431542) was set as 1, and the data are presented as fold induction. (B) The effect of SB431542 on RARE-LacZ activity in COCs. Data are shown for activity of LacZ enzyme driven by RARE promoter in cumulus cells of COCs with or without 2 μM of SB431542 for 20 hours. Values are RARE-LacZ activity at 595 nm after the protein samples were incubated for 2 hours at 37°C with chlorophenol red-β-d-galactopyranoside. Values are given as mean ± standard error of the mean (SEM) of more than three replicates. (C) The effect of RA or SB431542 on the expression of Lhcgr in cumulus cells of COCs. COCs were collected from ovaries of immature mice primed with eCG for 48 hours. COCs (n = 50) were cultured in DMEM/F12 medium containing sodium pyruvate (0.23 mM), BSA (3 mg/mL), IBMX (0.2 mM), and estradiol 17β (10 μM) for 20 hours. RA: COCs were cultured for 20 hours with RA (5 μM). Levels of mRNA were normalized to that of L19. (D) The effect of RA or SB431542 on methylation of the Lhcgr promoter region in cumulus cells of COCs. COCs were collected from ovary of immature mice primed with eCG for 48 hours. COCs (n = 100) were cultured in DMEM/F12 medium containing sodium pyruvate (0.23 mM), BSA (3 mg/mL), IBMX (0.2 mM), and estradiol 17β (10 μM) for 20 hours. Sequence analysis was performed on more than five colonies for each treatment of three replicates. Values are given as mean ± SEM. SB431542: COCs were cultured for 20 hours with SB431542 (2 μM). RA: COCs were cultured for 20 hours with RA (5 μM). *P < 0.05 compared with control. C, control.
When COCs were cultured with RA, the expression of Lhcgr was significantly increased (Fig. 6B) and the degree of DNA methylation in Lhcgr promoter region was significantly decreased as compared with that in control (Fig. 6C).
Discussion
The expression pattern of Lhcgr is specific in each ovarian somatic cell type during follicular development; in theca cells, Lhcgr is constitutively expressed; in granulosa cells, Lhcgr is induced by FSH; and in cumulus cells, Lhcgr expression is low and is not induced by FSH (20, 21). The differences in Lhcgr expression have been thought to be regulated primarily by oocyte-secreted factors, based on the evidence that the addition of denuded oocytes to granulosa cell cultures significantly decreases Lhcgr expression (6) and because oocytectomy induced Lhcgr expression in cumulus cells (19). Although the oocyte-derived factors GDF9 and BMP15 affect the function and phenotype of cumulus cells (22), whether the same or related factors or other hormones control Lhcgr expression in granulosa cells remain to be clearly defined.
In the current study, we document that eCG treatment in vivo dramatically induced Lhcgr expression in granulosa cells and that the induction of Lhcgr expression was associated with reduced methylation of the Lhcgr promoter region in granulosa cells. By contrast, Lhcgr was not induced in cumulus cells, where >50% of the cytosines in the Lhcgr promoter region remained methylated. One transcription factor that facilitates Lhcgr expression is Sp1, which binds to CpG islands within promoter regions (14). For example, CpG islands are present in the Lhcgr promoter region and have been functionally linked to Lhcgr expression. Specifically, in the highly methylated JAR cell line, the CpG islands in the Lhcgr promoter region are methylated, Lhcgr expression is low, and treatment with the histone deacetylase inhibitor trichostatin A does not induce Lhcgr transcription (23). On the other hand, in MCF7 cells, which are a hypomethylated cell line, Lhcgr expression is detected and further induced by trichostatin A treatment (23), indicating that Lhcgr expression in follicular somatic cells might be associated the methylation status of the Lhcgr promoter region.
To test this hypothesis, we used two approaches in the current study: (1) Using in vitro culture of granulosa cells, we examined the relationship between the DNA methylation status in Lhcgr promoter region and the expression of Lhcgr mRNA; and (2) we used in vivo studies to analyze RA de novo synthesis and the demethylation of the Lhcgr promoter region. We observed that when granulosa cells were cultured with the DNA methyltransferase inhibitor 5azadC or with FSH alone, Lhcgr expression was not induced. However, Lhcgr expression was significantly increased and DNA methylation of the Lhcgr promoter was significantly reduced when FSH and 5azadC treatments were combined. Previously, we observed that RA is synthesized in granulosa cells during follicular development, is dependent on the induction of ADH and ALDH family members by FSH, and that RA synthesis influences Lhcgr expression (13).
Herein, we show that the injection of the ADH inhibitor 4MP with eCG suppressed the FSH-induced demethylation of the Lhcgr promoter region in granulosa cells and prevented induction of Lhcgr mRNA. Furthermore, the addition of RA to FSH-containing medium induced Lhcgr expression and demethylation of its promoter region in granulosa cell cultures. More importantly, like 5azadC alone, neither FSH alone nor RA alone increased Lhcgr expression, strongly suggesting that the demethylation of the Lhcgr promoter involves FSH-dependent RA biosynthesis that facilitates the ability to FSH to subsequently induce Lhcgr expression. The enhanced ability of FSH to induce Lhcgr in the presence of RA likely involves the transcription factors, Sp1 and β-catenin, which are activated by FSH signaling (7) and bind to the unmethylated Lhcgr promoter region. The fact that FSH rapidly activates Lhcgr promoter-reporter constructs in cultured granulosa cells (7), whereas >24 hours of culture or after eCG injection in vivo are required for the induction of Lhcgr expression, supports the hypothesis that demethylation of the Lhcgr promoter preceded, and is obligatory for, FSH induction of this gene (11, 12).
Cumulus cells secrete GDF9 and BMP15, which regulate metabolic pathways in cumulus cells and granulosa cells (24, 25). In cumulus cells, the expression of enzymes regulating RA biosynthesis, ADH, and ALDH was lower than that in FSH-stimulated granulosa cells and appears to be related to the suppression of the RA pathway by transforming growth factor β factors GDF9 and BMP15 SMAD-signaling events (26) for two reasons: (1) The SMAD inhibitor induced ADH and ALDH enzymes in cumulus cells and (2) the addition of denuded oocytes suppressed FSH-changed ADH and ALDH expression in granulosa cells. Moreover, the addition of RA to COC cultures induced Lhcgr expression in cumulus cells and decreased DNA methylation of the Lhcgr promoter region. The negative functional interplay between RA and SMAD signaling pathways is also operative in mouse embryonic palate mesenchymal cell proliferation (27, 28), supporting our observations. Thus, the cell-specific synthesis of RA in granulosa cells of antral follicles appears to be a key regulator of preovulatory follicular development via cell-specific epigenetic regulations.
Using transcriptome analyses, the abnormal expression of genes involved in RA synthesis and SMAD signaling pathways was reported in granulosa cells of patients with PCOS (29–31). Specifically, higher expression of LH receptor is observed in theca cells and in granulosa cells of PCOS ovaries at early stages of follicular development, which results in premature luteinization of the granulosa cells (32, 33). Ovulation is not induced in patients with PCOS, and the quality of oocytes collected from preovulatory follicles in hormonal stimulation cycle is lower than that in normal patients (34). Some patients with PCOS have a single nucleotide polymorphism in Bmp15 (35); single nucleotide polymorphism in Bpm15 also increases the risk of ovarian hyperstimulation syndrome (36). Ovarian hyperstimulation syndrome may also be related to Lhcgr expression or responses to LH and hCG, suggesting that reduced or altered oocyte functions (i.e., reduced GDF9 or BMP15) may lead to abnormal follicular functions due to incomplete or altered epigenetic regulation of Lhcgr and likely other genes in granulosa cells and cumulus cells. Furthermore, with increasing age, oocyte quality is reduced and follicular development is impaired not only during natural cycles but also in controlled ovarian stimulation as compared with those in younger women (37). Because the secretion levels of oocyte-derived factors are decreased with increasing age (38), one reason why the success rate of assisted reproductive technology is decreased in older patients may be associated with abnormal epigenetic regulation of granulosa cell and cumulus cell genes. DNA methylation status in ovarian somatic cells might provide a novel marker to determine whether matured oocytes are of high developmental competence. To clear this hypothesis, we are trying to comprehensively understand the changes of DNA methylation status, not only Lhcgr but also in the whole genome in granulosa cells during follicular development, and the relationship between the changes and the functions of oocytes.
In conclusion, the expression of Lhcgr in each type of ovarian somatic cell is associated with the level of DNA methylation within the Lhcgr promoter. Demethylation of the Lhcgr promoter region is enhanced by RA, which is specifically synthesized de novo sin granulosa cells in response to FSH and treatment with eCG. In cumulus cells, RA synthesis is suppressed and DNA methylation of the Lhcgr promoter region is maintained by oocyte-secreted factors. Thus, the cell type–specific synthesis of RA and the regulation of DNA demethylation are key factors that control the cell type–specific differentiation of ovarian somatic cells in antral follicles and their ability to respond to the ovulatory LH surge to release matured oocytes.
Supplementary Material
Acknowledgments
Ovine follicle-stimulating hormone was kindly provided by Dr. A. F. Parlow of the National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Disease.
Financial Support: This work was supported in part by the Japan Society for the Promotion of Science (Grant JP 16H05017 to M.S.), the Japan Agency for Medical Research and Development (Grant 16gk0110015h0001 to M.S.), and by National Institutes of Health (Grant HD-076980 to J.S.R.).
Author Contributions: M.S. and J.S.R. designed the study. T.K. performed the experiments, analyzed the data, and wrote the manuscript. J.S.R. and M.S. revised the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 4MP
4-methylpyrazole
- 5azadC
5-aza-2ʹ-deoxycytidine
- ADH
alcohol dehydrogenase
- ALDH
aldehyde dehydrogenase
- COC
cumulus-oocyte complex
- DMEM
Dulbecco’s modified Eagle medium
- eCG
equine chorionic gonadotropin
- FCS
fetal calf serum
- FSH
follicle-stimulating hormone
- GV
germinal vesicle
- hCG
human chorionic gonadotropin
- IBMX
3-isobutyl-1-methylxanthine
- LH
luteinizing hormone
- LHCGR
luteinizing hormone receptor
- mRNA
messenger RNA
- PCOS
polycystic ovary syndrome
- PCR
polymerase chain reaction
- RA
retinoic acid
- RAR
retinoic acid receptor
- RARE
retinoic acid responsive element
- T
testosterone
References
- 1. Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15(6):725–751. [DOI] [PubMed] [Google Scholar]
- 2. Themmen APN, Huhtaniemi IT: Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21(5):551–583. [DOI] [PubMed] [Google Scholar]
- 3. Hillier SG. Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod. 1994;9(2):188–191. [DOI] [PubMed] [Google Scholar]
- 4. Balla A, Danilovich N, Yang Y, Sairam MR. Dynamics of ovarian development in the FORKO immature mouse: structural and functional implications for ovarian reserve. Biol Reprod. 2003;69(4):1281–1293. [DOI] [PubMed] [Google Scholar]
- 5. Minegishi T, Nakamura K, Takakura Y, Miyamoto K, Hasegawa Y, Ibuki Y, Igarashi M. Cloning and sequencing of human LH/hCG receptor cDNA. Biochem Biophys Res Commun. 1990;172(3):1049–1054. [DOI] [PubMed] [Google Scholar]
- 6. Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod. 1997;56(4):976–984. [DOI] [PubMed] [Google Scholar]
- 7. Law NC, Weck J, Kyriss B, Nilson JH, Hunzicker-Dunn M. Lhcgr expression in granulosa cells: roles for PKA-phosphorylated β-catenin, TCF3, and FOXO1. Mol Endocrinol. 2013;27(8):1295–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23(2):141–174. [DOI] [PubMed] [Google Scholar]
- 9. Pal S, Claffey KP, Cohen HT, Mukhopadhyay D. Activation of Sp1-mediated vascular permeability factor/vascular endothelial growth factor transcription requires specific interaction with protein kinase C zeta. J Biol Chem. 1998;273(41):26277–26280. [DOI] [PubMed] [Google Scholar]
- 10. Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal. 2006;18(9):1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawai T, Mihara T, Kawashima I, Fujita Y, Ikeda C, Negishi H, Richards JS, Shimada M. Endogenous acetaldehyde toxicity during antral follicular development in the mouse ovary. Reprod Toxicol. 2012;33(3):322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Selig JI, Troppmann B, Gromoll J. Impact of DNA methylation on the regulation of the luteinizing hormone/choriogonadotropin receptor expression. Exp Clin Endocrinol Diabetes. 2013;121(3). Abstract OP5_28. [Google Scholar]
- 13. Kawai T, Yanaka N, Richards JS, Shimada M. De novo-synthesized retinoic acid in ovarian antral follicles enhances FSH-mediated ovarian follicular cell differentiation and female fertility. Endocrinology. 2016;157(5):2160–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gebhard C, Benner C, Ehrich M, Schwarzfischer L, Schilling E, Klug M, Dietmaier W, Thiede C, Holler E, Andreesen R, Rehli M. General transcription factor binding at CpG islands in normal cells correlates with resistance to de novo DNA methylation in cancer cells. Cancer Res. 2010;70(4):1398–1407. [DOI] [PubMed] [Google Scholar]
- 15. Zhu JQ, Zhu L, Liang XW, Xing FQ, Schatten H, Sun QY. Demethylation of LHR in dehydroepiandrosterone-induced mouse model of polycystic ovary syndrome. Mol Hum Reprod. 2010;16(4):260–266. [DOI] [PubMed] [Google Scholar]
- 16. Schenk T, Stengel S, Zelent A. Unlocking the potential of retinoic acid in anticancer therapy. Br J Cancer. 2014;111(11):2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Angrisano T, Sacchetti S, Natale F, Cerrato A, Pero R, Keller S, Peluso S, Perillo B, Avvedimento VE, Fusco A, Bruni CB, Lembo F, Santoro M, Chiariotti L. Chromatin and DNA methylation dynamics during retinoic acid-induced RET gene transcriptional activation in neuroblastoma cells. Nucleic Acids Res. 2011;39(6):1993–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5(8):1333–1344. [DOI] [PubMed] [Google Scholar]
- 19. Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol. 2007;305(1):300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeppesen JV, Kristensen SG, Nielsen ME, Humaidan P, Dal Canto M, Fadini R, Schmidt KT, Ernst E, Yding Andersen C. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab. 2012;97(8):E1524–E1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129(6):3200–3207. [DOI] [PubMed] [Google Scholar]
- 22. Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15(6):854–866. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Fatima N, Dufau ML. Coordinated changes in DNA methylation and histone modifications regulate silencing/derepression of luteinizing hormone receptor gene transcription. Mol Cell Biol. 2005;25(18):7929–7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sugiura K, Pendola FL, Eppig JJ. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol. 2005;279(1):20–30. [DOI] [PubMed] [Google Scholar]
- 25. Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pangas SA. Bone morphogenetic protein signaling transcription factor (SMAD) function in granulosa cells. Mol Cell Endocrinol. 2012;356(1-2):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakabe M, Kokubo H, Nakajima Y, Saga Y. Ectopic retinoic acid signaling affects outflow tract cushion development through suppression of the myocardial Tbx2-Tgfβ2 pathway. Development. 2012;139(2):385–395. [DOI] [PubMed] [Google Scholar]
- 28. Lu J, Tan L, Li P, Gao H, Fang B, Ye S, Geng Z, Zheng P, Song H. All-trans retinoic acid promotes neural lineage entry by pluripotent embryonic stem cells via multiple pathways. BMC Cell Biol. 2009;10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wood JR, Nelson VL, Ho C, Jansen E, Wang CY, Urbanek M, McAllister JM, Mosselman S, Strauss JF III. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J Biol Chem. 2003;278(29):26380–26390. [DOI] [PubMed] [Google Scholar]
- 30. Wickenheisser JK, Nelson-DeGrave VL, Hendricks KL, Legro RS, Strauss JF III, McAllister JM. Retinoids and retinol differentially regulate steroid biosynthesis in ovarian theca cells isolated from normal cycling women and women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(8):4858–4865. [DOI] [PubMed] [Google Scholar]
- 31. Khalaf M, Morera J, Bourret A, Reznik Y, Denoual C, Herlicoviez M, Mittre H, Benhaim A. BMP system expression in GCs from polycystic ovary syndrome women and the in vitro effects of BMP4, BMP6, and BMP7 on GC steroidogenesis. Eur J Endocrinol. 2013;168(3):437–444. [DOI] [PubMed] [Google Scholar]
- 32. Dumesic DA, Richards JS. Ontogeny of the ovary in polycystic ovary syndrome. Fertil Steril. 2013;100(1):23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanamarlapudi V, Gordon UD, López Bernal A. Luteinizing hormone/chorionic gonadotrophin receptor overexpressed in granulosa cells from polycystic ovary syndrome ovaries is functionally active. Reprod Biomed Online. 2016;32(6):635–641. [DOI] [PubMed] [Google Scholar]
- 34. Dumesic DA, Padmanabhan V, Abbott DH. Polycystic ovary syndrome and oocyte developmental competence. Obstet Gynecol Surv. 2008;63(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wei LN, Huang R, Li LL, Fang C, Li Y, Liang XY. Reduced and delayed expression of GDF9 and BMP15 in ovarian tissues from women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31(11):1483–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abir R, Fisch B. Invited commentary: a single nucleotide polymorphism in BMP15 is associated with high response to controlled ovarian hyperstimulation. Reprod Biomed Online. 2011;23(1):77–80. [DOI] [PubMed] [Google Scholar]
- 37. Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, Grunfeld L. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337(8754):1375–1377. [DOI] [PubMed] [Google Scholar]
- 38. Shen M, Qi C, Kuang YP, Yang Y, Lyu QF, Long H, Yan ZG, Lu YY. Observation of the influences of diosgenin on aging ovarian reserve and function in a mouse model. Eur J Med Res. 2017;22(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.